Residues from Water Precipitation via Ferric Hydroxide Threaten Soil Fertility
Abstract
1. Introduction
2. Methodology
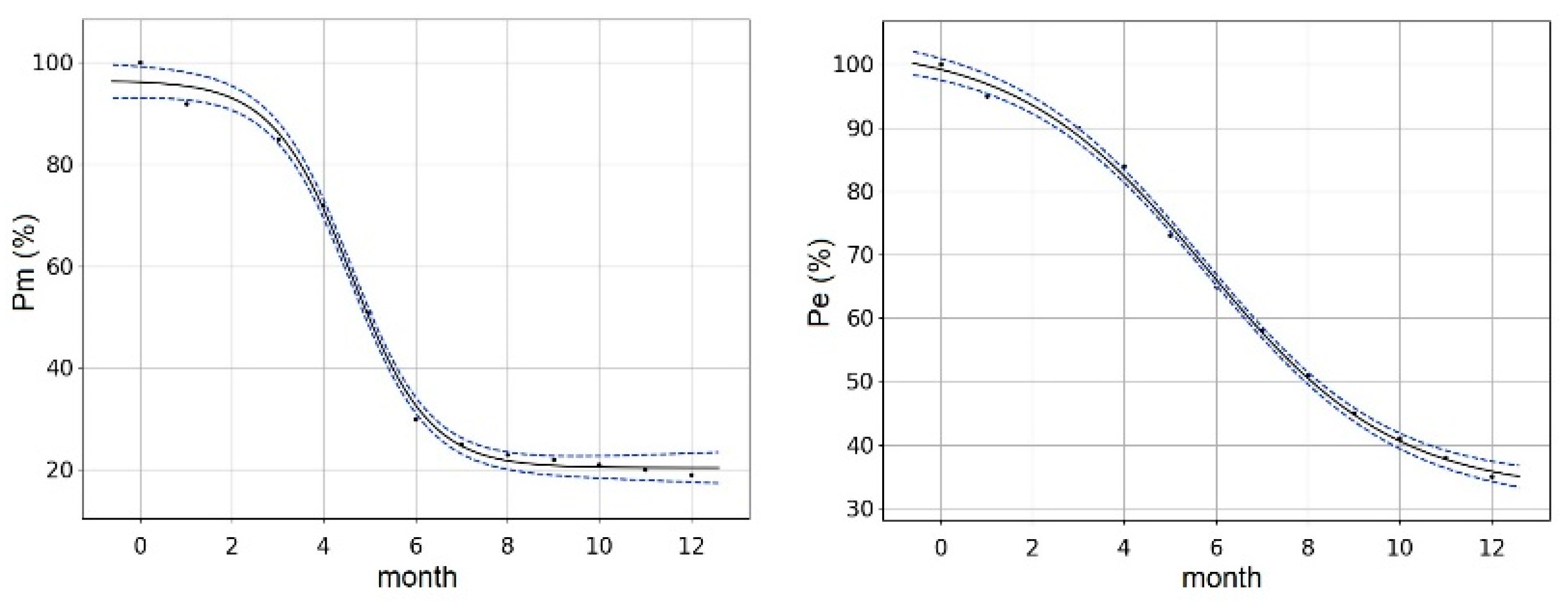
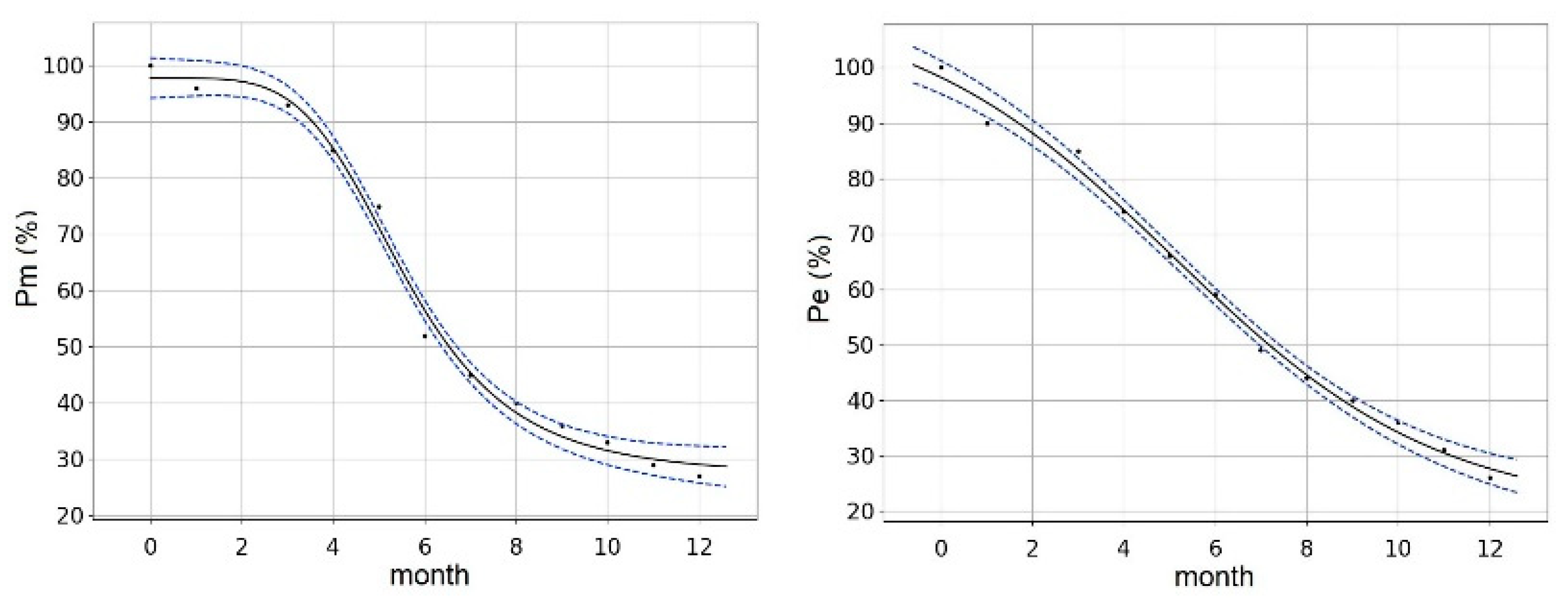
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kliestik, T.; Nica, E.; Musa, H.; Poliak, M.; Mihai, E.A. Networked, Smart, and Responsive Devices in Industry 4.0 Manufacturing Systems. Econ. Manag. Financ. Mark. 2020, 15, 23–29. [Google Scholar]
- Beever, J.; Brightman, A.O. Reflexive Principlism as an Effective Approach for Developing Ethical Reasoning in Engineering. Sci. Eng. Ethics 2015, 22, 275–291. [Google Scholar] [CrossRef] [PubMed]
- Winn, M.L.; Angell, L.C. Towards a Process Model of Corporate Greening. Organ. Stud. 2000, 21, 1119–1147. [Google Scholar] [CrossRef]
- Maroušek, J.; Kolář, L.; Strunecký, O.; Kopecký, M.; Bartoš, P.; Maroušková, A.; Cudlínová, E.; Konvalina, P.; Šoch, M. Modified biochars present an economic challenge to phosphate management in wastewater treatment plants. J. Clean. Prod. 2020, 272, 123015. [Google Scholar] [CrossRef]
- Maroušek, J.; Rowland, Z.; Valášková, K.; Král, P. Techno-economic assessment of potato waste management in developing economies. Clean Technol. Environ. Policy 2020, 22, 937–944. [Google Scholar] [CrossRef]
- Kliestik, T.; Valaskova, K.; Nica, E.; Kovacova, M.; Lazaroiu, G. Advanced methods of earnings management: Monotonic trends and change-points under spotlight in the Visegrad countries. Oecon. Copernic. 2020, 11, 371–400. [Google Scholar] [CrossRef]
- Kliestik, T.; Misankova, M.; Valaskova, K.; Svabova, L. Bankruptcy Prevention: New Effort to Reflect on Legal and Social Changes. Sci. Eng. Ethics 2017, 24, 791–803. [Google Scholar] [CrossRef]
- Delmas, M.A.; Burbano, V.C. The Drivers of Greenwashing. Calif. Manag. Rev. 2011, 54, 64–87. [Google Scholar] [CrossRef]
- Mardoyan, A.; Braun, P. Analysis of Czech Subsidies for Solid Biofuels. Int. J. Green Energy 2015, 12, 405–408. [Google Scholar] [CrossRef]
- Kovacova, M.; Kliestik, T.; Valaskova, K.; Durana, P.; Juhaszova, Z. Systematic review of variables applied in bankruptcy prediction models of Visegrad group countries. Oecon. Copernic. 2019, 10, 743–772. [Google Scholar] [CrossRef]
- O’Grady, P.F. Thales of Miletus: The Beginnings of Western Science and Philosophy; Routledge: London, UK, 2017. [Google Scholar]
