Sustainable Utilization of Sewage Sludge through the Synthesis of Liquid Fertilizer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. P conversion Methods
2.3. Analytical Methods
2.4. Wet Chemical Methods
2.5. Synthesis Liquid Fertilizer and Plant Availability
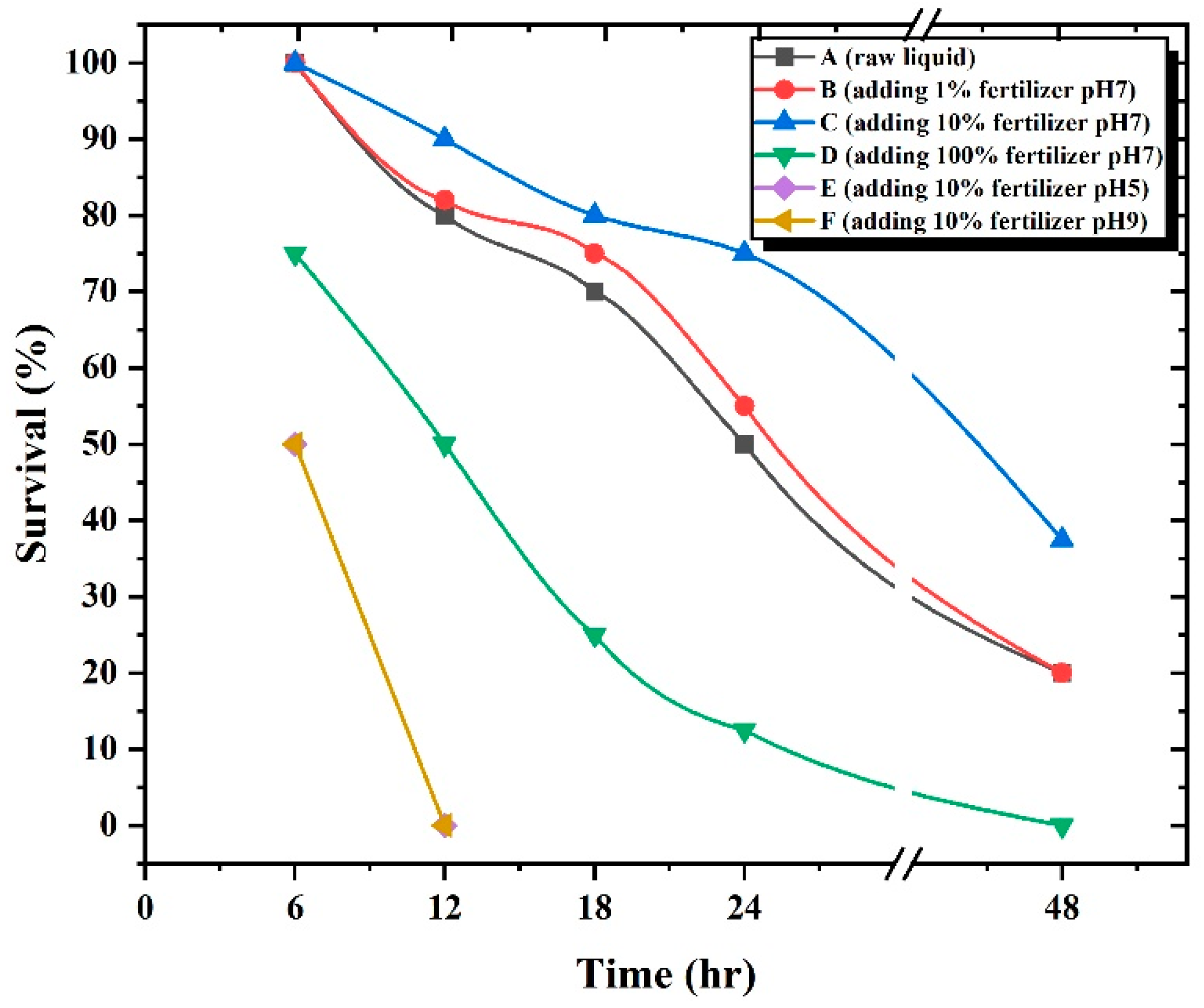
2.6. Liquid Fertilizer Toxicity Test
3. Results
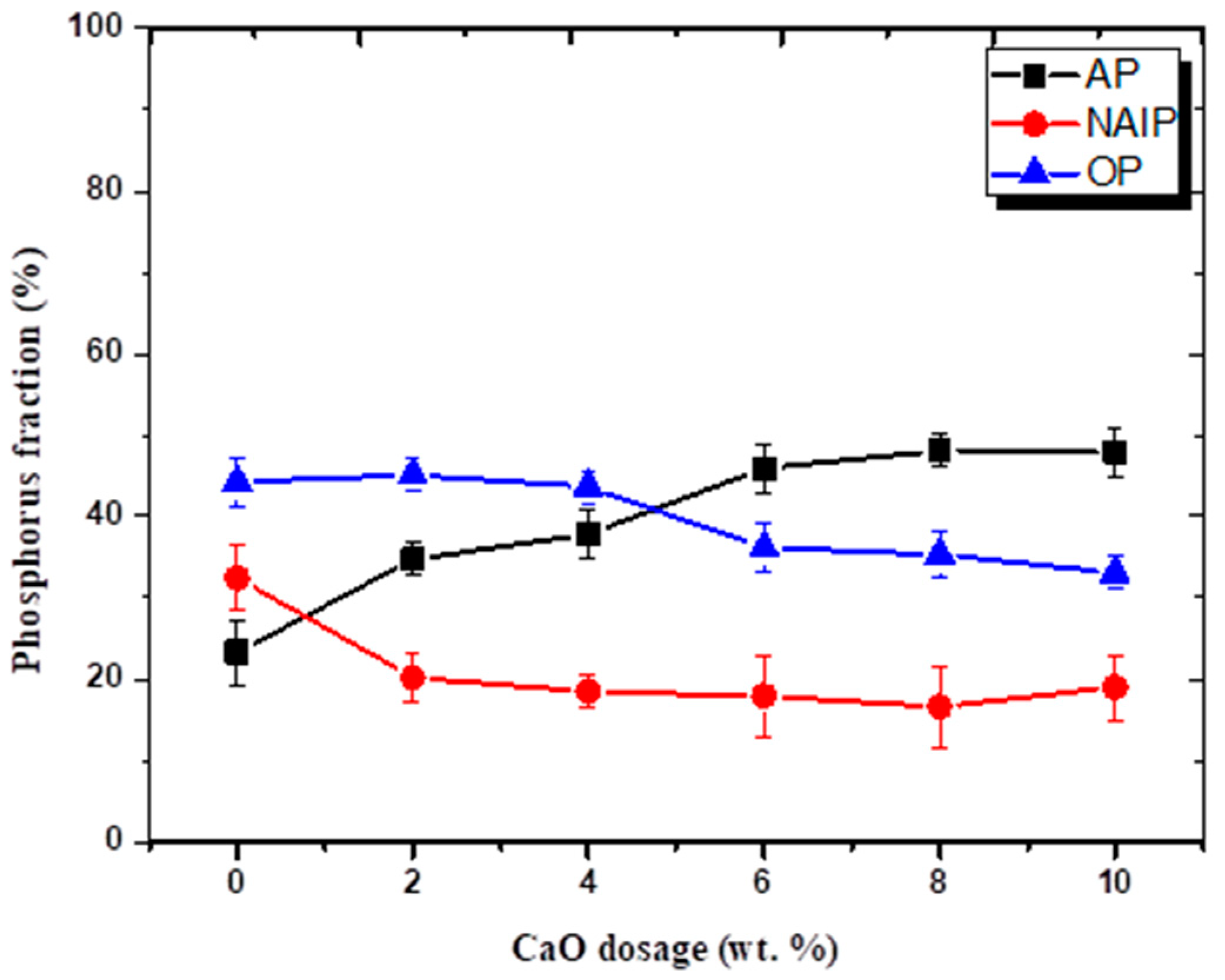
3.1. Effect of CaO Fraction
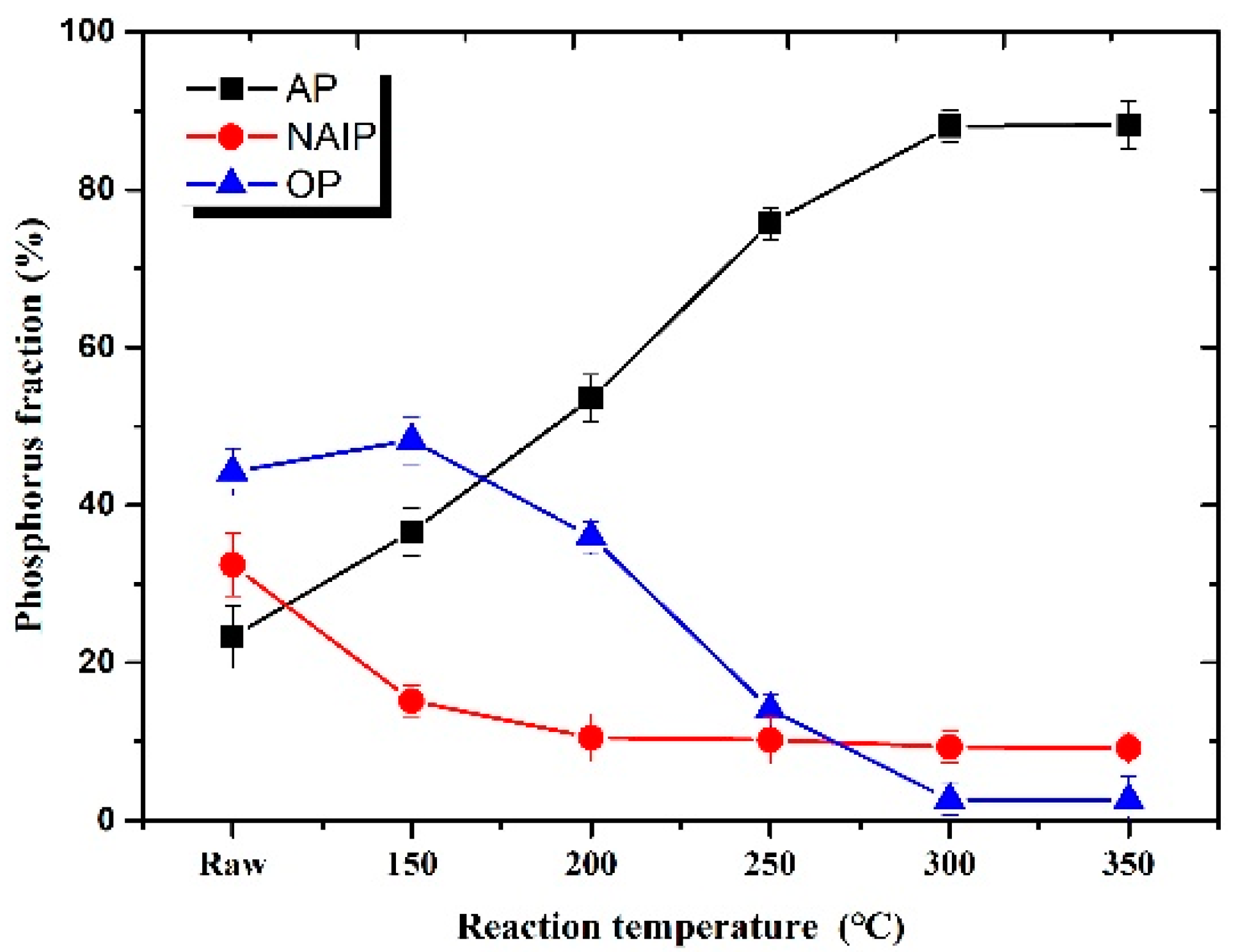
3.2. Effect of Reaction Temperature
3.3. XRD Analysis of Pre- and Post-Conversion SS
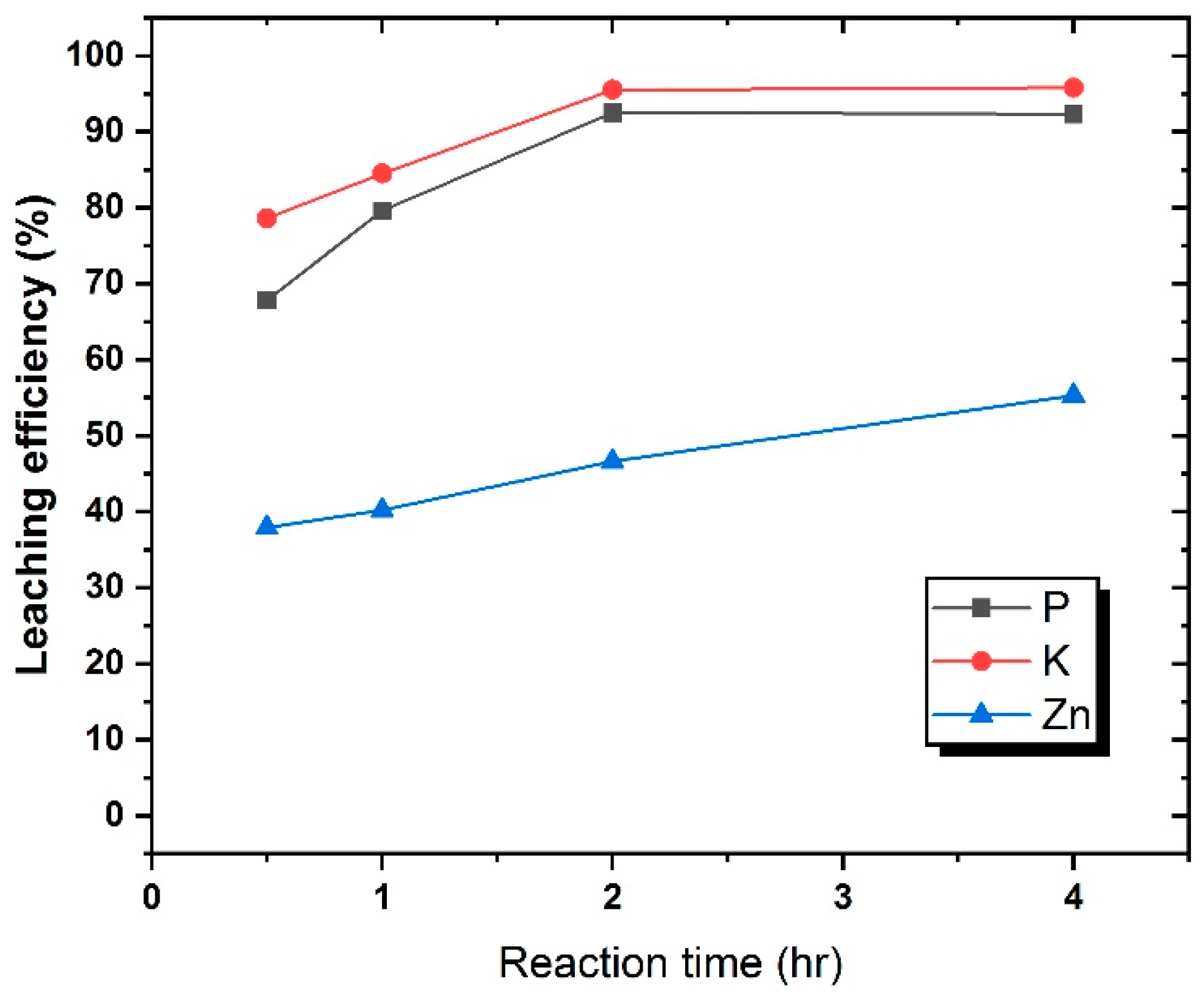
3.4. Leaching of P from Pre- and Post-Conversion SS by Using an Acid and Alkali
3.5. Leaching of Heavy Metals from Post-Conversion SS by Using an Acid and Alkali
3.6. Synthesis of Liquid Fertilizer
3.7. Liquid Fertilizer Toxicity Test with D. magna
4. Discussion
4.1. Discussion on Conversion Results
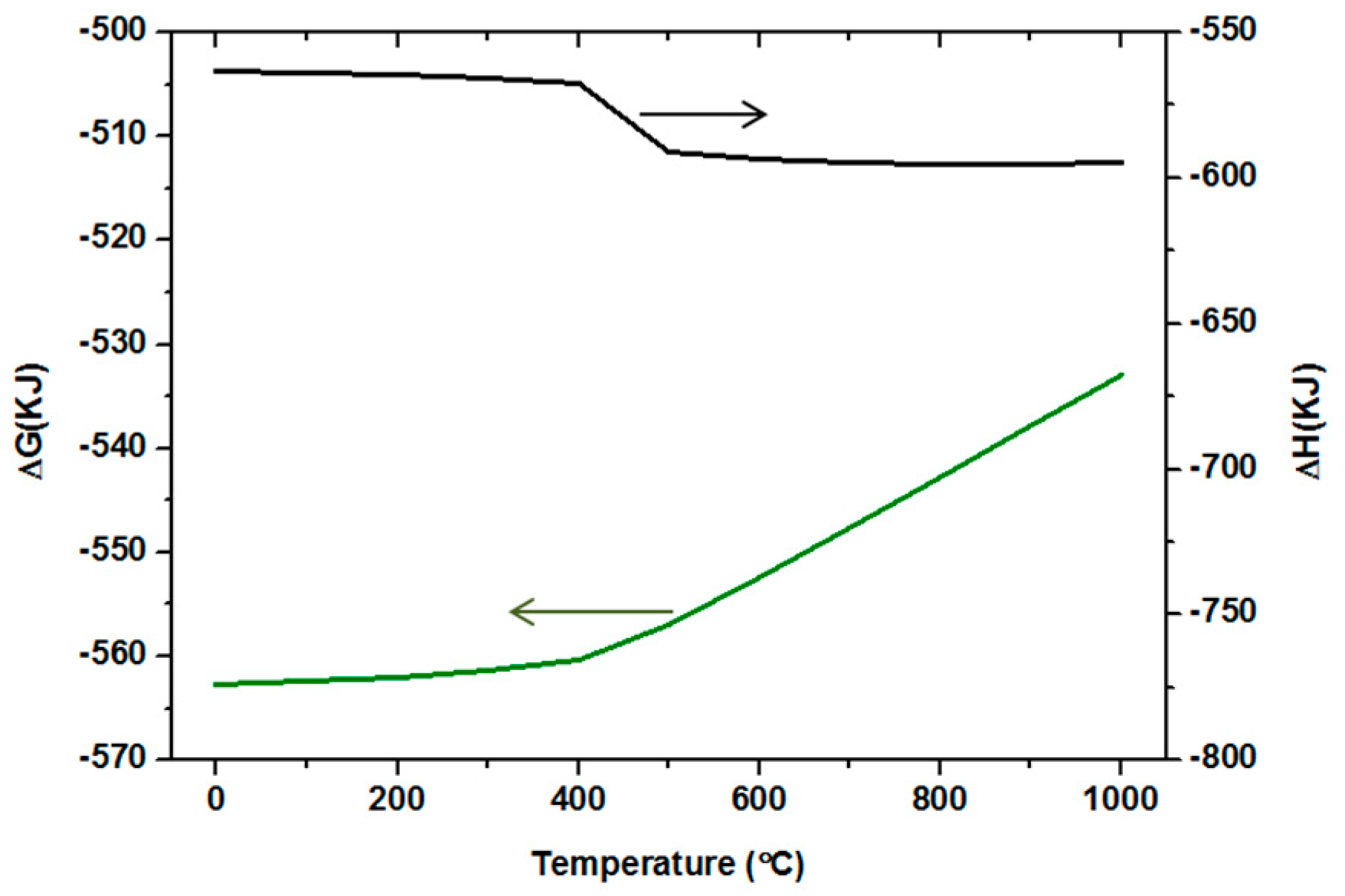
4.2. The Mechanism of Conversion by Addition of CaO
→ 5Ca2P2O7 + 2MgAl2(PO4)28H2O
4.3. Comprehensive Comparison of Combined Organic Acid, Inorganic Acid, and Alkali for Recovery of P and Leaching of Heavy Metals from Pre- or Postconversion SS
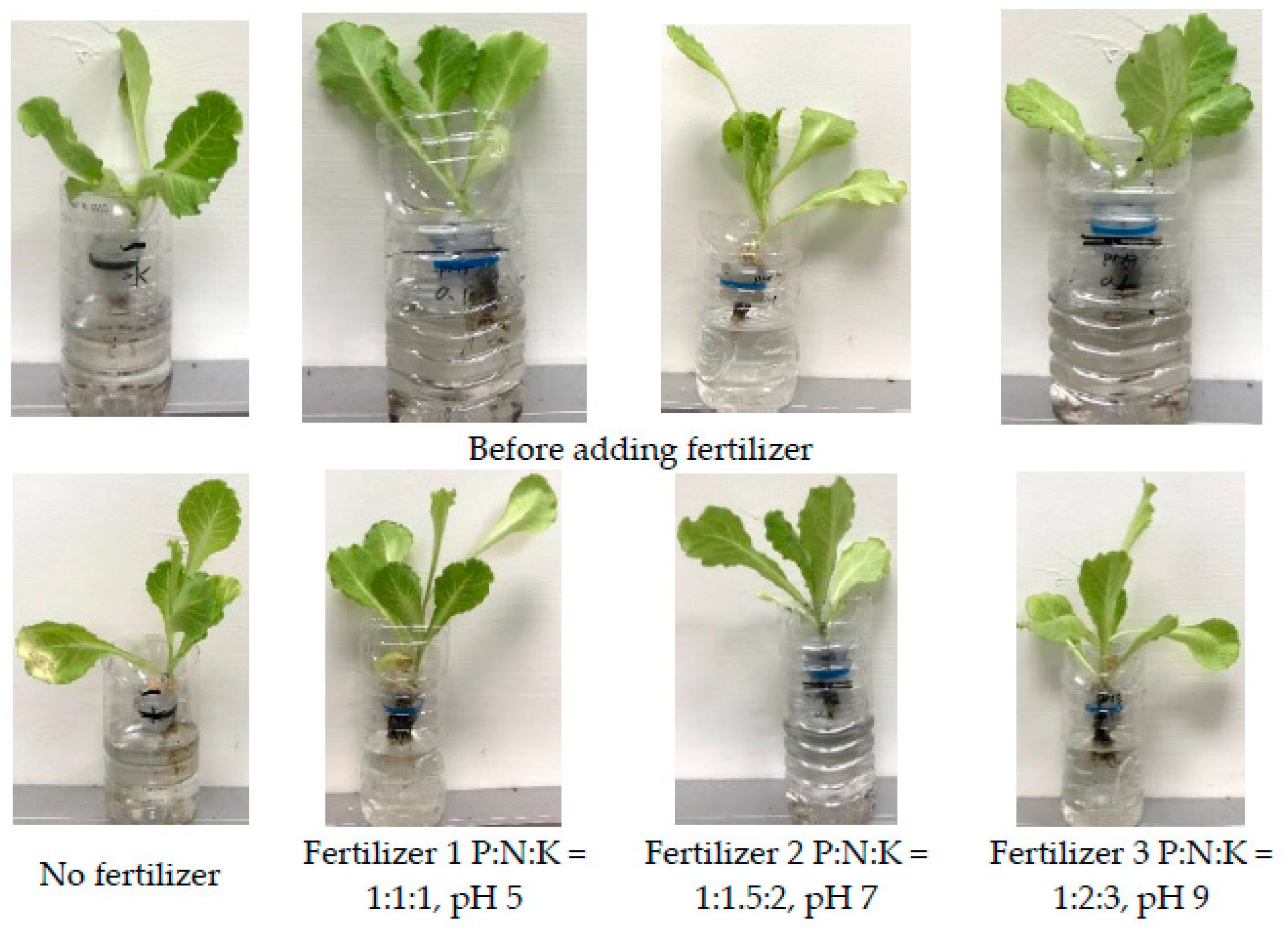
4.4. Application of Synthesis Liquid Fertilizer Grow Plants
4.5. Liquid Fertilizer Toxicity
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Clarke, J.I.; Macbeth, H.; Collinson, P. The growing concentration of world population from 1950 to 2050. In Human Population Dynamics; Pew Research Center: Washington, DC, USA, 2014; pp. 41–64. [Google Scholar]
- Hall, J.E. Alternative Uses for Sewage Sludge. Waste Manag. 1992, 12, 100. [Google Scholar]
- Hus, C.W.; Chen, C.T. Strength development of cement pastes with alkali-activated dehydrated sewage sludge. Constr. Build. Mater. 2020, 255, 119243. [Google Scholar]
