Investigation of Corrosion Rate and Rust Expansion Form of Segment Reinforcement for Shield Tunnel by Combined Action of Soil Loading, Chloride Ion and Stray Current
Abstract
1. Introduction
2. Numerical Model of 3D Shield Tunnel
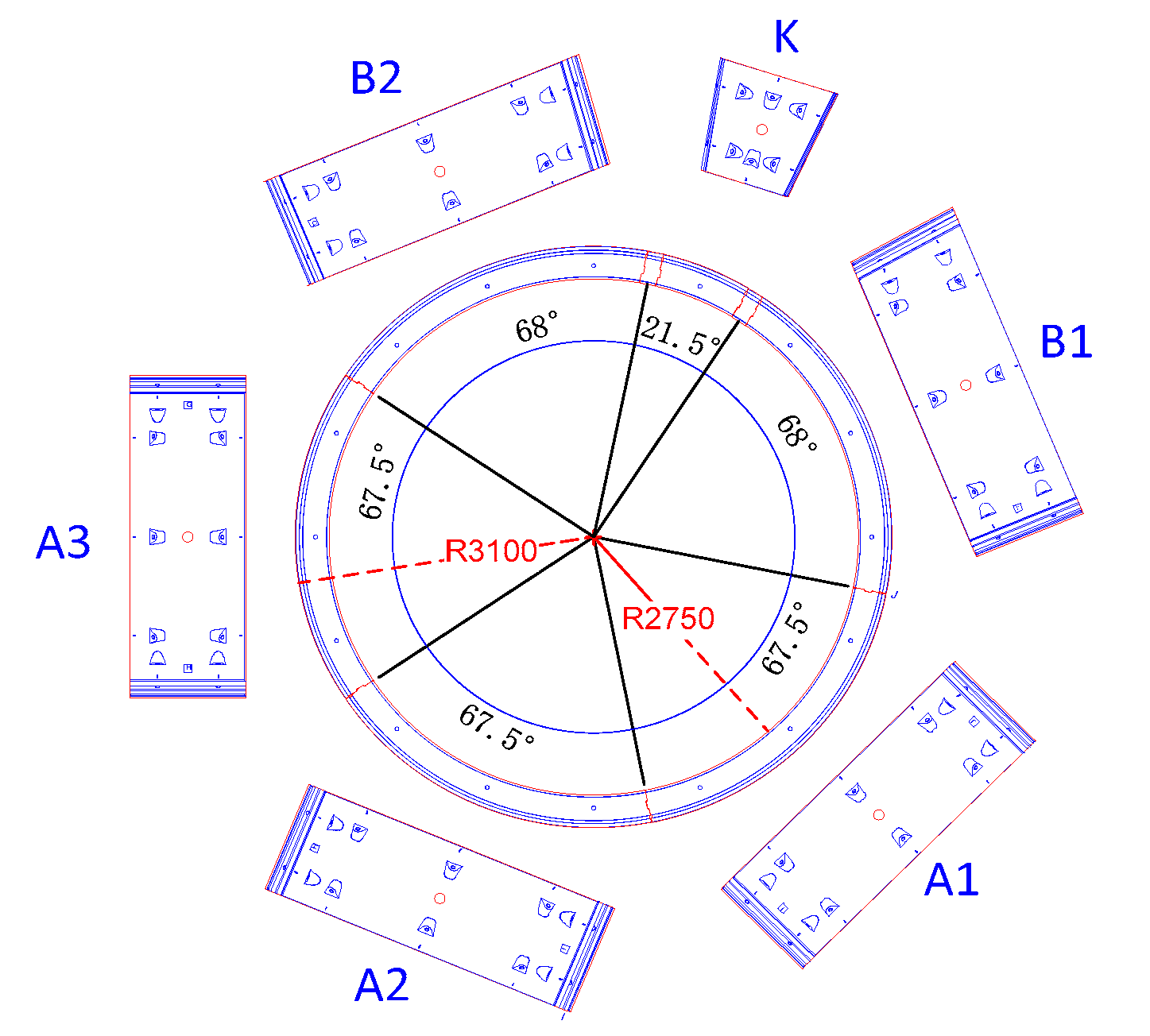
2.1. Segment Details
2.2. Model Configuration
