Application of Oxalic Acid and 1-Methylcyclopropane (1-Mcp) with Low and High-Density Polyethylene on Post-Harvest Storage of Litchi Fruit
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection of Litchi Fruits
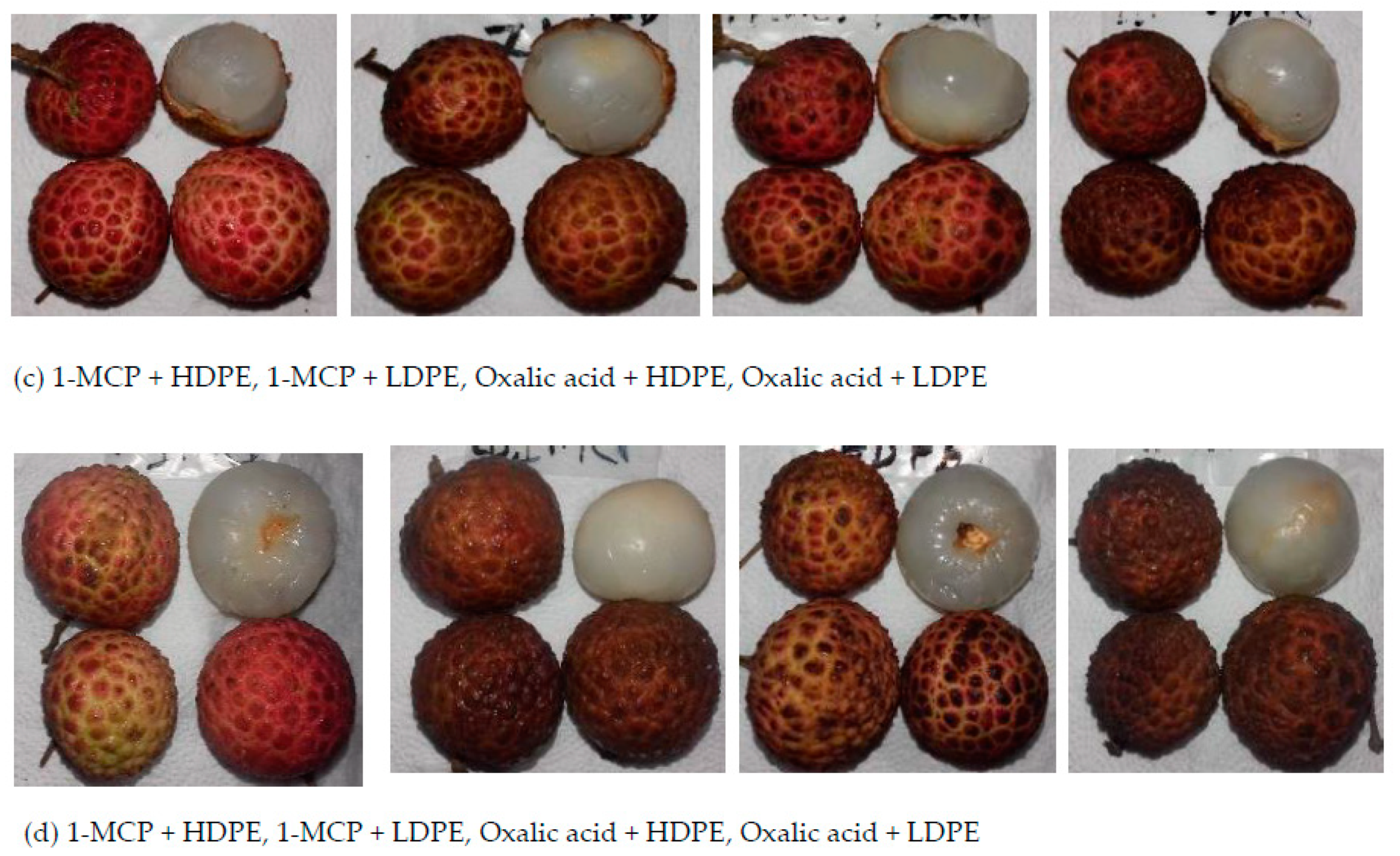
2.2. Fruit Treatments and Storage
2.3. Analysis of Physicochemical Properties
2.3.1. Determination of Weight Loss
2.3.2. Determination of Pericarp Color, pH, and Titratable Acidity
2.3.3. Determination of Total Sugar Contents
2.3.4. Determination of Vitamin C (Ascorbic Acid) Content
2.3.5. Evaluation of Total Anthocyanin Content
2.3.6. Assessment of Total Phenol Content
2.3.7. Estimation of Flavonoid Content
2.3.8. Antioxidant Activity Measured by DPPH Scavenging
2.3.9. Antioxidant Activity Measured by Reducing Power
2.3.10. Measurement of Superoxide Dismutase (SOD) Enzyme Assay
2.3.11. Statistical Analysis
3. Results and Discussions
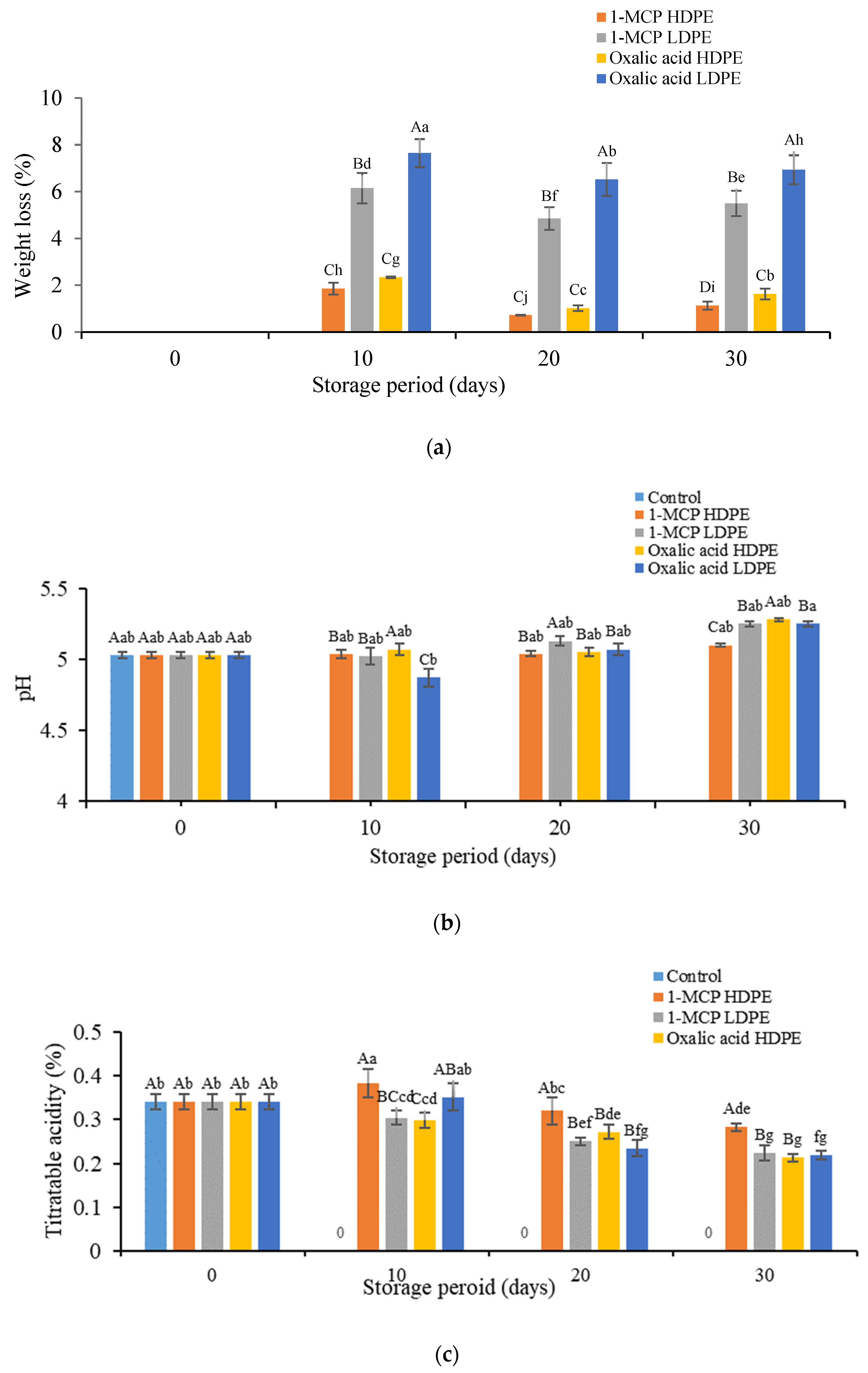
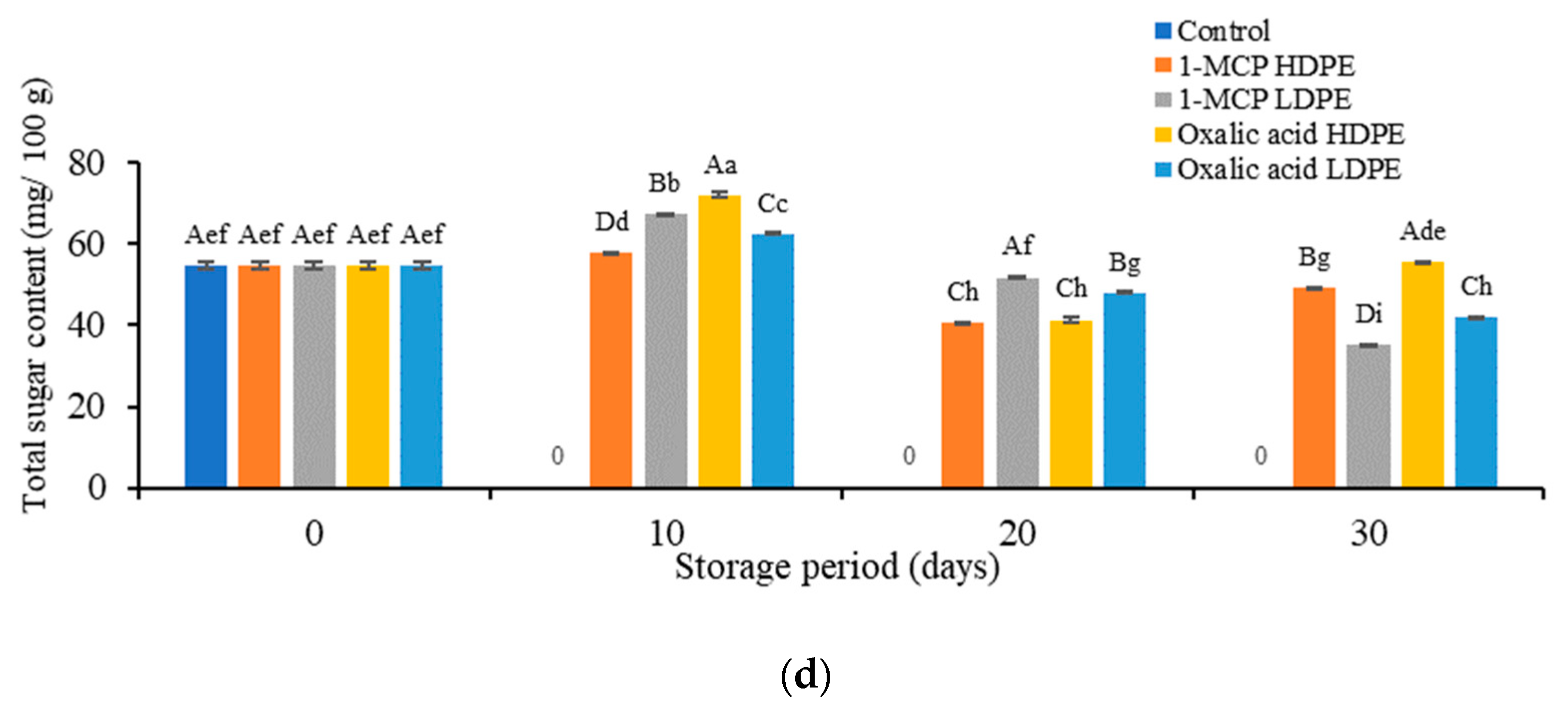
3.1. Effects of Treatment on the Physicochemical Properties
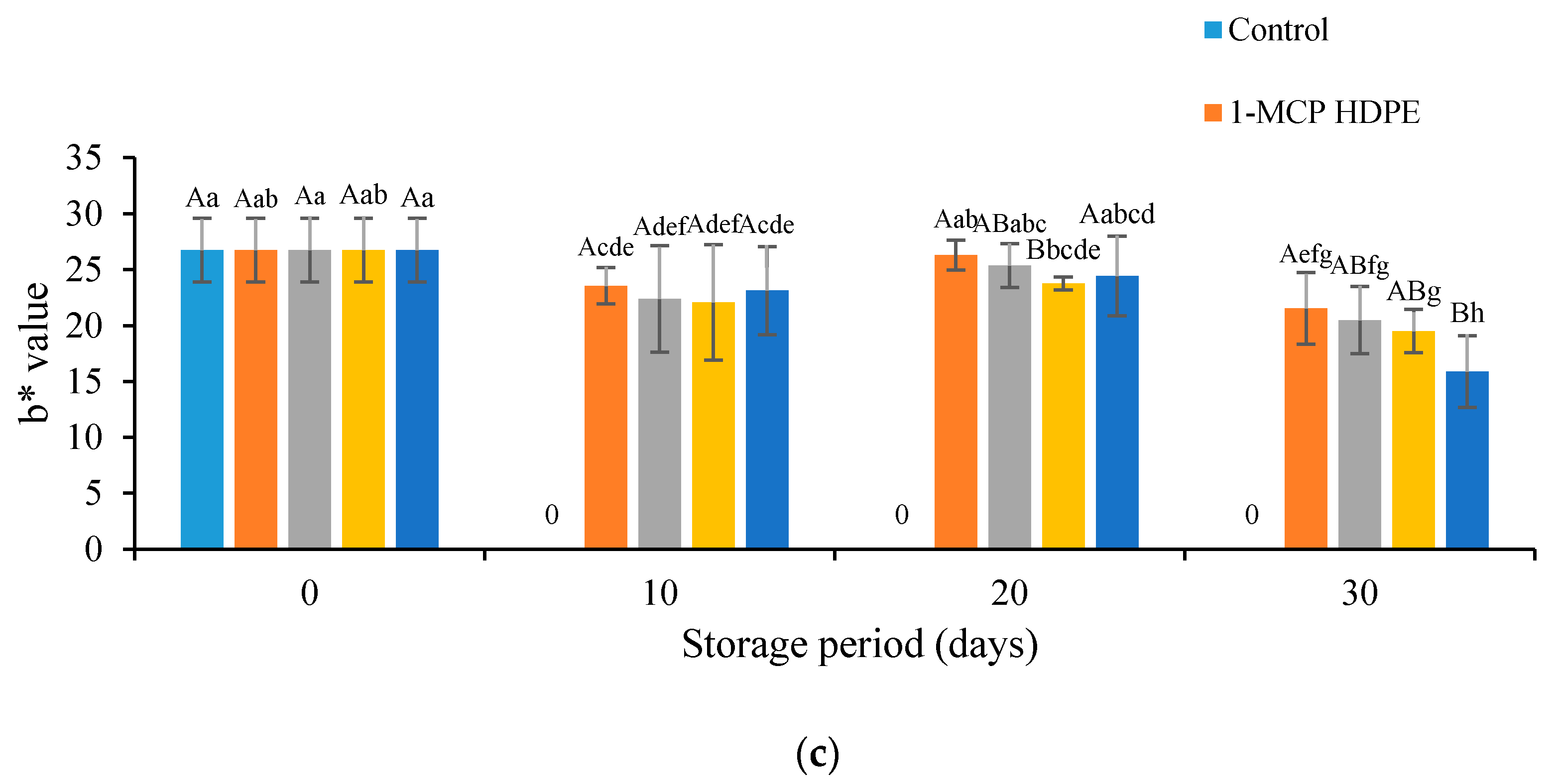
3.2. Effects of Treatment on the Color Values
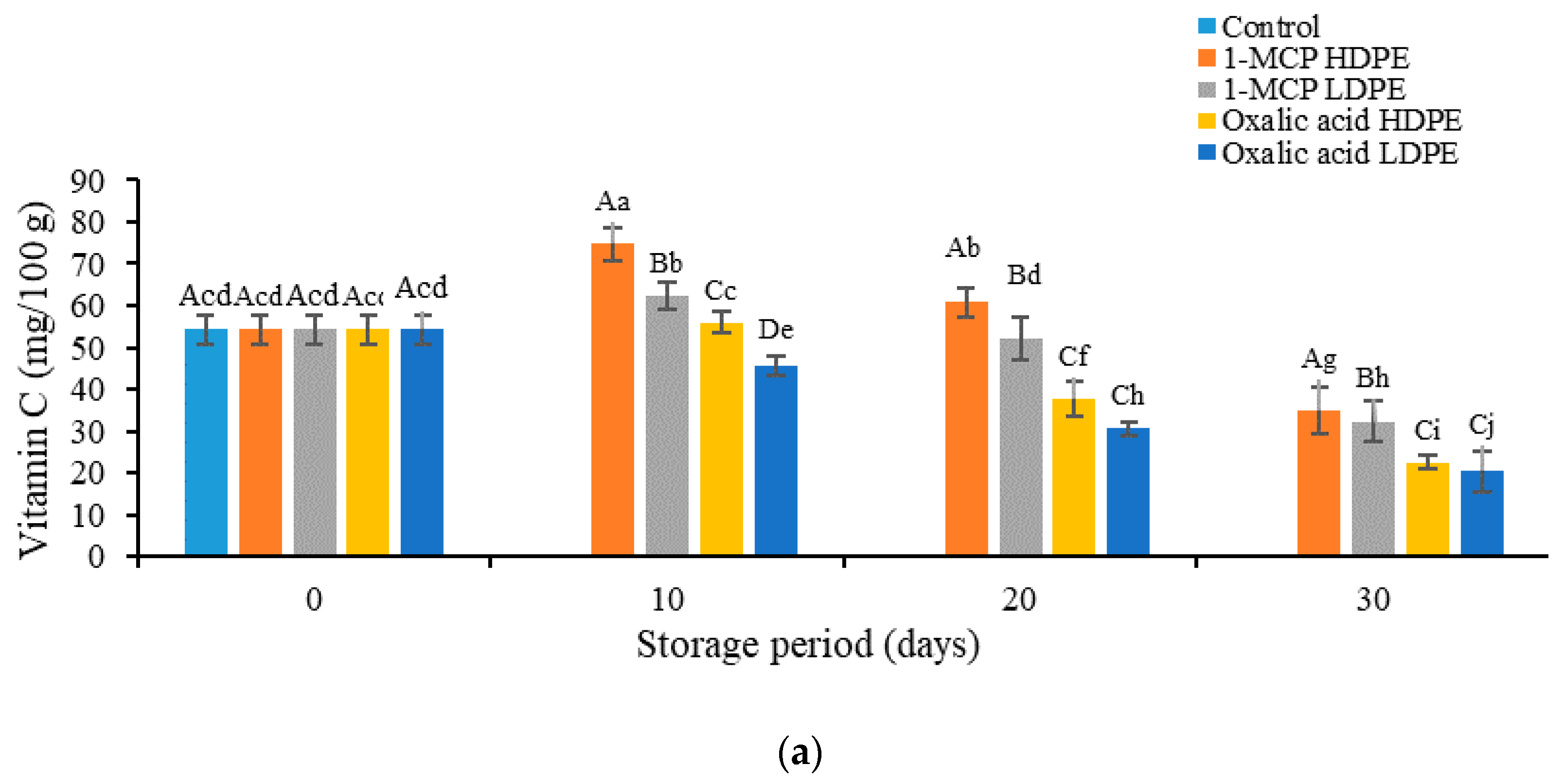
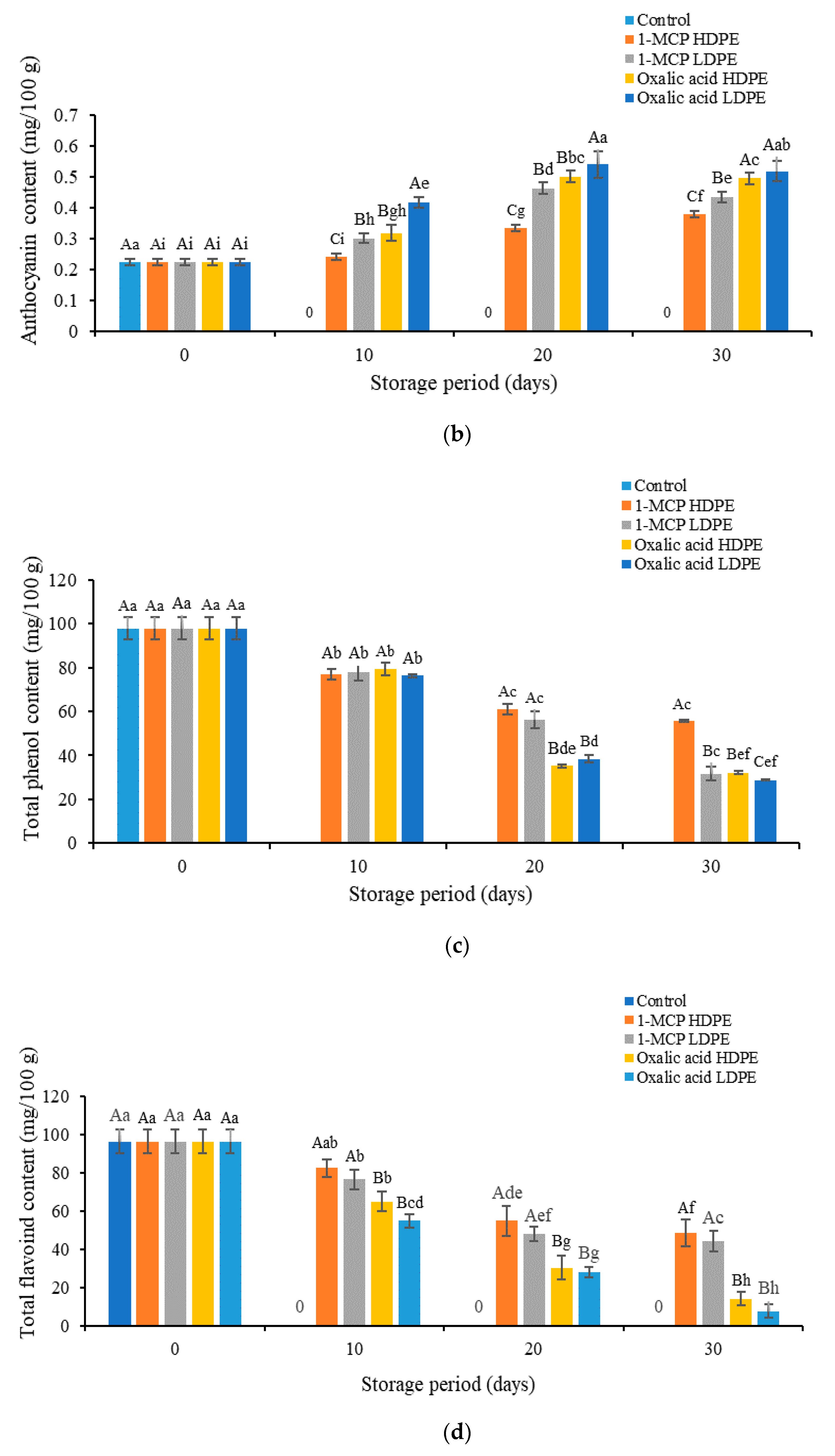
3.3. Effects of Treatment on the Content of Vitamin C, Anthocyanins, Phenol, and Flavonoids
3.4. Effects of Treatments on DPPH Scavenging Activity
3.5. Effects of Treatments on Superoxide Dismutase (SOD) Activity
3.6. Correlation among Acidity, Bioactive Compounds, Antioxidant, and Enzyme Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A

References
- Neog, M.; Saikia, L. Control of postharvest pericarp browning of litchi (Litchi chinensis Sonn). J. Food Sci. Technol. 2010, 47, 100–104. [Google Scholar] [CrossRef]
- Ramma, I. Postharvest Sulphur Dioxide Fumigation and Low Acid Dip for Pericarp Colour Retention and Decay Prevention on Litchi. In Report of the Food and Agricultural Research Council; AMAS: Reduit, Mauritius, 2003; pp. 41–48. [Google Scholar]
