Abstract
A large amount of wastewater from various and discharged sources that are not treated in any way could affect properties of both land and water, causing severe problems for the environment. Advanced oxidation processes seem to be a feasible option to address effluent treatment with regard to salvation of the environment. In this work, a CdO/ZnO/Yb2O3 composite composed of trimetallic semiconductors was synthesized through a simple one-pot hydrophile approach at low temperatures and then was employed as a photocatalyst. The degradation of phenol, a common organic persistent pollutant in industrial wastewater, was efficiently catalyzed in the presence of simulated sunlight. It was demonstrated that the synthesized CdO/ZnO/Yb2O3 photocatalyst was significantly active after 15 min of reaction and facilitated the effective degradation of the phenol. The CdO/ZnO/Yb2O3 photocatalyst achieved 71.5% and 97.81% degradation of the phenol without and with the existence of H2O2, correspondingly. The results show that the synthesized composite possesses high oxygen reduction capability and is available for rapid and potent photodegradation of the organic persistent pollutants such as phenol, with minimal damage to the environment.
1. Introduction
Contamination of aquatic resources has been recognized among the main concerns of developing and other developed nations. Large amounts of biological contaminants identified in aquatic bodies (drinkable water processing factories) usually come from distinct segments of industry (food, textile, oil and gas, printing, and medical industries). In particular, it is the textile industry that is one of the most polluting sectors for water bodies, because it uses large amounts of water, consumes high quantities of synthetic dyes, and produces a great deal of organic-rich wastewater. In addition to this, the textile industry is severely colored by dyes that do not adhere to the tissue’s fabric in the processing [1,2,3].
Different approaches to water treatment are deployed to alleviate disposition of industrial polluted wastewater and reduce the influence of organic persistent pollutants from water to the environment to a minimum. These can be mentioned among others as biological treatment, which requires extended periods of time to bring the wastewater up to specification, filtration processes with the utilization of membranes [4], electrochemical methods [5], adsorption procedures [6], and advanced oxidation processes (AOP) [7]. Specifically, there has been much interest in the heterogeneous photocatalytic process as an advanced oxidation process due to its greater speed and effectiveness compared to other processes in the purification process.
In the photocatalytic process, the semiconductor material gets exposed to a light resource that has energies equal to or larger than their strip gaps. The radiation therefore fosters the stimulation of electrons that facilitate the generation of hydroxyl radicals (∙OH) and other intervening species such as and that have high oxidation capacity and can function to degrade organic contaminants of concern with even lower levels (<100 ppm) [8,9]. Wide-bandgap semiconductor oxides, comprising TiO2, SnO2, ZnO, and WO3 [8,9,10,11,12,13,14], and metal salts (CdS and PbS) [15,16] have been utilized in light-mediated photosensitizers for the oxygenation and reduction of organic molecules. Nevertheless, there are certain practical inconveniences associated with the implementation of high concentrations of such elements to rapidly mineralize chemical species of environmental concern, in addition to causing detriment to humans and aquatic ecosystems, given their temporary stability. Altogether, these prevent a majority of photocatalysts from being cemented as alternatives towards environmental solutions [17].
Accordingly, transitional metal oxides represent highly prospective candidates for use in photo-oxidation procedures, in particular, because of their redox characteristics and the fact that they are relatively more friendly to the environment than other metal systems. So far, there are various kinds of metal oxides that are used as electron mediators towards photocatalysts, including TiO2, SnO2, WO3, In2O3, Fe2O3, and ZnO [14]. In addition, there are bimetallic nanomaterials, which include In2O3/ZnO nanocomposites [18]. CuO/SnO2 nanoparticles [19], Ga2O3/In2O3 nanocomposites [20], and SnO2/ZnO nanostructures [21], are already studied as competent and effective photocatalysts endowed with n-type wide band gaps and resistivities of 10−4 to 1012 Ω cm, and ZnO has been regarded as a critical material for photocatalysts.
Electron/hole recombination restricts photocatalysis and hence must be prevented. As an additional trap for photogenerated charge generation, the metal cation avoids or decreases the electron/hole reorganization under any circumstances. Semiconductor metal oxides with ZnO as a bimetallic oxide pair, for instance, SnO2, V2O5, WO3, and MoO3 [22], were examined in order to enhance the photocatalytic properties of ZnO [23]. Additionally, combinations of trimetallic oxides, like Zn0.4/Co0.6/Al2O4, ZnO/Al2O3/TiO2, and ZnO/SnO2/TiO2 were also realized and demonstrated good photocatalytic active properties [24,25].
The photocatalyst is the core of photocatalysis; thus, developing highly efficient and stable photocatalytic materials is one of the emphases of photocatalytic research. Cadmium sulfide has attracted much attention for its excellent visible light response, and the band structure of CdS semiconductor perfectly satisfies the thermodynamic requirements for many photocatalytic reactions [26,27]. Yb2O3 is a kind of semiconductor material with strong heat resistance and good stability. It is widely used in coatings, fuel, ceramics, energy materials, environmental protection, and environmental catalysis and so on [28,29].
In this context, the objective of this study was to prepare sustainable substances on the basis of CdO/ZnO/Yb2O3 by means of a one-pot hydrothermal method and apply them to the photocatalytic degradation of phenols driven by heterogeneous visible lighting. Phenol, which is considered to be durable, organic pollutants, and regular target molecules in wastewater are extensively exposed to the agate-dyeing, animal-feeding, and textile industries. Furthermore, phenol serves as a water-accessible fluorogenic tracer for the manufacture of inkjet and laser printer cartridges [30]. Notably, there are no studies on the photocatalytic properties of visible light for CdO/ZnO/Yb2O3 hydrothermally synthesized for phenol or other organic dyes.
2. Experiment
2.1. Materials and Methodology
A chemical of analytical grade was purchased from Sigma-Aldrich USA for use with no extra purification. State-of-the-art and extremely reliable equipment, known as the Thermo Scientific Nicolet iS50 FTIR Spectrometer (Madison, WI, USA) and 300 UV/Vis spectrophotometer, was applied to document the UV/Vis and FTIR spectra of CdO/ZnO/Yb2O3 nanosheets (NSs). Analysis of CdO/ZnO/Yb2O3 NSs was carried out as an XPS study with the aim of identifying binding energy (eV) amongst Cd, Zn, Yb, and O, with a K-α1 spectrometer (Thermo Scientific, K-α1 1066), using the excitation source of radiation (A1 Kα1, spot size of ¼ 300.0 mm, flux energy of ¼ 200.0 eV, pressure of ~10−8 Torr). Examination of the molecular alignment, elemental analysis, morphology, particle size, and other optical properties of CdO/ZnO/Yb2O3 NSs was conducted via a FESEM device (JEOL, JSM-7600F, Tokyo, Japan) installed with XEDS. XRD evaluations were carried out under environmental conditions for the purpose of analyzing the crystallinity of CdO/ZnO/Yb2O3 NSs. The surface areas of Brunauer–Emmett–Teller (BET) were acquired on an instrument of Micromeritics Tristar 3000 by means of a nitrogen physisorption isotherm at 77 K (adsorption–desorption branch).
2.2. Synthesis of CdO/ZnO/Yb2O3 NSs
CdO/ZnO/Yb2O3 NSs were generated through a hydrothermal method with 0.1 M of CdCl2, ZnCl2, YbCl3, and NH4OH as reaction precursors. The hydrothermal technique, which is a classic solid-state system, has been broadly implemented in making doped nanomaterials, and the resulting nanomaterials exhibit minor size and rational grain size smaller than the size of the phase formation. According to the method [31], a mixture of CdCl2, YbCl3, and ZnCl2 was solubilized with distilled water (50.0 mL) and stirred constantly in a tapered flask (150.0 mL). After adjusting a pH of 11 to the obtained solution through the addition of NH4OH, it was subjected to a constant magnet stirring regime by putting the entire solution in an oven at 180.0 °C. Eventually, the resulting NSs attained were exposed to a muffle oven and calcined at 600 °C for about 4 h. The calcined samples were then described by UV, FTIR, XPS, XRD, and FESEM.