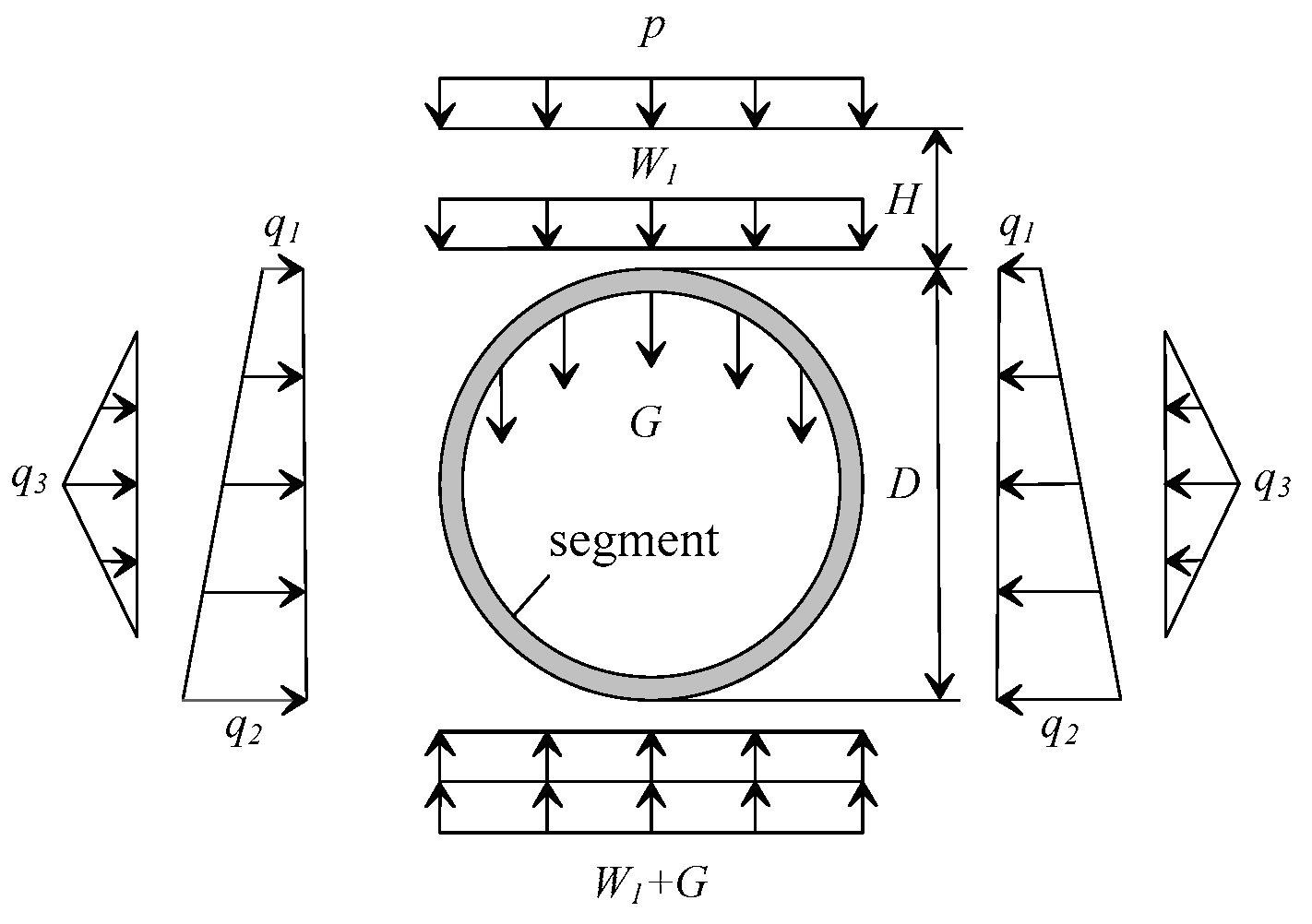
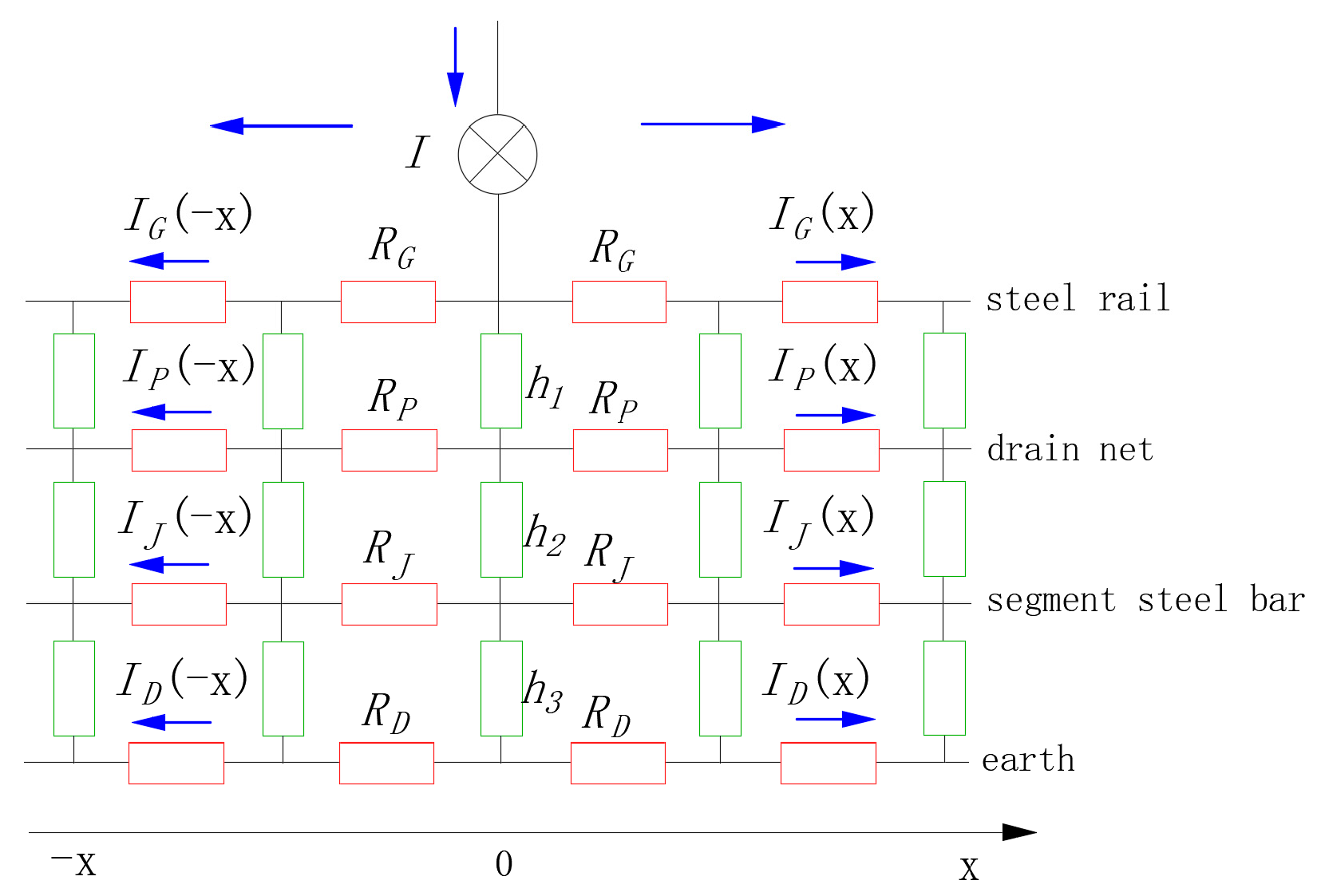
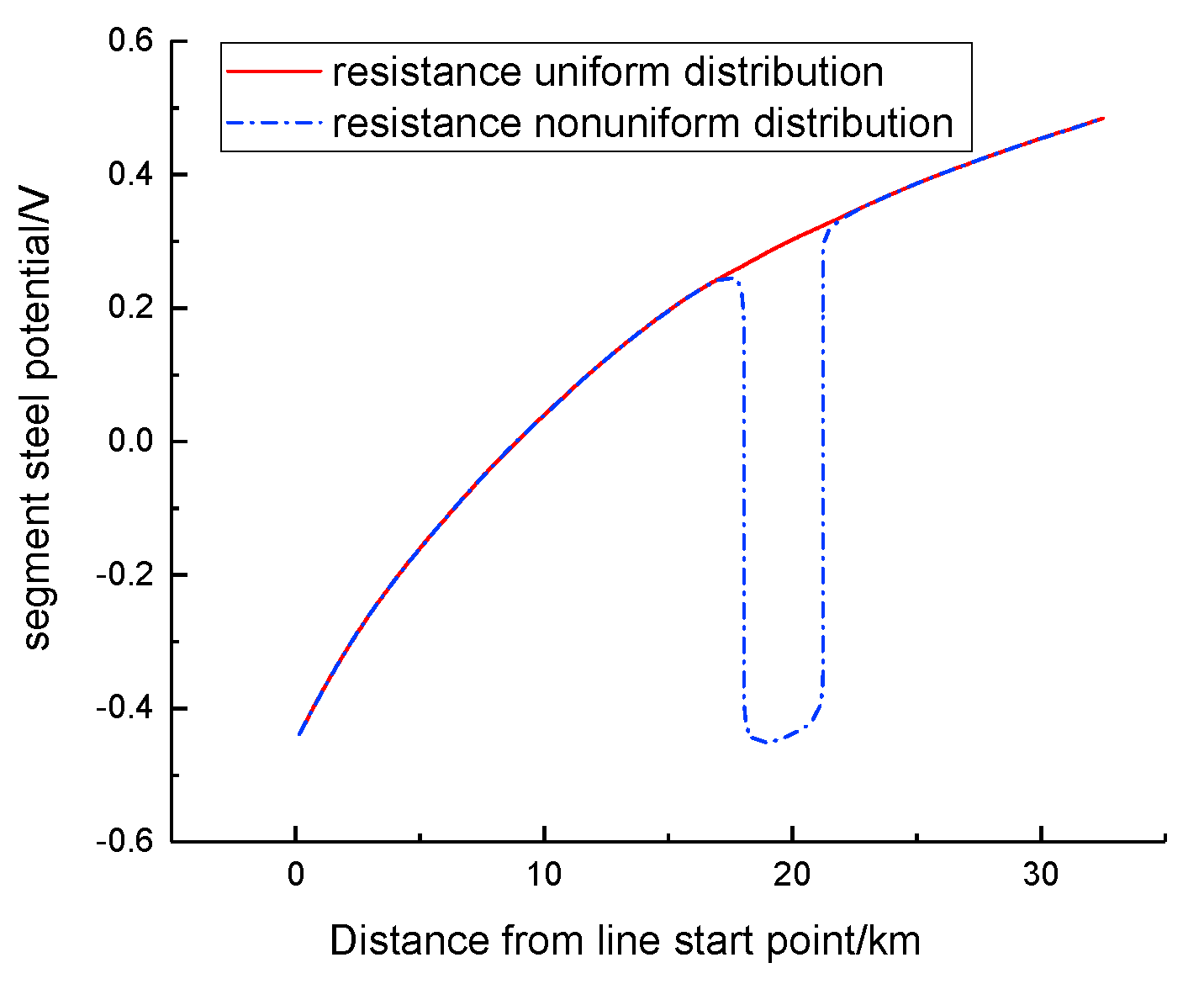
2.3. Boundary Conditions
