Kinetic and Isotherm Studies of Ni2+ and Pb2+ Adsorption from Synthetic Wastewater Using Eucalyptus camdulensis—Derived Biochar
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation and Characterization of Eucalyptus camdulensis (EU)-Biochar
2.2. Materials
2.3. Batch Experiments and Adsorption Performance
2.4. Adsorption Kinetic and Equilibrium Isotherm Models
3. Results and Discussion
3.1. Characteristics of EU-Biochar
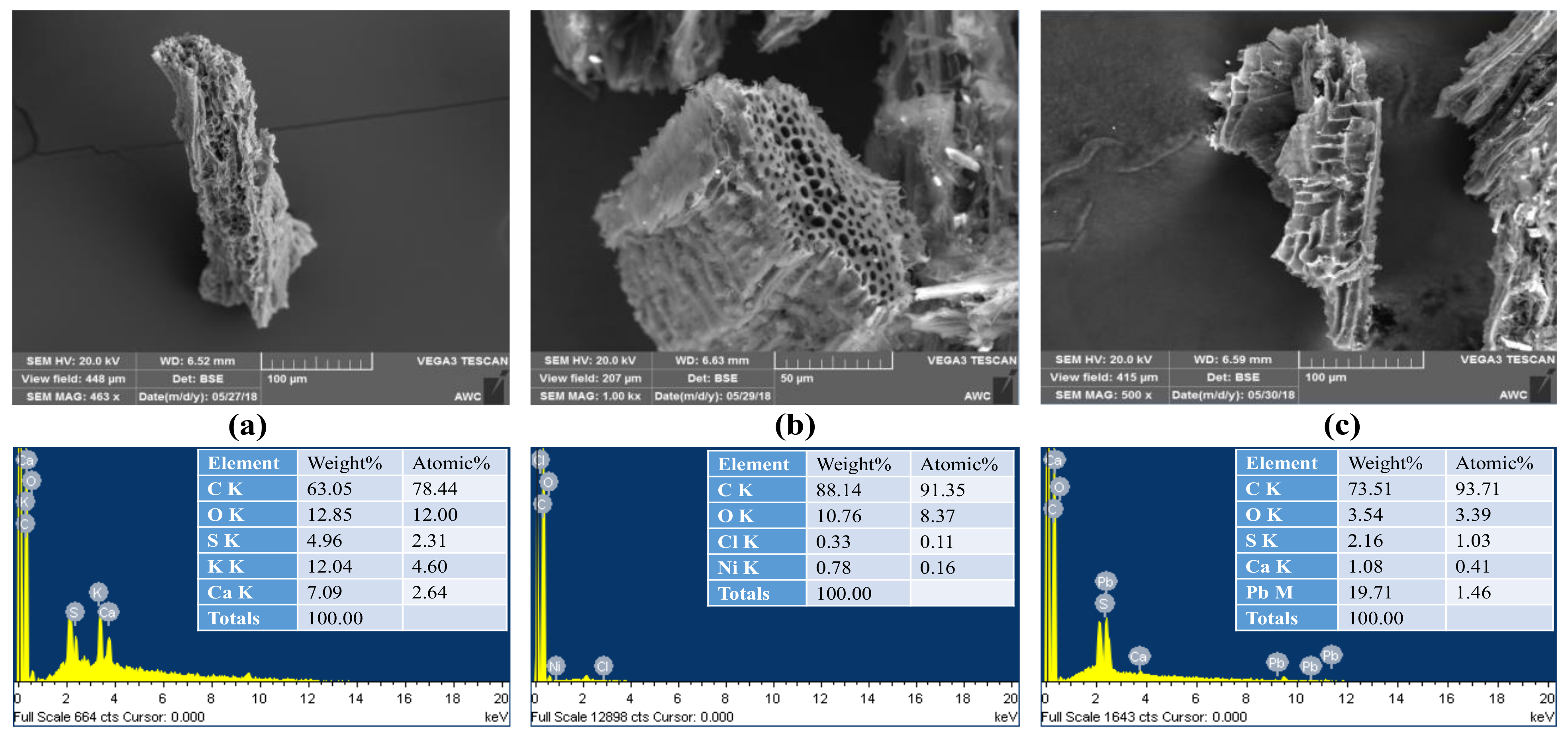
3.1.1. Scanning Electron Microscopy (SEM) and Energy-Dispersive X-ray (EDX) Spectroscopy Analysis
3.1.2. Fourier Transform Infrared (FTIR) Spectroscopy Analysis
3.2. Adsorption Equilibrium and Optimization of Contact Time, Initial Metal Ion Concentrations, EU-Biochar Dose, and Solution pH
3.3. Evaluation of the Adsorption Data through Kinetic Fitting Models
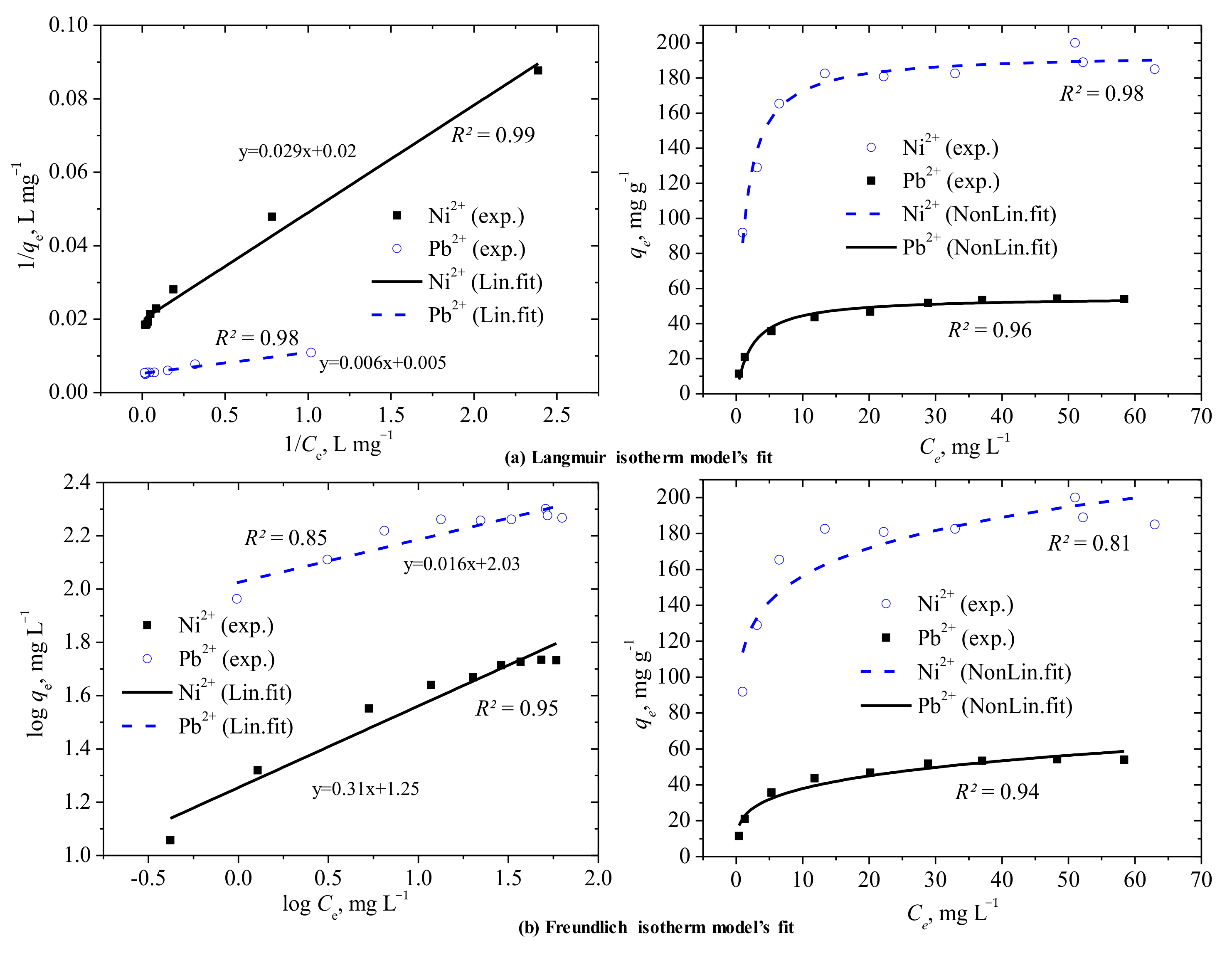
3.4. Evaluation of the Adsorption Data through Isotherm Fitting Models
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhang, P.; Yang, D.; Zhang, Y.; Li, Y.; Liu, Y.; Cen, Y.; Zhang, W.; Geng, W.; Rong, T.; Liu, Y.; et al. Re-Examining the Drive Forces of China’s Industrial Wastewater Pollution Based on GWR Model at Provincial Level. J. Clean. Prod. 2020, 262, 121309. [Google Scholar] [CrossRef]
- Shafiq, M.; Alazba, A.A.; Amin, M.T. Removal of Heavy Metals from Wastewater Using Date Palm as a Biosorbent: A Comparative Review. Sains Malays. 2018, 47, 35–49. [Google Scholar] [CrossRef]
- Saritas, O.; Proskuryakova, L.N. Water Resources—an Analysis of Trends, Weak Signals and Wild Cards with Implications for Russia. Foresight 2017, 19, 152–173. [Google Scholar] [CrossRef]
- Qi, M.; Yang, Y.; Zhang, X.; Zhang, X.; Wang, M.; Zhang, W.; Lu, X.; Tong, Y. Pollution Reduction and Operating Cost Analysis of Municipal Wastewater Treatment in China and Implication for Future Wastewater Management. J. Clean. Prod. 2020, 253, 120003. [Google Scholar] [CrossRef]
- Mohan, D.; Sarswat, A.; Ok, Y.S.; Pittman, C.U. Organic and Inorganic Contaminants Removal from Water with Biochar, a Renewable, Low Cost and Sustainable Adsorbent—A Critical Review. Bioresour. Technol. 2014, 160, 191–202. [Google Scholar] [CrossRef]
- Islam, M.A.; Awual, M.R.; Angove, M.J. A Review on Nickel(II) Adsorption in Single and Binary Component Systems and Future Path. J. Environ. Chem. Eng. 2019, 7, 103–305. [Google Scholar] [CrossRef]
- Hua, M.; Zhang, S.; Pan, B.; Zhang, W.; Lv, L.; Zhang, Q. Heavy Metal Removal from Water/Wastewater by Nanosized Metal Oxides: A Review. J. Hazard. Mater. 2012, 211–212, 317–331. [Google Scholar] [CrossRef]