NH4OH(s) → NH4(aq)+ + OH(aq)−
ZnCl2 → Zn(aq)2+ + 2Cl(aq)−
CdCl2 → Cd(aq)2+ + 2Cl(aq)−
YbCl3 → Yb(aq)3+ + 3Cl(aq)−
NH4(aq)+ + 7OH(aq)− + Zn(aq)2+ + Cd(aq)2+ + Yb(aq)3+ + Cl(aq)− → Zn(OH)2(aq)↓ + Cd(OH)2(aq)↓ + Yb(OH)3(aq)↓ + NH4Cl(aq)
Zn(OH)2(aq)↓ + Cd(OH)2(aq)↓ + 2Yb(OH)3(aq)↓ → CdO/ZnO/Yb2O3(s)↓ + 5H2O(aq)
From Equations (1)–(6) above, the progression of the reaction was found to be slow. The pH of the reaction medium acts as an important factor in producing the tertiary metallic oxide CdO/ZnO/Yb2O3 NSs. At a defined pH, ZnCl2 is promptly hydrolyzed to generate zinc hydroxide in an alkaline solution (ammonium hydroxide) based on Equation (5). For this reason, NH4OH served to adjust the pH (alkaline phase) and the slow transport of hydroxyl ions (OH−) into the reaction mediator. On approaching a cut-off value for the concentration of Zn2+ and OH− ions, nuclei begin to form with a solution of Zn(OH)2. As the level of Yb3+ and Cd2+ ions gets higher (reaction (3–5)), soluble Zn(OH)2 crystals nucleate at a slower rate due to the lower energy of activation of Zn(OH)2 and the formation of heterogeneous nuclei with those of other oxides, typically Cd(OH)2, Yb(OH)3, or Zn(OH)2. Reaction regimes with high concentrations of Yb3+ and Cd2+ trigger some bigger Cd(OH)2/Zn(OH)2/Yb(OH)3 crystals to develop immiscible agglomerates featuring a lamellar formation as per reactions (5) and (6). The resulting crystals were then cleaned completely with acetone, ethyl alcohol, and aqueous one after another and kept dry at indoor temperatures. At last, they were calcined by putting the immiscible Cd(OH)2/Zn(OH)2/Yb(OH)3 nanocrystals into a stove (Barnstead Thermoelectric, 6000 furnace, US) at 600.0 °C for 4 h. Based on the nanoparticle growth theory of the Ostwald-ripening approach, initially, the CdO/ZnO/Yb2O3 NSs nuclei advance through self- and inter-agglomeration and subsequently recombine a second time to constitute CdO/ZnO/Yb2O3 NSs. The internal order of molecules in the nanocrystals corresponds to the other via Van der Waals forces.
2.3. Photocatalytic and Absorption Experiment
Photocatalytic effectiveness of CdO/ZnO/Yb2O3 NSs on phenol solutions was assessed. The tests were carried out in a custom-made photoreactor, which incorporated a UV and visually illuminated light source positioned overhead. Irradiation of the samples was carried out with a PHILIPS cleo 90 W TL-D Actinic BL for various times at a maximal emission wavelength of 365 nm. The combined UV radiation on cells at wavelengths in the 290–400 nm range was 90 ± 2 Wm−2. Once irradiated, specimens were passed over a 0.45 µm filter and then analyzed using appropriate analytical technologies.
Throughout the reaction, a distance of 10 cm was kept between the lamp and the solution. To conduct the experiments, 10.0 mg of oxide (667 mg·L−1) was scattered in petri plates that held 15.0 mL of a pre-prepared phenol stock solution with a concentration of 5.0 mg·L−1 at 22 °C. The suspension (solution and photocatalyst) was placed in darkness and stirred magnetically for 60 min to create an adsorption–desorption equilibrium.
All experiments were all undertaken at pH 7.0 (the normal pH of a phenol concentration of 7.0 mg·L−1) and were performed in triplicate. In the same circumstances, photolysis tests were carried out without the photocatalyst, as opposed to adsorption tests, which were conducted in darkness, by applying the identical method outlined in an earlier report [32].
It was necessary to include H2O2 (30 mg·L−1) and isopropyl alcohol in the reaction container for examining the effect of hydroxyl radicals (OH∙) on photocatalysis. According to literature reports, it was found that 0.5 mL of H2O2 suffices to further improve the forming of OH∙ and increase the material’s catalytic efficiency.
Consequently, it was determined that in order to assess the photolysis, adsorption capability, and photocatalytic effectiveness of the oxide both with or without the presence of H2O2, an equal amount of solution was gathered from a Petri dish for 15 min after the reaction, followed by centrifugation and filtration. Subsequently, with the use of a Shimadzu UV-1800 spectrophotometer, it was possible to analyze the resulting solution based on UV-Vis absorption spectroscopy over a wavelength scan ranging from 240 to 800 nm. As a result, the ultimate concentration of the solution could be derived through observing the largest absorption band of phenol at 271 nm following Equation (7), proposed by Rahman et al. [33] and Chantelle et al. [34]:
in which C0 represents the original concentration at 0 min and C represents the changing concentration as time progresses.
3. Results and Discussion
3.1. Photocatalytic Characteristics
3.1.1. Analysis of Optics and Structures
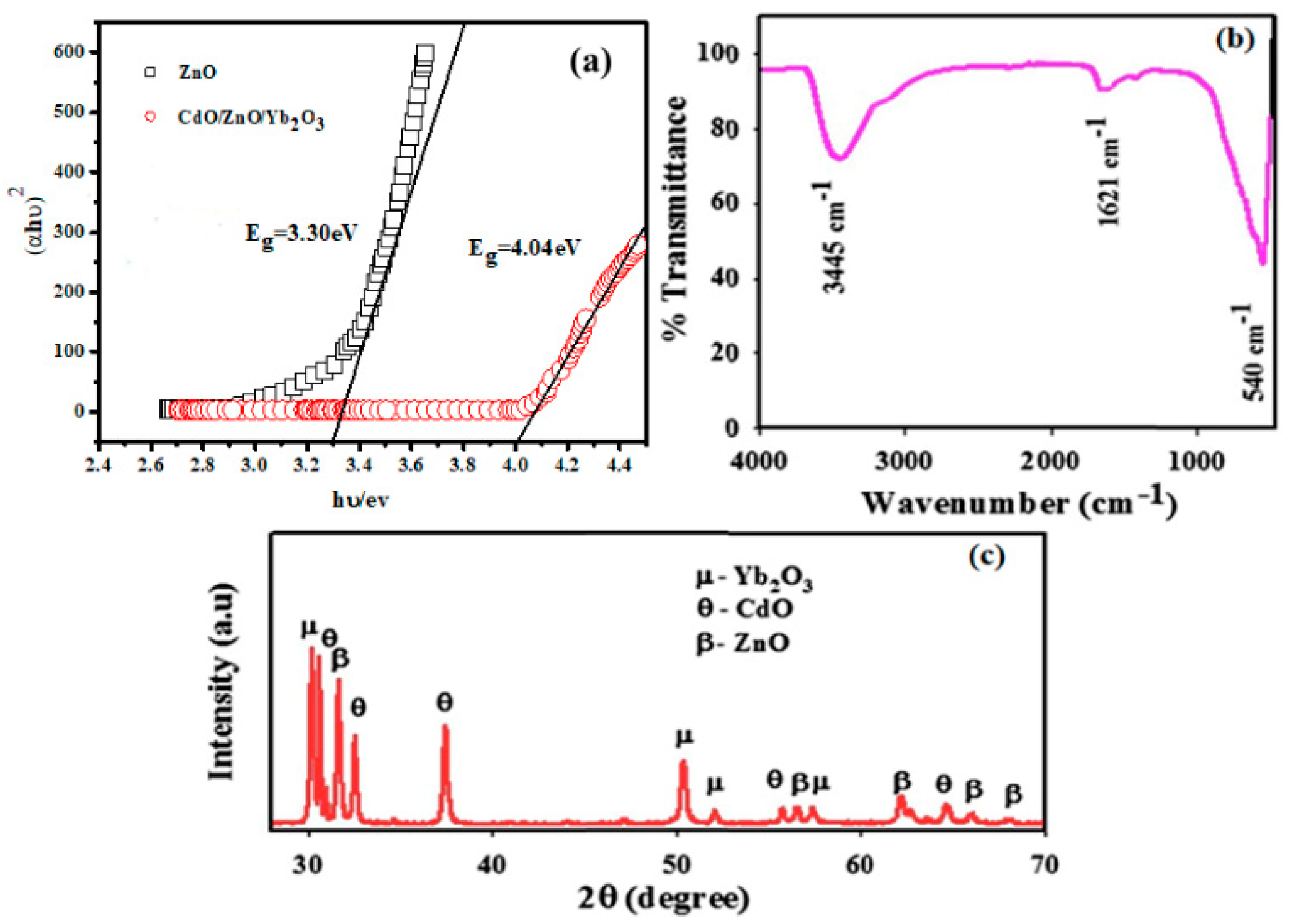
One of the key features in evaluating the photocatalytic efficiency of CdO/ZnO/Yb2O3 NSs is their optical properties. The spectrum presents an intense, consistent, and extensive band at 307 nm, which indicates the valence band electronic transition from lower to higher energy levels for the CdO/ZnO/Yb2O3. Figure 1a illustrates the data derived from the Tauc plot with the use of a UV-Vis spectrophotometer. The photon energy and absorption coefficient could be achieved with the Tauc model by means of a relation, as shown in Equation (8), in which the notations, α, hυ, A, Eg, and n denote the absorption coefficient, proportionality constant, photon energy, optical bandgap energy, and an integral number, correspondingly. The band-gap values are derived through additional plotting of Tauc curves to the energy axis (X-axis), as shown in the figure. The band gap of CdO/ZnO/Yb2O3 was 4.04 eV, which is higher than ZnO (3.30 eV). The CdO/ZnO/Yb2O3 ternary composite could induce more electronic species that are placed at certain higher Fermi sites compared to the single materials, thus expanding the energy band gap [27]. The newly prepared ternary composite with the higher band gap was able to absorb a higher portion of irradiated UV from the lamp than ZnO, and thus, photocatalytic performances could be improved.
