Sustainable Biodiesel Production by Transesterification of Waste Cooking Oil and Recycling of Wastewater Rich in Glycerol as a Feed to Microalgae
Abstract
:1. Introduction
2. Materials and Methods
2.1. Transesterification of Vegetable Oils
2.2. Transesterification of Waste-Cooking Oils
- Study of the reaction rate at lab scale in discontinuous operating mode, to find the main factors influencing kinetics, compute conversion and yields, estimate kinetic parameters, and choose the optimal values of the main factors (reactants ratio, catalyst load, temperature) influencing the process;
- Verification of the quality of products and by-products, analysis of the produced waste and wastewater, identification and test of possible processes to recycle and/or reuse by-products and waste;
- Experimental tests at pilot-scale in discontinuous operating mode, by considering all the other phenomena influencing kinetics, as heat and mass transfer;
- Experimental tests and simulation at pilot-scale in continuous operating mode, to optimize residence times and yields.
2.2.1. Lab-Scale
2.2.2. Pilot-Scale
2.3. Wastewater Treatment by Microalgae
2.4. Instrumentation
2.4.1. Transesterification of Waste Oil
2.4.2. Wastewater Recycling in Microalgae Cultivation
3. Results
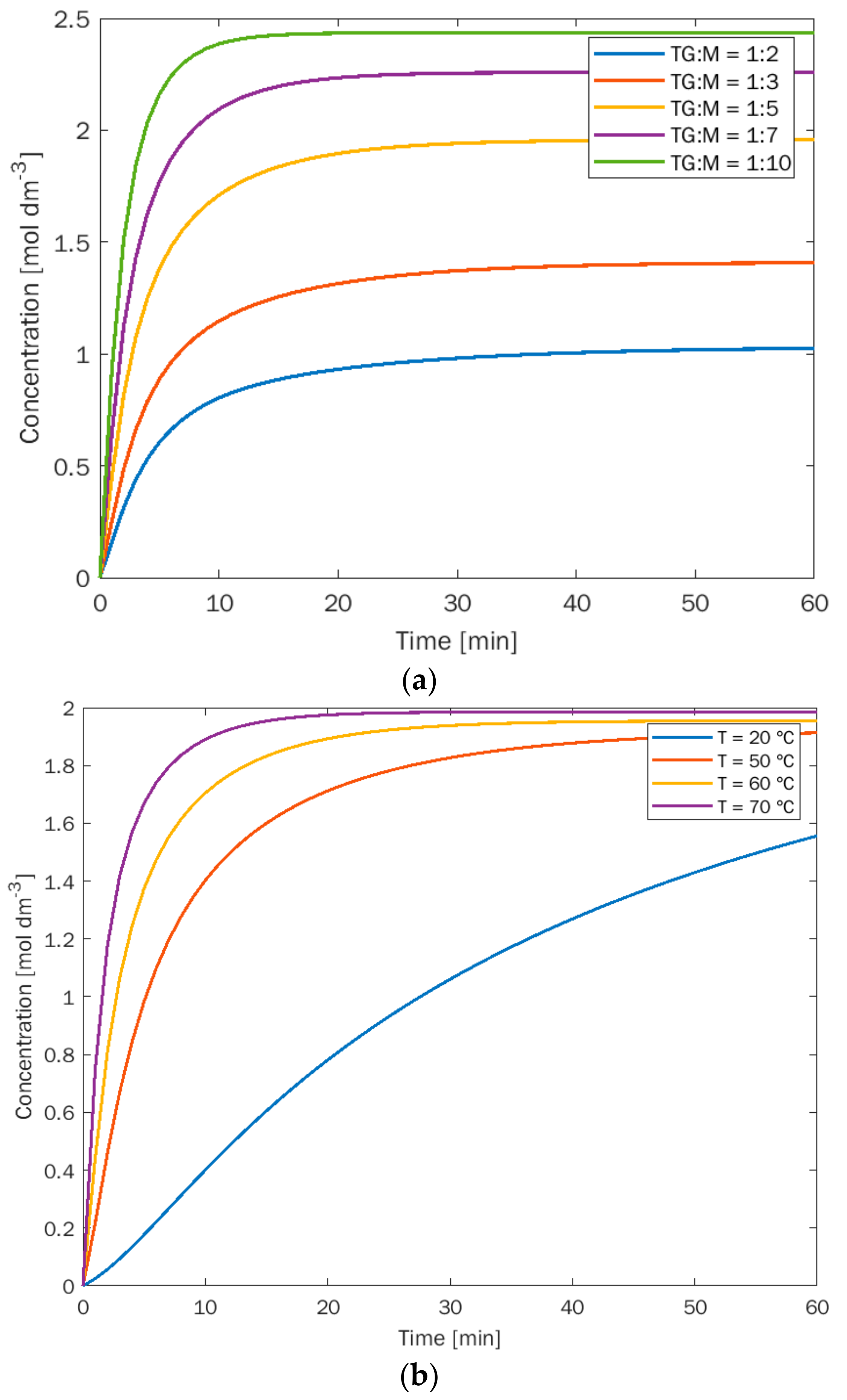
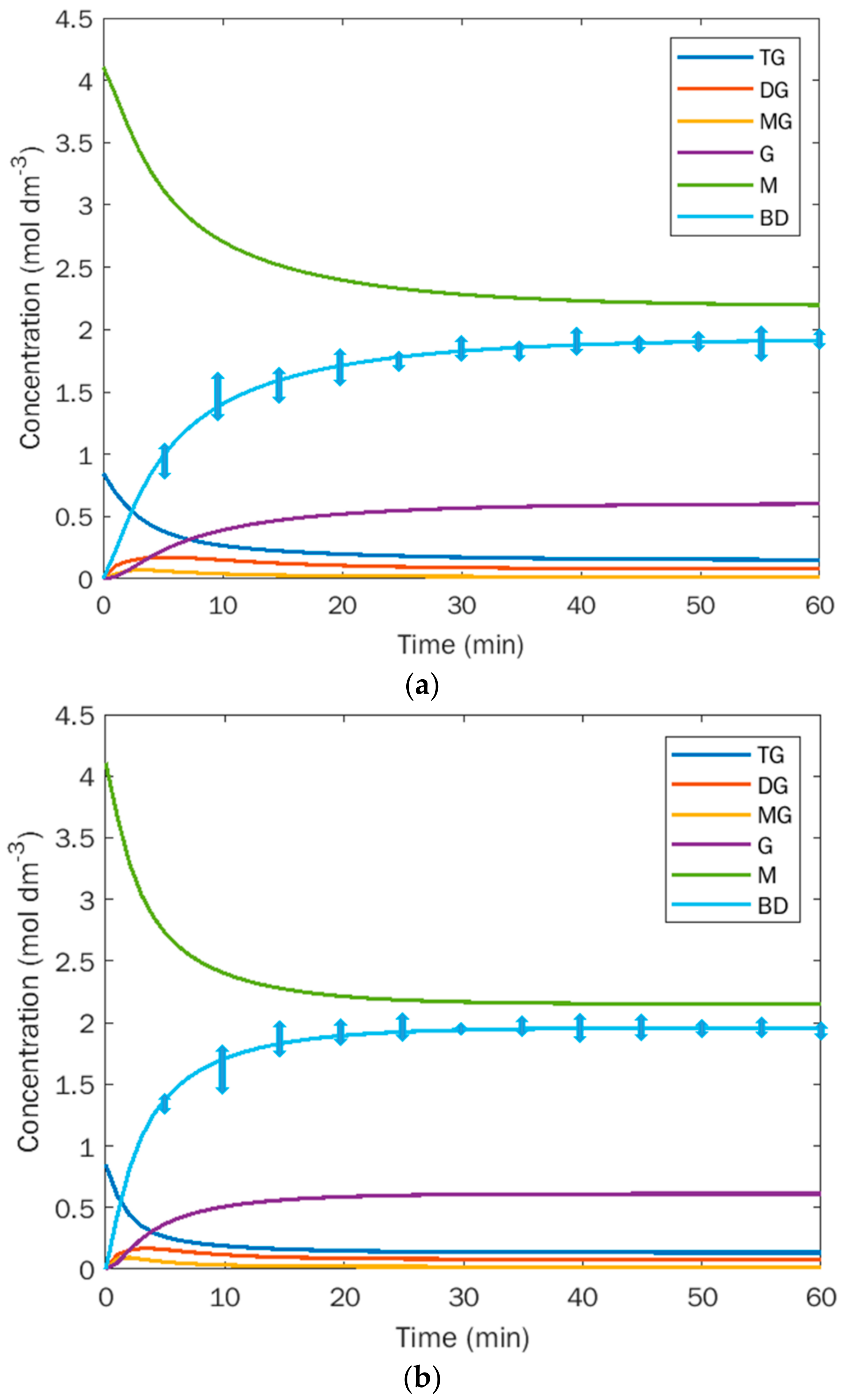
3.1. Experiments at the Lab Scale: Kinetics
- Factor N.1: type of oils, at four levels (waste sunflower oil, uncooked sunflower, olive, and rapeseed oil);
- Factor N.2: molar reactants ratio, at three levels (oil/alcohol = 1:3, 1:5, 1:7);
- Factor N.3: temperature, at four levels (20 °C, 50 °C, 60 °C, 70 °C);
- Factor N.4: catalyst load at three levels (0.3%, 0.6%, 1.2% w/w in methanol).
