Adsorption Behavior of Acid-Treated Brazilian Palygorskite for Cationic and Anionic Dyes Removal from the Water
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Acid Treatment
2.3. Characterizations
2.4. Adsorption Experiments
2.5. Isothermal, Kinetic, and Thermodynamic Studies
2.6. Regeneration of Adsorbents
3. Results and Discussion
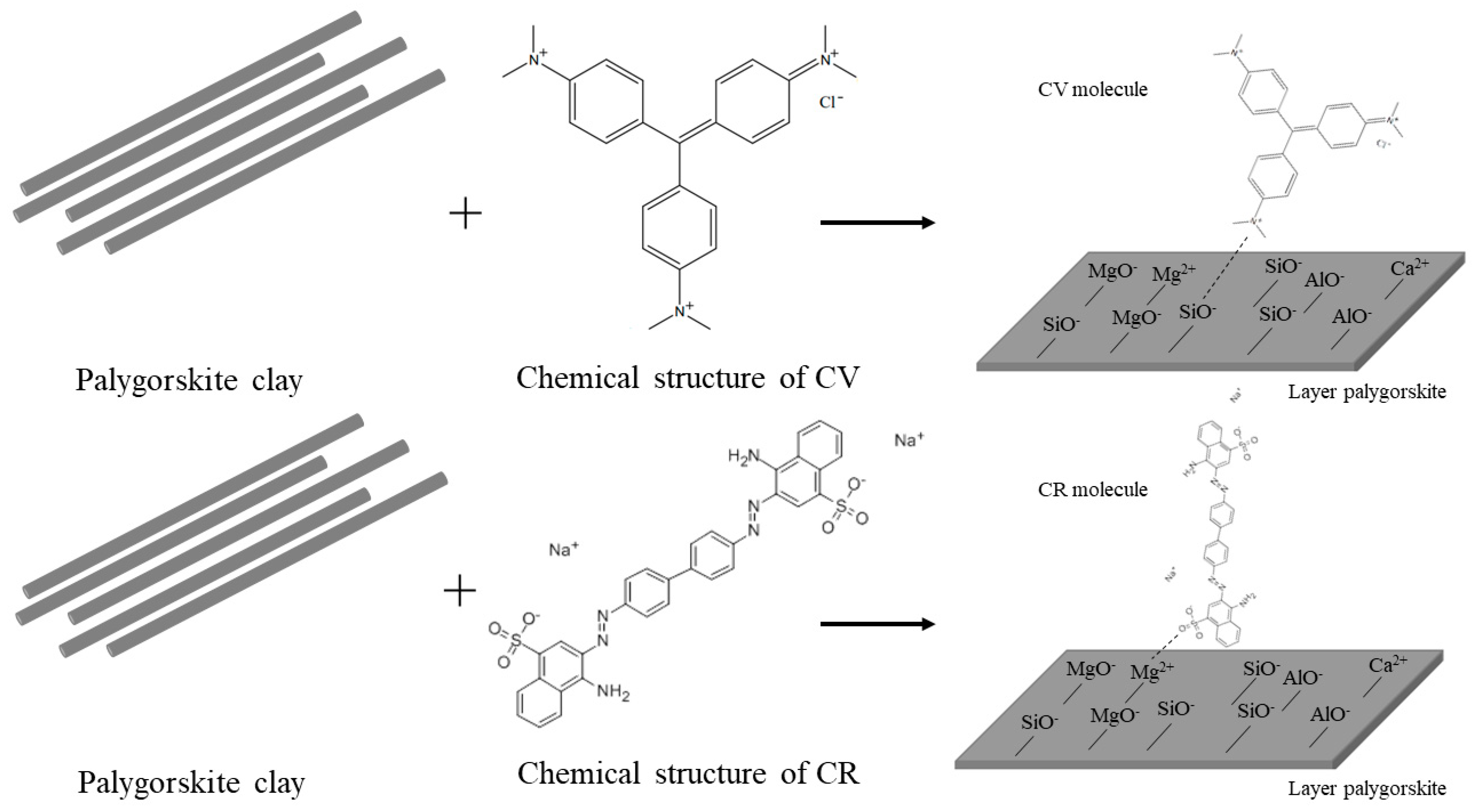
3.1. Characterization of Raw and Acid-Treated Palygorskite
3.2. Optimization of Adsorption Conditions
3.2.1. Effect of Dye Concentrations
3.2.2. Effect of Contact Time
3.2.3. Effect of pH Variation
3.2.4. Effect of Adsorbent Dosage
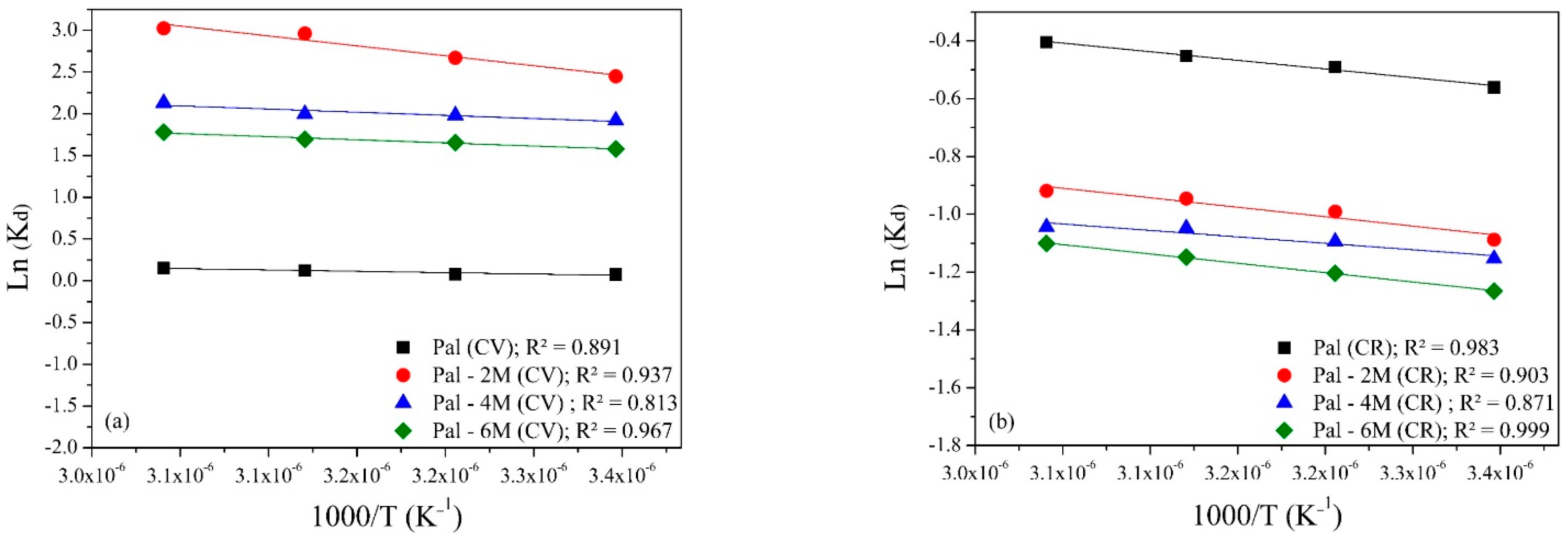
3.2.5. Effect of Temperature
3.3. Adsorption Isotherms
3.4. Adsorption Kinetics
3.5. Adsorption Thermodynamics
3.6. Characterization after Adsorption
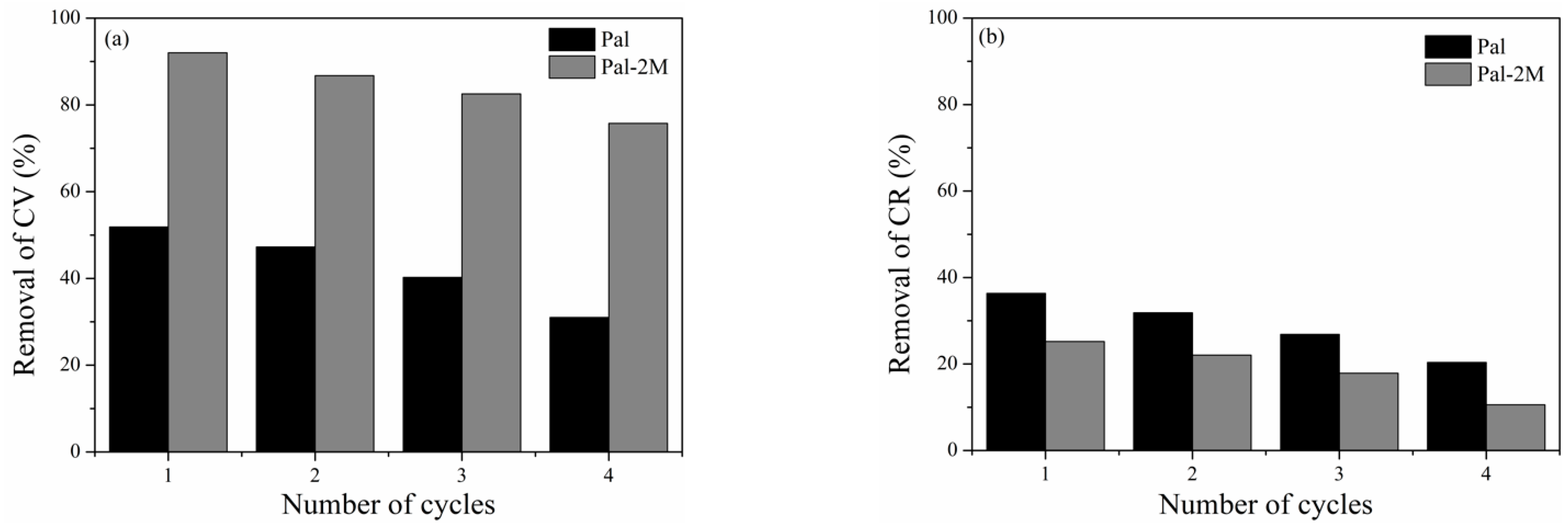
3.7. Regeneration Study
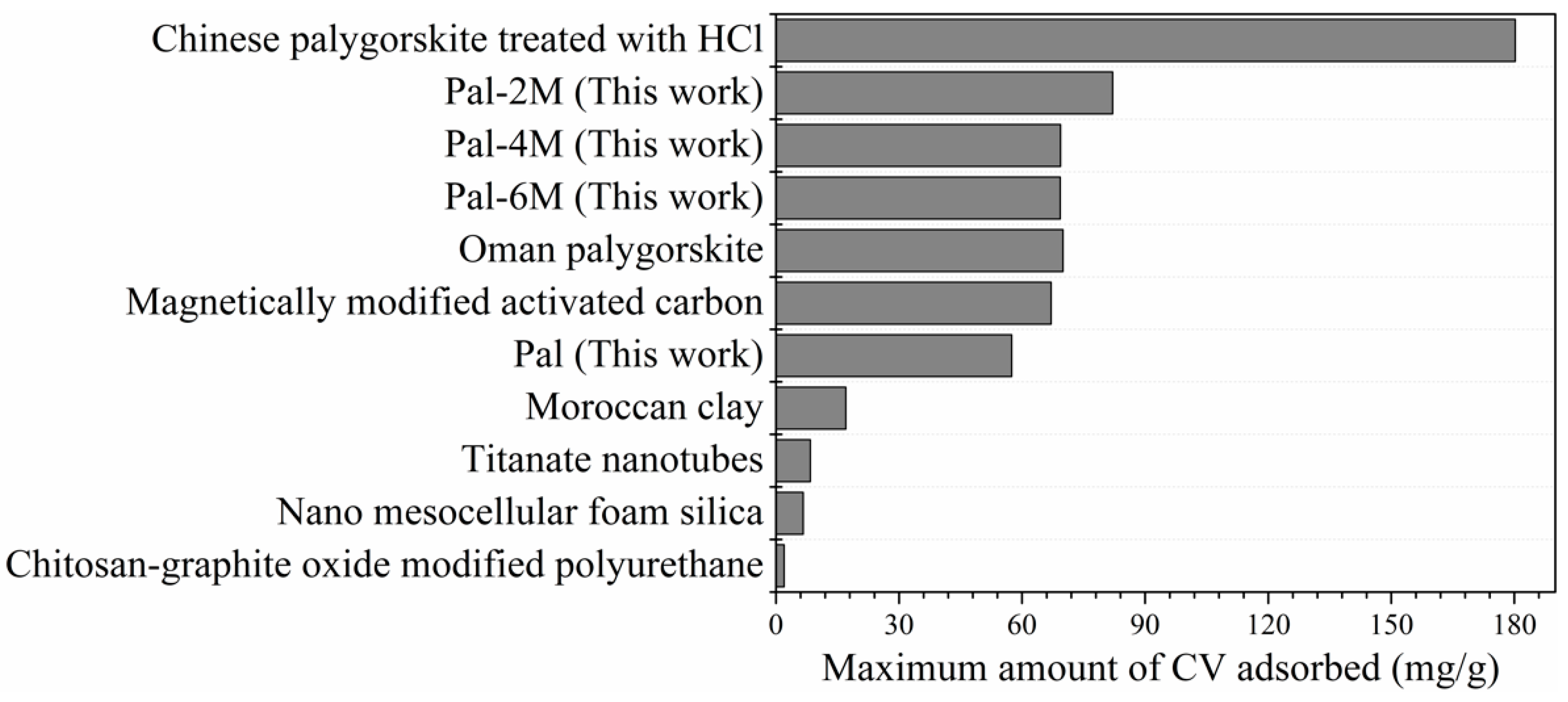
3.8. Comparison with Other Adsorbents
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Goh, P.S.; Ismail, A.F. A review on inorganic membranes for desalination and wastewater treatment. Desalination 2018, 434, 60–80. [Google Scholar] [CrossRef]
- Mirza, A.; Ahmad, R. An efficient sequestration of toxic crystal violet dye from aqueous solution by Alginate/Pectin nanocomposite: A novel and ecofriendly adsorbent. Groundw. Sustain. Dev. 2020, 11, 100373. [Google Scholar] [CrossRef]
- Tyagi, U. Adsorption of dyes using activated carbon derived from pyrolysis of vetiveria zizanioides in a fixed bed reactor. Groundw. Sustain. Dev. 2020, 10, 100303. [Google Scholar] [CrossRef]
- Sirajudheen, P.; Meenakshi, S. Lanthanum (III) incorporated chitosan-montmorillonite composite as flexible material for adsorptive removal of azo dyes from water. Mater. Today Proc. 2020, 27, 318–326. [Google Scholar] [CrossRef]
- Elella, M.H.A.; Sabaa, M.W.; Abd ElHafeez, E.; Mohamed, R.R. Crystal violet dye removal using crosslinked grafted xanthan gum. Int. J. Biol. Macromol. 2019, 137, 1086–1101. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, R.; Ansari, K. Polyacrylamide-Grafted Actinidia deliciosa peels powder (PGADP) for the sequestration of crystal violet dye: Isotherms, kinetics and thermodynamic studies. Appl. Water Sci. 2020, 10, 1–15. [Google Scholar] [CrossRef]
- Cheruiyot, G.K.; Wanyonyi, W.C.; Kiplimo, J.J.; Maina, E.N. Adsorption of toxic crystal violet dye using coffee husks: Equilibrium, kinetics and thermodynamics study. Sci. Afr. 2019, 5, e00116. [Google Scholar] [CrossRef]
- Ebrahimpour, M.; Hassaninejad-Darzi, S.K.; Zavvar Mousavi, H. Adsorption of ternary toxic crystal violet, malachite green and methylene blue onto synthesised SBA-15 mesoporous nanoparticles. Int. J. Environ. Anal. Chem. 2020, 1–24. [Google Scholar] [CrossRef]
- Pang, X.; Sellaoui, L.; Franco, D.; Dotto, G.L.; Georgin, J.; Bajahzar, A.; Belmabrouk, H.; Lamine, A.B.; Bonilla-Petriciolet, A.; Li, Z. Adsorption of crystal violet on biomasses from pecan nutshell, para chestnut husk, araucaria bark and palm cactus: Experimental study and theoretical modeling via monolayer and double layer statistical physics models. Chem. Eng. J. 2019, 378, 122101. [Google Scholar] [CrossRef]
- Sadeghi, S.; Nasehi, Z. Simultaneous determination of Brilliant Green and Crystal Violet dyes in fish and water samples with dispersive liquid-liquid micro-extraction using ionic liquid followed by zero crossing first derivative spectrophotometric analysis method. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 201, 134–142. [Google Scholar] [CrossRef]
- Ciobanu, G.; Harja, M.; Diaconu, M.; Cimpeanu, C.; Teodorescu, R.; Bucur, D. Crystal violet dye removal from aqueous solution by nanohydroxyapatite. J. Food Agric. Environ. 2014, 12, 499–502. [Google Scholar]
- Mani, S.; Bharagava, R.N. Exposure to crystal violet, its toxic, genotoxic and carcinogenic effects on environment and its degradation and detoxification for environmental safety. Rev. Environ. Contamin. Toxicol. 2016, 237, 71–104. [Google Scholar]