- Zheng, X.; Tian, S. Effect of oxalic acid on control of postharvest browning of litchi fruit. Food Chem. 2006, 96, 519–523. [Google Scholar] [CrossRef]
- Candan, A.P.; Graell, J.; Crisosto, C.; Larrigaudiere, C. Improvement of storability and shelf-life of ‘Blackamber’ plums treated with 1-methylcyclopropene. Food Sci. Technol. Int. 2006, 15, 437–444. [Google Scholar] [CrossRef]
- De Reuck, K.; Sivakumar, D.; Korsten, L. Integrated application of 1-methylcyclopropene and modified atmosphere packaging to improve quality retention of litchi cultivars during storage. Postharharvest Biol. Technol. 2009, 52, 71–77. [Google Scholar] [CrossRef]
- Sisler, E.C.; Serek, M. Inhibitors of ethylene responses in plant at the receptor level: Recent development. Physiol. Plantar. 1997, 100, 577–582. [Google Scholar] [CrossRef]
- Yadav, S.; Kumar, A.; Saini, R.S. Biochemical and organoleptic quality changes during storage of ber fruit transported with different packaging materials. Haryana J. Hortic. Sci. 2005, 34, 25–28. [Google Scholar]
- Kudachikar, V.B.; Kulkarni, S.G.; Vasantha, M.S.; Prasad, B.A. Effect of modified atmosphere packaging on shelf- life and fruit quality of banana stored at low temperature. J. Food Sci. Technol. 2007, 44, 74–78. [Google Scholar]
- Ranganna, S. Plant Pigments in Manual Analysis of Fruit and Vegetable Products; Ranganna, S., Ed.; TaTa McGraw-Hill Publishing Co., Ltd.: New Delhi, India, 1997; pp. 72–93. [Google Scholar]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.T.; Smith, F. Colorimetric method for determination of sugars and related substances. Anaytical Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Calorimetry of total phenolic with phosphomolybdic phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Kim, D.O.; Jeong, S.W.; Lee, C.Y. Antioxidant capacity of phenolic phytochemicals from various cultivars of plums. Food Chem. 2003, 81, 321–326. [Google Scholar] [CrossRef]