| Kinetic Constant [dm3 mol−1 min−1] | Activation Energy [J mol−1] | Pre-Exponential Factor [dm3 mol−1 min−1] | |||
|---|---|---|---|---|---|
| 0.050 | 55,000 | 1.9 × 107 | |||
| 0.110 | 41,556 | 3.35 × 105 | |||
| 0.215 | 83,094 | 1.99 × 1012 | |||
| 1.228 | 61,250 | 4.43 × 109 | |||
| 0.242 | 26,866 | 3.77 × 103 | |||
| 0.007 | 44,116 | 5.36 × 104 | |||
3.2. Biodiesel Production at the Pilot Scale
3.3. Biodiesel Quality Analysis
3.3.1. FAME Distribution and Cetane Number
3.3.2. Lower Heating Value
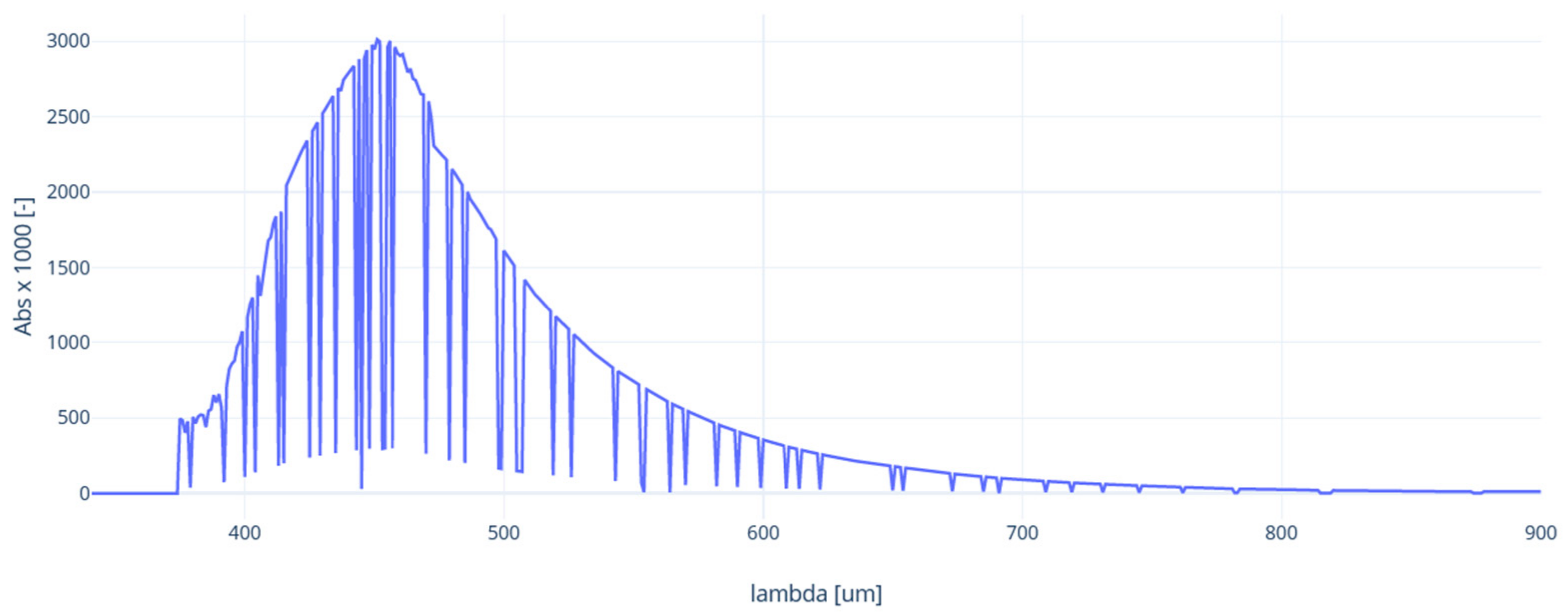
3.3.3. UV-VIS Analysis
3.4. The Recycling of Washing Wastewater
3.5. The Potential of Glycerol, a By-Product of the Production of Bioesters
4. Discussion
4.1. Kinetics of Transesterification
- Similar yields in biodiesel are obtained for the waste and the virgin sunflower oil using for the former an almost doubled quantity of sodium hydroxide catalyst; the probable reason being that in the exhaust oil more free fatty acids are present;
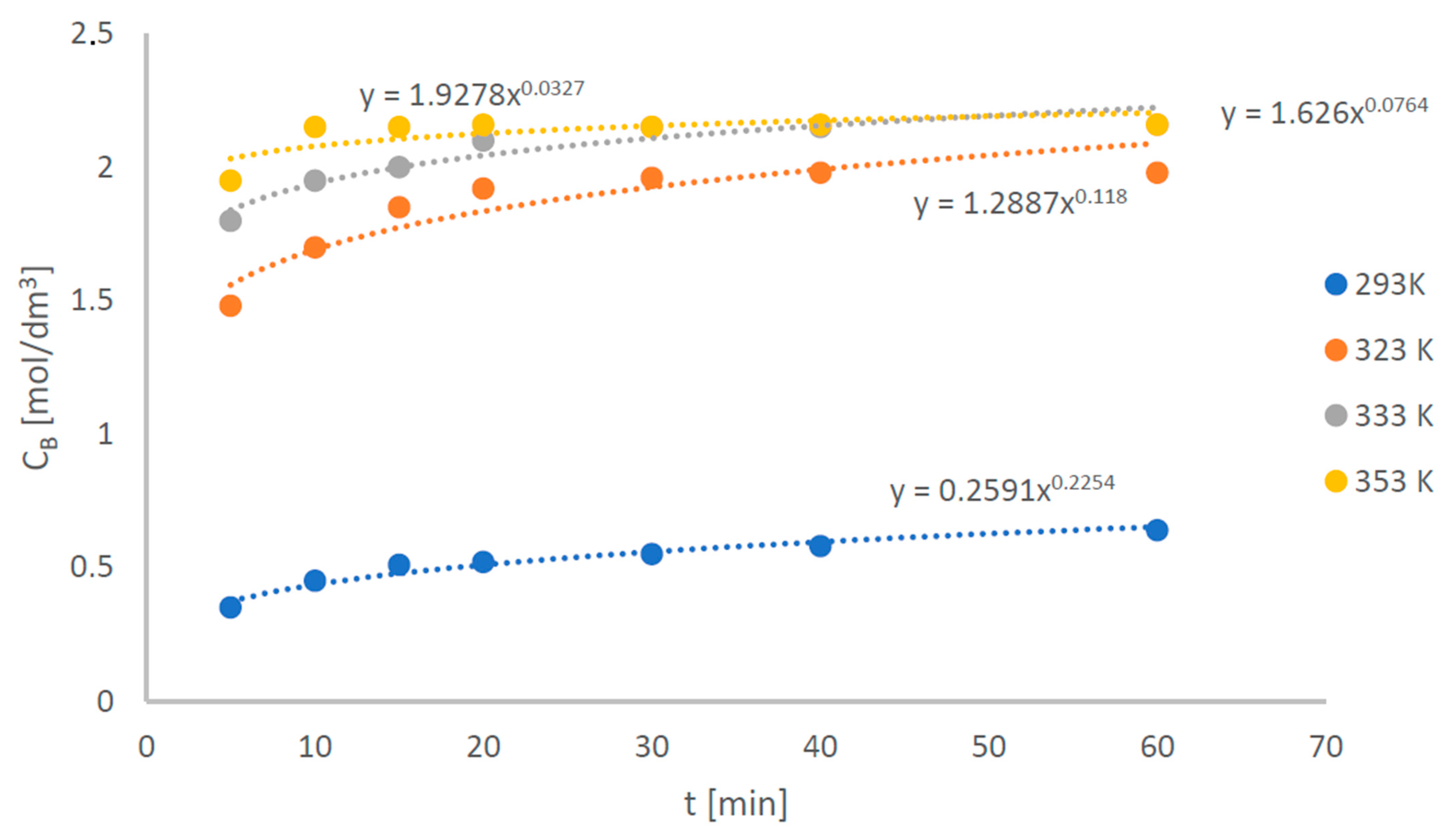
- A pseudo-first-order reaction scheme can be adopted to model the transesterification reaction of different kinds of oils in the chosen temperature ranges, only paying attention that the system is reaction controlled for almost all the reaction time. This condition is assured at = 8000 at lab-scale;
- The values of the activation energy estimated from the experimental data do not differ a lot by changing the type of oil (sunflower, rapeseed, and olive oil) and this is in agreement with the literature, where similar values of activation energy are found for different waste/virgin oils. Conversely, the estimated values of the pre-exponential factor highly depend on oil type and operating conditions.
4.2. Quality of Biodiesel
4.3. Microalgae Using Washing Wastewater
4.4. Feasibility of the Coupled Process
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A

References
- Ritchie, H.; Roser, M. Energy: Fossil Fuels. Available online: https://ourworldindata.org/fossil-fuels (accessed on 1 November 2021).
- Bardi, U. Peak Oil, 20 Years Later: Failed Prediction or Useful Insight? Energy Res. Soc. Sci. 2019, 48, 257–261. [Google Scholar] [CrossRef]
- Welsby, D.; Price, J.; Pye, S.; Ekins, P. Unextractable Fossil Fuels in a 1.5 °C World. Nature 2021, 597, 230–234. [Google Scholar] [CrossRef]
- Varone, A.; Ferrari, M. Power to Liquid and Power to Gas: An Option for the German Energiewende. Renew. Sustain. Energy Rev. 2015, 45, 207–218. [Google Scholar] [CrossRef] [Green Version]
- Araújo, K.; Mahajan, D.; Kerr, R.; da Silva, M. Global Biofuels at the Crossroads: An Overview of Technical, Policy, and Investment Complexities in the Sustainability of Biofuel Development. Agriculture 2017, 7, 32. [Google Scholar] [CrossRef] [Green Version]
