Application of Full Factorial Design Method for Optimization of Heavy Metal Release from Lead Smelting Slag
Abstract
1. Introduction
2. Materials and Methods
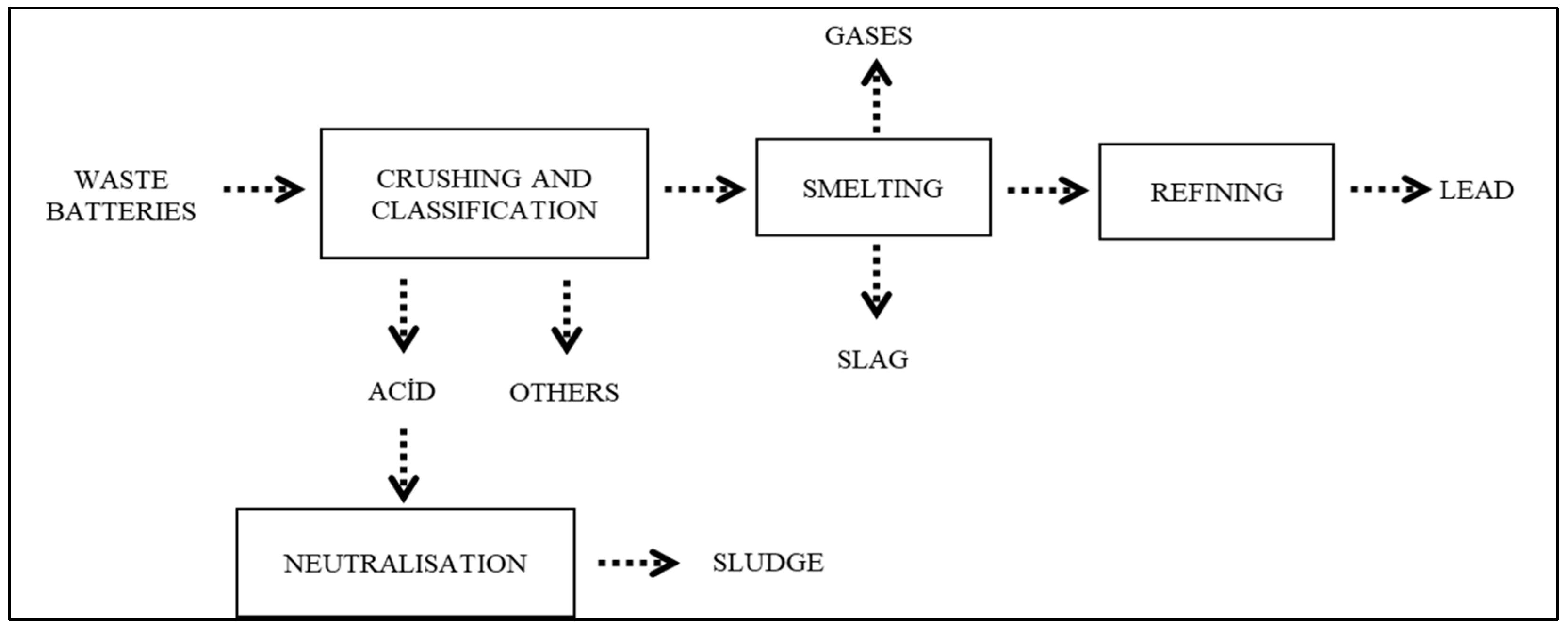
2.1. Materials
Characterization of Chemical Properties of Lead Smelting Slag
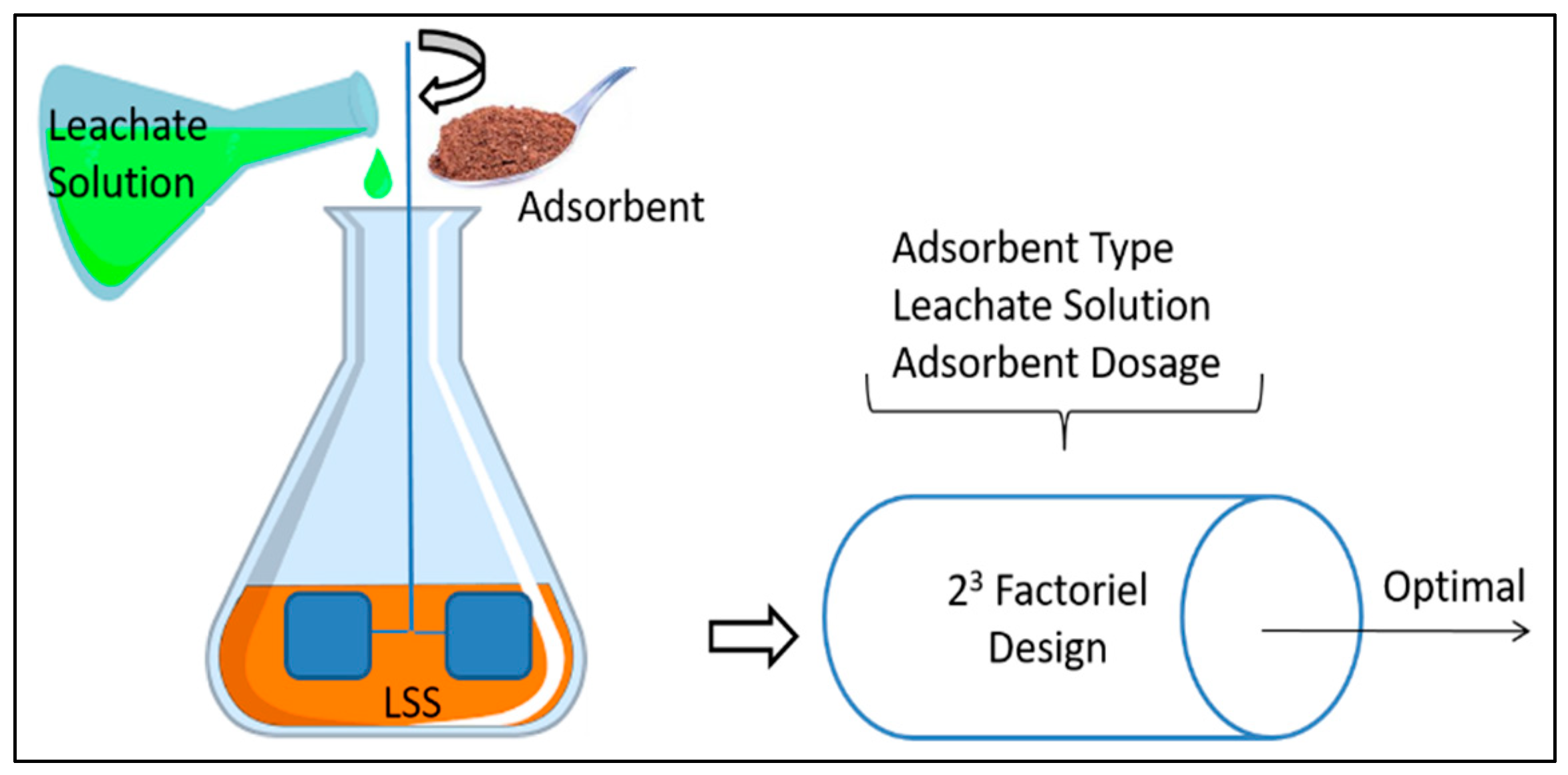
2.2. Experimental Procedure
2.3. Factorial Design
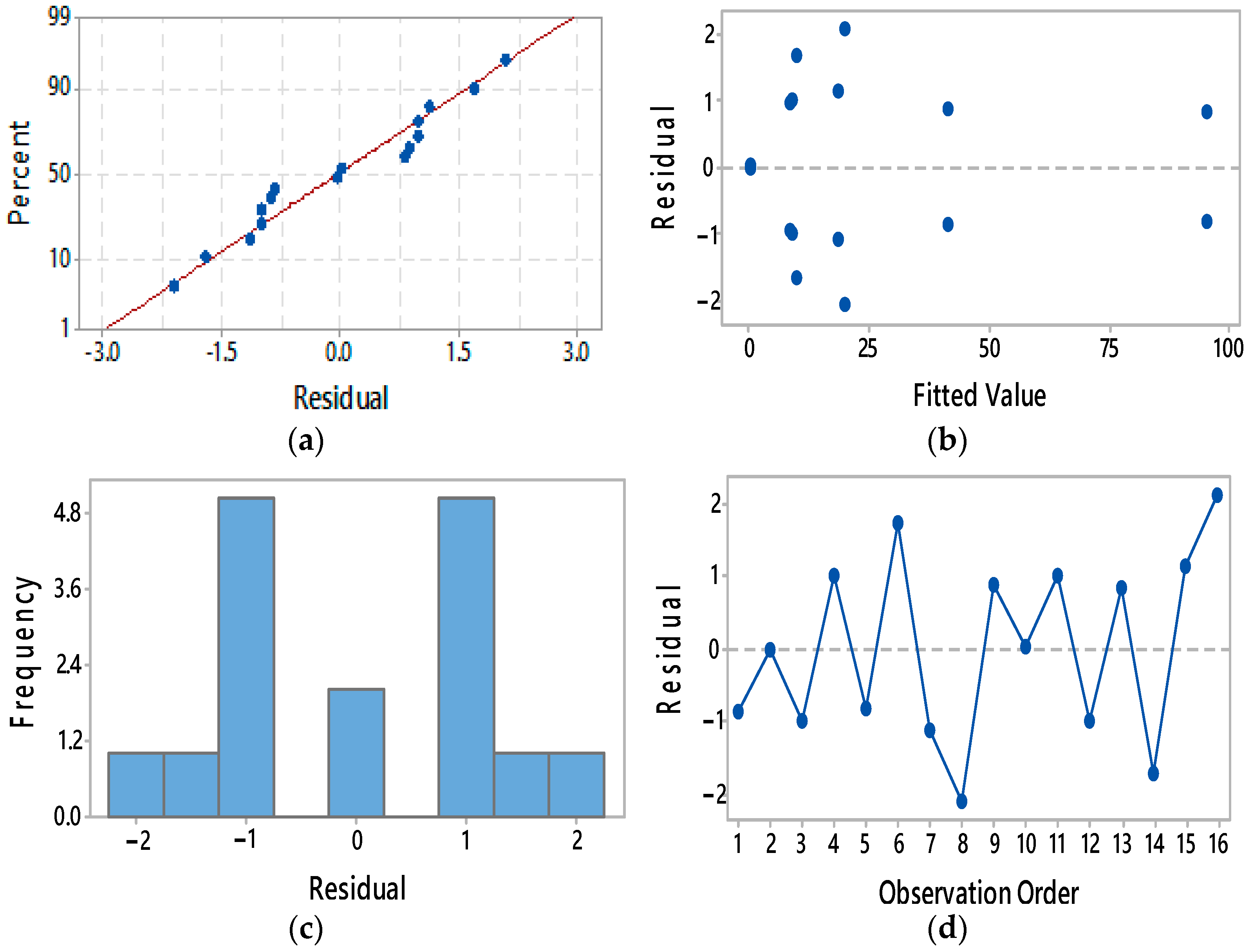
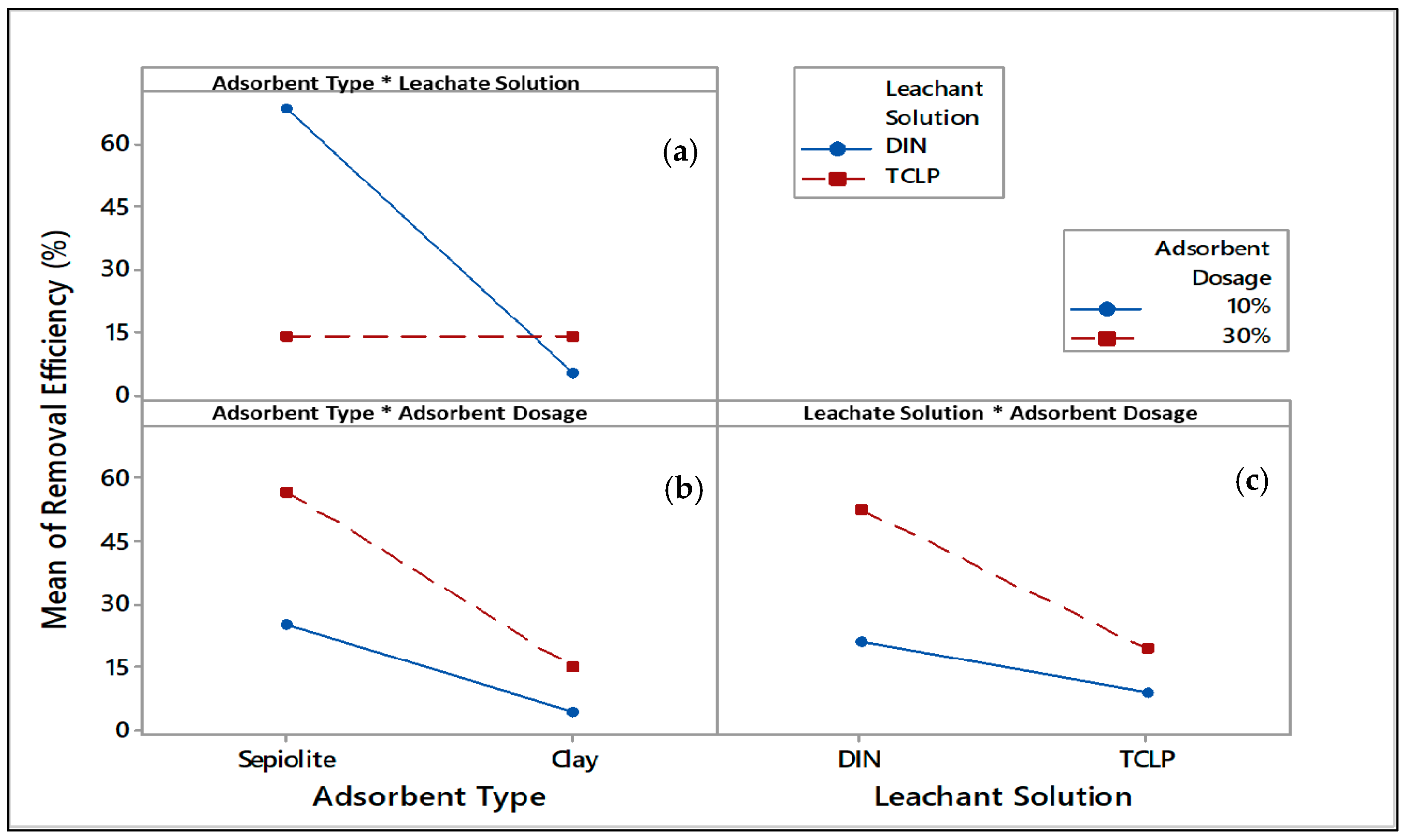
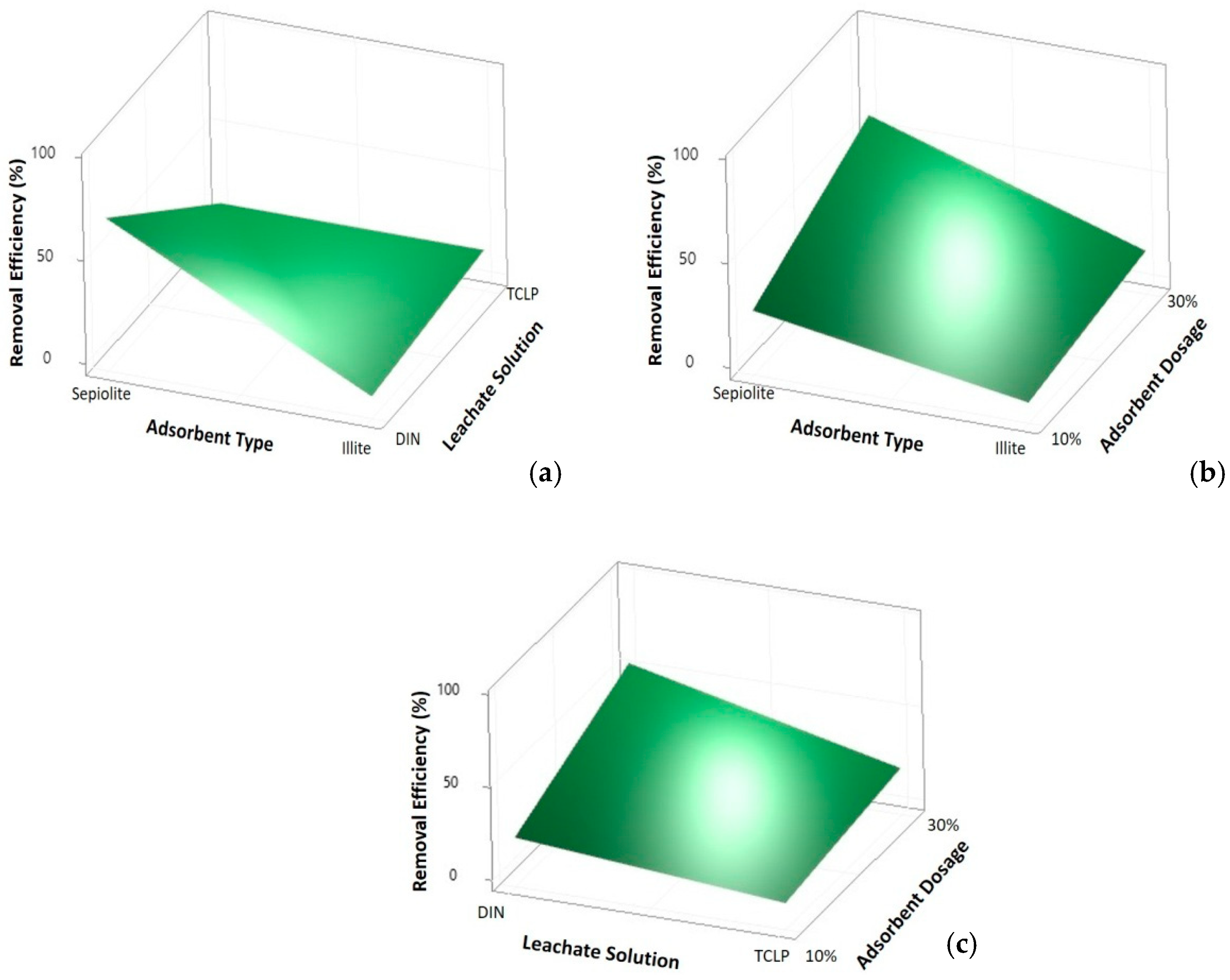
3. Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wani, A.A.; Usmani, J.A. Lead toxicity: A review. Interdiscip. Toxicol. 2015, 8, 55–64. [Google Scholar] [CrossRef]
- Gorini, F.; Muratori, F.; Morales, M.A. The Role of Heavy Metal Pollution in Neurobehavioral Disorders: A Focus on Autism. Rev. J. Autism Dev. Disord. 2014, 1, 354–372. [Google Scholar] [CrossRef]
- May, G.J.; Davidson, A.; Monahov, B. Lead batteries for utility energy storage: A review. J. Energy Storage 2018, 158, 145–157. [Google Scholar] [CrossRef]
- Pillot, C. The recharge able battery market and main trends. In Proceedings of the 15th European Lead Battery Conference, Valletta, Malta, 13–16 September 2016; pp. 2014–2025. [Google Scholar]
- Kurt, O. Interviewer; Mutlu Akü ve Malzemeleri San. A.Ş.: Tuzla, İstanbul, 2012. [Google Scholar]
- Jiang, W.Y. Global and Chinese Lead-Acid Battery Industry Report. Forward-Industrial Research Institute. Available online: https://www.qianzhan.com/analyst/detail/220/181101-627d5bc4.html (accessed on 30 March 2021).
- TÜİK—VeriPortalı. Available online: https://data.tuik.gov.tr/Kategori/GetKategori?p=sanayi-114&dil=1 (accessed on 31 March 2021).
- Milton, B.A.; Juan, V.Z.; Maria, M.V.; Alejandro, A.; Juan Jose, P.C. Chemical characterization and local dispersion of slag generated by a lead recovery plant in Central Mexico. Afr. J. Biotechnol. 2014, 19, 1973–1976. [Google Scholar]