- Zaman, S.; Begum, A.; Rabbani, K.S.; Bari, L. Low cost and sustainable surface water purification methods using Moringa seeds and scallop powder followed by bio-sand filtration. Water Supply 2016, 17, 125–137. [Google Scholar] [CrossRef]
- Singh, N.; Nagpal, G.; Agrawal, S. Rachna Water purification by using Adsorbents: A Review. Environ. Technol. Innov. 2018, 11, 187–240. [Google Scholar] [CrossRef]
- Foster, J.E. Plasma-based water purification: Challenges and prospects for the future. Phys. Plasmas 2017, 24, 055501. [Google Scholar] [CrossRef]
- Tortajada, C.; van Rensburg, P. Drink more recycled wastewater. Nature 2020, 577, 26–28. [Google Scholar] [CrossRef]
- Maroušek, J.; Kwan, J.T.H. Use of pressure manifestations following the water plasma expansion for phytomass disintegration. Water Sci. Technol. 2013, 67, 1695–1700. [Google Scholar] [CrossRef] [PubMed]
- Marasova, J.; Vallušová, A.; Vasileva, E.S.; de Saint Julien, D.P. CSR Practices: The Case of Veolia in Three European Countries. In Responsible Organizations in the Global Context; Palgrave Macmillan: Cham, Switzerland, 2019; pp. 175–197. [Google Scholar]
- Peters, E.; Kliestik, T.; Musa, H.; Durana, P. Product decision-making information systems, real-time big data analytics, and deep learning-enabled smart process planning in sustainable industry 4.0. J. Self-Gov. Manag. Econ. 2020, 8, 16–22. [Google Scholar]
- Bolisetty, S.; Peydayesh, M.; Mezzenga, R. Sustainable technologies for water purification from heavy metals: Review and analysis. Chem. Soc. Rev. 2019, 48, 463–487. [Google Scholar] [CrossRef]
- Bull, R.J.; Birnbaum, L.; Cantor, K.P.; Rose, J.B.; Butterworth, B.E.; Pegram, R.; Tuomisto, J. Water Chlorination: Essential Process or Cancer Hazard? Toxicol. Sci. 1995, 28, 155–166. [Google Scholar] [CrossRef]
- Roccaro, P.; Mancini, G.; Vagliasindi, F.G. Water intended for human consumption—Part I: Compliance with European water quality standards. Desalination 2005, 176, 1–11. [Google Scholar] [CrossRef]
- Exley, C. Aluminum Should Now Be Considered a Primary Etiological Factor in Alzheimer’s Disease. J. Alzheimer’s Dis. Rep. 2017, 1, 23–25. [Google Scholar] [CrossRef] [PubMed]
- Yin, R.; Fan, C.; Sun, J.; Shang, C. Oxidation of iron sulfide and surface-bound iron to regenerate granular ferric hydroxide for in-situ hydrogen sulfide control by persulfate, chlorine and peroxide. Chem. Eng. J. 2018, 336, 587–594. [Google Scholar] [CrossRef]