- Anastopoulos, G.; Zannikou, Y.; Stournas, S.; Kalligeros, S. Transesterification of Vegetable Oils with Ethanol and Characterization of the Key Fuel Properties of Ethyl Esters. Energies 2009, 2, 362–376. [Google Scholar] [CrossRef]
- Ferella, F.; Mazziotti Di Celso, G.; De Michelis, I.; Stanisci, V.; Vegliò, F. Optimization of the Transesterification Reaction in Biodiesel Production. Fuel 2010, 89, 36–42. [Google Scholar] [CrossRef]
- Ma, F.; Clements, L.D.; Hanna, M.A. Biodiesel Fuel from Animal Fat. Ancillary Studies on Transesterification of Beef Tallow. Ind. Eng. Chem. Res. 1998, 37, 3768–3771. [Google Scholar] [CrossRef] [Green Version]
- Janaun, J.; Ellis, N. Perspectives on Biodiesel as a Sustainable Fuel. Renew. Sustain. Energy Rev. 2010, 14, 1312–1320. [Google Scholar] [CrossRef]
- Pasqualino, J.C.; Montané, D.; Salvadó, J. Synergic Effects of Biodiesel in the Biodegradability of Fossil-Derived Fuels. Biomass Bioenergy 2006, 30, 874–879. [Google Scholar] [CrossRef]
- Sharma, Y.C.; Singh, B.; Upadhyay, S.N. Advancements in Development and Characterization of Biodiesel: A Review. Fuel 2008, 87, 2355–2373. [Google Scholar] [CrossRef]
- Labeckas, G.; Slavinskas, S. The Effect of Rapeseed Oil Methyl Ester on Direct Injection Diesel Engine Performance and Exhaust Emissions. Energy Convers. Manag. 2006, 47, 1954–1967. [Google Scholar] [CrossRef]
- Naylor, R.L.; Higgins, M.M. The Political Economy of Biodiesel in an Era of Low Oil Prices. Renew. Sustain. Energy Rev. 2017, 77, 695–705. [Google Scholar] [CrossRef]
- Lamers, P.; Hamelinck, C.; Junginger, M.; Faaij, A. International Bioenergy Trade—A Review of Past Developments in the Liquid Biofuel Market. Renew. Sustain. Energy Rev. 2011, 15, 2655–2676. [Google Scholar] [CrossRef]
- Smyth, S.J.; Lubieniechi, S. The Food Versus Feed/Fuel Debate. In Plant Bioproducts; Chen, G., Weselake, R., Singer, S., Eds.; Springer: New York, NY, USA, 2018; pp. 219–244. ISBN 978-1-4939-8616-3. [Google Scholar]
- Ayoub, M.; Abdullah, A.Z. Critical Review on the Current Scenario and Significance of Crude Glycerol Resulting from Biodiesel Industry towards More Sustainable Renewable Energy Industry. Renew. Sustain. Energy Rev. 2012, 16, 2671–2686. [Google Scholar] [CrossRef]
- Ramesh kumar, V.; Narendrakumar, G.; Thyagarajan, R.; Melchias, G. A Comparative Analysis of Biodiesel Production and Its Properties from Leptolyngbya Sp. BI-107 and Chlorella Vulgaris under Heat Shock Stress. Biocatal. Agric. Biotechnol. 2018, 16, 502–506. [Google Scholar] [CrossRef]