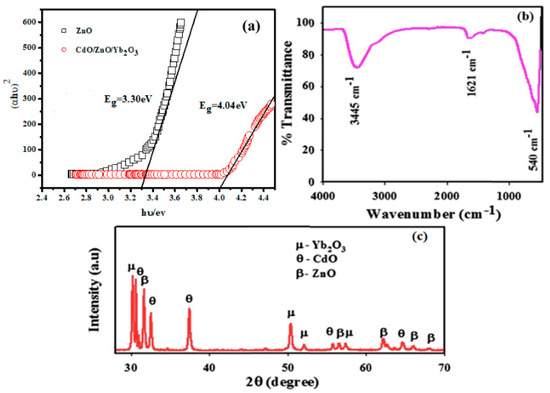
Figure 1.
Optical and morphological assessment via (a) UV-vis DRS, (b) FTIR, and (c) XRD modes of CdO/ZnO/Yb2O3 NSs.
FTIR offers a modern tool for analysis, enabling the identification of the functional characteristics of atoms or molecules through their appropriate vibrances of the atoms and molecules. Accordingly, the NSs synthesized by CdO/ZnO/Yb2O3 were researched through FTIR analysis, which was carried out within the 450–4000 cm−1 region, as shown in Figure 1b. Based on the FTIR spectra, the primary peaks identified were found at 3445, 1621, and 540 cm−1. The peaks at 3445 and 1621 cm−1 are, respectively, correlated with the standard OH stretched and H2O bent modes. At 540 cm−1, the peak is associated with Cd-O or Zn-O or Yb-O stretching [35,36], X-ray powder diffraction (XRD) being considered among the standard techniques of analysis and extensively adopted to identify the phase of crystalline nanomaterials, which conveys information on the size of the single cell. To investigate the structural characteristics and crystalline phases of the synthesized CdO/ZnO/Yb2O3 NSs, the XRD method is applied using Cu-K1 radiation (=1.54178 Å) at a 2° min−1 scan rate in the range of 10–80°. The XRD pattern observed demonstrated that the NSs possessed good crystalline properties and presented a mixed phase of Cd-O, Zn-O, and Yb2O3, as illustrated in Figure 1c. The pronounced diffraction reflexes with ZnO Index of β were (100), (110), (103), (112), and (200), comparable to the hexagonal phases of ZnO bulk and ZnO [37].
The observations report showed significant resemblance to the JCPDS standard data card (JCPDS No. 36-1451), with several additional peaks for CdO index, as θ were (111), (200), (220), (311), and (222) respectively, which are in excellent agreement with the literature reports [38].
In addition to this, several other sharp peaks with a Yb2O3 index of µ were identified in the mode of XRD diffraction, respectively, denoted as (110), (101), (112), and (200), in full agreement with the literature reports [39,40].
Furthermore, sharpness of the peaks indicates excellent crystallization of the product NSs.
3.1.2. Analysis of Morphology and Elements
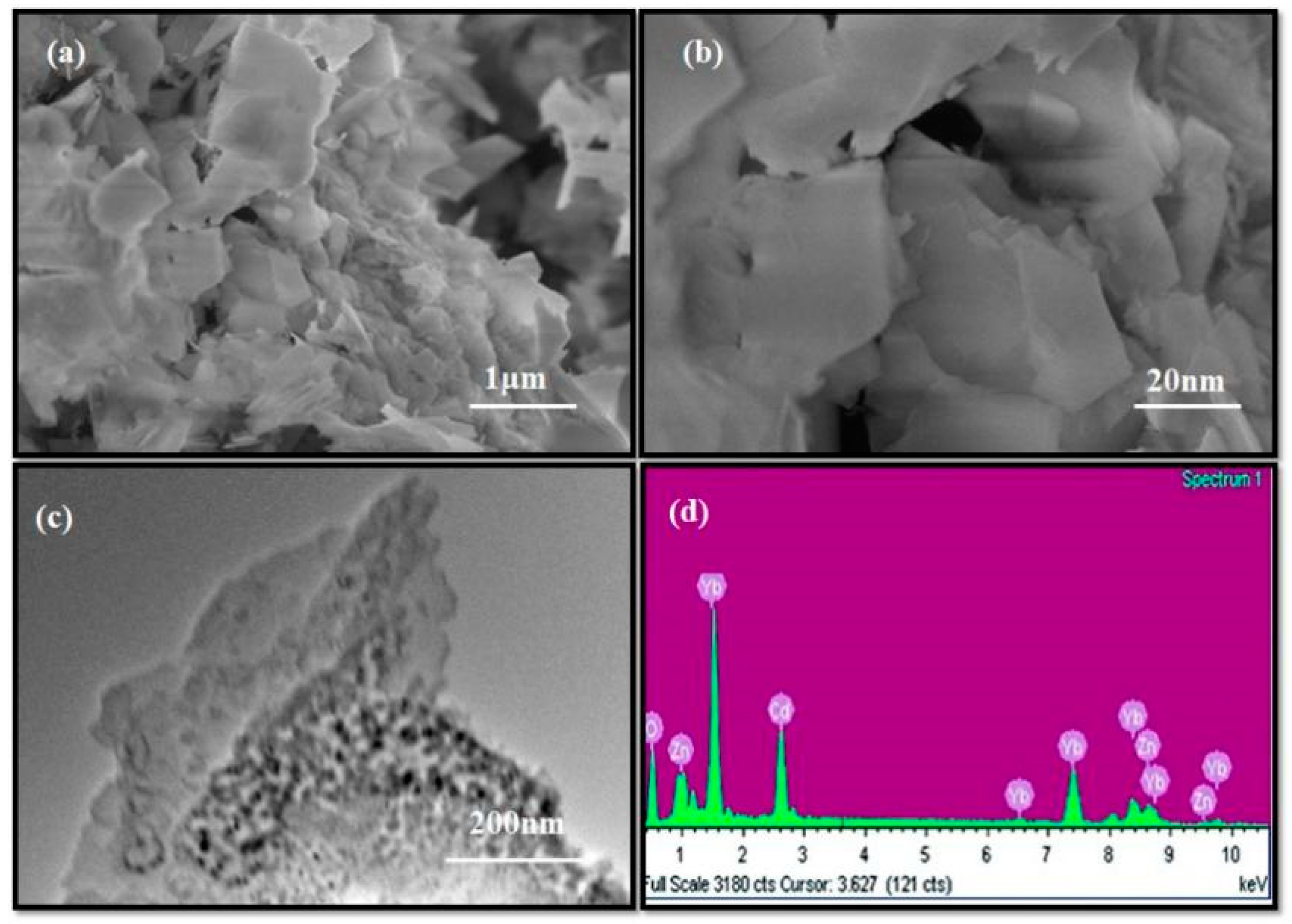
The morphological studies of the synthesized CdO/ZnO/Yb2O3 NSs were probed by FESEM, resulting in representative low and high magnification FESEM images illustrated in Figure 2a,b. Typical TEM images of the sample are shown in Figure 2c. These images clearly demonstrate homogeneous and arranged CdO/ZnO/Yb2O3 nanoflakes [41]. Energy-dispersive X-ray spectroscopy (EDS) probing of the CdO/ZnO/Yb2O3 NSs showed the presence of aligned Cd, Yb, Zn, and O among the pure calcined–doped materials of the nanosheets and explicitly revealed the organization of the calcined–prepared materials with Cd, Zn, Yb, and oxygen elements, as shown in Figure 2d. The contents of Cd, Yb, Zn, and O are 4.67%, 9.24%, 61.22%, and 24.87%, respectively. The absence of any other peaks in the EDS indicates the CdO/ZnO/Yb2O3 NSs product is exclusively composed of Cd, Yb, Zn, and O [34].