- Cerná, M. Use of Solvent Extraction for the Removal of Heavy Metals from Liquid Wastes. Environ. Monit. Assess. 1995, 34, 151–162. [Google Scholar] [CrossRef] [PubMed]
- Bashir, A.; Malik, L.A.; Ahad, S.; Manzoor, T.; Bhat, M.A.; Dar, G.N.; Pandith, A.H. Removal of Heavy Metal Ions from Aqueous System by Ion-Exchange and Biosorption Methods. Environ. Chem. Lett. 2019, 17, 729–754. [Google Scholar] [CrossRef]
- Barakat, M.A. New Trends in Removing Heavy Metals from Industrial Wastewater. Arab. J. Chem. 2011, 4, 361–377. [Google Scholar] [CrossRef]
- Oncel, M.S.; Muhcu, A.; Demirbas, E.; Kobya, M. A Comparative Study of Chemical Precipitation and Electrocoagulation for Treatment of Coal Acid Drainage Wastewater. J. Environ. Chem. Eng. 2013, 1, 989–995. [Google Scholar] [CrossRef]
- Ahmad, J.; Naeem, S.; Ahmad, M.; Usman, A.R.A.; Al-Wabel, M.I. A Critical Review on Organic Micropollutants Contamination in Wastewater and Removal through Carbon Nanotubes. J. Environ. Manag. 2019, 246, 214–228. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.S.; Miao, H.H.; He, W.; Shen, H.L. Competitive Adsorption of Pb(II), Cu(II), and Cd(II) Ions on Wheat-Residue Derived Black Carbon. J. Chem. Eng. Data 2011, 56, 444–449. [Google Scholar] [CrossRef]
- Yu, J.-X.; Wang, L.-Y.; Chi, R.-A.; Zhang, Y.-F.; Xu, Z.-G.; Guo, J. Competitive Adsorption of Pb2+ and Cd2+ on Magnetic Modified Sugarcane Bagasse Prepared by Two Simple Steps. Appl. Surf. Sci. 2013, 268, 163–170. [Google Scholar] [CrossRef]
- Reddy, N.; Yang, Y. Biofibers from Agricultural Byproducts for Industrial Applications. Trends Biotechnol. 2005, 23, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Oh, T.K.; Choi, B.; Shinogi, Y.; Chikushi, J. Characterization of Biochar Derived from Three Types of Biomass. J. Fac. Agric. Kyushu Univ. 2012, 57, 61–66. [Google Scholar] [CrossRef]
- Sciban, M.; Radetic, B.; Kevresan, Z.; Klasnja, M. Adsorption of Heavy Metals from Electroplating Wastewater by Wood Sawdust. Bioresour. Technol. 2007, 98, 402–409. [Google Scholar] [CrossRef]
- Sud, D.; Mahajan, G.; Kaur, M. Agricultural Waste Material as Potential Adsorbent for Sequestering Heavy Metal Ions from Aqueous Solutions—A Review. Bioresour. Technol. 2008, 99, 6017–6027. [Google Scholar] [CrossRef]
- Muramatsu, H.; Kim, Y.A.; Yang, K.-S.; Cruz-Silva, R.; Toda, I.; Yamada, T.; Terrones, M.; Endo, M.; Hayashi, T.; Saitoh, H. Rice Husk-Derived Graphene with Nano-Sized Domains and Clean Edges. Small 2014, 10, 2766–2770. [Google Scholar] [CrossRef]
- Suman; Kardam, A.; Gera, M.; Jain, V.K. A Novel Reusable Nanocomposite for Complete Removal of Dyes, Heavy Metals and Microbial Load from Water Based on Nanocellulose and Silver Nano-Embedded Pebbles. Environ. Technol. 2015, 36, 706–714. [Google Scholar] [CrossRef]