- Perez-Garcia, O.; Escalante, F.M.E.; de-Bashan, L.E.; Bashan, Y. Heterotrophic Cultures of Microalgae: Metabolism and Potential Products. Water Res. 2011, 45, 11–36. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Zheng, H.; Addy, M.; Anderson, E.; Liu, Y.; Chen, P.; Ruan, R. Cultivation of Chlorella Vulgaris in Wastewater with Waste Glycerol: Strategies for Improving Nutrients Removal and Enhancing Lipid Production. Bioresour. Technol. 2016, 207, 252–261. [Google Scholar] [CrossRef]
- Choi, H.-J.; Yu, S.-W. Influence of Crude Glycerol on the Biomass and Lipid Content of Microalgae. Biotechnol. Biotechnol. Equip. 2015, 29, 506–513. [Google Scholar] [CrossRef]
- Paladino, O.; Neviani, M. Airlift Photo-Bioreactors for Chlorella Vulgaris Cultivation in Closed-Loop Zero Waste Biorefineries. Biomass Bioenergy 2021, 144, 105926. [Google Scholar] [CrossRef]
- Otera, J. Transesterification. Chem. Rev. 1993, 93, 1449–1470. [Google Scholar] [CrossRef]
- Wright, H.J.; Segur, J.B.; Clark, H.V.; Coburn, S.K.; Langdon, E.E.; DuPuis, R.N. A Report on Ester Interchange. Oil Soap 1944, 21, 145–148. [Google Scholar] [CrossRef]
- Freedman, B.; Butterfield, R.O.; Pryde, E.H. Transesterification Kinetics of Soybean Oil 1. J. Am. Oil Chem. Soc. 1986, 63, 1375–1380. [Google Scholar] [CrossRef]
- Schuchardt, U.; Sercheli, R.; Vargas, R.M. Transesterification of Vegetable Oils: A Review. J. Braz. Chem. Soc. 1998, 9, 199–210. [Google Scholar] [CrossRef] [Green Version]
- Freedman, B.; Pryde, E.H.; Mounts, T.L. Variables Affecting the Yields of Fatty Esters from Transesterified Vegetable Oils. J. Am. Oil Chem. Soc. 1984, 61, 1638–1643. [Google Scholar] [CrossRef]
- Schwab, A.W.; Bagby, M.O.; Freedman, B. Preparation and Properties of Diesel Fuels from Vegetable Oils. Fuel 1987, 66, 1372–1378. [Google Scholar] [CrossRef]
- Aksoy, H.A.; Becerik, I.; Karaosmanoğlu, F.; Yatmaz, H.C.; Civelekoğlu, H. Utilization Prospects of Turkish Raisin Seed Oil as an Alternative Engine Fuel. Fuel 1990, 69, 600–603. [Google Scholar] [CrossRef]
- Filip, V.; Zajic, J.; Smidrkal, J. Methanolysis of Rapeseed Oil Triglycerides. Rev. Fr. Corps Gras 1992, 39, 91–94. [Google Scholar]
- Duran, E.A.; Tinoco, R.; Pérez, A.; Berrones, R.; Eapen, D.; Sebastián, P.J. A Conparative Study of Biodiesel Purification with Magnesium Silicate and Water. J. New Mater. Electrochem. Syst. 2014, 17, 105–111. [Google Scholar] [CrossRef] [Green Version]
- Saengprachum, N.; Pengprecha, S.; Poothongkam, J. Glycerine Removal in Biodiesel Purification Process by Adsorbent from Rice Husk. Int. J. Sci. Eng. Technol. 2013, 2, 474–478. [Google Scholar]
- Sims, B. Finding the Right Purification Approach. Trends in Biodiesel Washing and Polishing. Available online: http://www.biodieselmagazine.com/articles/7661/finding-the-right-purification-approach (accessed on 7 July 2021).