- Kulkarni, M.R.; Revanth, T.; Acharya, A.; Bhat, P. Removal of Crystal Violet dye from aqueous solution using water hyacinth: Equilibrium, kinetics and thermodynamics study. Resour. Technol. 2017, 3, 71–77. [Google Scholar] [CrossRef]
- Li, Z.; Hanafy, H.; Zhang, L.; Sellaoui, L.; Netto, M.S.; Oliveira, M.L.S.; Seliem, M.K.; Dotto, G.L.; Bonilla-Petriciolet, A.; Li, Q. Adsorption of congo red and methylene blue dyes on an ashitaba waste and a walnut shell-based activated carbon from aqueous solutions: Experiments, characterization and physical interpretations. Chem. Eng. J. 2020, 388, 124263. [Google Scholar] [CrossRef]
- Gupta, V.K.; Agarwal, S.; Ahmad, R.; Mirza, A.; Mittal, J. Sequestration of toxic congo red dye from aqueous solution using ecofriendly guar gum/activated carbon nanocomposite. Int. J. Biol. Macromol. 2020, 158, 1310–1318. [Google Scholar] [CrossRef]
- Swan, N.B.; Zaini, M.A.A. Adsorption of malachite green and congo red dyes from water: Recent progress and future outlook. Ecol. Chem. Eng. S 2019, 26, 119–132. [Google Scholar] [CrossRef]
- Elwakeel, K.Z.; Elgarahy, A.M.; Elshoubaky, G.A.; Mohammad, S.H. Microwave assist sorption of crystal violet and Congo red dyes onto amphoteric sorbent based on upcycled Sepia shells. J. Environ. Health Sci. Eng. 2020, 18, 35–50. [Google Scholar] [CrossRef]
- Zhang, Q.; Yan, Z.; Ouyang, J.; Zhang, Y.; Yang, H.; Chen, D. Chemically modified kaolinite nanolayers for the removal of organic pollutants. Appl. Clay Sci. 2018, 157, 283–290. [Google Scholar] [CrossRef]
- Kirankumar, V.S.; Sumathi, S. Copper and cerium co-doped cobalt ferrite nanoparticles: Structural, morphological, optical, magnetic, and photocatalytic properties. Environ. Sci. Pollut. Res. 2019, 26, 19189–19206. [Google Scholar] [CrossRef]
- Cao, X.-L.; Yan, Y.-N.; Zhou, F.-Y.; Sun, S.-P. Tailoring nanofiltration membranes for effective removing dye intermediates in complex dye-wastewater. J. Membr. Sci. 2020, 595, 117476. [Google Scholar] [CrossRef]
- Bouras, H.D.; Isik, Z.; Arikan, E.B.; Bouras, N.; Chergui, A.; Yatmaz, H.C.; Dizge, N. Photocatalytic oxidation of azo dye solutions by impregnation of ZnO on fungi. Biochem. Eng. J. 2019, 146, 150–159. [Google Scholar] [CrossRef]
- Zhou, F.; Cheng, Y.; Gan, L.; Chen, Z.; Megharaj, M.; Naidu, R. Burkholderia vietnamiensis C09V as the functional biomaterial used to remove crystal violet and Cu (II). Ecotoxicol. Environ. Saf. 2014, 105, 1–6. [Google Scholar] [CrossRef]
- Baddouh, A.; El Ibrahimi, B.; Rguitti, M.M.; Mohamed, E.; Hussain, S.; Bazzi, L. Electrochemical removal of methylene bleu dye in aqueous solution using Ti/RuO2–IrO2 and SnO2 electrodes. Sep. Sci. Technol. 2019, 1–10. [Google Scholar] [CrossRef]
- Yang, H.-C.; Gong, J.-L.; Zeng, G.-M.; Zhang, P.; Zhang, J.; Liu, H.-Y.; Huan, S.-Y. Polyurethane foam membranes filled with humic acid-chitosan crosslinked gels for selective and simultaneous removal of dyes. J. Colloid Interface Sci. 2017, 505, 67–78. [Google Scholar] [CrossRef]
- Wang, Y.; Su, X.; Xu, Z.; Wen, K.; Zhang, P.; Zhu, J.; He, H. Preparation of surface-functionalized porous clay heterostructures via carbonization of soft-template and their adsorption performance for toluene. Appl. Surf. Sci. 2016, 363, 113–121. [Google Scholar] [CrossRef]
- Sana, D.; Jalila, S. A comparative study of adsorption and regeneration with different agricultural wastes as adsorbents for the removal of methylene blue from aqueous solution. Chin. J. Chem. Eng. 2017, 25, 1282–1287. [Google Scholar] [CrossRef]
- Shi, C.; Tao, F.; Cui, Y. Evaluation of nitriloacetic acid modified cellulose film on adsorption of methylene blue. Int. J. Biol. Macromol. 2018, 114, 400–407. [Google Scholar] [CrossRef]
- Tang, J.; Mu, B.; Zong, L.; Wang, A. From waste hot-pot oil as carbon precursor to development of recyclable attapulgite/carbon composites for wastewater treatment. J. Environ. Sci. 2019, 75, 346–358. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.-L.; Xu, M.-Y.; Xie, Z.-W.; Hai, W.; Xie, X.-L.; He, F.-A. Selective adsorption of anionic dyes from aqueous solution by a novel β-cyclodextrin-based polymer. J. Mol. Struct. 2020, 1203, 127373. [Google Scholar] [CrossRef]