- Çelebi, S.; Yetiş, Ü.; Ünlü, K. Identification of management strategies and generation factors for spent lead acid battery recovery plant wastes in Turkey. Waste Manag. Res. J. Sustain. Circ. Econ. 2019, 37, 199–209. [Google Scholar] [CrossRef]
- U.S. Environmental Protection Agency (EPA). Water: Monitoring & Assessment-5.9 Conductivity; U.S. Environmental Protection Agency (EPA): Washington, DC, USA, 2012.
- Ballantyne, A.D.; Hallett, J.P.; Riley, J.; Shah, N.; Payne, D.J. Lead acid battery recycling for the twenty-first century. R. Soc. Open Sci. 2018, 5, 171368. [Google Scholar] [CrossRef]
- Boxall, N.J.; Adamekab, N.; Chengac, K.Y.; Haqued, N.; Bruckardd, W.; Kaksonen, A.H. Multi stage leaching of metals from spent lithiumi on battery waste using electrochemically generated acidic lixiviant. Waste Manag. 2018, 74, 435–445. [Google Scholar] [CrossRef] [PubMed]
- Marotz, C.A.; Sanders, J.G.; Zuniga, C.; Zaramela, L.S.; Knight, R.; Zengler, K. Improving Saliva Shotgun Metagenomics By Chemical Host Dna Depletion. Microbiome 2018, 6, 42. [Google Scholar] [CrossRef]
- Jaria, G.; Silva, C.P.; Ferreira, C.I.A.; Otero, M. Sludge from paper mill effluent treatment as raw material to produce carbon adsorbents: An alternative waste management strategy. J. Environ. Manag. 2017, 188, 203–211. [Google Scholar] [CrossRef]
- Malakootian, M.; Hossaini, H.; Asadipour, A.; Daneshkhah, M. Preparation and characterization of modified sepiolite for the removal of Acid green 20 from aqueous solutions: Isotherm, kinetic and process optimization. Appl. Water Sci. 2018, 8, 174. [Google Scholar] [CrossRef]