2.4. Segment Steel Corrosion Process
- (a)
- Dissolution of iron at the anodic sites:
- (b)
- Reaction of dissolved oxygen in the pore water with the electrons on the cathode:
- (c)
- Transport of hydroxyl ions to the anode, where corrosion products forms:
2.4.1. Oxygen Diffusion and Consumption
2.4.2. Polarization of Anode and Cathode
3. Corrosion Rate of the Steel Bar Surface
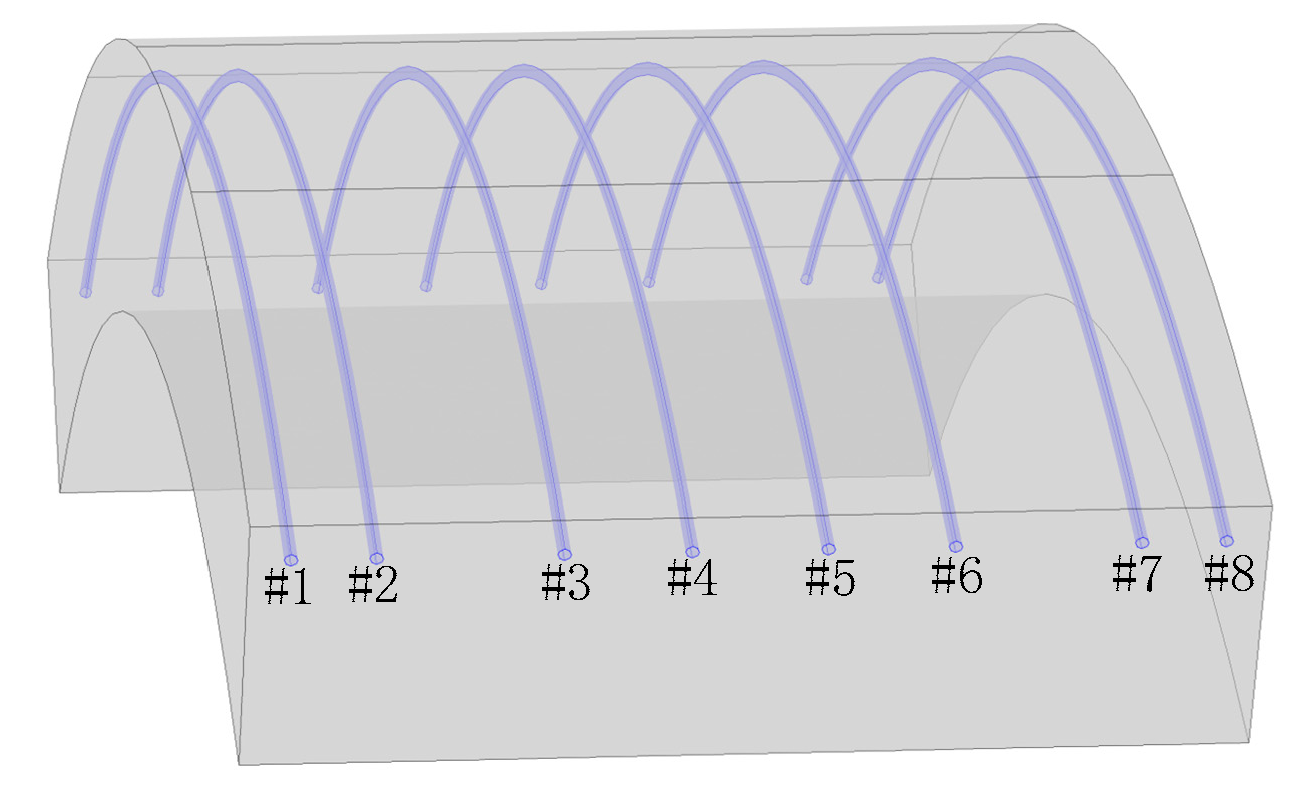
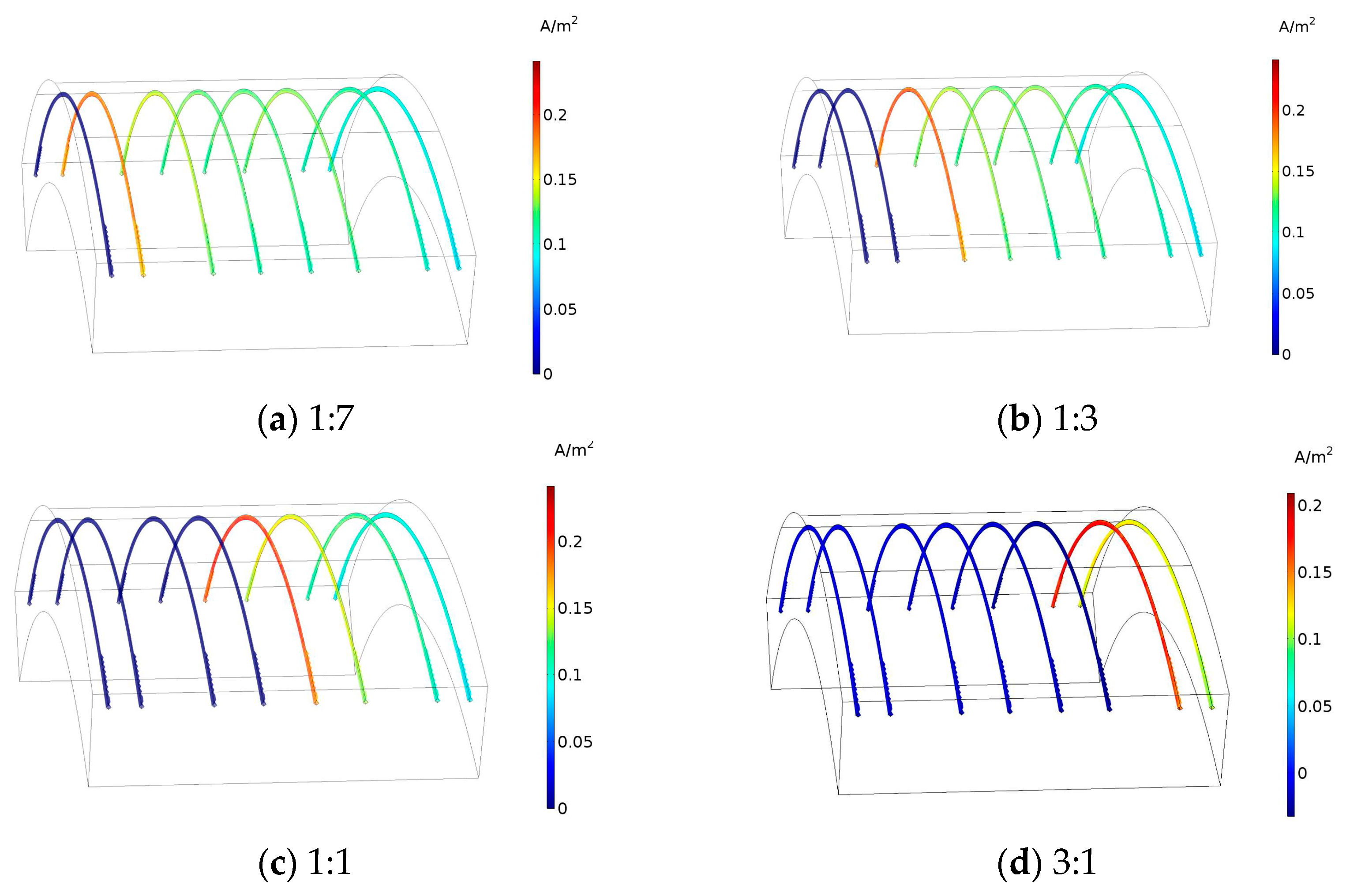