- Fernandes, J.V.; Rodrigues, A.M.; Menezes, R.R.; Neves, G.D.A. Adsorption of Anionic Dye on the Acid-Functionalized Bentonite. Materials 2020, 13, 3600. [Google Scholar] [CrossRef]
- Buburuzan, A.M.; Catrinescu, C.; Macoveanu, M. Comparative study of the adsorption-desorption cycles of hexane over hypercrosslinked polymeric adsorbents and activated carbon. Environ. Eng. Manag. J. 2010, 9, 125–132. [Google Scholar] [CrossRef]
- Zhu, J.; Zhang, P.; Wang, Y.; Wen, K.; Su, X.; Zhu, R.; He, H.; Xi, Y. Effect of acid activation of palygorskite on their toluene adsorption behaviors. Appl. Clay Sci. 2018, 159, 60–67. [Google Scholar] [CrossRef]
- Yang, R.; Li, D.; Li, A.; Yang, H. Adsorption properties and mechanisms of palygorskite for removal of various ionic dyes from water. Appl. Clay Sci. 2018, 151, 20–28. [Google Scholar] [CrossRef]
- Azha, S.F.; Sellaoui, L.; Yunus, E.H.E.; Yee, C.J.; Bonilla-Petriciolet, A.; Lamine, A.B.; Ismail, S. Iron-modified composite adsorbent coating for azo dye removal and its regeneration by photo-Fenton process: Synthesis, characterization and adsorption mechanism interpretation. Chem. Eng. J. 2019, 361, 31–40. [Google Scholar] [CrossRef]
- Piri, F.; Mollahosseini, A.; Hosseini, M.M. Enhanced adsorption of dyes on microwave-assisted synthesized magnetic zeolite-hydroxyapatite nanocomposite. J. Environ. Chem. Eng. 2019, 7, 103338. [Google Scholar] [CrossRef]
- Xinguo, X.; Jiling, Z.; Ruiyu, J.; Qi, X. Application of Modified Attapulgite Clay as the Adsorbent in Gasoline Desulfurization. China Pet. Process. Petrochem. Technol. 2014, 16, 63–68. [Google Scholar]
- Han, J.; Liang, X.; Xu, Y.; Xu, Y. Removal of Cu2+ from aqueous solution by adsorption onto mercapto functionalized palygorskite. J. Ind. Eng. Chem. 2015, 23, 307–315. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, W.; Zhang, J.; Liu, P.; Wang, A. A comparative study about adsorption of natural palygorskite for methylene blue. Chem. Eng. J. 2015, 262, 390–398. [Google Scholar] [CrossRef]
- Câmara, A.B.F.; Sales, R.V.; Bertolino, L.C.; Furlanetto, R.P.P.; Rodríguez-Castellón, E.; De Carvalho, L.S. Novel application for palygorskite clay mineral: A kinetic and thermodynamic assessment of diesel fuel desulfurization. Adsorption 2020, 26, 267–282. [Google Scholar] [CrossRef]
- Huang, D.; Zheng, Y.; Zhang, Z.; Quan, Q.; Qiang, X. Synergistic effect of hydrophilic palygorskite and hydrophobic zein particles on the properties of chitosan films. Mater. Des. 2020, 185, 108229. [Google Scholar] [CrossRef]
- Al-Futaisi, A.; Jamrah, A.; Al-Hanai, R. Aspects of cationic dye molecule adsorption to palygorskite. Desalination 2007, 214, 327–342. [Google Scholar] [CrossRef]
- Youcef, L.D.; Belaroui, L.S.; López-Galindo, A. Adsorption of a cationic methylene blue dye on an Algerian palygorskite. Appl. Clay Sci. 2019, 179, 105145. [Google Scholar] [CrossRef]
- Abidi, N.; Duplay, J.; Jada, A.; Errais, E.; Ghazi, M.; Semhi, K.; Trabelsi-Ayadi, M. Removal of anionic dye from textile industries’ effluents by using Tunisian clays as adsorbents. Ζeta potential and streaming-induced potential measurements. Comptes Rendus Chim. 2019, 22, 113–125. [Google Scholar] [CrossRef]
- Silva, M.P.D.; Santos, M.d.S.F.; Santos, M.R.M.C.; Santos Júnior, L.D.S.; Fonseca, M.G.D.; Silva Filho, E.C.D. Natural palygorskite as an industrial dye remover in single and binary systems. Mater. Res. 2016, 19, 1232–1240. [Google Scholar] [CrossRef]
- Hu, Z.; Shi, F.; Liu, G.; Zhang, B.; Zhang, H. Removal of Methylene Blue from Aqueous Solution by Adsorption Onto Modified Attapulgite Clay. Energy Environ. Focus 2015, 4, 316–323. [Google Scholar] [CrossRef]
- da Costa, F.P.; da Silva Morais, C.R.; Pinto, H.C.; Rodrigues, A.M. Microstructure and physico-mechanical properties of Al2O3-doped sustainable glass-ceramic foams. Mater. Chem. Phys. 2020, 256, 123612. [Google Scholar] [CrossRef]