- Li, S.; Huang, X.; Muhammad, F.; Yu, L.; Xia, M.; Zhao, J.; Jiao, B.; Shiau, Y.C.; Li, D. Waste solidification/stabilization of lead zinc slag by utilizing fly ash based geopolymers. Econ. Environ. Stud. 2016, 16, 819–830. [Google Scholar] [CrossRef]
- Townsend, T.; Jang, Y.C.; Tolaymat, T.; Jambeck, J.A. Final Report on Leaching Tests for Evaluating Risk in Solid Waste Management Decision Making; Department of Environmental Engineering Sciences, University of Florida: Gainesville, FL, USA, 2003. [Google Scholar]
- Uddin, M.K. A review on the adsorption of heavy metals by clay minerals, with special focus on the past decade. Chem. Eng. J. 2017, 308, 438–462. [Google Scholar] [CrossRef]
- Yuan, G.; Theng, B.K.G.; Churchman, J.; Gates, W.P. Clay sand Clay Minerals for Pollution Control. Dev. Clay Sci. 2006, 1, 625–675. [Google Scholar]
- Srinivasan, R. Advances in Application of Natural Clayand Its Composites in Removal of Biological, Organic, and Inorganic Contaminants from Drinking Water. Adv. Mater. Sci. Eng. 2011, 2011, 1687–843417. [Google Scholar] [CrossRef]
- Nohales, A.; Solar, L.; Porcar, I.; Vallo, C.I.; Go’mez, C.M. Morphology, flexural, and thermal properties of sepiolite modified epoxy resins with different curing agents. Eur. Polym. J. 2006, 42, 3093–3101. [Google Scholar] [CrossRef]
- Liu, H.; Chen, W. Magnetic mesoporous imprinted adsorbent based on Fe3O4-modified sepiolite for organic micro pollutant removal from aqueous solution. R. Soc. Chem. 2015, 5, 27034–27042. [Google Scholar]
- Bauer, A.; Velde, B.; Gaupp, R. Experimental constraints on illite crystal morphology. Clay Miner. 2000, 35, 587–597. [Google Scholar] [CrossRef]
- Day-Stirrat, R.J.; Aplin, A.C.; Kurtev, K.D. Latedia genesis of illite-smectite in the Podhale Basin, southern Poland: Chemistry, morphology, and preferred orientation. Geosphere 2017, 13, 2137–2153. [Google Scholar] [CrossRef]