- Inyang, M.; Gao, B.; Yao, Y.; Xue, Y.; Zimmerman, A.; Mosa, A.; Pullammanappallil, P.; Ok, Y.S.; Cao, X. A Review of Biochar as a Low-Cost Adsorbent for Aqueous Heavy Metal Removal. Crit. Rev. Environ. Sci. Technol. 2016, 46, 406–433. [Google Scholar] [CrossRef]
- Huang, G.; Wang, D.; Ma, S.; Chen, J.; Jiang, L.; Wang, P. A New, Low-Cost Adsorbent: Preparation, Characterization, and Adsorption Behavior of Pb(II) and Cu(II). J. Colloid Interface Sci. 2015, 445, 294–302. [Google Scholar] [CrossRef]
- Xie, Y.; Yuan, X.; Wu, Z.; Zeng, G.; Jiang, L.; Peng, X.; Li, H. Adsorption Behavior and Mechanism of Mg/Fe Layered Double Hydroxide with Fe3O4-Carbon Spheres on the Removal of Pb(II) and Cu(II). J. Colloid Interface Sci. 2019, 536, 440–455. [Google Scholar] [CrossRef]
- Bouhamed, F.; Elouear, Z.; Bouzid, J.; Ouddane, B. Batch Sorption of Pb(II) Ions from Aqueous Solutions Using Activated Carbon Prepared from Date Stones: Equilibrium, Kinetic, and Thermodynamic Studies. Desalination Water Treat. 2014, 52, 2261–2271. [Google Scholar] [CrossRef]
- Yang, R.; Li, H.; Huang, M.; Yang, H.; Li, A. A Review on Chitosan-Based Flocculants and Their Applications in Water Treatment. Water Res. 2016, 95, 59–89. [Google Scholar] [CrossRef]
- Hegazi, H.A. Removal of Heavy Metals from Wastewater Using Agricultural and Industrial Wastes as Adsorbents. HBRC J. 2013, 9, 276–282. [Google Scholar] [CrossRef]
- Sočo, E.; Kalembkiewicz, J. Adsorption of Nickel(II) and Copper(II) Ions from Aqueous Solution by Coal Fly Ash. J. Environ. Chem. Eng. 2013, 1, 581–588. [Google Scholar] [CrossRef]
- Song, M.; Wei, Y.; Cai, S.; Yu, L.; Zhong, Z.; Jin, B. Study on Adsorption Properties and Mechanism of Pb2+ with Different Carbon Based Adsorbents. Sci. Total Environ. 2018, 618, 1416–1422. [Google Scholar] [CrossRef] [PubMed]
- Jindo, K.; Mizumoto, H.; Sawada, Y.; Sanchez-Monedero, M.A.; Sonoki, T. Physical and Chemical Characterization of Biochars Derived from Different Agricultural Residues. Biogeosciences 2014, 11, 6613–6621. [Google Scholar] [CrossRef]
- Zhao, J.; Shen, X.-J.; Domene, X.; Alcañiz, J.-M.; Liao, X.; Palet, C. Comparison of Biochars Derived from Different Types of Feedstock and Their Potential for Heavy Metal Removal in Multiple-Metal Solutions. Sci. Rep. 2019, 9, 9869. [Google Scholar] [CrossRef]
- Ding, Z.; Hu, X.; Wan, Y.; Wang, S.; Gao, B. Removal of Lead, Copper, Cadmium, Zinc, and Nickel from Aqueous Solutions by Alkali-Modified Biochar: Batch and Column Tests. J. Ind. Eng. Chem. 2016, 33, 239–245. [Google Scholar] [CrossRef]