- Homer, I.; Hunter, E. Comparison of Different Artisan Methods to Obtaining Biodiesel. Int. J. Sci. Environ. Technol. 2014, 3, 33–47. [Google Scholar]
- Paladino, O.; Fissore, F.; Neviani, M. A Low-Cost Monitoring System and Operating Database for Quality Control in Small Food Processing Industry. J. Sens. Actuator Netw. 2019, 8, 52. [Google Scholar] [CrossRef] [Green Version]
- Paladino, O.; Neviani, M. Scale-up of Photo-Bioreactors for Microalgae Cultivation by π-Theorem. Biochem. Eng. J. 2020, 153, 107398. [Google Scholar] [CrossRef]
- Noureddini, H.; Zhu, D. Kinetics of Transesterification of Soybean Oil. J. Am. Oil Chem. Soc. 1997, 74, 1457–1463. [Google Scholar] [CrossRef]
- Reyero, I.; Arzamendi, G.; Zabala, S.; Gandía, L.M. Kinetics of the NaOH-Catalyzed Transesterification of Sunflower Oil with Ethanol to Produce Biodiesel. Fuel Process. Technol. 2015, 129, 147–155. [Google Scholar] [CrossRef]
- Vicente, G.; Martínez, M.; Aracil, J.; Esteban, A. Kinetics of Sunflower Oil Methanolysis. Ind. Eng. Chem. Res. 2005, 44, 5447–5454. [Google Scholar] [CrossRef]
- McCurry, J.D.; Wang, C.-X.; Zone, W.F. Analysis of Glycerin and Glycerides in Biodiesel (B100) Using ASTM D6584 and EN14105. 2007. Available online: citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.468.9267&rep=rep1&type=pdf (accessed on 1 November 2021).
- Gopinath, A.; Puhan, S.; Nagarajan, G. Effect of Biodiesel Structural Configuration on Its Ignition Quality. Int. J. Energy Environ. 2010, 1, 295–306. [Google Scholar]
- Knothe, G. Dependence of Biodiesel Fuel Properties on the Structure of Fatty Acid Alkyl Esters. Fuel Process. Technol. 2005, 86, 1059–1070. [Google Scholar] [CrossRef]
- Murphy, M.; Taylor, J.; McCormick, R. Compendium of Experimental Cetane Number Data. In National Renewable Energy Laboratory; US Department of Energy: Columbus, OH, USA, 2004; NREL/SR-540-36805. [Google Scholar]
- Paladino, O.; Neviani, M. A Closed Loop Biowaste to Biofuel Integrated Process Fed with Waste Frying Oil, Organic Waste and Algal Biomass: Feasibility at Pilot Scale. Renew. Energy 2018, 124, 61–74. [Google Scholar] [CrossRef]
- Bischoff, H.W.; Bold, H.C. Phycological Studies IV. Some Soil Algae from Enchanted Rock and Related Algal Species; University of Texas Publication N. 6318: Austin, TX, USA, 1963. [Google Scholar]
- Stelmachowski, M. Utilization of Glycerol, a by-Product of the Transestrification Process of Vegetable Oils: A Review. Ecol. Chem. Eng. S 2011, 18, 9–30. [Google Scholar]
- Valliyappan, T.; Bakhshi, N.N.; Dalai, A.K. Pyrolysis of Glycerol for the Production of Hydrogen or Syn Gas. Bioresour. Technol. 2008, 99, 4476–4483. [Google Scholar] [CrossRef]
- Chaudhari, S.; Bhakshi, N. Steam Gasification of Chars and Bio-Oil; Report to Bioenergy Development Program Renewable Energy Branch; Energy, Mines and Resources: Ottawa, ON, Canada, 2002; pp. 396–436. [Google Scholar]
- Fernández, Y.; Arenillas, A.; Díez, M.A.; Pis, J.J.; Menéndez, J.A. Pyrolysis of Glycerol over Activated Carbons for Syngas Production. J. Anal. Appl. Pyrolysis 2009, 84, 145–150. [Google Scholar] [CrossRef]
- Douette, A.M.D.; Turn, S.Q.; Wang, W.; Keffer, V.I. Experimental Investigation of Hydrogen Production from Glycerin Reforming. Energy Fuels 2007, 21, 3499–3504. [Google Scholar] [CrossRef]