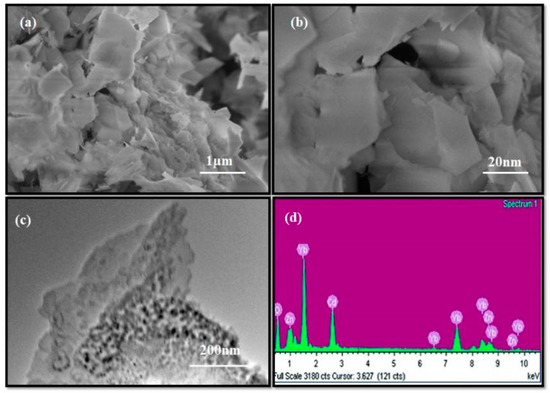
Figure 2.
(a,b) FESEM analysis of low to high magnetic images, (c,d) TEM image and EDS of the CdO/ZnO/Yb2O3 NSs.
3.1.3. Analysis of XPS
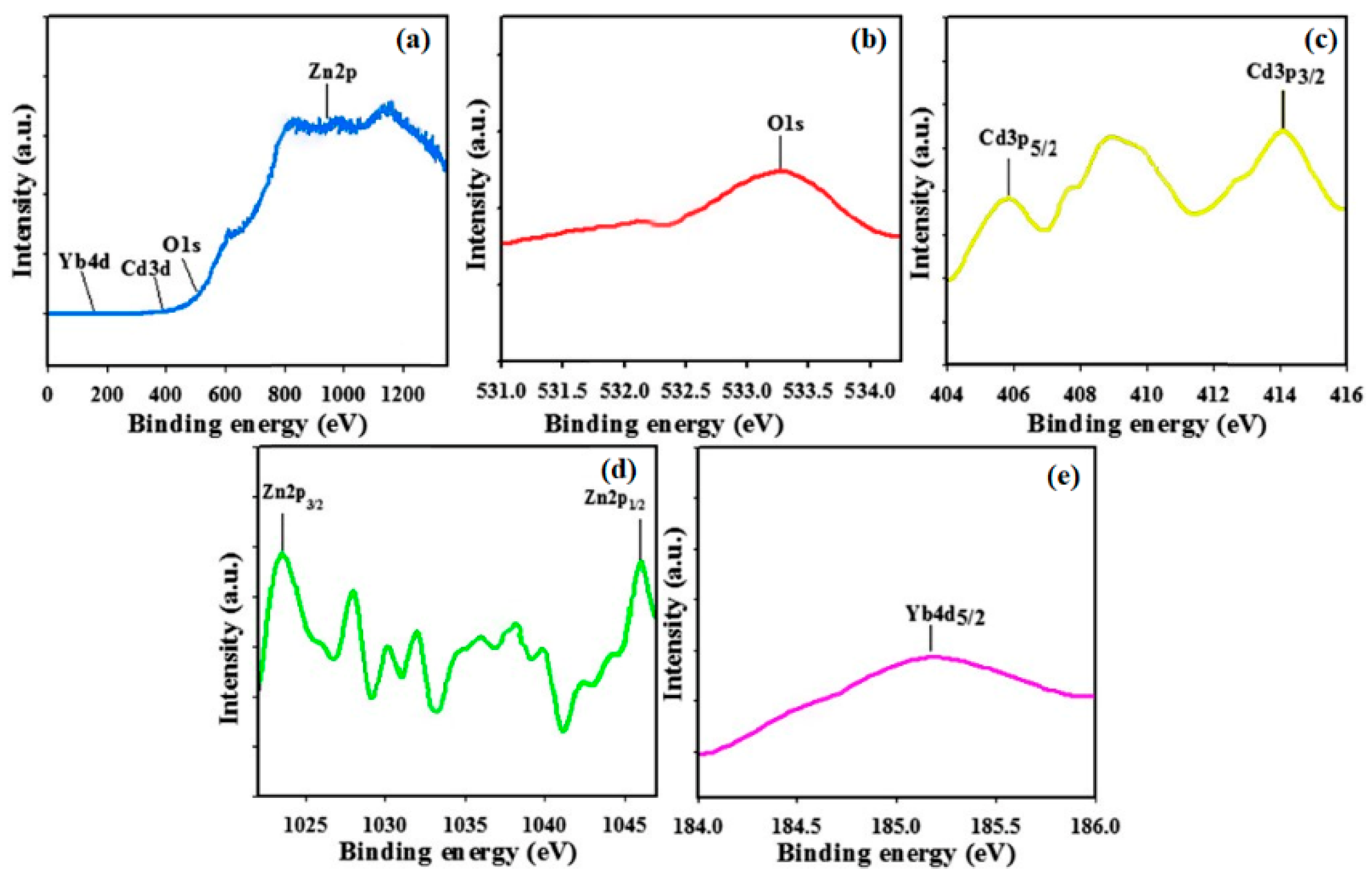
In order to pre-prove the compositional configuration present in the prepared specimens CdO/ZnO/Yb2O3 NSs, XPS, a quantitative spectroscopy technology, was practiced for the NSs. The implications of XPS detection allow the chemical nature and kinetic energy of the elements present to be expected, which includes the amount of electrons present in the specimen at the time of X-ray beam irradiation [42]. Figure 3 shows high resolution XPS spectra of Cd 3p, O 1s, Yb 4d, and Zn 2p. With respect to the primary two same peaks of the Cd 3d orbital, no significant variation is observed between the two peaks, which both are indicative of an equal Cd oxidation status. In Figure 3c, the binding energies are 406.08 and 414.08 eV for Cd 3d5/2 and Cd 3d3/2, separately, which are eigenvalues of Cd2+ in CdO. The O 1s, which is wide and asymmetric in Figure 3b, illustrates the existence of hyper-component oxygen species [43]. In Figure 3d, binding energies of 1023.08 and 1046.08 eV were observed for the Zn 2p3/2 and Zn 2p1/2 peaks, corresponding to eigenvalues of Zn2+ oxidation in ZnO [44]. In addition, the apparent peak of the Yb 4d spectrum from Figure 3e is orientated at 185.08 eV and could be identified as Yb 4d5/2, in considerable agreement with the Yb 4d binding energy of Yb2O3 [45,46].