- Gomes, S.D.C.; Zhou, J.L.; Li, W.; Long, G. Progress in manufacture and properties of construction materials incorporating water treatment sludge: A review. Resour. Conserv. Recycl. 2019, 145, 148–159. [Google Scholar] [CrossRef]
- Maroušek, J.; Strunecký, O.; Stehel, V. Biochar farming: Defining economically perspective applications. Clean Technol. Environ. Policy 2019, 21, 1389–1395. [Google Scholar] [CrossRef]
- George, T.S.; Hinsinger, P.; Turner, B.L. Phosphorus in soils and plants–facing phosphorus scarcity. Plant Soil 2016, 401, 1–6. [Google Scholar] [CrossRef]
- Cordell, D. Global phosphorus scarcity: A food secure future? J. Nutr. Intermed. Metab. 2017, 8, 61–62. [Google Scholar] [CrossRef]
- Maroušek, J.; Stehel, V.; Vochozka, M.; Kolář, L.; Maroušková, A.; Strunecký, O.; Peterka, J.; Kopecký, M.; Shreedhar, S. Ferrous sludge from water clarification: Changes in waste management practices advisable. J. Clean. Prod. 2019, 218, 459–464. [Google Scholar] [CrossRef]
- DeSmidt, E.; Ghyselbrecht, K.; Zhang, Y.; Pinoy, L.; Van Der Bruggen, B.; Verstraete, W.; Rabaey, K.; Meesschaert, B. Global Phosphorus Scarcity and Full-Scale P-Recovery Techniques: A Review. Crit. Rev. Environ. Sci. Technol. 2015, 45, 336–384. [Google Scholar] [CrossRef]
- Busch, D.; Kammann, C.; Grünhage, L.; Muller, C. Simple Biotoxicity Tests for Evaluation of Carbonaceous Soil Additives: Establishment and Reproducibility of Four Test Procedures. J. Environ. Qual. 2012, 41, 1023–1032. [Google Scholar] [CrossRef] [PubMed]
- Maroušek, J. Novel technique to enhance the disintegration effect of the pressure waves on oilseeds. Ind. Crop. Prod. 2014, 53, 1–5. [Google Scholar] [CrossRef]
- Valaskova, K.; Throne, O.; Kral, P.; Michalkova, L. Deep learning-enabled smart process planning in cyber-physical system-based manufacturing. J. Self-Gov. Manag. Econ. 2020, 8, 121–127. [Google Scholar]
- Stávková, J.; Maroušek, J. Novel sorbent shows promising financial results on P recovery from sludge water. Chemosphere 2021, 276, 130097. [Google Scholar] [CrossRef] [PubMed]
- Cordell, D.; White, S. Life’s bottleneck: Sustaining the world’s phosphorus for a food secure future. Annu. Rev. Environ. Resour. 2014, 39, 161–188. [Google Scholar] [CrossRef]
- Menezes-Blackburn, D.; Giles, C.D.; Darch, T.; George, T.S.; Blackwell, M.S.A.; Stutter, M.; Shand, C.; Lumsdon, D.; Cooper, P.; Wendler, R.; et al. Opportunities for mobilizing recalcitrant phosphorus from agricultural soils: A review. Plant Soil 2018, 427, 5–16. [Google Scholar] [CrossRef] [PubMed]