- Pereira da Costa, F.; Rodrigues da Silva Morais, C.; Rodrigues, A.M. Sustainable glass-ceramic foams manufactured from waste glass bottles and bentonite. Ceram. Int. 2020, 46, 17957–17961. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of gases in multimolecular layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Lima, A.; Bruno, C.; Luna, B.; Fl, A.; Alves, S.; Lucena, S.; Margareti, S.; Meneghetti, P. From Disposal to Reuse: Production of Sustainable Fatty Acid Alkyl Esters Derived from Residual Oil Using a Biphasic Magnetic Catalyst. Sustainability 2020, 12, 10159. [Google Scholar]
- Aljeboree, A.M.; Alshirifi, A.N.; Alkaim, A.F. Kinetics and equilibrium study for the adsorption of textile dyes on coconut shell activated carbon. Arab. J. Chem. 2017, 10, S3381–S3393. [Google Scholar] [CrossRef]
- Kuang, Y.; Zhang, X.; Zhou, S. Adsorption of methylene blue in water onto activated carbon by surfactant modification. Water 2020, 12, 587. [Google Scholar] [CrossRef]
- Sahu, O.; Singh, N. Significance of bioadsorption process on textile industry wastewater. In The Impact and Prospects of Green Chemistry for Textile Technology; Elsevier: Amsterdam, The Netherlands, 2019; pp. 367–416. [Google Scholar]
- Doğan, M.; Özdemir, Y.; Alkan, M. Adsorption kinetics and mechanism of cationic methyl violet and methylene blue dyes onto sepiolite. Dye. Pigment. 2007, 75, 701–713. [Google Scholar] [CrossRef]
- Garba, Z.N. The Relevance of Isotherm and Kinetic Models to Chlorophenols Adsorption: A Review. Avicenna J. Environ. Health Eng. 2019, 6, 55–65. [Google Scholar] [CrossRef]
- Simonin, J.-P. On the comparison of pseudo-first order and pseudo-second order rate laws in the modeling of adsorption kinetics. Chem. Eng. J. 2016, 300, 254–263. [Google Scholar] [CrossRef]
- Staroń, P.; Chwastowski, J.; Banach, M. Sorption and desorption studies on silver ions from aqueous solution by coconut fiber. J. Clean. Prod. 2017, 149, 290–301. [Google Scholar] [CrossRef]
- Mohammadi, N.; Khani, H.; Gupta, V.K.; Amereh, E.; Agarwal, S. Adsorption process of methyl orange dye onto mesoporous carbon material–kinetic and thermodynamic studies. J. Colloid Interface Sci. 2011, 362, 457–462. [Google Scholar] [CrossRef]
- Suarez, M.; García-Romero, E.; Del Río, M.S.; Martinetto, P.; Dooryhee, E. The effect of the octahedral cations on the dimensions of the palygorskite cell. Clay Miner. 2007, 42, 287–297. [Google Scholar] [CrossRef]
- Garciá-Romero, E.; Suárez, M. On the chemical composition of sepiolite and palygorskite. Clays Clay Miner. 2010, 58, 1–20. [Google Scholar] [CrossRef]
- Suárez, M.; García-Rivas, J.; Sánchez-Migallón, J.M.; García-Romero, E. Spanish palygorskites: Geological setting, mineralogical, textural and crystal-chemical characterization. Eur. J. Mineral. 2018, 30, 733–746. [Google Scholar] [CrossRef]
- Wang, Y.; Shen, Y.; Qin, Z.; Li, S.; Zhang, T. Preparation of three-dimensional palygorskite based carrier. MethodsX 2020, 7, 100815. [Google Scholar] [CrossRef]
- Augsburger, M.S.; Strasser, E.; Perino, E.; Mercader, R.C.; Pedregosa, J.C. FTIR and Mössbauer investigation of a substituted palygorskite: Silicate with a channel structure. J. Phys. Chem. Solids 1998, 59, 175–180. [Google Scholar] [CrossRef]
- Chen, H.; Zhao, Y.; Wang, A. Removal of Cu (II) from aqueous solution by adsorption onto acid-activated palygorskite. J. Hazard. Mater. 2007, 149, 346–354. [Google Scholar] [CrossRef]
- Boudriche, L.; Calvet, R.; Hamdi, B.; Balard, H. Effect of acid treatment on surface properties evolution of attapulgite clay: An application of inverse gas chromatography. Colloids Surf. A Physicochem. Eng. Asp. 2011, 392, 45–54. [Google Scholar] [CrossRef]
- Moulton, B.J.A.; Rodrigues, A.M.; Sampaio, D.V.; Silva, L.D.; Cunha, T.R.; Zanotto, E.D.; Pizani, P.S. The origin of the unusual DSC peaks of supercooled barium disilicate liquid. CrystEngComm 2019, 21, 2768–2778. [Google Scholar] [CrossRef]
- Moulton, B.J.A.; Rodrigues, A.M.; Pizani, P.S.; Sampaio, D.V.; Zanotto, E.D. A Raman investigation of the structural evolution of supercooled liquid barium disilicate during crystallization. Int. J. Appl. Glas. Sci. 2018, 9, 510–517. [Google Scholar] [CrossRef]