- Hoffmann, G.; Schingnitz, D.; Bilitewski, B. Comparing different methods of analysing sewage sludge, dewatered sewage sludge and sewage sludge ash. Desalination 2010, 250, 399–403. [Google Scholar] [CrossRef]
- Moreira, M.J.; Andrea, A.; Feijoo, G. Life Cycle Assessment of Municipal Wastewater and Sewage Sludge Treatment Book; CRC Press: Boca Raton, FL, USA, 2018; pp. 33–48. [Google Scholar]
- Rudolfs, W. Sewage Sludge as Fertilizer. Can. J. Soil Sci. 1972, 52, 270–273. [Google Scholar] [CrossRef]
- Wang, S.; Jin, X.; Zhao, H.; Wu, F. Phosphorus fractions and its release in the sediments from the shallow lakes in the middle and lower reaches of Yangtze River area in China. Colloids Surf. A Physicochem. Eng. Asp. 2006, 273, 109–116. [Google Scholar] [CrossRef]
- Zhang, B.; Fang, F.; Guo, J.; Chen, Y.; Li, Z.; Guo, S. Phosphorus fractions and phosphate sorption-release characteristics relevant to the soil composition of water-level-fluctuating zone of three gorges reservoir. Ecol. Eng. 2012, 40, 153–159. [Google Scholar] [CrossRef]
- Barbosa, S.G.; Peixoto, L.; Meulman, B.; Alves, M.M.; Pereira, M.A. A design of experiments to assess phosphorous removal and crystal properties in struvite precipitation of source separated urine using different Mg sources. Chem. Eng. J. 2016, 298, 146–153. [Google Scholar] [CrossRef] [Green Version]
- Shashvatt, U.; Benoit, J.; Aris, H.; Blaney, L. CO2-assisted phosphorus extraction from poultry litter and selective recovery of struvite and potassium struvite. Water Res. 2018, 143, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; An, J.; Wan, Y.; Du, Q.; Wang, X.; Cheng, X.; Li, N. Phosphorus competition in bioinduced vivianite recovery from wastewater. Environ. Sci. Technol. 2018, 52, 13863–13870. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Li, J.; Li, B.; Zhang, D.; Zhao, N.; Tang, S. Comparison of different K-struvite crystallization processes for simultaneous potassium and phosphate recovery from source-separated urine. Sci. Total Environ. 2019, 651, 787–795. [Google Scholar] [CrossRef] [PubMed]
- Qian, T.T.; Jiang, H. Migration of phosphorus in sewage sludge during different thermal treatment processes. ACS Sust. Chem. Eng. 2014, 2, 1411–1419. [Google Scholar] [CrossRef]
- Li, R.; Zhang, Z.; Li, Y.; Teng, W.; Wang, W.; Yang, T. Transformation of apatite phosphorus and non-apatite inorganic phosphorus during incineration of sewage sludge. Chemosphere 2015, 141, 57–61. [Google Scholar] [CrossRef]
- Li, R.; Teng, W.; Li, Y.; Wang, W.; Cui, R.; Yang, T. Potential recovery of phosphorus during the fluidized bed incineration of sewage sludge. J. Clean. Prod. 2017, 140, 964–970. [Google Scholar] [CrossRef]
- Yu, B.; Luo, J.; Xie, H.; Yang, H.; Chen, S.; Liu, J.; Zhang, R.; Li, Y.Y. Species, fractions, and characterization of phosphorus in sewage sludge: A critical review from the perspective of recovery. Sci. Total Environ. 2021, 786, 147437. [Google Scholar] [CrossRef]
- Adam, C.; Peplinski, B.; Michaelis, M.; Kley, G.; Simon, F.G. Thermochemical treatment of sewage sludge ashes for phosphorus recovery. Waste Manag. 2009, 29, 1122–1128. [Google Scholar] [CrossRef]
- Li, R.; Yin, J.; Wang, W.; Li, Y.; Zhang, Z. Transformation of phosphorus during drying and roasting of sewage sludge. Waste Manag. 2014, 34, 1211–1216. [Google Scholar] [CrossRef]
- Singhal, A.; Tewari, V.K.; Prakash, S. Utilization of treated spent liquor sludge with fly ash in cement and concrete Build. Build Environ. 2008, 43, 991–998. [Google Scholar] [CrossRef]