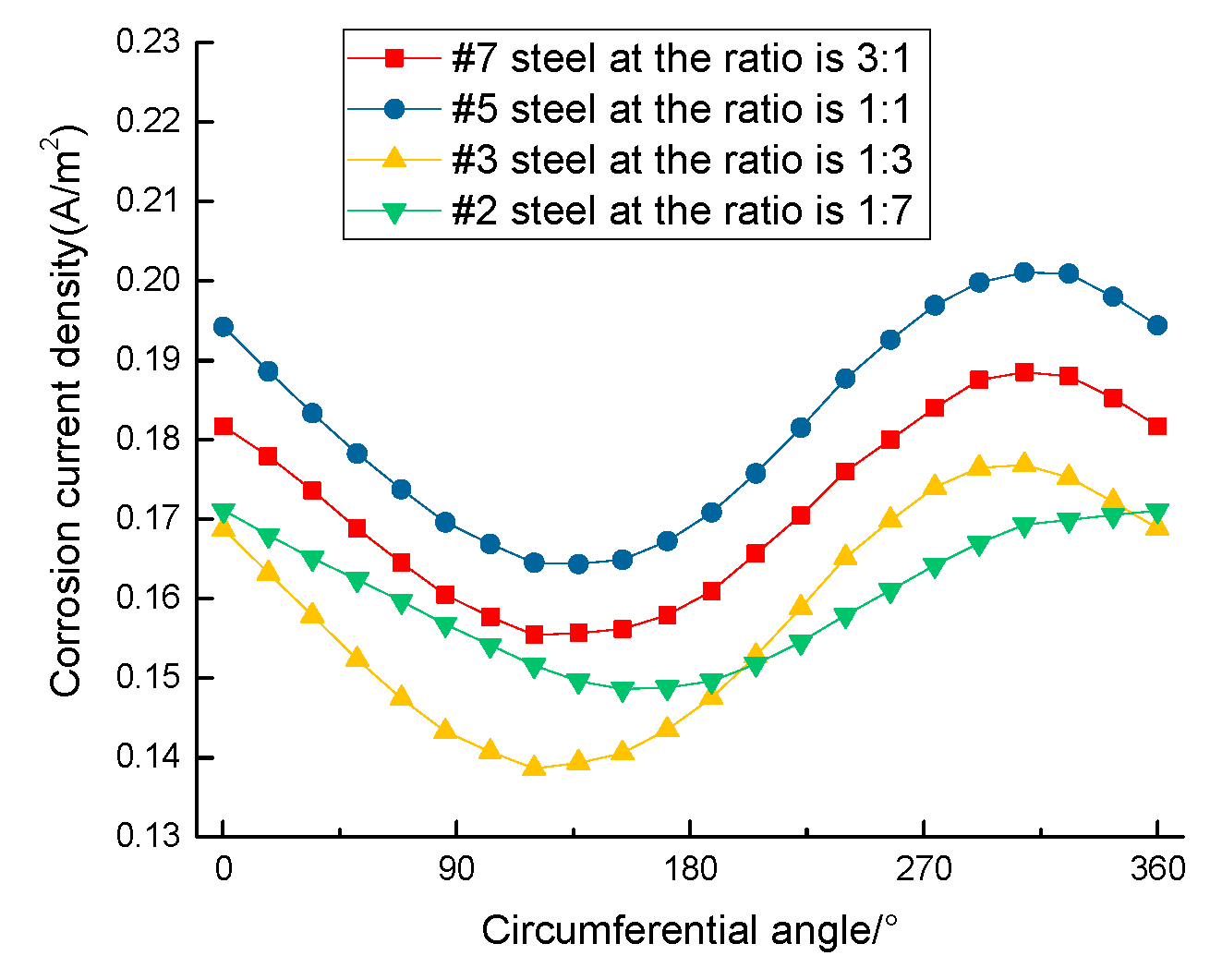
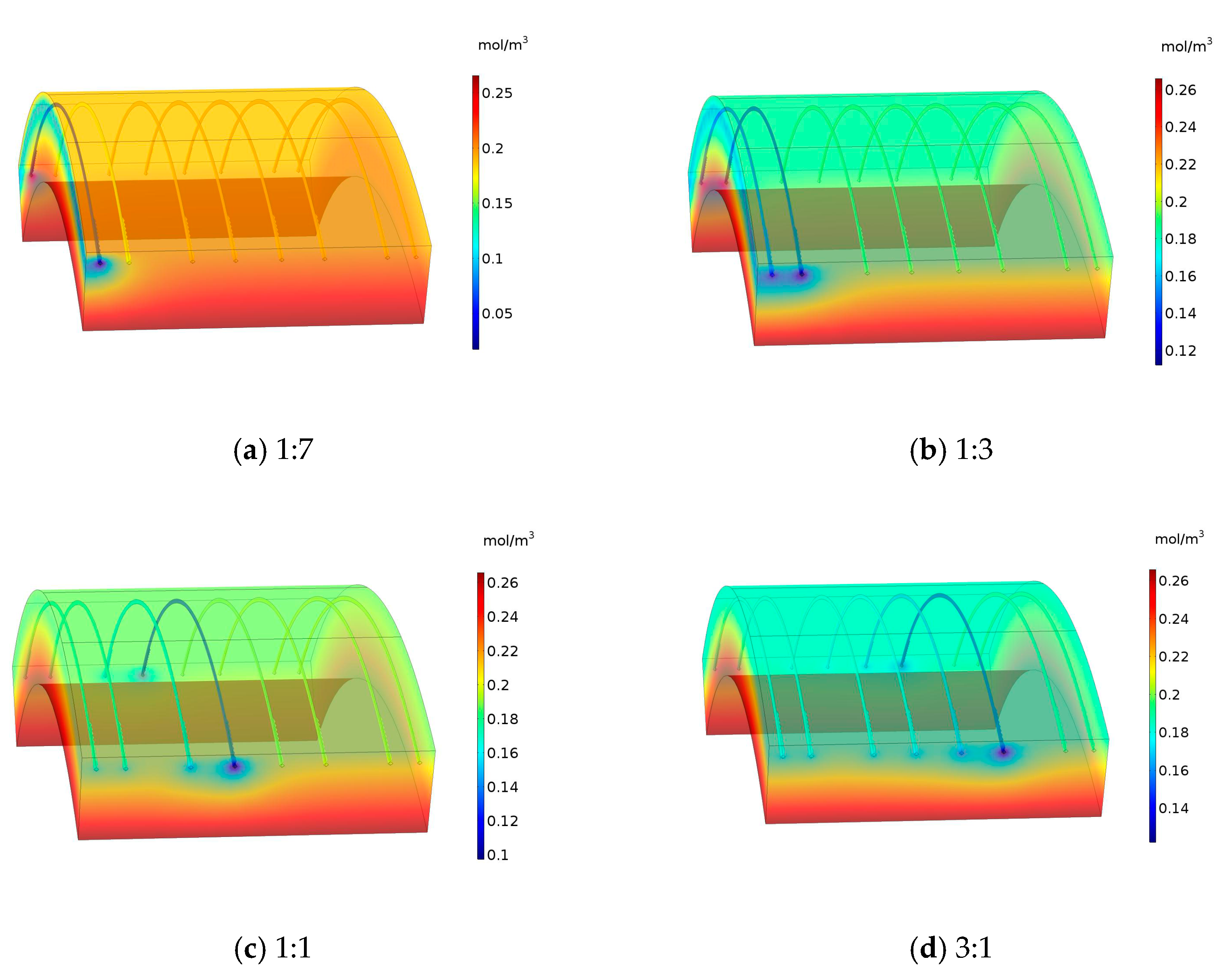
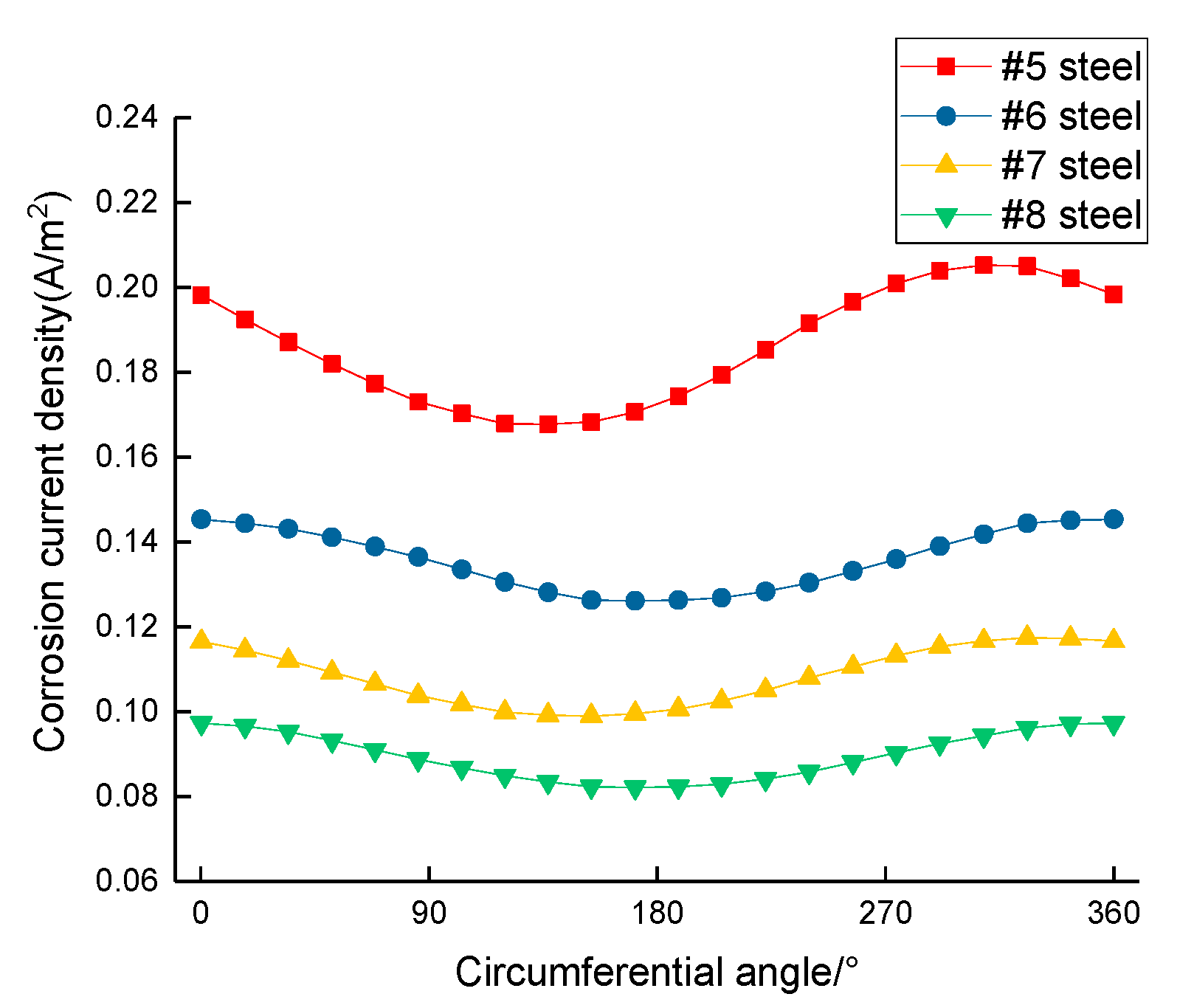
3.1. Corrosion Rate of Steel Bar under