- Yin, J.; Deng, C.; Yu, Z.; Wang, X.; Xu, G. Effective Removal of Lead Ions from Aqueous Solution Using NanoIllite/Smectite Clay: Isotherm, Kinetic, and Thermodynamic. Model. Adsorpt. Water 2018, 10, 210. [Google Scholar] [CrossRef]
- Elhalil, A.; Tounsadi, H.; Elmoubarki, R.; Mahjoubi, F.Z.; Farnane, M.; Sadiq, M.; Abdennouri, M.; Qourzal, S.; Barka, N. Factorial experimental design for the optimization of catalytic degradation of malachite green dye in aqueous solution by Fenton process. Water Resour. Ind. 2016, 15, 41–48. [Google Scholar] [CrossRef]
- Brasil, J.L.; Martins, L.C.; Ev, R.R.; Dupont, J.; Dias, S.L.P.; Sales, J.A.A.; Airoldi, C.; Lima, E.C. Factorial design for optimization of flow injection pre concentration procedure for copper(II) determination in natural waters, using 2-aminomethylpyridine grafted silica gel as adsorbent and spectrophotometric detection. Int. J. Environ. Anal. Chem. 2005, 15, 475–491. [Google Scholar] [CrossRef]
- Montgomery, D.C. Design and Analysis of Experiments, to Design, Data Analysis and Model Building; John Wiley and Sons: New York, NY, USA, 1997. [Google Scholar]
- Zhao, Y.; Gu, W.; Li, Y. Molecular design of 1,3,5,7-tetracn derivatives with reduced bioconcentration using 3D-QSAR modeling, full factorial design, and molecular docking. J. Mol. Graph. Model. 2018, 84, 197–214. [Google Scholar] [CrossRef]
- Bounouri, Y.; Berkani, M.; Zamouche, A.; Ycerz, L. Optimization and modeling of synthesis parameters of neodymium(III) bromide by dry method using full factorial design analysis. Arab. J. Chem. 2020, 13, 366–376. [Google Scholar] [CrossRef]