- Adhikari, S.; Fernando, S.; Gwaltney, S.R.; Filip To, S.D.; Mark Bricka, R.; Steele, P.H.; Haryanto, A. A Thermodynamic Analysis of Hydrogen Production by Steam Reforming of Glycerol. Int. J. Hydrogen Energy 2007, 32, 2875–2880. [Google Scholar] [CrossRef]
- Birla, A.; Singh, B.; Upadhyay, S.N.; Sharma, Y.C. Kinetics Studies of Synthesis of Biodiesel from Waste Frying Oil Using a Heterogeneous Catalyst Derived from Snail Shell. Bioresour. Technol. 2012, 106, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Bajpai, D.; Tyagi, V.K. Biodiesel: Source, Production, Composition, Properties and Its Benefits. J. Oleo Sci. 2006, 55, 487–502. [Google Scholar] [CrossRef] [Green Version]
- John, M.; Abdullah, M.O.; Hua, T.Y.; Nolasco-Hipólito, C. Techno-Economical and Energy Analysis of Sunflower Oil Biodiesel Synthesis Assisted with Waste Ginger Leaves Derived Catalysts. Renew. Energy 2021, 168, 815–828. [Google Scholar] [CrossRef]
- Naik, B.D.; Meivelu, U. Experimental Studies on Sodium Methoxide Supported Bentonite Catalyst for Biodiesel Preparation from Waste Sunflower Oil. Environ. Prog. Sustain. Energy 2020, 39, e13390. [Google Scholar] [CrossRef]
- Fazil Khan, M.; Garg, A.; Jain, S.; Dwivedi, G.; Nath Verma, T. Optimization of Low-Temperature Transesterification of Low FFA Blend of Sunflower Oil and Algae Oil. Fuel 2020, 279, 118459. [Google Scholar] [CrossRef]
- Sayed, M.R.; Abukhadra, M.R.; Abdelkader Ahmed, S.; Shaban, M.; Javed, U.; Betiha, M.A.; Shim, J.-J.; Rabie, A.M. Synthesis of Advanced MgAl-LDH Based Geopolymer as a Potential Catalyst in the Conversion of Waste Sunflower Oil into Biodiesel: Response Surface Studies. Fuel 2020, 282, 118865. [Google Scholar] [CrossRef]

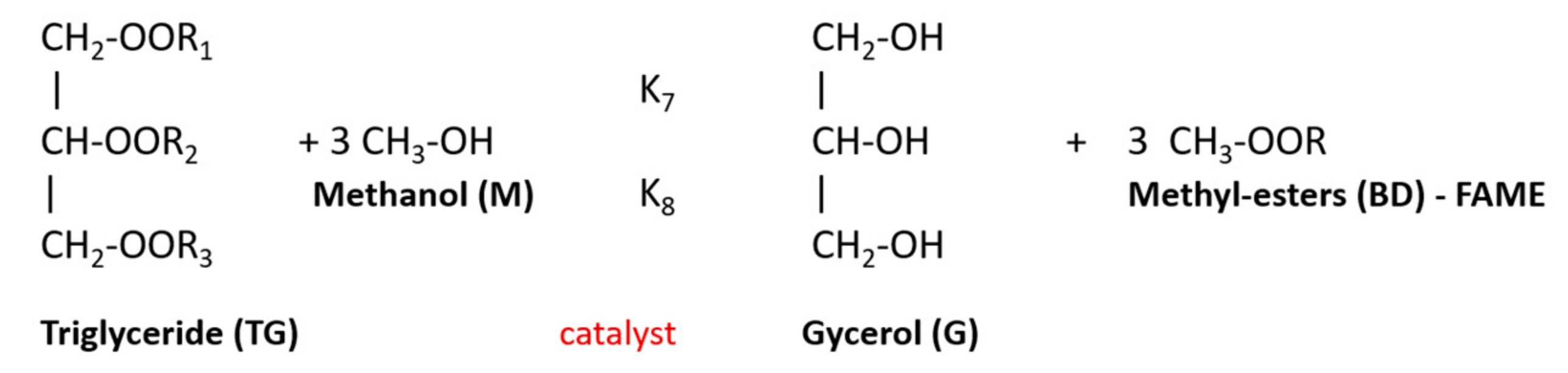


| Impurity | Effect |
|---|---|
| Water | Oxidation, corrosion, and bacteriological growth (filter blockage). |
| Glycerol | Deposits in the injectors (carbon residue), high viscosity, crystallization, settling problems. |
| Methanol | Low values of density and viscosity, low flash point (transport, storage, and usage problems), corrosion of Al and Zn. |
| Soap | Deposits in the injectors (carbon residue), filter blockage (sulfated ashes), engine weakening. |
| TG:M | 5 min | 10 min | 15 min | 20 min | 30 min | 40 min | 60 min |
|---|---|---|---|---|---|---|---|
| 1:3 | 0.35 | 0.45 | 0.51 | 0.52 | 0.55 | 0.58 | 0.64 |
| 1:5 | 1.50 | 1.72 | 1.85 | 1.92 | 1.97 | 1.98 | 2.01 |
| 1:7 | 1.80 | 1.95 | 2.00 | 2.10 | 2.15 | 2.15 | 2.16 |
| 5 min | 10 min | 15 min | 20 min | 30 min | 40 min | 60 min | |
|---|---|---|---|---|---|---|---|
| 20 °C | 0.15 | 0.21 | 0.35 | 0.50 | 0.65 | 0.80 | 1.15 |
| 50 °C | 1.35 | 1.60 | 1.80 | 1.88 | 1.92 | 1.98 | 1.98 |
| 70 °C | 1.90 | 2.08 | 2.10 | 2.15 | 2.15 | 2.16 | 2.16 |
| Waste Oil [kg] | Methanol [kg] | NaOH [kg] | Resid. Time in DSTRs [min] | Produced Biodiesel [kg] | Crude Glycerol [kg] |
| 11 | 2 | 0.12 | 50 | 8.8–9 | 1.7–1.9 |
| Wastewater to Microalgae [L] | Residual Glycerol [g L−1] | Residual Methanol [g L−1] | Residual NaOH [g L−1] | Soaps [g L−1] | Salts of Fatty Acids [g L−1] |
| 1.2 | 4–12 | 1–2 | 3 | 1 | 2 |
| Inlet Waste Oil [kg h−1] | Inlet Methanol [kg h−1] | Inlet NaOH [kg h−1] | Mean Resid. Time in CSTRs [min] | Inlet Oil Flow Rate [L h−1] |
| 11 | 2 | 0.12 | 60 | 0.25 |
| Produced Biodiesel [kg h−1] | Crude Glycerol [kg h−1] | Wastewater to Microalgae [L h−1] | Waste Oil Conversion 1st unit | Waste Oil Conversion 2nd unit |
| 8.6–8.9 | 1.7–1.8 | 1–2 | 0.54 | 0.78 |
| FAME Common Name | % Total | Average CN |