- Ahmad, M.; Ahmad, M.; Usman, A.R.A.; Al-Faraj, A.S.; Ok, Y.S.; Hussain, Q.; Abduljabbar, A.S.; Al-Wabel, M.I. An Efficient Phosphorus Scavenging from Aqueous Solution Using Magnesiothermally Modified Bio-Calcite. Environ. Technol. 2017, 1–12. [Google Scholar] [CrossRef]
- Wang, L.; Wang, Y.; Ma, F.; Tankpa, V.; Bai, S.; Guo, X.; Wang, X. Mechanisms and Reutilization of Modified Biochar Used for Removal of Heavy Metals from Wastewater: A Review. Sci. Total Environ. 2019, 668, 1298–1309. [Google Scholar] [CrossRef]
- Ahmad, M.; Rajapaksha, A.U.; Lim, J.E.; Zhang, M.; Bolan, N.; Mohan, D.; Vithanage, M.; Lee, S.S.; Ok, Y.S. Biochar as a Sorbent for Contaminant Management in Soil and Water: A Review. Chemosphere 2014, 99, 19–33. [Google Scholar] [CrossRef]
- Li, H.; Dong, X.; da Silva, E.B.; de Oliveira, L.M.; Chen, Y.; Ma, L.Q. Mechanisms of Metal Sorption by Biochars: Biochar Characteristics and Modifications. Chemosphere 2017, 178, 466–478. [Google Scholar] [CrossRef]
- Xu, X.; Cao, X.; Zhao, L. Comparison of Rice Husk- and Dairy Manure-Derived Biochars for Simultaneously Removing Heavy Metals from Aqueous Solutions: Role of Mineral Components in Biochars. Chemosphere 2013, 92, 955–961. [Google Scholar] [CrossRef]
- Kloss, S.; Zehetner, F.; Dellantonio, A.; Hamid, R.; Ottner, F.; Liedtke, V.; Schwanninger, M.; Gerzabek, M.H.; Soja, G. Characterization of Slow Pyrolysis Biochars: Effects of Feedstocks and Pyrolysis Temperature on Biochar Properties. J. Environ. Qual. 2012, 41, 990–1000. [Google Scholar] [CrossRef]
- Suliman, W.; Harsh, J.B.; Abu-Lail, N.I.; Fortuna, A.-M.; Dallmeyer, I.; Garcia-Perez, M. Influence of Feedstock Source and Pyrolysis Temperature on Biochar Bulk and Surface Properties. Biomass Bioenergy 2016, 84, 37–48. [Google Scholar] [CrossRef]
- Amin, M.T.; Alazba, A.A.; Shafiq, M. Removal of Copper and Lead Using Banana Biochar in Batch Adsorption Systems: Isotherms and Kinetic Studies. Arab. J. Sci. Eng. 2017, 1–12. [Google Scholar] [CrossRef]
- Rajput, M.S.; Sharma, A.; Sharma, S.; Verma, S. Removal of Lead (II) from Aqueous Solutions by Orange Peel. Int. J. Appl. Res. 2015, 1, 411–413. [Google Scholar]
- Ahmad, M.; Ahmad, M.; Usman, A.R.A.; Al-Faraj, A.S.; Abduljabbar, A.S.; Al-Wabel, M.I. Biochar Composites with Nano Zerovalent Iron and Eggshell Powder for Nitrate Removal from Aqueous Solution with Coexisting Chloride Ions. Environ. Sci. Pollut. Res. Int. 2017. [Google Scholar] [CrossRef]
- Usman, A.R.A.; Abduljabbar, A.; Vithanage, M.; Ok, Y.S.; Ahmad, M.; Ahmad, M.; Elfaki, J.; Abdulazeem, S.S.; Al-Wabel, M.I. Biochar Production from Date Palm Waste: Charring Temperature Induced Changes in Composition and Surface Chemistry. J. Anal. Appl. Pyrolysis 2015, 115, 392–400. [Google Scholar] [CrossRef]