- Marosz, M.; Kowalczyk, A.; Gil, B.; Chmielarz, L. Acid-treated Clay Minerals as Catalysts for Dehydration of Methanol and Ethanol. Clays Clay Miner. 2020, 68, 23–37. [Google Scholar] [CrossRef]
- Xavier, K.C.M.; Santos, M.D.S.F.D.; Santos, M.R.M.C.; Oliveira, M.E.R.; Carvalho, M.W.N.C.; Osajima, J.A.; Silva Filho, E.C.D. Effects of acid treatment on the clay palygorskite: XRD, surface area, morphological and chemical composition. Mater. Res. 2014, 17, 3–8. [Google Scholar] [CrossRef]
- Oliveira, R.N.; Acchar, W.; Soares, G.D.A.; Barreto, L.S. The increase of surface area of a Brazilian palygorskite clay activated with sulfuric acid solutions using a factorial design. Mater. Res. 2013, 16, 924–928. [Google Scholar] [CrossRef]
- Dhanya, B.A.P.; Pushpaletha, P. Metal supported and metal ion exchanged catalysts from palygorskite for acetylation reaction. Indian J. Chem. A 2020, 57, 649–654. [Google Scholar]
- Madejova, J.; Komadel, P. Baseline studies of the clay minerals society source clays: Infrared methods. Clays Clay Miner. 2001, 49, 410–432. [Google Scholar] [CrossRef]
- Suarez, M.; Garcia-Romero, E. FTIR spectroscopic study of palygorskite: Influence of the composition of the octahedral sheet. Appl. Clay Sci. 2006, 31, 154–163. [Google Scholar] [CrossRef]
- Tian, G.; Wang, W.; Kang, Y.; Wang, A. Palygorskite in sodium sulphide solution via hydrothermal process for enhanced methylene blue adsorption. J. Taiwan Inst. Chem. Eng. 2016, 58, 417–423. [Google Scholar] [CrossRef]
- Frost, R.L.; Locos, O.B.; Ruan, H.; Kloprogge, J.T. Near-infrared and mid-infrared spectroscopic study of sepiolites and palygorskites. Vib. Spectrosc. 2001, 27, 1–13. [Google Scholar] [CrossRef]
- Frost, R.L.; Bahfenne, S.; Graham, J. Infrared and infrared emission spectroscopic study of selected magnesium carbonate minerals containing ferric iron—Implications for the geosequestration of greenhouse gases. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2008, 71, 1610–1616. [Google Scholar] [CrossRef][Green Version]
- Liu, Q.; Yao, X.; Cheng, H.; Frost, R.L. An infrared spectroscopic comparison of four Chinese palygorskites. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2012, 96, 784–789. [Google Scholar] [CrossRef]
- Rhouta, B.; Zatile, E.; Bouna, L.; Lakbita, O.; Maury, F.; Daoudi, L.; Lafont, M.C.; Amjoud, M.; Senocq, F.; Jada, A. Comprehensive physicochemical study of dioctahedral palygorskite-rich clay from Marrakech High Atlas (Morocco). Phys. Chem. Miner. 2013, 40, 411–424. [Google Scholar] [CrossRef]
- Tian, G.; Wang, W.; Mu, B.; Kang, Y.; Wang, A. Facile fabrication of carbon/attapulgite composite for bleaching of palm oil. J. Taiwan Inst. Chem. Eng. 2015, 50, 252–258. [Google Scholar] [CrossRef]
- Ouyang, J.; Zhao, Z.; Suib, S.L.; Yang, H. Degradation of Congo Red dye by a Fe2O3@ CeO2-ZrO2/Palygorskite composite catalyst: Synergetic effects of Fe2O3. J. Colloid Interface Sci. 2019, 539, 135–145. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Q.; Chen, H.; Wang, A. XRF and nitrogen adsorption studies of acid-activated palygorskite. Clay Miner. 2010, 45, 145–156. [Google Scholar] [CrossRef]
- Rusmin, R.; Sarkar, B.; Biswas, B.; Churchman, J.; Liu, Y.; Naidu, R. Structural, electrokinetic and surface properties of activated palygorskite for environmental application. Appl. Clay Sci. 2016, 134, 95–102. [Google Scholar] [CrossRef]
- Frinisrasra, N.; Srasra, E. Effect of heating on palygorskite and acid treated palygorskite properties. Электронная обработка материалов 2008, 44, 43–49. [Google Scholar]
- Xavier, K.C.M.; Santos, M.S.F.; Osajima, J.A.; Luz, A.B.; Fonseca, M.G.; Silva Filho, E.C. Thermally activated palygorskites as agents to clarify soybean oil. Appl. Clay Sci. 2016, 119, 338–347. [Google Scholar] [CrossRef]
- Ogorodova, L.; Vigasina, M.; Melchakova, L.; Krupskaya, V.; Kiseleva, I. Thermochemical study of natural magnesium aluminum phyllosilicate: Palygorskite. J. Chem. Thermodyn. 2015, 89, 205–211. [Google Scholar] [CrossRef]