- Madhujith, T.; Shahidi, F. Optimization of the extraction of antioxidative constituents of six barley cultivars and their antioxidant properties. J. Agric. Food Chem. 2006, 54, 8048–8057. [Google Scholar] [CrossRef]
- Oyaizu, M. Studies on products of browning reaction: Antioxidative activity of products of browning reaction. Jpn. J. Nutr. 1986, 44, 307–315. [Google Scholar] [CrossRef]
- Kumar, R.R.; Goswami, S.; Sharma, S.K.; Singh, K.; Gadpayle, K.A.; Kumar, N.; Manorama, S.; Rai, R.D. Protection against heat stress in wheat involves change in cell membrane stability, antioxidant enzymes, osmolyte, H2O2 and transcript of heat shock protein. Int. J. Plant Physiol. Biochem. 2012, 4, 83–91. [Google Scholar]
- Sakhale, B.K.; Gaikwad, S.S.; Chavan, R.F. Application of 1-methylcyclopropene on mango fruit (Cv. Kesar): Potential for shelf life enhancement and retention of quality. J. Food Sci. Technol. 2018, 55, 776–781. [Google Scholar] [CrossRef] [PubMed]
- Borkar, P.A.; Jadhao, S.D.; Bakane, P.H.; Borkar, S.L.; Murumkar, R.P. Effect of ethylene absorbent and different packaging materials on storage life of Banana. Asian J. Biochem. Sci. 2008, 3, 233–236. [Google Scholar]
- Poudel, S.; Shrestha, A.K.; Mishra, K.; Gotame, T.P.; Regmi, R. Influence of Postharvest Treatment of Oxalic Acid on Shelf Life and Quality of Litchi Fruit cv. Muzaffarpur. Int. J. Environ. Agric. Biotech. 2016, 1, 896–901. [Google Scholar] [CrossRef]
- Ayranci, E.; Tunc, S. A method for measurement of the oxygen permeability and the development of edible films to reduce the rate of oxidative reaction of fresh foods. Food Chem. 2003, 80, 423–431. [Google Scholar] [CrossRef]