|---|---|---|
| Waste sunflower oil | ||
| Linoleic Acid (di-unsaturated 9–12) | 56.5–56.6% | 39.0 ± 0.2 |
| Oleic Acid (unsaturated) | 28.2–28.4% | 56.6 ± 0.2 |
| Palmitic Acid (saturated) | 7.4–7.6% | 76.6 ± 0.3 |
| Stearic Acid (saturated) | 4.5–4.6% | 85.9 ± 0.3 |
| Tricosanoic Acid (saturated) | 1.0–1.2% | 71.6 ± 0.2 |
| Total Cetane number | 47.9 ± 0.1 | |
| Virgin sunflower oil | ||
| Linoleic Acid (di-unsaturated 9–12) | 58.3–58.5% | 39.2 ± 0.1 |
| Oleic Acid (unsaturated) | 27.1–27.3% | 56.6 ± 0.1 |
| Palmitic Acid (saturated) | 7.2–7.4% | 76.6 ± 0.2 |
| Stearic Acid (saturated) | 4.4–4.6% | 85.9 ± 0.3 |
| Behenic acid (saturated) | 1.0–1-1% | 74.1 ± 0.1 |
| Total Cetane number | 47.8 ± 0.2 | |
| Olive oil | ||
| Oleic Acid (unsaturated) | 80.7–80.9% | 56.6 ± 0.2 |
| Palmitic Acid (saturated) | 12.3–12.4% | 76.6 ± 0.2 |
| Stearic Acid (saturated) | 3.6–3.8% | 85.9 ± 0.3 |
| Total Cetane number | 58.4 ± 0.2 | |
| Rapeseed oil | ||
| Oleic Acid (unsaturated) | 78.4–78.5% | 56.6 ± 0.2 |
| Linoleic Acid (di-unsaturated 9–12) | 8.4–8.6% | 39.2 ± 0.3 |
| Palmitic Acid (saturated) | 5.0–5.2% | 76.6 ± 0.3 |
| Stearic Acid (saturated) | 2.1–2-2% | 85.9 ± 0.2 |
| Eicosanoic acid (unsaturated) | 1.7–1.8% | 52.5 ± 0.2 |
| Total Cetane number | 53.4 ± 0.2 | |
| Biodiesel Source | LHV [kJ kg−1] |
|---|---|
| Olive oil | 36,660 ± 120 |
| Rapeseed oil | 36,992 ± 110 |
| Virgin sunflower oil | 36,079 ± 110 |
| Waste sunflower oil | 36,557 ± 100 |
| Operating Conditions | [d−1] | [g L−1] | Average Biomass * [g L−1 d−1] | R2 |
|---|---|---|---|---|
| Outdoor, Fed-batch | Min: 0.382 ± 0.218 Max: 0.425 ± 0.253 | Min: 1.629 ± 0.073 Max: 1.775 ± 0.082 | 233 254 | 0.742 0.743 |
| Outdoor, airlift | Min: 0.273 ± 0.066 Max: 0.326 ± 0.043 | Min: 1.493 ± 0.043 Max: 1.596 ± 0.050 | 249 266 | 0.982 0.953 |
| Light Conditions | Regime | Recycled Wastewater | Required Sparging | Recycled Flue-Gas | Extraction + Feeding | Brought to Volume |
|---|---|---|---|---|---|---|
| Summer daylight | Churn flow Reriser = 11,000 | 28.8 L d−1 | 20 m3 h−1 × 12 | 90 m3 h−1 | Day 3, 6 = 6.2 L × 12 | Day 1, 2, 4, 5 |
| Winter daylight | Churn flow Reriser = 10,300 | 28.8 L d−1 | 20 m3 h−1 × 12 | 90 m3 h−1 | Day 6 = 9.1 L × 12 | Day 1, 2, 3, 4, 5 |
| Night conditions | Bubble flow Reriser = 5200 | 28.8 L d−1 | 3–4 m3 h−1 × 12 air | Storage tank | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paladino, O.; Neviani, M. Sustainable Biodiesel Production by Transesterification of Waste Cooking Oil and Recycling of Wastewater Rich in Glycerol as a Feed to Microalgae. Sustainability 2022, 14, 273. https://doi.org/10.3390/su14010273
Paladino O, Neviani M. Sustainable Biodiesel Production by Transesterification of Waste Cooking Oil and Recycling of Wastewater Rich in Glycerol as a Feed to Microalgae. Sustainability. 2022; 14(1):273. https://doi.org/10.3390/su14010273
Chicago/Turabian StylePaladino, Ombretta, and Matteo Neviani. 2022. "Sustainable Biodiesel Production by Transesterification of Waste Cooking Oil and Recycling of Wastewater Rich in Glycerol as a Feed to Microalgae" Sustainability 14, no. 1: 273. https://doi.org/10.3390/su14010273
APA StylePaladino, O., & Neviani, M. (2022). Sustainable Biodiesel Production by Transesterification of Waste Cooking Oil and Recycling of Wastewater Rich in Glycerol as a Feed to Microalgae. Sustainability, 14(1), 273. https://doi.org/10.3390/su14010273