- Bayram, H.; Önal, M.; Üstünışık, G.; Sarıkaya, Y. Some thermal characteristics of a mineral mixture of palygorskite, metahalloysite, magnesite and dolomite. J. Therm. Anal. Calorim. 2007, 89, 169–174. [Google Scholar] [CrossRef]
- Amorim, K.B.; Angélica, R.S. Mineralogy and geochemistry of occurrence of palygorskite of Alcântara, S. Luís-Grajaú basin, Maranhão, Brazil. Cerâmica 2011, 57, 483–490. [Google Scholar] [CrossRef]
- Wang, K.; Wang, L.; Zhang, Y.; Zhang, Y.; Liang, J. Microstructural evolution and sintering properties of palygorskite nanofibers. Int. J. Appl. Ceram. Technol. 2020, 17, 1833–1842. [Google Scholar] [CrossRef]
- Bouna, L.; Rhouta, B.; Amjoud, M.; Maury, F.; Lafont, M.-C.; Jada, A.; Senocq, F.; Daoudi, L. Synthesis, characterization and photocatalytic activity of TiO2 supported natural palygorskite microfibers. Appl. Clay Sci. 2011, 52, 301–311. [Google Scholar] [CrossRef]
- Kadïr, S.; Eren, M.; Atabey, E. Dolocretes and associated palygorskite occurrences in siliciclastic red mudstones of the Sariyer formation (Middle Miocene), southeastern side of the Çanakkale strait, Turkey. Clays Clay Miner. 2010, 58, 205–219. [Google Scholar] [CrossRef]
- Myriam, M.; Suarez, M.; Martin-Pozas, J.M. Structural and textural modifications of palygorskite and sepiolite under acid treatment. Clays Clay Miner. 1998, 46, 225–231. [Google Scholar] [CrossRef]
- Gonzalez, F.; Pesquera, C.; Benito, I.; Mendioroz, S.; Pajares, J.A. Mechanism of acid activation of magnesic palygorskite. Clays Clay Miner. 1989, 37, 258–262. [Google Scholar] [CrossRef]
- Omer, O.S.; Hussein, B.H.M.; Ouf, A.M.; Hussein, M.A.; Mgaidi, A. An organified mixture of illite-kaolinite for the removal of Congo red from wastewater. J. Taibah Univ. Sci. 2018, 12, 858–866. [Google Scholar] [CrossRef]
- Wang, W.; Tian, G.; Zhang, Z.; Wang, A. A simple hydrothermal approach to modify palygorskite for high-efficient adsorption of methylene blue and Cu (II) ions. Chem. Eng. J. 2015, 265, 228–238. [Google Scholar] [CrossRef]
- Dong, W.; Lu, Y.; Wang, W.; Zong, L.; Zhu, Y.; Kang, Y.; Wang, A. A new route to fabricate high-efficient porous silicate adsorbents by simultaneous inorganic-organic functionalization of low-grade palygorskite clay for removal of Congo red. Microporous Mesoporous Mater. 2019, 277, 267–276. [Google Scholar] [CrossRef]
- Sousa, H.R.; Silva, L.S.; Sousa, P.A.A.; Sousa, R.R.M.; Fonseca, M.G.; Osajima, J.A.; Silva-Filho, E.C. Evaluation of methylene blue removal by plasma activated palygorskites. J. Mater. Res. Technol. 2019, 8, 5432–5442. [Google Scholar] [CrossRef]
- Zhang, S.; Zhong, L.; Yang, H.; Tang, A.; Zuo, X. Magnetic carbon-coated palygorskite loaded with cobalt nanoparticles for Congo Red removal from waters. Appl. Clay Sci. 2020, 198, 105856. [Google Scholar] [CrossRef]
- Wong, S.; Abd Ghafar, N.; Ngadi, N.; Razmi, F.A.; Inuwa, I.M.; Mat, R.; Amin, N.A.S. Effective removal of anionic textile dyes using adsorbent synthesized from coffee waste. Sci. Rep. 2020, 10, 1–13. [Google Scholar] [CrossRef]
- Wekoye, J.N.; Wanyonyi, W.C.; Wangila, P.T.; Tonui, M.K. Kinetic and equilibrium studies of Congo red dye adsorption on cabbage waste powder. Environ. Chem. Ecotoxicol. 2020, 2, 24–31. [Google Scholar] [CrossRef]
- Puri, C.; Sumana, G. Highly effective adsorption of crystal violet dye from contaminated water using graphene oxide intercalated montmorillonite nanocomposite. Appl. Clay Sci. 2018, 166, 102–112. [Google Scholar] [CrossRef]
- Tang, H.; Li, W.; Zhang, T.; Li, Q.; Xing, J.; Liu, H. Improvement in diesel desulfurization capacity by equilibrium isotherms analysis. Sep. Purif. Technol. 2011, 78, 352–356. [Google Scholar] [CrossRef]
- Litefti, K.; Freire, M.S.; Stitou, M.; González-Álvarez, J. Adsorption of an anionic dye (Congo red) from aqueous solutions by pine bark. Sci. Rep. 2019, 9, 1–11. [Google Scholar] [CrossRef]