- Barka, N.; Abdennouri, M.; Boussaoud, A.; Galadi, A.; Baâlala, M.; Bensitel, M.; Sahibed-Dine, A.; Nohair, K.; Sadiq, M. Full factorial experimental design applied to oxalicacid photo catalytic degradation in TiO2 aqueous suspension. Arab. J. Chem. 2014, 7, 752–757. [Google Scholar] [CrossRef]
- Torrades, F.; García-Montaño, J. Using central composite experimental design to optimize the degradation of realdye waste water by Fenton and photo-Fenton reactions. Dyes Pigment 2014, 100, 184–189. [Google Scholar] [CrossRef]
- Montgomery, D.C. Design and Analysis of Experiments, 8th ed.; John Wiley & Sons: New York, NY, USA, 2012. [Google Scholar]
- Mendonça, D.R.; Andrade, H.M.C.; Guimarães, P.R.B.; Vianna, R.F.; Meneghetti, S.M.P.; Pontes, L.A.M.; Teixeira, L.S.G. Application of full factorial design and Doehlert matrix for the optimisation of beef tallow methanolysis via homogeneous catalysis. Fuel Process. Technol. 2011, 92, 342–348. [Google Scholar] [CrossRef]
- Gygi, C.; Decarlo, N.; Williams, B. Six Sigma for Dummies; International Lead and Zinc Study Group. Annual Lead and Zinc Statistics Report; Wiley Publishing Inc.: Indianapolis, IN, USA, 2005. [Google Scholar]
- Ismail, A.A.; El-Midany, A.A.; Ibrahim, I.A.; Matsunaga, H. Heavy metal removal using SiO2-TiO2 binary oxide: Experimental design approach. Adsorption 2008, 14, 21–29. [Google Scholar] [CrossRef]
- Budka, A.; Łacka, A.; Gaj, R.; Jajor, E.; Korbas, M. Predicting winter wheat yields by comparing regression equations. Crop Prot. 2015, 78, 84–91. [Google Scholar] [CrossRef]
- Padilla, E.; Ramos, R.L.; Barron, J.M.; Leyva, R. Adsorption of Heavy Metal Ions from Aqueous Solution onto Sepiolite. Adsorpt. Sci. Technol. 2011, 29, 569–584. [Google Scholar] [CrossRef]
- Marques Fernandes, M.; Baeyens, B. Cation exchange and surface complexation of lead on montmorillonite and illite including competitive adsorption effects. Appl. Geochem. 2019, 100, 190–202. [Google Scholar] [CrossRef]