- Doğan, M.; Alkan, M.; Demirbaş, Ö.; Özdemir, Y.; Özmetin, C. Adsorption Kinetics of Maxilon Blue GRL onto Sepiolite from Aqueous Solutions. Chem. Eng. J. 2006, 124, 89–101. [Google Scholar] [CrossRef]
- Gunay, A. Application of Nonlinear Regression Analysis for Ammonium Exchange by Natural (Bigadiç) Clinoptilolite. J. Hazard. Mater. 2007, 148, 708–713. [Google Scholar] [CrossRef]
- Manohar, D.M.; Noeline, B.F.; Anirudhan, T.S. Adsorption Performance of Al-Pillared Bentonite Clay for the Removal of Cobalt(II) from Aqueous Phase. Appl. Clay Sci. 2006, 31, 194–206. [Google Scholar] [CrossRef]
- Zaghouane-Boudiaf, H.; Boutahala, M.; Arab, L. Removal of Methyl Orange from Aqueous Solution by Uncalcined and Calcined MgNiAl Layered Double Hydroxides (LDHs). Chem. Eng. J. 2012, 187, 142–149. [Google Scholar] [CrossRef]
- Rajoriya, R.K.; Prasad, B.; Mishra, I.M.; Wasewar, K.L. Adsorption of Benzaldehyde on Granular Activated Carbon: Kinetics, Equilibrium, and Thermodynamic. Chem. Biochem. Eng. Q. 2007, 21, 219–226. [Google Scholar]
- Tang, H.; Zhou, W.; Zhang, L. Adsorption Isotherms and Kinetics Studies of Malachite Green on Chitin Hydrogels. J. Hazard. Mater. 2012, 209–210, 218–225. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, S.; Mishra, R.; Saha, P.; Kushwaha, P. Adsorption Thermodynamics, Kinetics and Isosteric Heat of Adsorption of Malachite Green onto Chemically Modified Rice Husk. Desalination 2011, 265, 159–168. [Google Scholar] [CrossRef]
- Taty-Costodes, V.C.; Fauduet, H.; Porte, C.; Delacroix, A. Removal of Cd(II) and Pb(II) Ions, from Aqueous Solutions, by Adsorption onto Sawdust of Pinus Sylvestris. J. Hazard. Mater. 2003, 105, 121–142. [Google Scholar] [CrossRef] [PubMed]
- Hamdaoui, O. Dynamic Sorption of Methylene Blue by Cedar Sawdust and Crushed Brick in Fixed Bed Columns. J. Hazard. Mater. 2006, 138, 293–303. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, F.-S. Removal of Lead from Water Using Biochars Prepared from Hydrothermal Liquefaction of Biomass. J. Hazard. Mater. 2009, 167, 933–939. [Google Scholar] [CrossRef]
- Mahdi, Z.; Yu, Q.J.; El Hanandeh, A. Removal of Lead(II) from Aqueous Solution Using Date Seed-Derived Biochar: Batch and Column Studies. Appl. Water Sci. 2018, 8, 181. [Google Scholar] [CrossRef]
- Yakkala, K.; Yu, M.-R.; Roh, H.; Yang, J.-K.; Chang, Y.-Y. Buffalo Weed (Ambrosia Trifida L. Var. Trifida) Biochar for Cadmium (II) and Lead (II) Adsorption in Single and Mixed System. Desalination Water Treat. 2013, 51, 7732–7745. [Google Scholar] [CrossRef]