- Liu, R.; Lai, T.; Xu, Y.; Tian, S. Changes in physiology and quality of laiying pear in long time storage. Sci. Hortic. 2013, 150, 31–35. [Google Scholar] [CrossRef]
- Hailu, M.; Workneh, T.S.; Belew, D. Effect of packaging materials on the quality of Guava cultivars (Musa spp.). J. Food Sci. Technol. 2014, 51, 2947–2963. [Google Scholar] [CrossRef] [PubMed]
- Divya, A.R.; Jayashree, S.; Bhogi, B. Effect of storage methods on national quality of sapota candy. Asian J. Dairy Food Res. 2014, 33, 104–108. [Google Scholar] [CrossRef]
- Shubham, M.N.K.; Kumar, R.; Dongariyal, A.; Rai, R.; Arya, M.K. Effect of edible coating and packaging on postharvest life and quality of litchi (Litchi chinensis Sonn.) fruits during storage. J. Pharmacogn. Phytochem. 2020, 9, 3018–3023. [Google Scholar]
- Rao, D.V.S.; Shivashankara, K.S. Individual shrink wrapping extends the storage life and maintains the antioxidants of mangoes (cvs ‘Alphonso and Baganapalli’) stored at 8 °C. J. Food Sci. Technol. 2015, 52, 4351–4359. [Google Scholar] [CrossRef]
- Zhang, Z.; Pang, X.; Zuoliang, J.; Jiang, Y. Role of anthocyanin degradation in litchi pericarp browning. Food Chem. 2001, 75, 217–221. [Google Scholar] [CrossRef]
- Liu, H.; Cao, J.; Jiang, W. Changes in phenolics and antioxidant property of peach fruit during ripening and responses to 1-methylcyclopropene. Postharvest Biol. Technol. 2015, 108, 111–118. [Google Scholar]
- Arthey, D.; Philip, R.A. Fruit Processing Nutrition, Product and Quality Management, 2nd ed.; Brijbasi Art Press Ltd.: Noida, India, 2005; p. 45. [Google Scholar]
- Alam, M.K.; Ahmed, M.; Akter, M.S.; Islam, N.; Eun, J. Effect of carboxymethylcellulose and starch as thickening agents on the quality of tomato ketchup. Pak. J. Nutr. 2009, 8, 1144–1149. [Google Scholar]