- Arenas, L.T.; Lima, E.C.; Santos, A.A.D.; Vaghetti, J.C.P.; Coasta, T.M.H.; Benvenutti, E.V. Use of statistical design of experiments to evaluate the sorption capacity of 1,4-diazoniabicycle[2,2,2]octane silica chloride for Cr(VI) adsorption. Colloids Surf. A Physiochem. Eng. Asp. 2006, 297, 240–248. [Google Scholar] [CrossRef]
- Chang, E.E.; Chiang, P.C.; Lu, P.H.; Ko, Y.W. Comparisons of metal leachability for various wastes by extraction and leaching methods. Chemosphere 2001, 45, 91–99. [Google Scholar] [CrossRef]
- Gálvez-Martos, J.L.; Styles, D.; Schoenberger, H.; Zeschmar-Lahl, B. Construction and demolition waste best management practice in Europe. Resour. Conserv. Recycl. 2018, 136, 166–178. [Google Scholar] [CrossRef]
- Gomes, G.M.F.; Mendes, T.F.; Wada, K. Reduction in toxicity and generation of slag in secondary lead process. J. Clean. Prod. 2011, 19, 1096–1103. [Google Scholar] [CrossRef]
- Huggett, J.M. Reference Module in Earth Systems and Environmental Sciences. Clay Miner. 2015. [Google Scholar] [CrossRef]
- Kreusch, M.A.; Ponte, M.J.J.S.; Ponte, H.A.; Kaminari, N.M.S.L.; Marino, C.E.B.; Mymrin, V. Techonologial improvements in automotive battery recycling. Resour. Conserv. Recycl. 2007, 52, 368–380. [Google Scholar] [CrossRef]
- Montgomery, D.C.; Runger, G.C.; Hubele, N.F. Engineering Statistics; John Wiley & Sons, Inc.: New York, NY, USA, 2001. [Google Scholar]
- Ponnusami, V.; Krithika, V.; Madhuram, R.; Srivastana, S.N. Biosorption of reactive dye using acid-treated rice husk: Factorial design analysis. J. Hazard. Mater. 2007, 142, 397–403. [Google Scholar] [CrossRef]