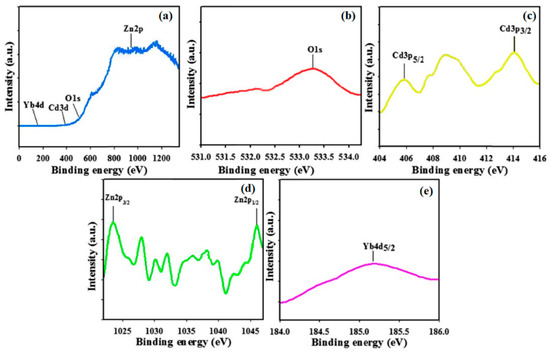
Figure 3.
Binding energy analysis of CdO/ZnO/Yb2O3 NSs studied by XPS: (a) full spectrum, (b) O 1s, (c) spin orbit Cd 3p level, (d) spin orbit Zn 2p level, and (e) spin orbit Yb 4d level.
3.2. Photocatalytic Experiments
3.2.1. Photocatalytic Activation and Potential Mechanism
In view of the photo-stability of phenol, it is necessary to apply a photocatalyst to facilitate the complete degradation. On this background, the photodegradation of phenol solutions (5 mg·L−1, pH 7) by CdO/ZnO/Yb2O3 NSs under UV-visible light for 15 min was carried out.
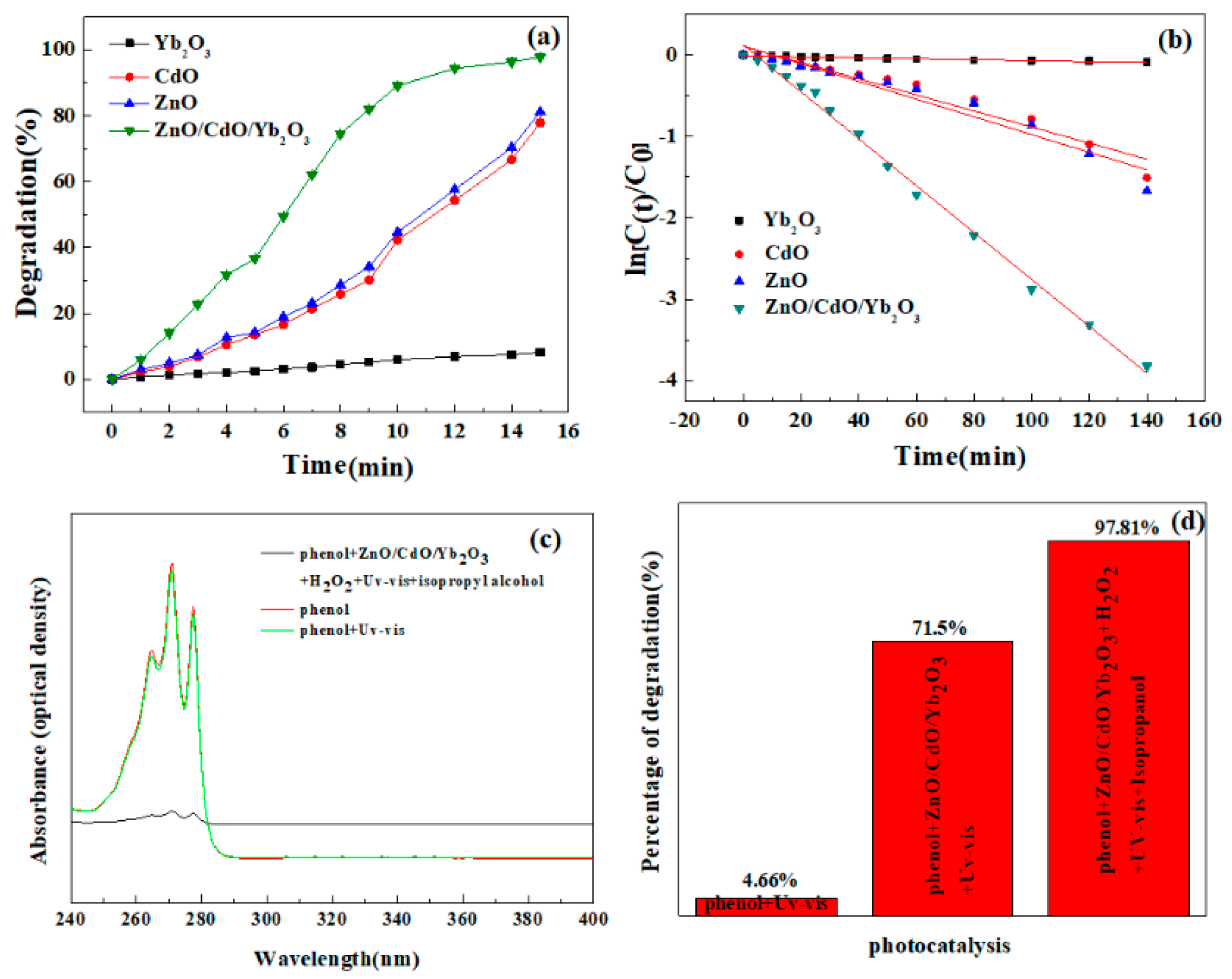
Two different mechanisms have been reported in the literature to characterize photocatalysis, i.e., a direct mechanism and an indirect mechanism. With respect to the direct mechanism, there is a transfer of electrons from the catalyst to the compound being oxidized, whereas in indirect photocatalysis the catalyst surface yields strong oxidants. Such oxidants have the ability to react uniformly with organic compounds to degrade them [47,48]. The adsorption on the catalyst is required for the indirect mechanism to take place. Therefore, the adsorption experiments were also carried out to better understand this point, as illustrated in Figure 4a. It was noticed that when CdO/ZnO/Yb2O3 NSs were employed, the adsorption of the phenol reached approximately 28.5%. On the other side, the BET surface area of NSs were 19.7 m2·g−1. The BET surface area of ZnO was 15.6 m2·g−1. In particular, it is possible that the distinct adsorption behavior of the CdO/ZnO/Yb2O3 NSs could account for the existence of various sorts of flaws on the catalyst surface, as the high specific surface areas under observation for both types of materials appear to play a significant role in this procedure, while we can assume that indirect mechanisms may be the most significant manner in which phenol photodegradation takes place in the existence of such catalysts.
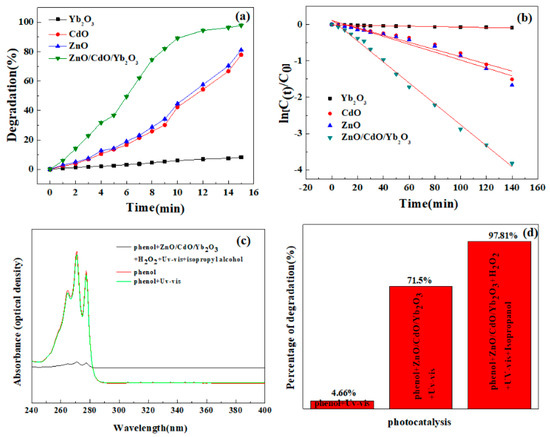
Figure 4.
(a) Comparison of the photocatalytic properties of pure ZnO, CdO, Yb2O3, and CdO/ZnO/Yb2O3 samples. (b) The corresponding rate constant k for different photocatalysts. (c) UV-Vis absorption spectra of phenol, phenol under UV-Vis, and phenol with CdO/ZnO/Yb2O3/H2O2/isopropanol under UV-Vis. (d) Degradation rate of the phenol solution with UV-Vis, catalysts/UV-Vis, and catalysts/UV-Vis/H2O2/isopropanol.
In order to quantify the reaction kinetics of phenol degradation in the experiment, we employed a pseudo-first order model for low concentrations.
ln(Ct/C0) = −kt
C0 and Ct are concentrations in the aqueous solution by time t = 0 and t and ka is the pseudo-first order rate constant, derived from the equation ln (Ct/C0) vs t plotted in Figure 4b. The largest reaction rate constant was 0.02882 min−1 for CdO/ZnO/Yb2O3 under UV-vis light. In this respect, the degradation of phenol by CdO/ZnO/Yb2O3 NSs attained 71.5% (Figure 4d).