- Hancock, J. Environmental Human Rights: Power, Ethics and Law; Routledge: London, UK, 2019. [Google Scholar]
- Maroušek, J.; Myšková, K.; Žák, J. Managing Environmental Innovation: Case Study on Biorefinery Concept; Revista Técnica de la Facultad de Ingeniería Universidad del Zulia: Caracas, Venezuela, 2015; Volume 38, pp. 216–220. [Google Scholar]
- Fan, D.; Lan, Y.; Tratnyek, P.G.; Johnson, R.L.; Filip, J.; O’Carroll, D.M.; Garcia, A.N.; Agrawal, A. Sulfidation of Iron-Based Materials: A Review of Processes and Implications for Water Treatment and Remediation. Environ. Sci. Technol. 2017, 51, 13070–13085. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.-H.; Wang, S.-Y. Application of water treatment sludge in the manufacturing of lightweight aggregate. Constr. Build. Mater. 2013, 43, 174–183. [Google Scholar] [CrossRef]
- Herreño, L.C.F.; Solano, D.M.V.; Sarabia, K.D.R.; Pérez, J.O.C.; Quintero, A.A.M. Drinking water treatment sludge as a partial substitute for clays in non-structural brick production. J. Phys. Conf. Ser. 2019, 1409, 012013. [Google Scholar] [CrossRef]
- Ralston, P.A.; Bays, C.L. Critical Thinking Development in Undergraduate Engineering Students from Freshman through Senior Year: A 3-Cohort Longitudinal Study. Am. J. Eng. Educ. 2015, 6, 85–98. [Google Scholar] [CrossRef]
- Maroušek, J.; Maroušková, A.; Kůs, T. Shower cooler reduces pollutants release in production of competitive cement substitute at low cost. Energy Sources Part A Recovery Util. Environ. Effects 2020, 1–10. [Google Scholar] [CrossRef]
- Glæsner, N.; Helming, K.; De Vries, W. Do Current European Policies Prevent Soil Threats and Support Soil Functions? Sustainability 2014, 6, 9538–9563. [Google Scholar] [CrossRef]
- Psacharopoulos, G. The social cost of an outdated law: Article 16 of the Greek constitution. Eur. J. Law Econ. 2003, 16, 123–137. [Google Scholar] [CrossRef]
- Maroušek, J. Study on commercial scale steam explosion of winter Brassica napus straw. Int. J. Green Energy 2013, 10, 944–951. [Google Scholar] [CrossRef]
- Wallace, J.D. Moral Relevance and Moral Conflict; Cornell University Press: Ithaca, NY, USA, 2019. [Google Scholar]
- Funk, C. Mixed messages about public trust in science. Issues Sci. Technol. 2017, 34, 86–88. [Google Scholar]