- Kessy, H.N.E.; Hu, Z.; Zhao, L.; Zhou, M. Effect of Steam Blanching and Drying on Phenolic Compounds of Litchi Pericarp. Molecules 2016, 21, 729. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.M.; Li, Y.B. Effects of chitosan coating on postharvest life and quality of longan fruit. Food Chem. 2000, 73, 139–143. [Google Scholar] [CrossRef]
- Razavi, F.; Hajilou, J.; Dehgan, G.; Hassani, R.N.B. Effect of postharvest oxalic acid treatment on ethylene production, quality parameters, and antioxidant potential of peach fruit during cold storage. Iran. J. Plant Physiol. 2017, 7, 2027–2036. [Google Scholar]
- Hoang, N.T.T.; Golding, J.B.; Wilkes, M.A. The effect of postharvest 1-MCP treatment and storage atmosphere on ‘Cripps Pink’ apple phenolic and antioxidant activity. Food Chem. 2011, 127, 1249–1256. [Google Scholar] [CrossRef]
- Cao, S.; Zheng, Y.; Yang, Z. Effect of 1-MCP treatment on nutritive and functional properties of loquat fruit during cold storage. N. Z. J. Crop Hortic. Sci. 2011, 39, 61–70. [Google Scholar] [CrossRef]
- Arnnok, P.; Ruangviriyachai, C.; Mahachai, R.; Techawongstein, S. Determination of total phenolics and anthocyanin contents in the pericarp of hot chilli pepper (Capsicum annuum L.). Int. Food Res. J. 2012, 19, 235–243. [Google Scholar]
- Altunkaya, A.; Gokmen, V. Effect of various inhibitors on enzymatic browning, antioxidant activity and total phenol content of fresh lettuce (Lactuca sativa). Food Chem. 2008, 107, 1173–1179. [Google Scholar] [CrossRef]
- Catro-Barquero, R.; Lamuela-Raventós, M.; Doménech, R.; Estruch, S.; Castro-Barquero, R.M.; Lamuela-Raventós, M.; Doménech, R. Estruch. Relationship between Mediterranean dietary polyphenol intake and obesity. Nutrients 2018, 10, 1523. [Google Scholar] [CrossRef]
- Martínez-Esplá, A.; Serrano, M.; Martínez-Romero, D.; Valeroa, D.; Zapata, P.J. Oxalic acid pre-harvest treatment increases antioxidant systems and improves plum quality at harvest and during postharvest storage. J. Sci. Food Agric. 2019, 99, 235–243. [Google Scholar] [CrossRef]