- Su, Y.; Zhu, W.; Gong, M.; Zhou, H.; Fan, Y.; Amuzu-Sefordzi, B. Interaction between sewage sludge components lignin (phenol) and proteins (alanine) in supercritical water gasification. Int. J. Hydrog. Energy 2015, 40, 9125–9136. [Google Scholar] [CrossRef]
- Chen, F.Y. The Characterization of Hong Kong Sewage Sludge for Its Ash Utilization in Cement Production; HKUST: Hong Kong, China, 2009. [Google Scholar]
- Xu, Y.; Hu, H.; Liu, J.; Luo, J.; Qian, G.; Wang, A. pH dependent phosphorus release from waste activated sludge: Contributions of phosphorus speciation. Chem. Eng. J. 2015, 267, 260–265. [Google Scholar] [CrossRef]
- Shiba, N.C.; Ntuli, F. Extraction and precipitation of phosphorus from sewage sludge. Waste Manag. 2017, 60, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Zhang, C.; Hu, H.; Liu, J.; Duan, T.; Luo, J. Phosphorus and short-chain fatty acids recovery from waste activated sludge by anaerobic fermentation: Effect of acid or alkali pretreatment. Bioresour. Technol. 2017, 240, 192–196. [Google Scholar] [CrossRef]
- Biswas, B.K.; Inoue, K.; Harada, H.; Ohto, K.; Kawakita, H. Leaching of phosphorus from incinerated sewage sludge ash by means of acid extraction followed by adsorption on orange waste gel. J. Environ. Sci. 2009, 21, 1753–1760. [Google Scholar] [CrossRef]
- Franz, M. Phosphate fertilizer from sewage sludge ash (SSA). Waste Manag. 2008, 28, 1809–1818. [Google Scholar] [CrossRef] [PubMed]
- Donatello, S.; Tong, D.; Cheeseman, C.R. Production of technical grade phosphoric acid from incinerator sewage sludge ash (ISSA). Waste Manag. 2010, 30, 1634–1642. [Google Scholar] [CrossRef] [Green Version]
- Xu, H.; He, P.; Gu, W.; Wang, G.; Shao, L. Recovery of phosphorus as struvite from sewage sludge ash. J. Environ. Sci. 2012, 24, 1533–1538. [Google Scholar] [CrossRef]