- Hu, X.; Xue, Y.; Liu, L.; Zeng, Y.; Long, L. Preparation and Characterization of Na2S-Modified Biochar for Nickel Removal. Environ. Sci. Pollut. Res. 2018, 25, 9887–9895. [Google Scholar] [CrossRef] [PubMed]
- Lyu, H.; Gao, B.; He, F.; Zimmerman, A.R.; Ding, C.; Huang, H.; Tang, J. Effects of Ball Milling on the Physicochemical and Sorptive Properties of Biochar: Experimental Observations and Governing Mechanisms. Environ. Pollut. 2018, 233, 54–63. [Google Scholar] [CrossRef]
- Aksu, Z.; Tezer, S. Exploited Application of Bacillus Sp. ETL-A & Pseudomonas Sp. ETL-B in Microbial Degradation of Orange 16 Dye. Process. Biochem. 2001, 36, 431–439. [Google Scholar]
- Chiou, M.S.; Li, H.Y. Equilibrium and Kinetic Modeling of Adsorption of Reactive Dye on Cross-Linked Chitosan Beads. J. Hazard. Mater. 2002, 93, 233–248. [Google Scholar] [CrossRef]
- Mestre, A.S.; Pires, J.; Nogueira, J.M.F.; Carvalho, A.P. Activated Carbons for the Adsorption of Ibuprofen. Carbon 2007, 45, 1979–1988. [Google Scholar] [CrossRef]
- Önal, Y.; Akmil-Başar, C.; Sarıcı-Özdemir, Ç. Elucidation of the Naproxen Sodium Adsorption onto Activated Carbon Prepared from Waste Apricot: Kinetic, Equilibrium and Thermodynamic Characterization. J. Hazard. Mater. 2007, 148, 727–734. [Google Scholar] [CrossRef]
- Hamdaoui, O.; Naffrechoux, E. Modeling of Adsorption Isotherms of Phenol and Chlorophenols onto Granular Activated Carbon: Part I. Two-Parameter Models and Equations Allowing Determination of Thermodynamic Parameters. J. Hazard. Mater. 2007, 147, 381–394. [Google Scholar] [CrossRef]
- Baccar, R.; Sarrà, M.; Bouzid, J.; Feki, M.; Blánquez, P. Removal of Pharmaceutical Compounds by Activated Carbon Prepared from Agricultural By-Product. Chem. Eng. J. 2012, 211–212, 310–317. [Google Scholar] [CrossRef]
- Tan, I.A.W.; Ahmad, A.L.; Hameed, B.H. Adsorption Isotherms, Kinetics, Thermodynamics and Desorption Studies of 2,4,6-Trichlorophenol on Oil Palm Empty Fruit Bunch-Based Activated Carbon. J. Hazard. Mater. 2009, 164, 473–482. [Google Scholar] [CrossRef]
- Foo, K.Y.; Hameed, B.H. Preparation, Characterization and Evaluation of Adsorptive Properties of Orange Peel Based Activated Carbon via Microwave Induced K2CO3 Activation. Bioresour. Technol. 2012, 104, 679–686. [Google Scholar] [CrossRef]
- Malik, P.K. Use of Activated Carbons Prepared from Sawdust and Rice-Husk for Adsorption of Acid Dyes: A Case Study of Acid Yellow 36. Dyes Pigment. 2003, 56, 239–249. [Google Scholar] [CrossRef]
- Kumar, P.S.; Ramakrishnan, K.; Gayathri, R. Removal of Nickel(II) from Aqueous Solutions by Ceralite IR 120 Cationic Exchange Resins. J. Eng. Sci. Technol. 2010, 5, 232–243. [Google Scholar]