For the assessment of the photostability of phenol under UV-Vis exposure, a photolysis test (control reaction) was conducted without a photocatalyst. The UV-Vis spectra of the phenol solutions prior to and after photolysis are indicated in Figure 4c. There was no significant drop in the maximal absorption band of phenol after 15 min of radiation. The maximal absorption at 271 nm (E = 4.57 eV) features the chromophore that is in charge of the coloration of the dye. The low reduction of this band following photolysis is equivalent to a discoloration of around 4.66%.
Diverse variables may influence the photocatalytic behavior of a material. The crystalline architecture and surface deficiencies, in combination with the particle size and surface area, for example, are essential factors influencing the photocatalysis that takes place as a result of the reaction between the phenol molecules and the catalyst [34]. It was noticed that the superior photocatalytic activities of CdO/ZnO/Yb2O3 NSs may be due to the structural flaws in the material itself or the existence of ionic deficiencies in the material. Consequently, high interaction between phenol and the surface of CdO/ZnO/Yb2O3 NSs arises, resulting in the enhanced adsorption capability of such oxides (Figure 4), as observed. Apart from this, it is possible that the morphology of the particles and the energy of the band gap might also facilitate the photogeneration of / pairs to contribute to the photodegradation of phenol in this system.
Variations in photocatalytic efficiency amongst those catalysts that have been prepared by these authors and the CdO/ZnO/Yb2O3 NSs synthesized from our investigations could be explained by the diverse patterns and electronic characteristics of the prototypes, which reflect variations in the terms of synthesis.
Considering the previous references, and on the basis of the assessed adsorption capabilities (Figure 4a), the potential indirect mechanisms of photodegradation of phenol can be interpreted with regard to the photocatalytic performances of the two substances produced in this work.
The mechanism, as investigated by Honorio et al. [49] and Ong et al. [50], is related to the interaction of the reactions described in the equations below (Equations (10)–(20)). To summarize, it is possible that photogenerated electrons could react with O2 to generate the anionic radical of superoxide and eventually the hydroxyl radical OH∙. Alternatively, the photo-generated vacancies would interact with water in the medium to produce the hydroxyl radical OH∙. An attack of the produced hydroxyl radicals on the dye molecules that adsorbed on the photocatalyst surface leads to the rapid generation of intermediate chemical compounds, and consequently to environmentally palatable green chemicals, namely H2O and CO2 (Equations (19) and (20)):
H2O2 + hυ → 2OH∙
Organic compounds + OH∙ → Intermediaries
Intermediaries → CO2 + H2O
3.2.2. Enhanced Photocatalytic Activity
Xiao et al. [51] and Miklos et al. [52] have implemented the integration of hydrogen peroxide (H2O2) and ultraviolet (UV) radiation to oxidize many biological contaminants, because H2O2 allows for improved generation of hydroxyl radicals (OH∙) to facilitate photodegradation. Nevertheless, the kinetics of the creation of these radicals is limited by an exclusive use of H2O2 and UV radiation. For this reason, to increase the photocatalyst production of hydroxyl radicals (OH∙) and the photodegradation of phenol, the photo-Fenton method was implemented.
To prove that hydroxyl radicals are the major reactive species, a photocatalytic test in the presence of isopropyl alcohol should be carried out. Following photocatalysis in the presence of H2O2 and isopropyl, the UV-vis spectrum of phenol is shown in Figure 4c. Photo degradation results in view of the existence of H2O2 and isopropyl are illustrated in Figure 4d. It is observable that a satisfactory improvement in the percentage degradation was recorded after 15 min of photocatalysis involving the catalyst/H2O2/isopropyl/UV-Vis, in contrast to the experiments carried out in the lack of H2O2 (Figure 4b). It could also be noted that a relatively low rate of degradation for phenol was realized without a photocatalyst (H2O2/visible light) (Figure 4b). The outcome could be related to the low quantities of hydroxyl radicals (OH∙) generated in the procedure, suggesting a restriction of the activity of hydrogen peroxide in the Fenton approach.
In the case of the CdO/ZnO/Yb2O3 NSs, greater efficiencies were obtained after about 15 min, attaining a photodegradation rate of 97.81% of phenol (Figure 4d). With of H2O2 present, CdO/ZnO/Yb2O3 NSs gained a much higher catalytic efficiency than that observed in the lack of H2O2 (Figure 4d). Improved hydroxyl radical (OH∙) production from the breakdown of the Cd2+, Zn2+, and Yb3 cations facilitated by H2O2 is attributable to this result, which is reproduced by the interaction between the cation and the photo-generated electrons and by the reaction of the vacancies with the water molecules present on the catalyst surface. It has been reported in the literature that hydrogen peroxide could also be catalyzed by Cd2+, ions; however, because of the low reaction of Cd (III) relative to H2O2, the decomposition of organic contaminants with photon Fenton reagents (Cd3+/H2O2) is considerably slower than that with Cd2+/H2O2 reagents [52].
According to the above, the application of H2O2 is a key factor in enhancing the rate of photogenerated hydroxyl radicals (OH•) and consequently the photodegradation of organic dyes. As a result, we demonstrate that the reorganization of electron–hole pairs in this study is significantly inhibited and the phenol/catalyst/H2O2/isopropyl/UV-visible light irradiation (Figure 4c) generates more oxidation radicals (OH•) compared to the normal photocatalytic phenol/catalyst/UV-visible light (Figure 4b). In addition, all the above-mentioned results together indicate that the photocatalytic performance of the synthesized oxide materials is significantly improved.
4. Conclusions
The simple, well-controlled, one-pot hydrothermal approach was adopted for the effective synthesis of CdO/ZnO/Yb2O3 catalyst at elevated temperatures. After calcination at 600 °C, well-crystallized CdO/ZnO/Yb2O3 was produced, which is described by XRD, XPS, FTIR, SEM, and EDS. With H2O2 present, CdO/ZnO/Yb2O3 showed a higher catalytic activity (97.81%) for phenol photodegradation. There is a considerable relationship between the effectiveness of the photocatalytic process and an indirect mechanism of heterogeneous photocatalysis. Our results demonstrate that light-driven photo-catalysis with the use of CdO/ZnO/Yb2O3 as catalyst may be regarded as more advantageous because of its financial feasibility and environmental durability, as solar light could be easily employed for such a process. Furthermore, the photocatalytic procedure could also facilitate the total decomposition of the organic pollutants (the mineralization of such chemical compounds in an environmentally acceptable manner, CO2 and H2O) and reduce their toxicities. The synthesized composite is therefore a prospective candidate for the quick disposal of wastewater pollution with persistent organic pollutants.