- Levlin, E. Phosphorus Recovery from Sewage Sludge—Two-Step Leaching Process With Acid and Base. In Proceedings of the Polish-Swedish SIOUSS, Cracow, Poland, 17–18 March 2005; pp. 51–60. [Google Scholar]
- Matsushita, K.; Toyama, H.; Tonouchi, N.; Okamoto-Kainuma, A. Acetic Acid Bacteria: Ecology and Physiology, Book; Springer: Berlin, Germany, 2016. [Google Scholar]
- Rahman, M.M.; Mostofa, M.G.; Rahman, M.A.; Islam, M.R.; Keya, S.S.; Das, A.K.; Miah, M.G.; Kawser, A.Q.M.R.; Ahsan, S.M.; Hashem, A.; et al. Acetic acid: A cost-effective agent for mitigation of seawater-induced salt toxicity in mung bean. Sci. Rep. 2019, 9, 15186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DiPalma, L.; Ferrantelli, P. Copper leaching from a sandy soil: Mechanism and parameters affecting EDTA extraction. J. Hazard. Mater. 2005, 122, 85–90. [Google Scholar] [CrossRef]
- Liu, C.C.; Lin, Y.C. Reclamation of copper-contaminated soil using EDTA or citric acid coupled with dissolved organic matter solution extracted from distillery sludge. Environ. Pollut. 2013, 178, 97–101. [Google Scholar] [CrossRef]
- Barbara, C.M.; Preston, C.M. A comparison of soil extraction procedures for 31P NMR spectroscopy. Soil Sci. 1996, 161, 770–785. [Google Scholar]
- Pardo, P.; Lopez-Sanchez, J.F.; Rauret, G. Relationships between phosphorus fractionation and major components in sediments using the SMT harmonized extraction procedure. Anal. Bioanal. Chem. 2003, 376, 248–254. [Google Scholar] [CrossRef]
- Xie, C.S.; Zhao, J.; Tang, J.; Xu, J.; Lin, X.Y.; Xua, X.H. The phosphorus fractions and alkaline phosphatase activities in sludge. Bioresour. Technol. 2011, 102, 2455–2461. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Albacete, M.; Martin, A.; Cartagena, M.C. Fractionation of phosphorus biowastes: Characterisation and environmental risk. Waste Manag. 2012, 32, 1061–1068. [Google Scholar] [CrossRef] [PubMed]