- Kaur, S.; Rani, S.; Mahajan, R.K. Adsorption kinetics for the removal of hazardous dye congo red by biowaste materials as adsorbents. J. Chem. 2012. [Google Scholar] [CrossRef]
- Khan, M.A.; Uddin, M.K.; Bushra, R.; Ahmad, A.; Nabi, S.A. Synthesis and characterization of polyaniline Zr (IV) molybdophosphate for the adsorption of phenol from aqueous solution. React. Kinet. Mech. Catal. 2014, 113, 499–517. [Google Scholar] [CrossRef]
- Zhu, Y.; Chen, T.; Liu, H.; Xu, B.; Xie, J. Kinetics and thermodynamics of Eu (III) and U (VI) adsorption onto palygorskite. J. Mol. Liq. 2016, 219, 272–278. [Google Scholar] [CrossRef]
- Sahu, S.; Pahi, S.; Tripathy, S.; Singh, S.K.; Behera, A.; Sahu, U.K.; Patel, R.K. Adsorption of methylene blue on chemically modified lychee seed biochar: Dynamic, equilibrium, and thermodynamic study. J. Mol. Liq. 2020, 315, 113743. [Google Scholar] [CrossRef]
- Abdi, M.; Balagabri, M.; Karimi, H.; Hossini, H.; Rastegar, S.O. Degradation of crystal violet (CV) from aqueous solutions using ozone, peroxone, electroperoxone, and electrolysis processes: A comparison study. Appl. Water Sci. 2020, 10, 1–10. [Google Scholar] [CrossRef]
- Cheriaa, J.; Khaireddine, M.; Rouabhia, M.; Bakhrouf, A. Removal of triphenylmethane dyes by bacterial consortium. Sci. World J. 2012. [Google Scholar] [CrossRef]
- Jabar, J.M.; Odusote, Y.A.; Alabi, K.A.; Ahmed, I.B. Kinetics and mechanisms of congo-red dye removal from aqueous solution using activated Moringa oleifera seed coat as adsorbent. Appl. Water Sci. 2020, 10, 1–11. [Google Scholar] [CrossRef]
- Zhang, J.; Yan, X.; Hu, M.; Hu, X.; Zhou, M. Adsorption of Congo red from aqueous solution using ZnO-modified SiO2 nanospheres with rough surfaces. J. Mol. Liq. 2018, 249, 772–778. [Google Scholar] [CrossRef]
- El-Harby, N.F.; Ibrahim, S.M.A.; Mohamed, N.A. Adsorption of Congo red dye onto antimicrobial terephthaloyl thiourea cross-linked chitosan hydrogels. Water Sci. Technol. 2017, 76, 2719–2732. [Google Scholar] [CrossRef]
- Zenasni, M.; Meroufel, B.; Merlin, A.; George, B. Adsorption of Congo red from aqueous solution using CTAB-kaolin from Bechar Algeria. J. Surf. Eng. Mater. Adv. Technol. 2014, 4, 332–341. [Google Scholar] [CrossRef]
- Farias, R.S.D.; Buarque, H.L.D.B.; Cruz, M.R.D.; Cardoso, L.M.F.; Gondim, T.D.A.; Paulo, V.R.D. Adsorption of congo red dye from aqueous solution onto amino-functionalized silica gel. Eng. Sanit. e Ambient. 2018, 23, 1053–1060. [Google Scholar] [CrossRef]
- Qin, J.; Qiu, F.; Rong, X.; Yan, J.; Zhao, H.; Yang, D. Adsorption behavior of crystal violet from aqueous solutions with chitosan–graphite oxide modified polyurethane as an adsorbent. J. Appl. Polym. Sci. 2015, 132, 41828. [Google Scholar] [CrossRef]
- Mohanty, S.; Moulick, S.; Maji, S.K. Adsorption/photodegradation of crystal violet (basic dye) from aqueous solution by hydrothermally synthesized titanate nanotube (TNT). J. Water Process Eng. 2020, 37, 101428. [Google Scholar] [CrossRef]
- Zhai, Q.-Z. Studies of adsorption of crystal violet from aqueous solution by nano mesocellular foam silica: Process equilibrium, kinetic, isotherm, and thermodynamic studies. Water Sci. Technol. 2020, 81, 2092–2108. [Google Scholar] [CrossRef] [PubMed]
- Miyah, Y.; Lahrichi, A.; Idrissi, M.; Anis, K.; Kachkoul, R.; Idrissi, N.; Lairini, S.; Nenov, V.; Zerrouq, F. Removal of cationic dye “crystal violet” in aqueous solution by the local clay. J. Mater. Environ. Sci. 2017, 8, 3570–3582. [Google Scholar]
- Hamidzadeh, S.; Torabbeigi, M.; Shahtaheri, S.J. Removal of crystal violet from water by magnetically modified activated carbon and nanomagnetic iron oxide. J. Environ. Health Sci. Eng. 2015, 13, 8. [Google Scholar] [CrossRef]


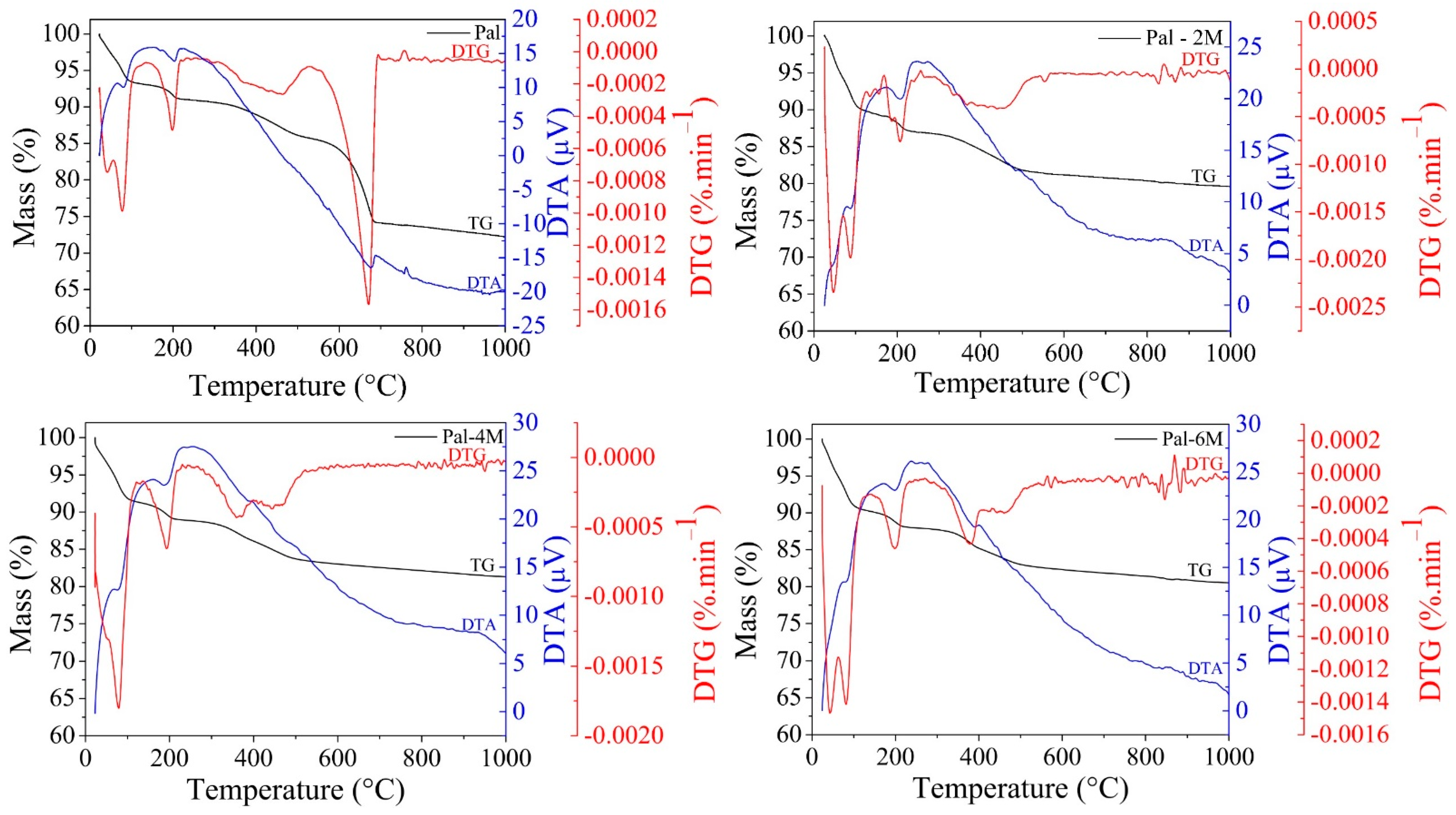
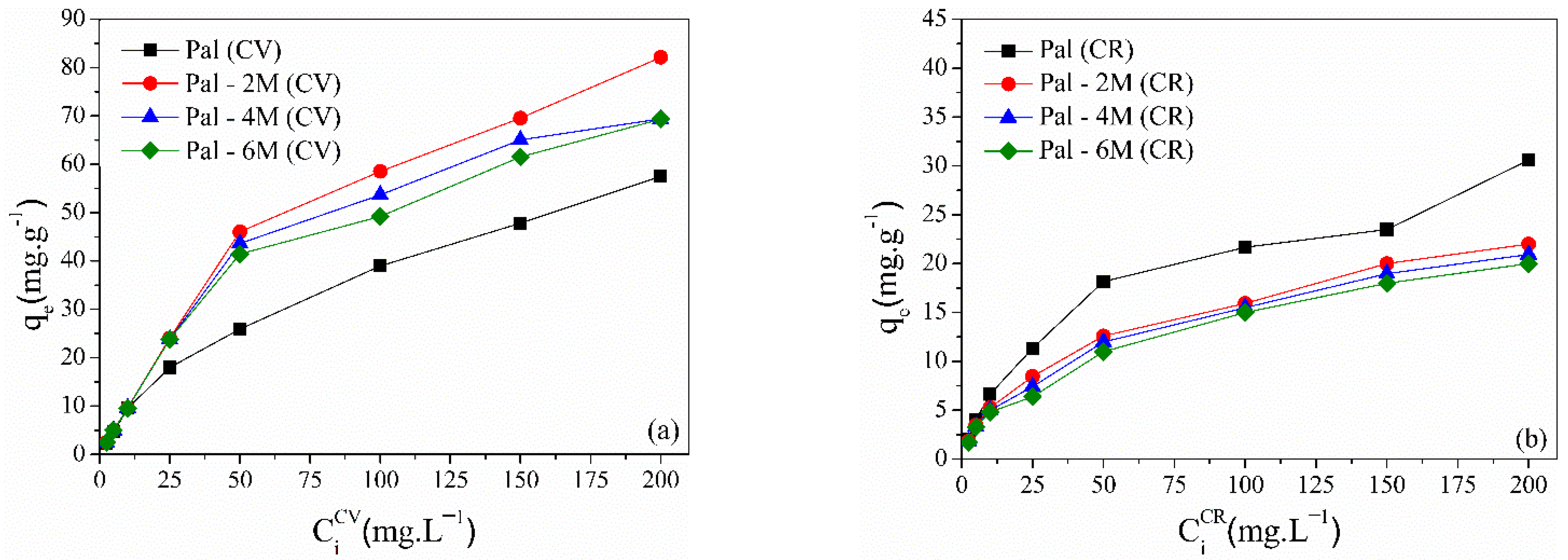
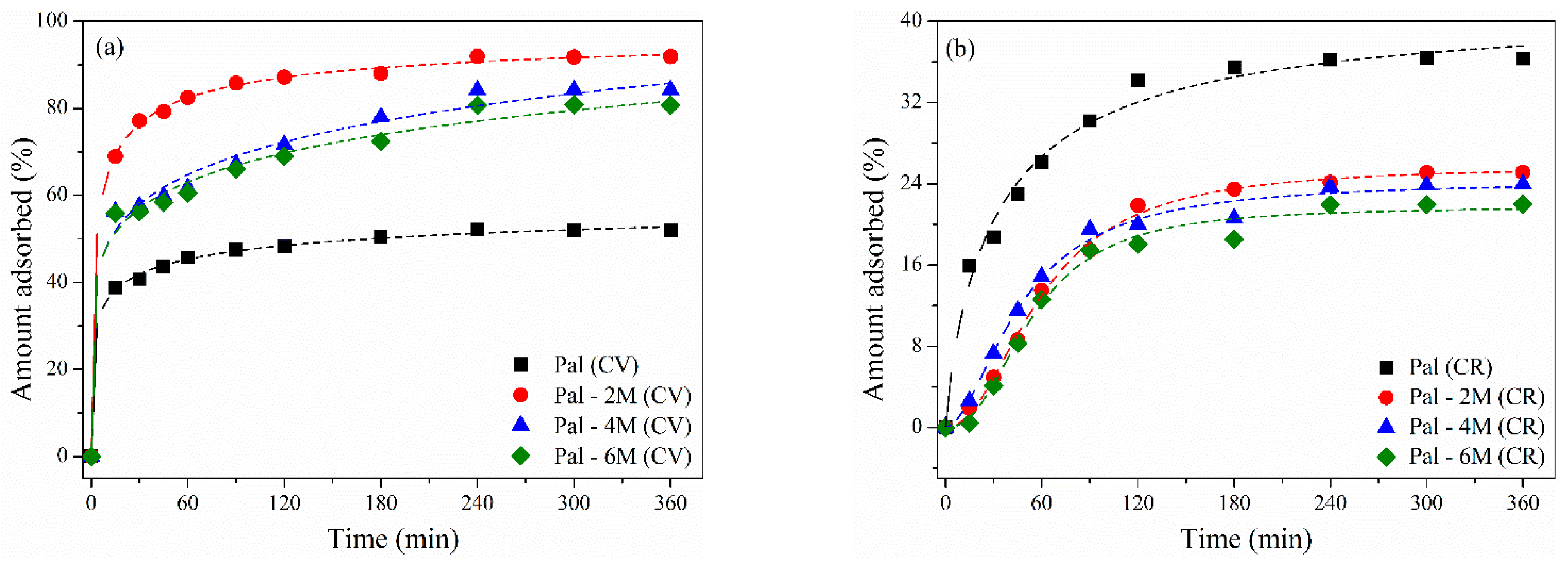

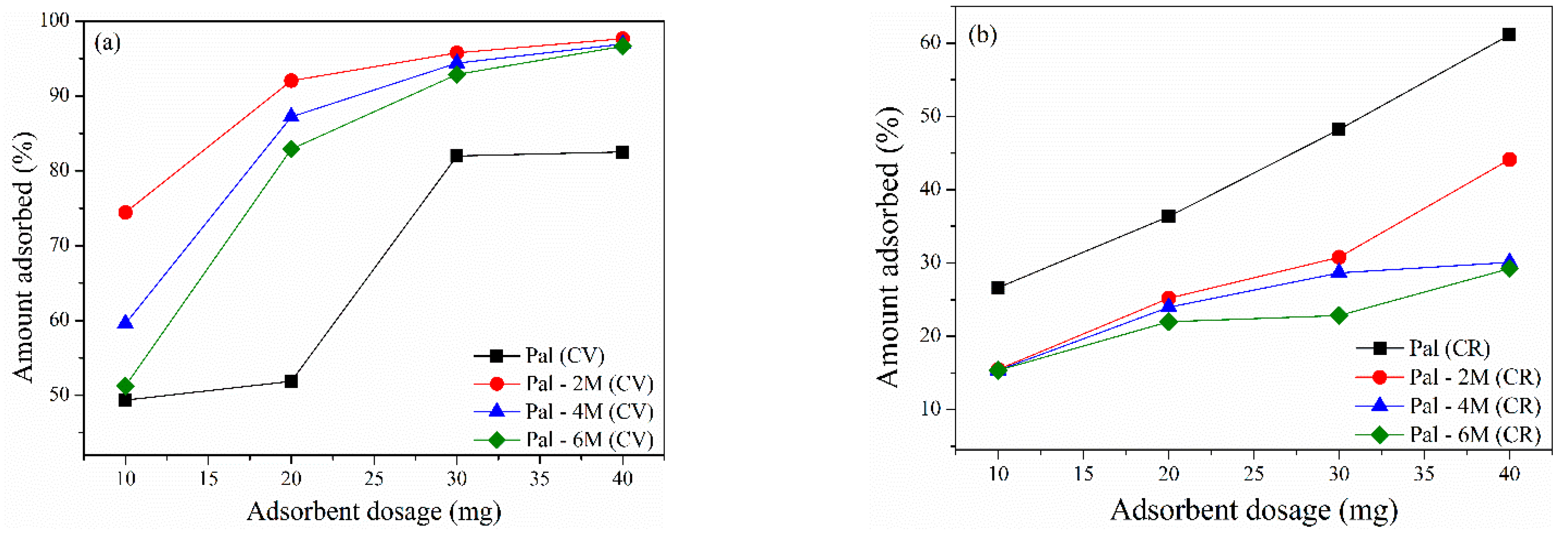







| Treatment | Acid Concentration | Sample Nomenclature |
|---|---|---|
| None | - | Pal |
| Acid | HCl–2 mol/L | Pal-2M |
| Acid | HCl–4 mol/L | Pal-4M |
| Acid | HCl–6 mol/L | Pal-6M |
| Sample | SiO2 | MgO | Al2O3 | CaO | Fe2O3 | K2O | Other Oxides |