- Maroušek, J. Finding the optimal parameters for the steam explosion process of hay. Revista Técnica de la Facultad de Ingeniería Universidad del Zulia 2012, 35, 170–178. [Google Scholar]
- Muhammad, Z.; Yi, F.; Naz, A.S.; Saleem, A. An investigation of justice in supply chain trust and relationship commitment-An empirical study of Pakistan. J. Compet. 2015, 7, 71–87. [Google Scholar]
- Zhu, R.; Chen, Q.; Zhou, Q.; Xi, Y.; Zhu, J.; He, H. Adsorbents based on montmorillonite for contaminant removal from water: A review. Appl. Clay Sci. 2016, 123, 239–258. [Google Scholar] [CrossRef]
- Ramus, C.A.; Montiel, I. When Are Corporate Environmental Policies a Form of Greenwashing? Bus. Soc. 2005, 44, 377–414. [Google Scholar] [CrossRef]
- Vatankhah, S.; Zarra-Nezhad, M.; Amirnejad, G. An empirical assessment of willingness to accept “low-cost” air transport services: Evidence from the Middle East. J. Tour. Serv. 2019, 10, 79–103. [Google Scholar] [CrossRef]
- Lenhard, R.; Malcho, M.; Jandačka, J. Modelling of heat transfer in the evaporator and condenser of the working fluid in the heat pipe. Heat Transf. Eng. 2019, 40, 215–226. [Google Scholar] [CrossRef]
- Sapolsky, R.M. Behave: The Biology of Humans at Our Best and Worst; Penguin: London, UK, 2017. [Google Scholar]
- Stutter, M.I.; Shand, C.A.; George, T.S.; Blackwell, M.S.A.; Dixon, L.; Bol, R.; Mackay, R.L.; Richardson, A.E.; Condron, L.M.; Haygarth, P.M. Land use and soil factors affecting accumulation of phosphorus species in temperate soils. Geoderma 2015, 257–258, 29–39. [Google Scholar] [CrossRef]
- Jandačka, J.; Mičieta, J.; Holubčík, M.; Nosek, R. Experimental Determination of Bed Temperatures during Wood Pellet Combustion. Energy Fuels 2017, 31, 2919–2926. [Google Scholar] [CrossRef]
- Bencsik, A.; Kosár, S.T.; Máchová, R. Corporate Culture in Service Companies that Support Knowledge Sharing. J. Tour. Serv. 2018, 9, 7–13. [Google Scholar] [CrossRef]
- Muo, I.; Azeez, A.A. Green entrepreneurship: Literature review and agenda for future research. Int. J. Entrep. Knowl. 2019, 7, 17–29. [Google Scholar] [CrossRef]
- Maroušek, J. Pretreatment of sunflower stalks for biogas production. Clean Technol. Environ. Policy 2013, 15, 735–740. [Google Scholar] [CrossRef]
- Hendriks, F.; Kienhues, D.; Bromme, R. Trust in science and the science of trust. In Trust and Communication in a Digitized World; Springer: Cham, Switzerland, 2016; pp. 143–159. [Google Scholar]
| A (13) | CZ (14) | F (9) | DE (16) | GB (3) | I (7) | CH (3) | |