- Kidd, P.S.; Domínguez-Rodríguez, M.J.; Díez, J.; Monterroso, C. Bioavailability and plant accumulation of heavy metals and phosphorus in agricultural soils amended by long-term application of sewage sludge. Chemosphere 2007, 66, 1458–1467. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Y.; Chen, H.; Xu, B.; Xiang, B.; Chen, Z.; Li, C.; Zeng, G. Influence of sewage sludge-based activated carbon and temperature on the liquefaction of sewage sludge: Yield and composition of bio-oil, immobilization and risk assessment of heavy metals. Bioresour. Technol. 2014, 159, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Aljerf, L.; Choukaife, A.E. Hydroxyapatite and Fluoroapatite Behavior with pH Change. Int. Med. J. 2017, 24, 407–410. [Google Scholar]
- Aljerf, L.; Choukaife, A.E. A Descriptive Study—In Vitro: New Validated Method for Checking HAp and FAp Behaviours. Int. Med. J. 2017, 24, 407–410. [Google Scholar]
- Steckenmesser, D.; Vogel, C.; Adam, C.; Steffens, D. Effect of various types of thermochemical processing of sewage sludges on phosphorus speciation, solubility, and fertilization performance. Waste Manag. 2017, 62, 194–203. [Google Scholar] [CrossRef]
- Karwowska, B. Optimalization of metals ions extraction from industrial wastewater sludge with chelating agents. Arch. Environ. Prot. 2012, 38, 15–21. [Google Scholar] [CrossRef]
- Wang, X.J.; Chen, J.; Yan, X.B.; Wang, X.; Zhang, J.; Huang, J.Y.; Zhao, J.F. Heavy metal chemical extraction from industrial and municipal mixed sludge by ultrasound-assisted citric acid. J. Ind. Eng. Chem. 2015, 27, 368–372. [Google Scholar] [CrossRef]
- Ebert, D. Ecology, Epidemiology, and Evolution of Parasitism in Daphnia, Book; National Center for Biotechnology Information: Bethesda, MD, USA, 2005. [Google Scholar]