- Fawbush, F.; Nock, J.F.; Watkins, C.B. Antioxidant contents and activities of peels and pulps from cv. Golden Delicious apples as related to their phenolic composition. Post. Biol. Technol. 2009, 52, 30–37. [Google Scholar] [CrossRef]
- MacLean, D.D.; Murr, D.P.; DeEll, J.R.; Horvath, C.R. Postharvest variation in apple (Malus Domestica Borkh.) flavonoids following harvest, storage and 1-MCP treatment. J. Agric. Food Chem. 2006, 54, 870–878. [Google Scholar] [CrossRef]
- Jiang, Y.; Li, Y.; Jianrong, L. Browning control, shelf life extension and quality maintenance of frozen litchi fruit by hydrochloric acid. J. Food Eng. 2004, 63, 147–151. [Google Scholar] [CrossRef]
- Hasan, F.A.S.; Mahfouz, S.A. Effect of 1-MCP on the postharvest senescence of coriander leaves during storage and its retention of antioxidant enzyme activity. Sci. Hortic. 2012, 17, 521–533. [Google Scholar]
- Kolniak-Ostek, J.; Wojdyło, A.; Markowski, J.; Siucin’ska, K. 1-Methylcyclopropene postharvest treatment and their effect on apple quality during long-term storage time. Eur. Food Res. Technol. 2014, 239, 603–612. [Google Scholar] [CrossRef]
- Ramli, R.; Ahmad, F.T. Effect of different storage conditions on the quality curry leaves (Murraya koenigii). In Proceedings of the International Conference of FASSA Jember, Jember, Indonesia, 1–3 August 2017. [Google Scholar]
- Duan, X.; Wu, G.; Jiang, Y. Evaluation of the Antioxidant Properties of Litchi Fruit Phenolics in Relation to Pericarp Browning Prevention. Molecules 2007, 12, 759–771. [Google Scholar] [CrossRef]
- Chang, L.W.; Yen, W.J.; Huang, S.C.; Duh, P.D. Antioxidant activity of sesame coat. Food Chem. 2002, 78, 347–354. [Google Scholar] [CrossRef]