Author Contributions
Conceptualization: W.J.; methodology: Z.L.; validation: H.Z.; formal analysis: Z.L., H.Z. and W.J.; data curation: Z.L.; writing—original draft: Z.L.; writing—review and editing: Z.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Nature Science Foundation of Heilongjiang province of China, grant number LH2019E113, and the Youth Doctor Foundation of Harbin university, grant number HUDF2017105.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
The authors acknowledge the School of Food Engineering of Harbin University and Harbin Institute of Technology. Z.L. would like to thank to the Youth Doctor Foundation of Harbin University (grant HUDF2017105). H.K.Z. would like to thank the NSF (grant LH2019E113).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Molinari, C.; Conte, S.; Zanelli, C.; Ardit, M.; Cruciani, G.; Dondi, M. Ceramic pigments and dyes beyond the inkjet revolution: From technological requirements to constraints in colorant design. Ceram. Int. 2020, 46, 21839–21872. [Google Scholar] [CrossRef]
- Wei, F.; Shahid, M.; Alnusairi, G.; Afzal, M.; Khan, A.; El-Esawi, M.; Abbas, Z.; Wei, K.; Zaheer, I.; Rizwan, M.; et al. Implementation of Floating Treatment Wetlands for Textile Wastewater Management: A Review. Sustainability 2020, 12, 5801. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, X.; Cong, S.; Geng, F.; Zhao, Z. Fusing electrochromic technology with other advanced technologies: A new roadmap for future development. Mater. Sci. Eng. R Rep. 2020, 140, 100524. [Google Scholar] [CrossRef]
- Ardakani, M.N.; Gholikandi, G.B. Microbial fuel cells (MFCs) in integration with anaerobic treatment processes (AnTPs) and membrane bioreactors (MBRs) for simultaneous efficient wastewater/sludge treatment and energy recovery—A state-of-the-art review. Biomass Bioenergy 2020, 141, 105726. [Google Scholar] [CrossRef]
- Liu, L.; Cheng, Q. Mass transfer characteristic research on electrodialysis for desalination and regeneration of solution: A comprehensive review. Renew. Sustain. Energy Rev. 2020, 134, 110115. [Google Scholar] [CrossRef]
- Rahmat, M.; Rehman, A. Highly efficient removal of crystal violet dye from water by MnO2 based nanofibrous mesh/photocatalytic process. J. Mater. Res. Technol. 2019, 8, 5149–5159. [Google Scholar] [CrossRef]
- Luo, T.; Wang, H.; Chen, L.; Li, J.; Wu, F.; Zhou, D. Visible light-driven oxidation of arsenite, sulfite and thiazine dyes: A new strategy for using waste to treat waste. J. Clean. Prod. 2021, 280, 124374. [Google Scholar] [CrossRef]
- Negishi, N.; Miyazaki, Y. Effect of HCO3−concentration in groundwater on TiO2 photocatalytic water purifica-tion. Appl. Catal. B-Environ. 2019, 242, 449–459. [Google Scholar] [CrossRef]
- Suthakaran, S.; Dhanapandian, S.; Krishnakumar, N.; Ponpandian, N. Surfactants assisted SnO2 nanoparticles synthesized by a hydrothermal approach and potential applications in water purification and energy conversion. J. Mater. Sci. Mater. Electron. 2019, 30, 13174–13190. [Google Scholar] [CrossRef]
- Youssef, Z.; Colombeau, L.; Yesmurzayeva, N.; Baros, F.; Vanderesse, R.; Hamieh, T.; Toufaily, J.; Frochot, C.; Roques-Carmes, T.; Acherar, S. Dye-sensitized nanoparticles for heterogeneous photocatalysis: Cases studies with TiO2, ZnO, fullerene and graphene for water purification. Dye. Pigment. 2018, 159, 49–71. [Google Scholar] [CrossRef]
- Chaudharyab, K.; Shaheena, N.; Zulfiqarc, S.; Sarwar, M.I.; Sulemane, M.; Agboola, P.O.; Shakirg, I.; Warsi, M.F. Binary WO3-ZnO nanostructures supported rGO ternary nanocomposite for visible light driven photocatalytic degradation of methylene blue. Synth. Met. 2020, 269, 116526. [Google Scholar] [CrossRef]
- Trawiński, J.; Skibiński, R. Multivariate comparison of photocatalytic properties of thirteen nanostructured metal oxides for water purification. J. Environ. Sci. Health Part A 2019, 54, 851–864. [Google Scholar] [CrossRef] [PubMed]
- Tayebee, R.; Esmaeili, E.; Maleki, B.; Khoshniat, A.; Chahkandi, M.; Mollania, N. Photodegradation of methylene blue and some emerging pharmaceutical micropollutants with an aqueous suspension of WZnO-NH2@H3PW12O40 nanocomposite. J. Mol. Liq. 2020, 317, 113928. [Google Scholar] [CrossRef]
- Karthikeyan, C.; Arunachalam, P.; Ramachandran, K.; Al-Mayouf, A.M.; Karuppuchamy, S. Recent advances in semiconductor metal oxides with enhanced methods for solar photocatalytic applications. J. Alloy. Compd. 2020, 828, 154281. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, H.; Tu, W.; Wu, S.; Liu, Y.; Tan, Y.Z.; Luo, H.; Yuan, X.; Chew, J.W. Petal-like CdS nanostructures coated with exfoliated sulfur-doped carbon nitride via chemically activated chain termination for enhanced visible-light–driven photocatalytic water purification and H2 generation. Appl. Catal. B Environ. 2018, 229, 181–191. [Google Scholar] [CrossRef]
- Sabet, M.; Mohammadi, M.; Googhari, F. Prominent Visible Light Photocatalytic and Water Purification Activity of PbS/CdS/CdO Nanocomposite Synthesized via Simple Co-Precipitation Method. Nanosci. Nanotechnol. Asia 2019, 9, 278–284. [Google Scholar] [CrossRef]
- Wang, X.; Brigante, M.; Dong, W.; Wu, Z.; Mailhot, G. Degradation of Acetaminophen via UVA-induced advanced oxidation processes (AOPs). Involvement of different radical species: HO∙, SO4∙−and HO2∙/O2∙−. Chemosphere 2020, 258, 127268. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, R.K.; Chottanahalli, S.P.K.; Madegowda, N.M.; Rai, V.R.; Ananda, S. Electrochemical synthesis of hierarchal flower-like hierarchical In2O3/ZnO nanocatalyst for textile industry effluent treatment, photo-voltaic, OH scavenging and anti-bacterial studies. Catal. Commun. 2017, 89, 25–28. [Google Scholar] [CrossRef]
- Kumar, M.R.; Murugadoss, G.; Pirogov, A.N.; Thangamuthu, R. A facile one step synthesis of SnO2/CuO and CuO/SnO2 nanocomposites: Photocatalytic application. J. Mater. Sci. Mater. Electron. 2018, 29, 13508–13515. [Google Scholar] [CrossRef]
- Zeleke, M.A.; Kuo, D.-H.; Ahmed, K.E.; Gultom, N.S. Facile synthesis of bimetallic (In,Ga)2(O,S)3 oxy-sulfide nanoflower and its enhanced photocatalytic activity for reduction of Cr(VI). J. Colloid Interface Sci. 2018, 530, 567–578. [Google Scholar] [CrossRef] [PubMed]
- Sudrajat, H.; Hartuti, S.; Babel, S.; Nguyen, T.K.; Tong, H.D. SnO2/ZnO heterostructured nanorods: Structural properties and mechanistic insights into the enhanced photocatalytic activity. J. Phys. Chem. Solids 2021, 149, 109762. [Google Scholar] [CrossRef]
- Yu, Y.; Yang, Q.; Ma, J.; Sun, W.; Yin, C.; Li, X.; Guo, J.; Jiang, Q.; Lu, Z. Construction of Strontium Titanate/Binary Metal Sulfide Heterojunction Photocatalysts for Enhanced Visible-Light-Driven Photocatalytic Activity. Nano 2018, 13, 11. [Google Scholar] [CrossRef]
- Sales, H.; Menezes, R.; Neves, G.; Souza, J.; Ferreira, J.; Chantelle, L.; De Oliveira, A.M.; Lira, H. Development of Sustainable Heterogeneous Catalysts for the Photocatalytic Treatment of Effluents. Sustainability 2020, 12, 7393. [Google Scholar] [CrossRef]