| Processing Technology | CaO Addition | Temperature | AP | NAIP | OP | References |
|---|---|---|---|---|---|---|
| Incineration | 0% | 900 °C | 40% | 60% | 0% | [14] |
| 10% | 90% | 10% | 0% | |||
| Fluidized bed | 0% | 900 °C | 40 | 60% | 0% | [15] |
| 10% | 90% | 10% | 0% | |||
| Thermal | 0% | 250 °C | 40% | 50% | 10% | [17] |
| Leaching Solution | Leaching Efficiency of P (%) | Separation Methods of P and Heavy Metals | Removal of Heavy Metals from P-Rich Solution (%) | Reference |
|---|---|---|---|---|
| 1 M H2SO4 | 90 | precipitation | 13 | [23] |
| ion exchange | 74 | |||
| 3 M H2SO4 | 99 | ion exchange + sulfide precipitation | 99 | [26] |
| 0.38 M H2SO4 | 85 | ion exchange | 99 | [27] |
| 0.8 M HCl | 95 | ion exchange | 99 | [28] |
| Content | SD | |
|---|---|---|
| Moisture (%) | 3.7 | 2.1 |
| Residue (%) | 23.4 | 1.6 |
| Combustible (%) | 72.9 | 1.1 |
| OC (%) | 35 | 5 |
| LOC (%) | 70 | 10 |
| HHV (MJ/kg) | 3500 | |
| Fe (g/kg-SS) | 36.3 | 2.4 |
| Si (g/kg-SS) | 32.6 | 1.4 |
| Ca (g/kg-SS) | 10.6 | 0.58 |
| P (g/kg-SS) | 7.64 | 0.7 |
| Al (g/kg-SS) | 6.98 | 0.2 |
| K (g/kg-SS) | 3.91 | 0.15 |
| Mg (g/kg-SS) | 2.11 | 0.44 |
| Zn (g/kg-SS) | 0.0153 | 0.08 |
| Cu (g/kg-SS) | 0.0048 | 0.008 |
| Cr (g/kg-SS) | 0.0015 | 0.005 |
| N (g/kg-SS) | 5.91 | 0.15 |
| Sample | Experimental Conditions | Leaching Efficiency (%) | ||
|---|---|---|---|---|
| NAIP | OP | AP | ||
| Raw SS | 1M acetic acid, at room temperature | 75.6 | 74.3 | 99.9 |
| 1 M acetic acid mix with 5 wt% citric acid, at room temperature | 90.8 | 73.6 | 99.9 | |
| 1 M Sulfuric acid, at 100 °C [22] | 91.2 | 89.2 | 99.9 | |
| 6 M Sodium hydroxide, at 100 °C [23] | 28.1 | 92.4 | 90.5 | |
| Conversion SS | 1 M acetic acid, at room temperature | 76.7 | - | 99.9 |
| 1 M acetic acid mix with 5 wt% citric acid, at room temperature | 90.1 | - | 99.9 | |