- Ramadoss, R.; Subramaniam, D. Removal of Divalent Nickel from Aqueous Solution Using Blue-Green Marine Algae: Adsorption Modeling and Applicability of Various Isotherm Models. Sep. Sci. Technol. 2019, 54, 943–961. [Google Scholar] [CrossRef]
- Saadi, R.; Saadi, Z.; Fazaeli, R. Determination of Axial Dispersion and Overall Mass Transfer Coefficients for Ni (II) Adsorption on Nanostructured γ-Alumina in a Fixed Bed Column: Experimental and Modeling Studies. Desalination Water Treat. 2015, 53, 2193–2203. [Google Scholar] [CrossRef]




| Model | Non-Linear Form | Linear Form |
|---|---|---|
| Kinetic | ||
| PFO | ||
| PSO | ||
| Elovich | ||
| ID–WM | ||
| Isotherm | ||
| Langmuir | ||
| Freundlich | ||
| Temkin | ||
| Halsey | ||
| D–R | ||
| H–J | ||
| Jovanovic | ||
| Biomass | Pyrolysis Temp (°C) | Holding Time | Pollutants | Qmax (mg g−1) | Isotherm | Kinetic Models | Reference |
| EU-biochar | 600 | 6 h | Ni2+ | 54 | Langmuir | PSO | This work |
| EU-biochar | 600 | 6 h | Pb2+ | 200 | Langmuir | PSO | This work |
| Rice husk biochar | 300 | 20 min | Pb2+ | 1.84 | Langmuir | PSO | [52] |
| Pine wood biochar | 300 | 20 min | Pb2+ | 3.89 | Langmuir | PSO | [52] |
| Hickory wood biochar | 600 | 2 h | Pb2+ | 11.2 | Langmuir | PSO | [31] |
| Date seed biochar | 550 | 3 h | Pb2+ | 74.60 | Freundlich, Langmuir | PSO | [53] |
| Buffalo weed biochar | 700 | 4 h | Pb2+ | 333.3 | Langmuir | PSO | [54] |
| Corn cob biochar | 600 | 2 h | Ni2+ | 15.40 | Langmuir | Elovich model | [55] |
| Sugar cane bagasse biochar | 600 | 2 h | Ni2+ | 38.15 | Redlich-Peterson | PFO | [56] |
| Kinetic Model | Parameter | Linear Form | Non-Linear Form | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Ni2+ (mg L−1) | Pb2+ (mg L−1) | Ni2+ (mg L−1) | Pb2+ (mg L−1) | ||||||
| 20 | 40 | 20 | 40 | 20 | 40 | 20 | 40 | ||
| qe exp (mg g−1) | 36.19 | 46.60 | 91.95 | 179.61 | 36.19 | 46.60 | 91.95 | 179.61 | |
| PFO | qe cal (mg g−1) | 2.90 | 5.39 | 5.27 | 34.15 | 34.98 | 46.79 | 89 | 159.84 |
| k1 (min−1) | 0.007 | 0.009 | 0.009 | 0.005 | 0.53 | 0.26 | 0.54 | 0.43 | |
| R2 | 0.24 | 0.36 | 0.2 | 0.32 | 0.76 | 0.92 | 0.46 | 0.64 | |
| PSO | qe cal (mg g−1) | 36.63 | 49.75 | 96.15 | 166.67 | 36.23 | 49.1 | 92.67 | 166.05 |
| k2 (g mg−1 min−1) | 0.019 | 0.007 | 0.0049 | 0.0082 | 0.027 | 0.009 | 0.0100 | 0.0047 | |
| h (mg g−1 min−1) | 24.88 | 17.39 | 45.66 | 227.27 | 34.98 | 21.24 | 86.22 | 128.21 | |
| R2 | 0.9999 | 0.9998 | 0.9999 | 0.9998 | 0.96 | 0.99 | 0.81 | 0.88 | |
| Elovich | α (mg g−1 min−1) | 5151.96 | 67.51 | 2160.36 | 1154.38 | 14,247.5 | 374.38 | 16,459.4 | 16,489 |
| β (g mg−1) | 2.76 | 5.48 | 7.62 | 14.26 | 0.36 | 0.18 | 0.13 | 0.07 | |
| R2 | 0.82 | 0.87 | 0.9 | 0.85 | 0.8 | 0.86 | 0.89 | 0.83 | |