- Nowacka, M.; Sledz, M.; Wiktor, A.; Witrowa-Rajchert, D. Changes of Radical Scavenging Activity and Polyphenols Content During Storage of Dried Apples. Int. J. Food Propert. 2014, 17, 1317–1331. [Google Scholar] [CrossRef]
- Yun, Z.; Gao, H.; Chen, X.; Chen, Z.; Zhang, Z.; Li, T.; Qu, H.; Jiang, Y. Effects of hydrogen water treatment on antioxidant system of litchi fruit during the pericarp browning. Food Chem. 2021, 336, 127618. [Google Scholar] [CrossRef]
- Massolo, J.F.; Concello´n, A.; Chaves, A.R. 1-methylcyclopropene (1-MCP) delays senescence, maintains quality and reduces browning of non-climacteric eggplant (Solanum melongena L.) fruit. Postharv. Biol. Technol. 2011, 59, 10–15. [Google Scholar] [CrossRef]
- Duan, X.W.; Liu, T.; Zhang, D.D.; Su, X.G.; Lin, H.T.; Jiang, Y.M. Effect of pure oxygen atmosphere on antioxidant enzyme and antioxidant activity of harvested litchi fruit during storage. Food Res. Int. 2011, 44, 1905–1911. [Google Scholar] [CrossRef]
- Singh, S.P.; Singh, Z. Controlled and modified atmospheres influence chilling injury, fruit quality and antioxidative system of Japanese plums (Prunus salicina Lindell). Int. J. Food Sci. Technol. 2013, 48, 363–374. [Google Scholar] [CrossRef]
- Lu, J.; Zhao, H.; Chen, J.; Fan, W.; Dong, J.; Kong, W.; Sun, J.; Cao, Y.; Cai, G. Evolution of Phenolic Compounds and Antioxidant Activity during Malting. J. Agric. Food Chem. 2007, 55, 10994–11001. [Google Scholar] [CrossRef]
- Samee, W.; Engkalohakul, M.; Nebbua, N.; Direkrojanavuti, P.; Sornchaithawatwong, C.; Kamkaen, N. Correlation Analysis between Total Acid, Total Phenolic and Ascorbic Acid Contents in Fruit Extracts and Their Antioxidant Activities. Thai. Pharm. Health Sci. J. 2006, 1, 196–203. [Google Scholar]
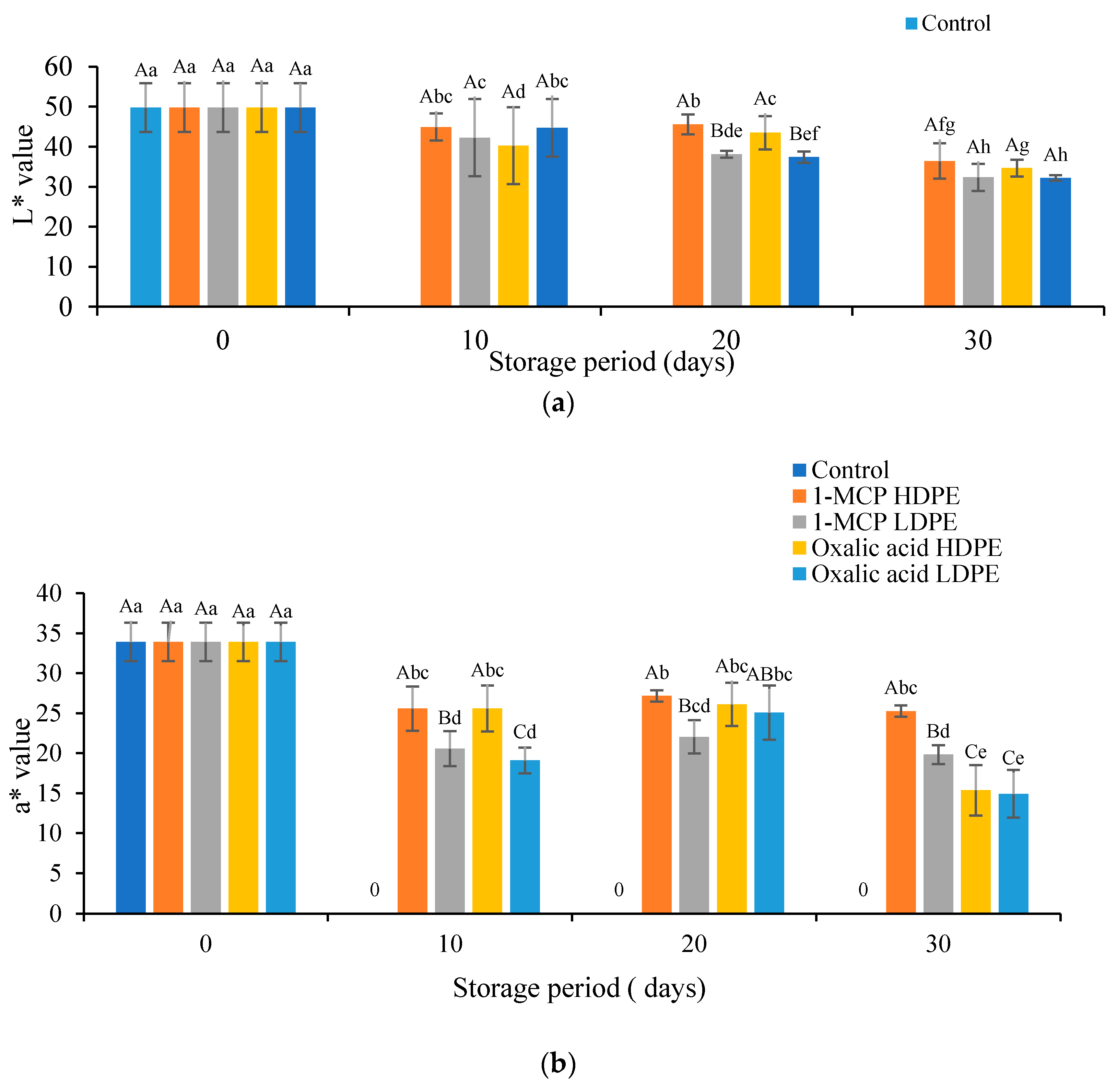


| Phenol | Flavonoid | Anthocyanin | Reducing Power | DPPH | SOD | Acidity | Vitamin C | |
|---|---|---|---|---|---|---|---|---|