- Al-Qaim, F.F.; Mussa, Z.H.; Yuzir, A.; Abdullah, M.P.; Othman, M.R. Full Factorial Experimental Design for Carbamazepine Removal Using Electrochemical Process: A Case Study of Scheming the Pathway Degradation. J. Braz. Chem. Soc. 2018, 29, 1721–1731. [Google Scholar] [CrossRef]
- Javanbakht, V.; Ghoreishi, S.M. Application of response surface methodology for optimization of lead removal from an aqueous solution by a novel superparamagnetic nanocomposite. Adsorpt. Sci. Technol. 2017, 35, 241–260. [Google Scholar] [CrossRef]
- Yilmaz, O.; Çokça, E.; Ünlü, K. Comparison of Two Leaching Tests to Assess the Effectiveness of Cement-Based Hazardous Waste Solidification/Stabilization. Turkish J. Eng. Environ. Sci. 2003, 27, 201–212. [Google Scholar]

| Operation Criteria | TCLP | DIN38414-S4 |
|---|---|---|
| Extraction Fluid | Acetic acid solution | Distilled water |
| pH | 4.93 ± 0.05 | - |
| Liquid to solid ratio | 20:1 | 10:1 |
| Extraction period | 18 h at 30 rpm | 24 h at 30 rpm |
| Temperature | Room temperature | Room temperature |
| Lead Smelting Slag | Sepiolite | Illite | |
|---|---|---|---|
| SiO2 Fe2O3 Al2O3 TiO2 CaO SO3 K2O MgO Na2O Mn2O3 PbO-PbS LOI Others | 15.41 40.77 3.01 0.24 2.08 0.61 0.46 0.42 5.35 0.46 11.50 6.32 13.37 | 53.47 0.16 0.19 - 0.71 - - 23.55 - - - - 21.92 | 76.90 1.50 12.00 0.20 0.80 - 4.00 <0.10 1.20 - - - 3.30 |
| Factor | Low Level (−1) | High Level (+1) |
|---|---|---|
| Adsorbent type (A) | Sepiolite | Illite |
| Leachate solution (B) | DIN | TCLP |
| Adsorbent dosage (%) (C) | 10 | 30 |
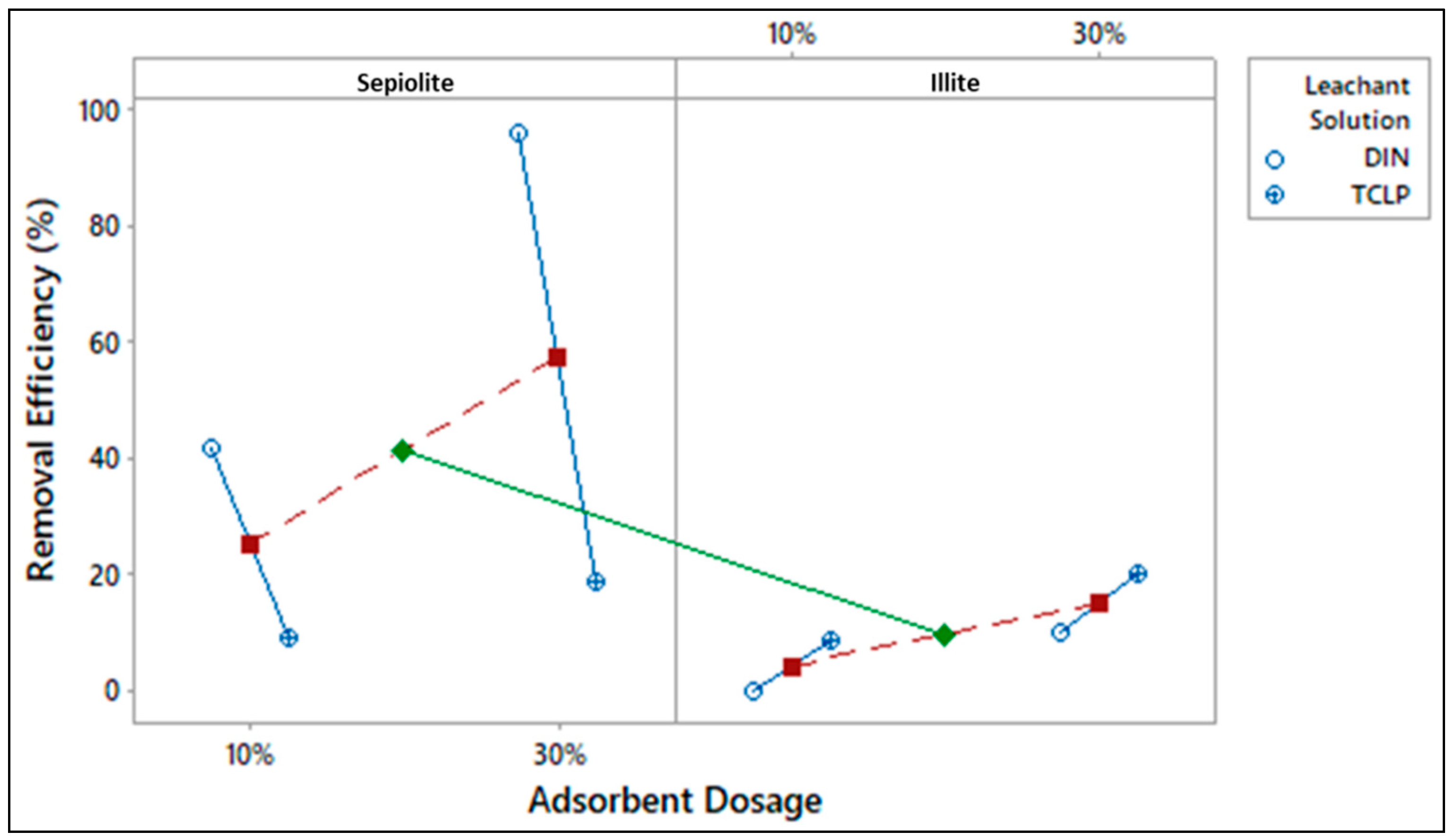
| Experiment | A | B | C | Removal Efficiency (%) | |
|---|---|---|---|---|---|
| Trial 1 | Trial 2 | ||||
| 1 | −1 | −1 | −1 | 40.80 | 42.56 |
| 2 | 1 | −1 | −1 | 0.14 | 0.20 |
| 3 | −1 | 1 | −1 | 8.03 | 10.01 |
| 4 | 1 | 1 | −1 | 9.41 | 7.45 |
| 5 | −1 | −1 | 1 | 94.71 | 96.36 |