|---|---|---|---|---|---|---|---|
| Al2O3 (mg.kg−1) | 0.8 ± 0.2 | 1.1 ± 0.4 | 1.0 ± 0.5 | 0.9 ± 0.4 | 1.0 ± 0.3 | 1.1 ± 0.3 | 0.9 ± 0.2 |
| CaO (mg.kg−1) | 0.3 ± 0.1 | 0.4 ± 0.3 | 0.6 ± 0.3 | 0.3 ± 0.2 | 0.4 ± 0.2 | 0.3 ± 0.2 | 0.9 ± 0.3 |
| Cell (mg.kg−1) | 2.0 ± 0.4 | 1.7 ± 0.3 | 2.2 ± 0.5 | 1.9 ± 0.3 | 1.9 ± 0.6 | 1.9 ± 0.3 | 2.1 ± 0.4 |
| Fe2O3 (mg.kg−1) | 8.9 ± 0.9 | 5.3 ± 1.2 | 9.8 ± 1.5 | 8.3 ± 3.6 | 9.9 ± 2.1 | 7.0 ± 2.4 | 8.3 ± 1.0 |
| Lign (mg.kg−1) | 1.4 ± 0.3 | 1.5 ± 0.3 | 1.5 ± 0.6 | 1.2 ± 0.4 | 1.6 ± 0.4 | 1.6 ± 0.6 | 1.5 ± 0.2 |
| MgO (mg.kg−1) | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.1 | 0.1 ± 0.0 | 0.1 ± 0.1 | 0.2 ± 0.1 | 0.1 ± 0.0 |
| MnO2 (mg.kg−1) | 0.1 ± 0.1 | 0.1 ± 0.0 | 0.2 ± 0.1 | 0.1 ± 0.0 | 0.2 ± 0.1 | 0.2 ± 0.2 | 0.1 ± 0.1 |
| Cox (mg.kg−1) | 2.1 ± 0.1 | 2.1 ± 0.1 | 2.2 ± 0.1 | 2.1 ± 0.1 | 2.1 ± 0.1 | 2.1 ± 0.2 | 2.2 ± 0.1 |
| P2O5 (mg.kg−1) | 0.1 ± 0.0 | 0.1 ± 0.1 | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.0 ± 0.0 |
| SiO2 (mg.kg−1) | 3.6 ± 0.3 | 3.7 ± 0.4 | 3.3 ± 0.5 | 3.4 ± 0.5 | 3.6 ± 0.3 | 3.3 ± 0.3 | 3.4 ± 0.4 |
| S (mg.kg−1) | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.1 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.1 ± 0.0 | 0.0 ± 0.0 |
| VS (mg.kg−1) | 21.0 ± 3.1 | 19.7 ± 4.4 | 22.5 ± 3.5 | 19.1 ± 2.9 | 16.7 ± 3.4 | 20.3 ± 2.2 | 19.5 ± 4.4 |
| I. | II. | III. | IV. | |
|---|---|---|---|---|
| HC0 (%) | 92 | 85 | 81 | 77 |
| HCfs (%) | 91 | 80 | 73 | 66 |
| GC0 (%) | 96 | 94 | 92 | 91 |
| GCfs (%) | 93 | 82 | 68 | 60 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brabenec, T.; Maroušková, A.; Zoubek, T.; Filip, M. Residues from Water Precipitation via Ferric Hydroxide Threaten Soil Fertility. Sustainability 2021, 13, 4327. https://doi.org/10.3390/su13084327
Brabenec T, Maroušková A, Zoubek T, Filip M. Residues from Water Precipitation via Ferric Hydroxide Threaten Soil Fertility. Sustainability. 2021; 13(8):4327. https://doi.org/10.3390/su13084327
Chicago/Turabian StyleBrabenec, Tomáš, Anna Maroušková, Tomáš Zoubek, and Martin Filip. 2021. "Residues from Water Precipitation via Ferric Hydroxide Threaten Soil Fertility" Sustainability 13, no. 8: 4327. https://doi.org/10.3390/su13084327
APA StyleBrabenec, T., Maroušková, A., Zoubek, T., & Filip, M. (2021). Residues from Water Precipitation via Ferric Hydroxide Threaten Soil Fertility. Sustainability, 13(8), 4327. https://doi.org/10.3390/su13084327