| Phenol | 1 | 0.936 ** | −0.894 ** | 0.935 ** | 0.828 ** | 0.714 ** | 0.822 ** | 0.751 ** |
| Flavonoid | 1 | −0.925 ** | 0.945 ** | 0.850 ** | 0.669 ** | 0.829 ** | 0.803 ** | |
| Anthocyanin | 1 | −0.903 ** | −0.817 ** | −0.677 ** | −0.803 ** | −0.802 ** | ||
| Reducing Power | 1 | 0.890 ** | 0.696 ** | 0.829 ** | 0.865 ** | |||
| DPPH | 1 | 0.877 ** | 0.856 ** | 0.975 ** | ||||
| SOD | 1 | 0.790 ** | 0.832 ** | |||||
| Acidity | 1 | 0.797 ** | ||||||
| Vitamin C | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hossain, M.S.; Ramachandraiah, K.; Hasan, R.; Chowdhury, R.I.; Kanan, K.A.; Ahmed, S.; Ali, M.A.; Islam, M.T.; Ahmed, M. Application of Oxalic Acid and 1-Methylcyclopropane (1-Mcp) with Low and High-Density Polyethylene on Post-Harvest Storage of Litchi Fruit. Sustainability 2021, 13, 3703. https://doi.org/10.3390/su13073703
Hossain MS, Ramachandraiah K, Hasan R, Chowdhury RI, Kanan KA, Ahmed S, Ali MA, Islam MT, Ahmed M. Application of Oxalic Acid and 1-Methylcyclopropane (1-Mcp) with Low and High-Density Polyethylene on Post-Harvest Storage of Litchi Fruit. Sustainability. 2021; 13(7):3703. https://doi.org/10.3390/su13073703
Chicago/Turabian StyleHossain, Md. Saddam, Karna Ramachandraiah, Rashidul Hasan, Rabiul Islam Chowdhury, Kawser Alam Kanan, Shakil Ahmed, Md. Aslam Ali, Md. Tariqul Islam, and Maruf Ahmed. 2021. "Application of Oxalic Acid and 1-Methylcyclopropane (1-Mcp) with Low and High-Density Polyethylene on Post-Harvest Storage of Litchi Fruit" Sustainability 13, no. 7: 3703. https://doi.org/10.3390/su13073703
APA StyleHossain, M. S., Ramachandraiah, K., Hasan, R., Chowdhury, R. I., Kanan, K. A., Ahmed, S., Ali, M. A., Islam, M. T., & Ahmed, M. (2021). Application of Oxalic Acid and 1-Methylcyclopropane (1-Mcp) with Low and High-Density Polyethylene on Post-Harvest Storage of Litchi Fruit. Sustainability, 13(7), 3703. https://doi.org/10.3390/su13073703