| 6 | 1 | −1 | 1 | 11.85 | 8.45 |
| 7 | −1 | 1 | 1 | 17.58 | 19.82 |
| 8 | 1 | 1 | 1 | 18.01 | 22.21 |
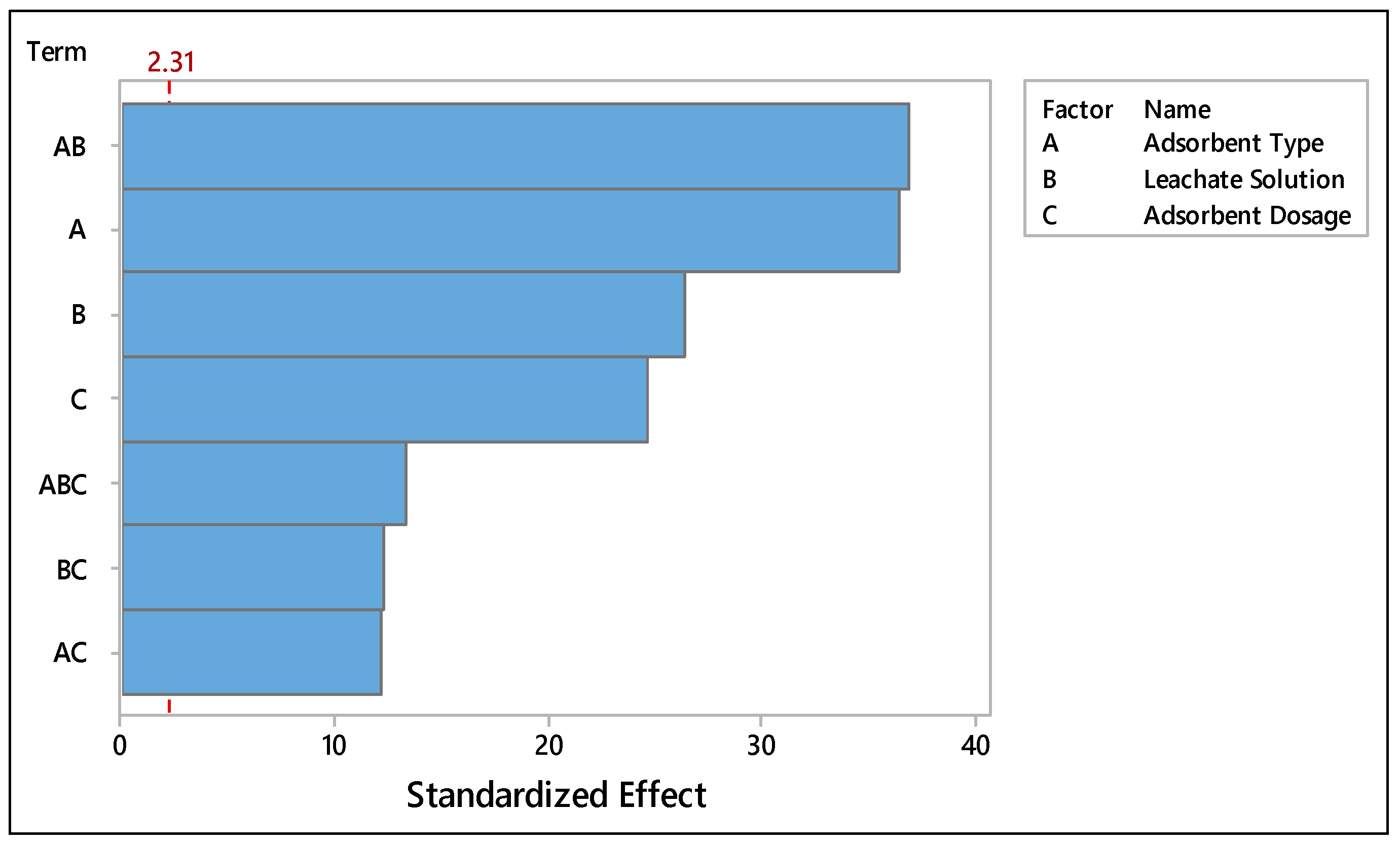
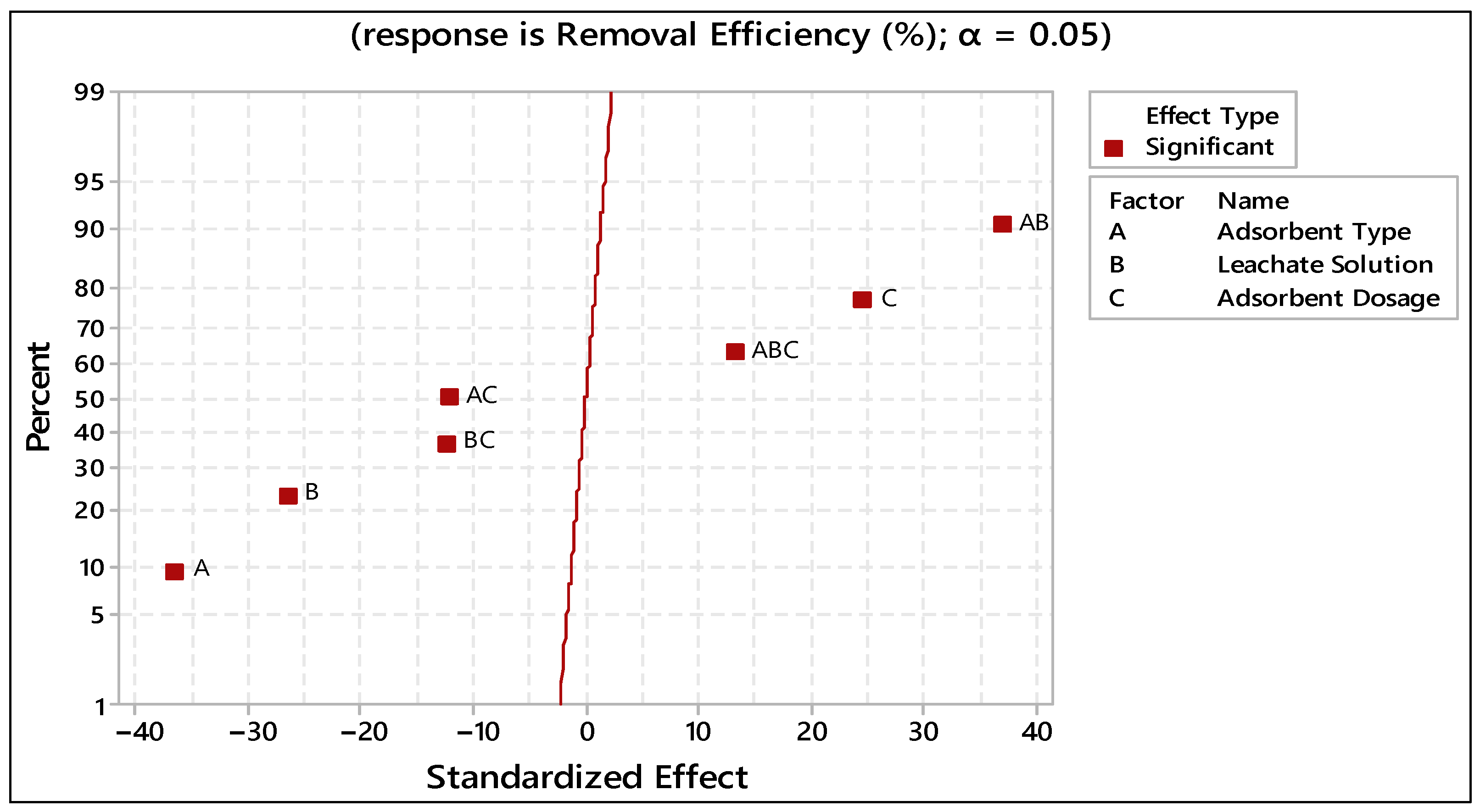
| Term | Effect | Coef | SE Coef | T | P |
|---|---|---|---|---|---|
| Constant | 25.474 | 0.432 | 58.95 | 0.000 | |
| Adsorbent Type | −31.519 | −15.759 | 0.432 | −36.47 | 0.000 |
| Leachate solution | −22.819 | −11.409 | 0.432 | −26.40 | 0.000 |
| Adsorbent Dosage | 21.299 | 10.649 | 0.432 | 24.64 | 0.000 |
| Adsorbent Type*Leachate Solution | 31.929 | 15.964 | 0.432 | 36.94 | 0.000 |
| Adsorbent Type*Adsorbent Dosage | −10.469 | −5.234 | 0.432 | −12.11 | 0.000 |
| Leachate solution*Adsorbent Dosage | −10.619 | −5.309 | 0.432 | −12.29 | 0.000 |
| Adsorbent Type*Leachate solution*Adsorbent Dosage | 11.469 | 5.734 | 0.432 | 13.27 | 0.000 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gürkan, E.H.; Tibet, Y.; Çoruh, S. Application of Full Factorial Design Method for Optimization of Heavy Metal Release from Lead Smelting Slag. Sustainability 2021, 13, 4890. https://doi.org/10.3390/su13094890
Gürkan EH, Tibet Y, Çoruh S. Application of Full Factorial Design Method for Optimization of Heavy Metal Release from Lead Smelting Slag. Sustainability. 2021; 13(9):4890. https://doi.org/10.3390/su13094890
Chicago/Turabian StyleGürkan, Elif Hatice, Yusuf Tibet, and Semra Çoruh. 2021. "Application of Full Factorial Design Method for Optimization of Heavy Metal Release from Lead Smelting Slag" Sustainability 13, no. 9: 4890. https://doi.org/10.3390/su13094890
APA StyleGürkan, E. H., Tibet, Y., & Çoruh, S. (2021). Application of Full Factorial Design Method for Optimization of Heavy Metal Release from Lead Smelting Slag. Sustainability, 13(9), 4890. https://doi.org/10.3390/su13094890