|---|---|---|---|---|---|---|---|
| Pal | 52.8 | 13.9 | 13.5 | 11.9 | 5.3 | 0.9 | 1.7 |
| Pal-2M | 69.3 | 8.3 | 13.5 | 0 | 5.2 | 0.9 | 2.8 |
| Pal-4M | 71.9 | 7.6 | 13.4 | 0 | 4.5 | 0.8 | 1.8 |
| Pal-6M | 73.4 | 7.1 | 13.4 | 0 | 4.0 | 0.8 | 1.2 |
| Sample | Specific Surface Area (m²/g) | Average Pore Diameter (nm) |
|---|---|---|
| Pal | 80.4 | 14.3 |
| Pal-2M | 141.2 | 15.2 |
| Pal-4M | 182.2 | 13.0 |
| Pal-6M | 176.9 | 13.3 |
| CV | CR | |||||||
|---|---|---|---|---|---|---|---|---|
| Models | Pal | Pal-2M | Pal-4M | Pal-6M | Pal | Pal-2M | Pal-4M | Pal-6M |
| Langmuir | ||||||||
| qmax(mg·g−1) | 60.2 | 83.3 | 69.9 | 69.0 | 30.3 | 25.3 | 22.2 | 21.3 |
| KL (L·mg−1) | 0.05 | 0.20 | 0.17 | 0.15 | 0.06 | 0.04 | 0.03 | 0.03 |
| RL | 0.09 | 0.03 | 0.02 | 0.03 | 0.08 | 0.11 | 0.14 | 0.14 |
| R2 | 0.967 | 0.976 | 0.990 | 0.975 | 0.956 | 0.967 | 0.966 | 0.955 |
| Error | 0.07 | 0.04 | 0.02 | 0.05 | 1.31 | 1.75 | 1.99 | 2.83 |
| Freundlich | ||||||||
| 1/n | 031 | 0.31 | 0.30 | 0.30 | 0.43 | 0.44 | 0.43 | 0.43 |
| Kf (mg1−1/n·Kg−1·L1/n) | 11.0 | 19.8 | 18.1 | 17.3 | 3.48 | 2.75 | 2.70 | 2.69 |
| R2 | 0.959 | 0.844 | 0.871 | 0.884 | 0.971 | 0.989 | 0.989 | 0.961 |
| Error | 0.09 | 0.08 | 0.06 | 0.06 | 0.03 | 0.01 | 0.01 | 0.02 |
| CV | CR | |||||||
|---|---|---|---|---|---|---|---|---|
| Models | Pal | Pal-2M | Pal-4M | Pal-6M | Pal | Pal-2M | Pal-4M | Pal-6M |
| Pseudo-first-order | ||||||||
| qe,exp (mg.g−1) | 25.97 | 45.98 | 42.08 | 40.36 | 18.17 | 12.56 | 11.99 | 11.01 |
| qe,cal (mg.g−1) | 77.31 | 194.17 | 126.30 | 112.06 | 126.46 | 208.40 | 190.55 | 199.52 |
| k1 (min−1) | 0.02 | 0.04 | 0.02 | 0.02 | 0.02 | 0.02 | 0.01 | 0.02 |
| R2 | 0.902 | 0.856 | 0.935 | 0.918 | 0.859 | 0.953 | 0.906 | 0.901 |
| Error | 0.51 | 5.17 | 0.53 | 0.81 | 2.67 | 3.96 | 7.26 | 4.66 |
| Pseudo-second-order | ||||||||
| qe,cal (mg.g−1) | 26.76 | 46.95 | 44.62 | 42.52 | 20.02 | 15.46 | 14.71 | 13.01 |
| k2 (min−1) | 3.87 | 2.74 | 1.05 | 1.17 | 0.04 | 0.02 | 0.02 | 0.02 |
| R2 | 0.999 | 0.999 | 0.996 | 0.996 | 0.998 | 0.992 | 0.991 | 0.993 |
| Error | 0.07 | 0.02 | 0.21 | 0.27 | 1.70 | 0.72 | 0.77 | 0.86 |
| Sample | ∆G (kJ mol−1) | ∆H (kJ mol−1) | ΔS (J k−1 mol −1) | |||
|---|---|---|---|---|---|---|
| 298 K | 308 K | 318 K | 328 K | |||
| Pal | −0.17 | −0.20 | −0.32 | −0.41 | 0.27 | 0.97 |
| Pal-2M | −6.07 | −6.83 | −7.82 | −8.23 | 1.98 | 9.11 |
| Pal-4M | −4.75 | −5.01 | −5.29 | −5.81 | 0.63 | 4.02 |
| Pal-6M | −3.91 | −4.25 | 4.47 | −4.82 | 0.62 | 3.67 |
| Sample | ∆G (kJ mol−1) | ∆H (kJ mol−1) | ΔS (J k−1 mol −1) | |||
|---|---|---|---|---|---|---|
| 298 K | 308 K | 318 K | 328 K | |||
| Pal | −1.41 | −1.56 | −1.69 | −1.83 | 0.49 | 1.11 |
| Pal-2M | −0.82 | −0.95 | −1.03 | −1.09 | 0.54 | 0.76 |
| Pal-4M | −0.77 | −0.84 | −0.92 | −0.95 | 0.37 | 0.11 |
| Pal-6M | −0.69 | −0.77 | −0.85 | −0.89 | 0.54 | 0.54 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, V.C.; Araújo, M.E.B.; Rodrigues, A.M.; Cartaxo, J.M.; Menezes, R.R.; Neves, G.A. Adsorption Behavior of Acid-Treated Brazilian Palygorskite for Cationic and Anionic Dyes Removal from the Water. Sustainability 2021, 13, 3954. https://doi.org/10.3390/su13073954
Silva VC, Araújo MEB, Rodrigues AM, Cartaxo JM, Menezes RR, Neves GA. Adsorption Behavior of Acid-Treated Brazilian Palygorskite for Cationic and Anionic Dyes Removal from the Water. Sustainability. 2021; 13(7):3954. https://doi.org/10.3390/su13073954
Chicago/Turabian StyleSilva, Vanderlane C., Maria Eduarda B. Araújo, Alisson M. Rodrigues, Juliana M. Cartaxo, Romualdo R. Menezes, and Gelmires A. Neves. 2021. "Adsorption Behavior of Acid-Treated Brazilian Palygorskite for Cationic and Anionic Dyes Removal from the Water" Sustainability 13, no. 7: 3954. https://doi.org/10.3390/su13073954
APA StyleSilva, V. C., Araújo, M. E. B., Rodrigues, A. M., Cartaxo, J. M., Menezes, R. R., & Neves, G. A. (2021). Adsorption Behavior of Acid-Treated Brazilian Palygorskite for Cationic and Anionic Dyes Removal from the Water. Sustainability, 13(7), 3954. https://doi.org/10.3390/su13073954