| Sample | Experimental Conditions | P | K | Cu | Zn | Cr | pH |
|---|---|---|---|---|---|---|---|
| Content (mg/kg) | |||||||
| Raw SS | 1M acetic acid mix with 5 wt% citric acid | 260.5 ± 1.9 | 36.63 ± 1.1 | ND | 7.65 ± 2.1 | ND | 2.56 |
| 1M Sulfuric acid [22] | 324.4 ± 1.1 | 37.24 ± 0.5 | 4.56 ± 1.1 | 14.5 ± 1.1 | 1.43 ± 2.6 | 0.37 | |
| 6M Sodium hydroxide [23] | 233.2 ± 2.2 | 20.94 ± 5.9 | ND | 7.89 ± 5.1 | 0.36 ± 0.05 | 11.5 | |
| Conversion SS | 1M acetic acid mix with 5 wt% citric acid | 315.5 ± 1.5 | 36.93 ± 0.8 | ND | 7.66 ± 2.1 | ND | 2.56 |
| Taiwanese regulations | - | - | 20 | 160 | 30 | - | |
| EU ECNo 2003/2003 | - | - | 10 | N/A | N/A | - | |
| Cu | Zn | Cr | Pb | Cd | |
|---|---|---|---|---|---|
| mg/kg | |||||
| Fertilizer 1 | 0.18 | 0.212 | 0.003 | ND | ND |
| Fertilizer 2 | ND | 0.046 | ND | ND | ND |
| Fertilizer 3 | ND | ND | ND | ND | ND |
| Taiwanese regulations | - | - | - | 0.3 | 0.2 |
| EU No 420-2011 | 0.1 | 0.2 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.-J.; Wang, L.-P.; Babel, S. Sustainable Utilization of Sewage Sludge through the Synthesis of Liquid Fertilizer. Sustainability 2022, 14, 387. https://doi.org/10.3390/su14010387
Chen Y-J, Wang L-P, Babel S. Sustainable Utilization of Sewage Sludge through the Synthesis of Liquid Fertilizer. Sustainability. 2022; 14(1):387. https://doi.org/10.3390/su14010387
Chicago/Turabian StyleChen, Yan-Jhang, Li-Pang Wang, and Sandhya Babel. 2022. "Sustainable Utilization of Sewage Sludge through the Synthesis of Liquid Fertilizer" Sustainability 14, no. 1: 387. https://doi.org/10.3390/su14010387
APA StyleChen, Y.-J., Wang, L.-P., & Babel, S. (2022). Sustainable Utilization of Sewage Sludge through the Synthesis of Liquid Fertilizer. Sustainability, 14(1), 387. https://doi.org/10.3390/su14010387