- Adnan, M.A.M.; Julkapli, N.M.; Hamid, S.B.A. Review on ZnO hybrid photocatalyst: Impact on photocatalytic activities of water pollutant degradation. Rev. Inorg. Chem. 2016, 36, 77–104. [Google Scholar] [CrossRef]
- Kumar, S.; Gupta, M.; Sathe, V.; Shripathi, T.; Phase, D.; Das, B. Study of the structural phase transformation, and optical behavior of the as synthesized ZnO–SnO2–TiO2 nanocomposite. Phase Transit. 2015, 88, 1122–1136. [Google Scholar] [CrossRef]
- Kumar, D.P.; Park, H.; Kim, E.H.; Hong, S.; Gopannagari, M.; Reddy, D.A.; Kim, T.K. Noble metal-free metal-organic framework-derived onion slice-type hollow cobalt sulfide nanostructures: Enhanced activity of CdS for improving photocatalytic hydrogen production. Appl. Catal. B Environ. 2018, 224, 230–238. [Google Scholar] [CrossRef]
- Vaizoğullar, A.I. Ternary CdS/MoS2/ZnO Photocatalyst: Synthesis, Characterization and Degradation of Ofloxacin under Visible Light Irradiation. J. Inorg. Organomet. Polym. Mater. 2020, 30, 4129–4141. [Google Scholar] [CrossRef]
- Ma, W.; Mack, D.; Malzbender, J.; Vaßen, R.; Stöver, D. Yb2O3 and Gd2O3 doped strontium zirconate for thermal barrier coatings. J. Eur. Ceram. Soc. 2008, 28, 3071–3081. [Google Scholar] [CrossRef]
- Liu, T.; Bai, X.; Miao, C.; Dai, Q.; Xu, W.; Yu, Y.; Chen, Q.; Song, H. Yb2O3/Au Upconversion Nanocomposites with Broad-Band Excitation for Solar Cells. J. Phys. Chem. C 2014, 118, 3258–3265. [Google Scholar] [CrossRef]
- Chiappetta, G.; Ndiaye, S.; Igbaria, A.; Kumar, C.; Vinh, J.; Toledano, M.B. Proteome Screens for Cys Residues Oxidation. Methods Enzymol. 2010, 473, 199–216. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.M.; Alam, M.M.; Asiri, A.M.; Islam, M.A. Ethanol sensor development based on ternary-doped metal oxides (CdO/ZnO/Yb2O3) nanosheets for environmental safety. RSC Adv. 2017, 7, 22627–22639. [Google Scholar] [CrossRef]
- Sales, H.B.; Bouquet, V.; Députier, S.; Ollivier, S.; Gouttefangeas, F.; Guilloux-Viry, M.; Dorcet, V.; Weber, I.T.; De Souza, A.G.; Dos Santos, I.M.G. Sr1−xBaxSnO3 system applied in the photocatalytic discoloration of an azo-dye. Solid State Sci. 2014, 28, 67–73. [Google Scholar] [CrossRef]
- Rahman, Q.I.; Ahmad, M.; Misra, S.K.; Lohani, M. Effective photocatalytic degradation of rhodamine B dye by ZnO nanoparticles. Mater. Lett. 2013, 91, 170–174. [Google Scholar] [CrossRef]
- Chantelle, L.; De Oliveira, A.L.M.; Kennedy, B.J.; Maul, J.; Da Silva, M.R.S.; Duarte, T.M.; Albuquerque, A.R.; Sambrano, J.R.; Landers, R.; Siu-Li, M.; et al. Probing the Site-Selective Doping in SrSnO3:Eu Oxides and Its Impact on the Crystal and Electronic Structures Using Synchrotron Radiation and DFT Simulations. Inorg. Chem. 2020, 59, 7666–7680. [Google Scholar] [CrossRef]
- Soylu, M.; Kader, H.S. Photodiode Based on CdO Thin Films as Electron Transport Layer. J. Electron. Mater. 2016, 45, 5756–5763. [Google Scholar] [CrossRef]
- Thema, F.; Beukes, P.; Gurib-Fakim, A.; Maaza, M. Green synthesis of Monteponite CdO nanoparticles by Agathosma betulina natural extract. J. Alloy. Compd. 2015, 646, 1043–1048. [Google Scholar] [CrossRef]
- Gutul, T.; Rusu, E.; Condur, N.; Ursaki, V.; Goncearenco, E.; Vlazan, P. Preparation of poly(N-vinylpyrrolidone)-stabilized ZnO colloid nanoparticles. Beilstein J. Nanotechnol. 2014, 5, 402–406. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, M.; Li, Y.; Du, Q.; Yuan, Y.; Ren, Y.; Liu, B.; Zhao, H.; Yang, H. Enhancing gas sensitivity of CdO octahedrons having {111} facets by hydrogenation and sensing mechanism of 3-coordinated Cd atoms as the reactive centers. Appl. Surf. Sci. 2020, 506, 144868. [Google Scholar] [CrossRef]
- Qian, C.; Zeng, T.; Liu, H. Synthesis and Downconversion Emission Property of Yb2O3:Eu3+Nanosheets and Nanotubes. Adv. Condens. Matter Phys. 2013, 2013, 1–5. [Google Scholar] [CrossRef]
- Shestakov, M.V.; Tikhomirov, V.K.; Kirilenko, D.; Kuznetsov, A.S.; Chibotaru, L.F.; Baranov, A.N.; Van Tendeloo, G.; Moshchalkov, V.V. Quantum cutting in Li (770 nm) and Yb (1000 nm) co-dopant emission bands by energy transfer from the ZnO nano-crystalline host. Opt. Express 2011, 19, 15955–15964. [Google Scholar] [CrossRef] [PubMed]
- Bagal, V.S.; Patil, G.P.; Deore, A.B.; Suryawanshi, S.R.; Late, D.J.; More, M.A.; Chavan, P.G. Surface modification of aligned CdO nanosheets and their enhanced field emission properties. RSC Adv. 2016, 6, 41261–41267. [Google Scholar] [CrossRef]
- Haeberle, J.; Henkel, K.; Gargouri, H.; Naumann, F.; Gruska, B.; Arens, M.; Tallarida, M.; Schmeißer, D. Ellipsometry and XPS comparative studies of thermal and plasma enhanced atomic layer deposited Al2O3-films. Beilstein J. Nanotechnol. 2013, 4, 732–742. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.L.; Mohammed, I.A.; Belmahi, M.; Assouar, M.B.; Rinnert, H.; Alnot, M. Thermal and Optical Properties of CdS Nanoparticles in Thermotropic Liquid Crystal Monomers. Materials 2010, 3, 2069–2086. [Google Scholar] [CrossRef]
- Xu, D.; Fan, D.; Shen, W. Catalyst-free direct vapor-phase growth of Zn1−xCuxO micro-cross structures and their optical properties. Nanoscale Res. Lett. 2013, 8, 46. [Google Scholar] [CrossRef]
- Zhang, F.; Wu, X.; Liang, C.; Li, X.; Wang, Z.; Li, H. Highly active, water-compatible and easily separable magnetic mesoporous Lewis acid catalyst for the Mukaiyama–Aldol reaction in water. Green Chem. 2014, 16, 3768–3777. [Google Scholar] [CrossRef]
- Yin, Q.; Jin, X.; Yang, G.; Jiang, C.; Song, Z.; Sun, G. Biocompatible folate-modified Gd3+/Yb3+-doped ZnO nanoparticles for dualmodal MRI/CT imaging. RSC Adv. 2014, 4, 53561–53569. [Google Scholar] [CrossRef]
- Duarte, A.A.L.S.; Cardoso, S.J.A.; Alçada, A.J. Emerging and Innovative Techniques for Arsenic Removal Applied to a Small Water Supply System. Sustainability 2009, 1, 1288–1304. [Google Scholar] [CrossRef]
- Molinari, R.; Argurio, P.; Poerio, T. Membrane Processes Based on Complexation Reactions of Pollutants as Sustainable Wastewater Treatments. Sustainability 2009, 1, 978–993. [Google Scholar] [CrossRef]
- Honorio, L.M.C.; de Oliveira, A.L.M.; Filho, E.C.D.S.; Osajima, J.A.; Hakki, A.; Macphee, D.E.; dos Santos, I.M.G. Supporting the photocatalysts on ZrO2: An effective way to enhance the photocatalytic activity of SrSnO3. Appl. Surf. Sci. 2020, 528, 146991. [Google Scholar] [CrossRef]
- Ong, C.B.; Ng, L.Y.; Mohammad, A.W. A review of ZnO nanoparticles as solar photocatalysts: Synthesis, mechanisms and applications. Renew. Sustain. Energy Rev. 2018, 81, 536–551. [Google Scholar] [CrossRef]
- Xiao, C.; Li, J.; Zhang, G. Synthesis of stable burger-like α-Fe2O3 catalysts: Formation mechanism and excellent photo-Fenton catalytic performance. J. Clean. Prod. 2018, 180, 550–559. [Google Scholar] [CrossRef]
- Miklos, D.B.; Remy, C.; Jekel, M.; Linden, K.G.; Drewes, J.E.; Hübner, U. Evaluation of advanced oxidation processes for water and wastewater treatment—A critical review. Water Res. 2018, 139, 118–131. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).