| ID–WM | Kip (mg g−1 min1/2) | 0.75 | 1.53 | 2.21 | 3.91 | 0.75 | 1.53 | 2.21 | 3.9 |
| C (mg g−1) | 27.48 | 30.46 | 68.08 | 120.09 | 27.48 | 30.46 | 68.1 | 120.1 | |
| R2 | 0.5 | 0.57 | 0.63 | 0.54 | 0.5 | 0.57 | 0.63 | 0.54 | |
| Isotherm Model | Parameter | Ni2+ | Pb2+ | ||
|---|---|---|---|---|---|
| Linear | Non-Linear | Linear | Non-Linear | ||
| qe exp, mg g−1 | 54 | 200 | |||
| Langmuir | qm, mg g−1 | 50.51 | 55.21 | 192.31 | 193.95 |
| KL, L mg−1 | 0.68 | 0.41 | 0.91 | 0.81 | |
| RL | 0.018 | 0.029 | 0.014 | 0.015 | |
| R2 | 0.99 | 0.98 | 0.98 | 0.96 | |
| Freundlich | qm, mg g−1 | 59.46 | 63.35 | 215.02 | 207.79 |
| KF, (mg g−1) (L mg−1)1/n | 15.54 | 21.48 | 106.28 | 114.00 | |
| 1/n | 0.306 | 0.247 | 0.161 | 0.137 | |
| R2 | 0.95 | 0.94 | 0.85 | 0.81 | |
| Temkin | KT, L mg−1 | 8.70 | 8.70 | 106.16 | 106.15 |
| Hads, kJ mol−1 | 277.50 | 277.5 | 110.75 | 110.75 | |
| R2 | 0.99 | 0.99 | 0.88 | 0.87 | |
| Halsey | qe cal, mg g−1 | 61.01 | 58.78 | 245.36 | 195.00 |
| nH | −3.27 | −4.05 | −6.22 | −7.30 | |
| KH | 0.323 | 0.000 | 0.264 | 0.000 | |
| R2 | 0.95 | 0.94 | 0.85 | 0.81 | |
| D–R | qm, mg g−1 | 45.95 | 49.31 | 179.68 | 181.64 |
| KDR, (mol kJ−1)2 | 2.0 × 10−7 | 4.0 × 10−7 | 2.0 × 10−7 | 2.6 × 10−7 | |
| E, kJ mol−1 | 1.58 | 1.12 | 1.58 | 1.39 | |
| R2 | 0.85 | 0.82 | 0.86 | 0.81 | |
| H–J | AHJ, mg g−1 | 357.14 | 147.39 | 25000 | 1076.7 |
| BHJ | 1.5357 | 3 | 2.25 | 4.47 | |
| R2 | 0.72 | 0.86 | 0.76 | 0.76 | |
| Jovanovic | qm, mg g−1 | 23.89 | 50.9 | 134.05 | 184.39 |
| Kj, L g−1 | −0.019 | −0.28 | −0.007 | −0.49 | |
| R2 | 0.55 | 0.91 | 0.46 | 0.87 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shafiq, M.; Alazba, A.A.; Amin, M.T. Kinetic and Isotherm Studies of Ni2+ and Pb2+ Adsorption from Synthetic Wastewater Using Eucalyptus camdulensis—Derived Biochar. Sustainability 2021, 13, 3785. https://doi.org/10.3390/su13073785
Shafiq M, Alazba AA, Amin MT. Kinetic and Isotherm Studies of Ni2+ and Pb2+ Adsorption from Synthetic Wastewater Using Eucalyptus camdulensis—Derived Biochar. Sustainability. 2021; 13(7):3785. https://doi.org/10.3390/su13073785
Chicago/Turabian StyleShafiq, Muhammad, Abdulrahman Ali Alazba, and Muhammad Tahir Amin. 2021. "Kinetic and Isotherm Studies of Ni2+ and Pb2+ Adsorption from Synthetic Wastewater Using Eucalyptus camdulensis—Derived Biochar" Sustainability 13, no. 7: 3785. https://doi.org/10.3390/su13073785
APA StyleShafiq, M., Alazba, A. A., & Amin, M. T. (2021). Kinetic and Isotherm Studies of Ni2+ and Pb2+ Adsorption from Synthetic Wastewater Using Eucalyptus camdulensis—Derived Biochar. Sustainability, 13(7), 3785. https://doi.org/10.3390/su13073785