Different Area Ratios of Cathode to Anode
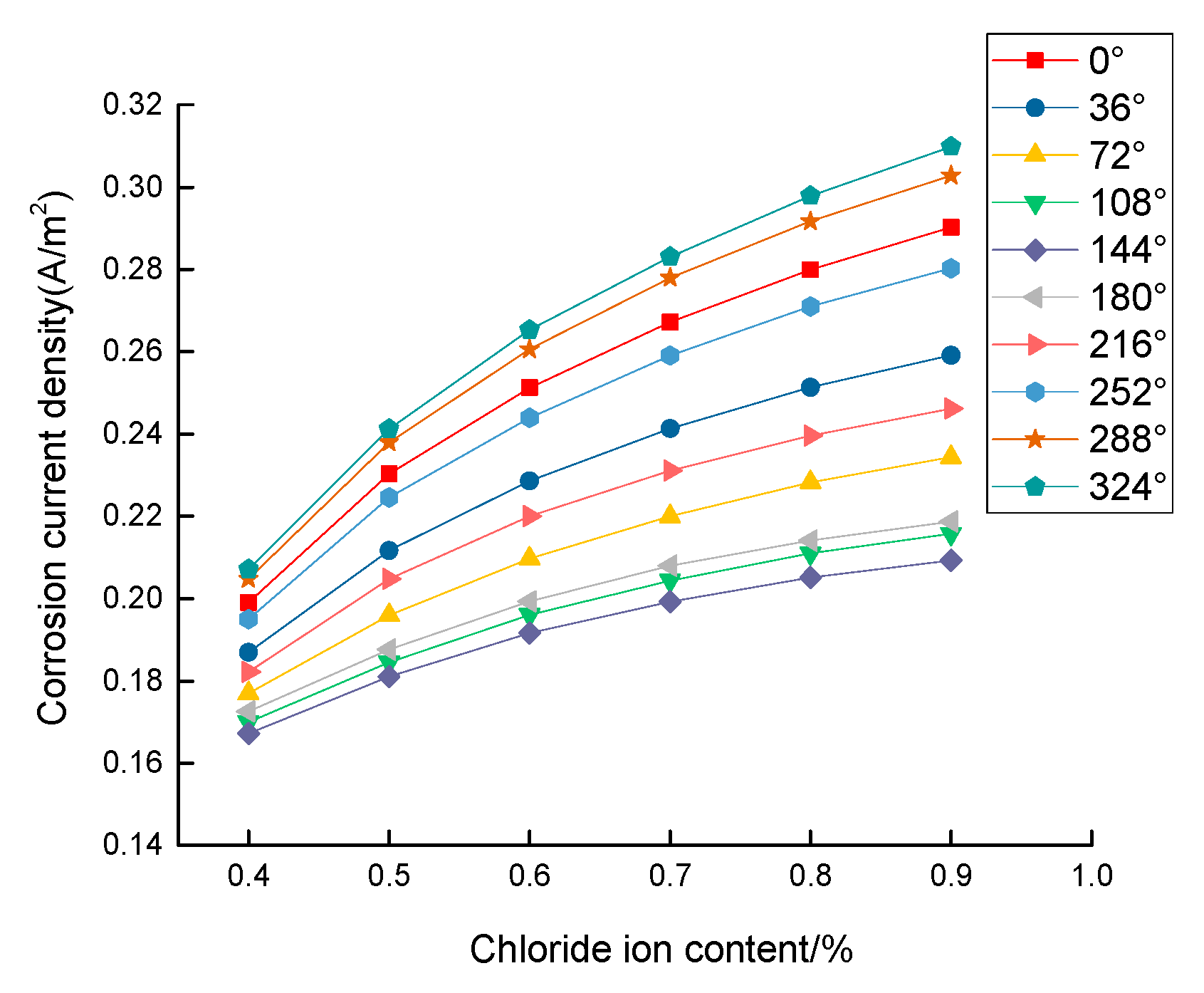
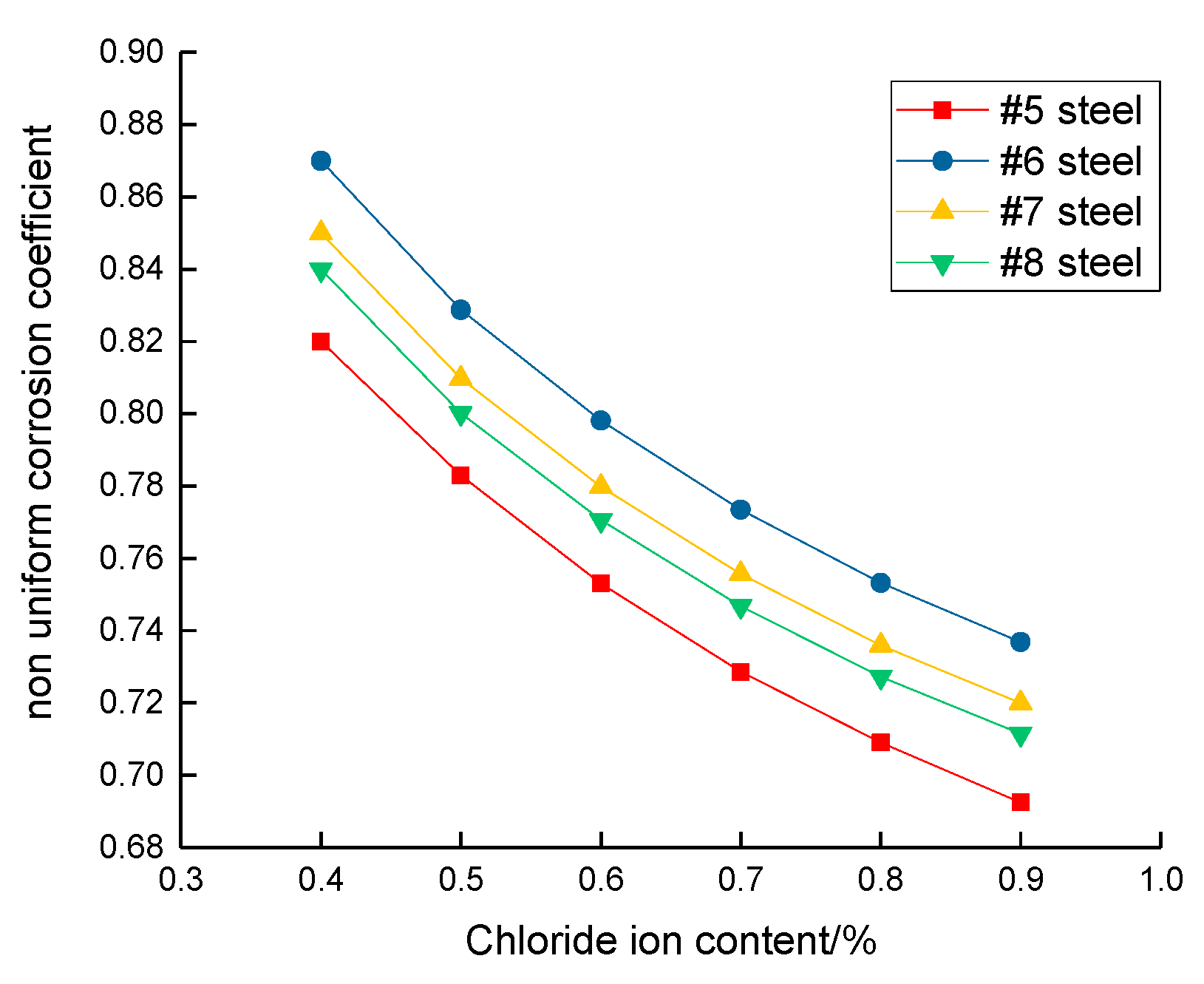
3.2. Corrosion Rate of Steel Bar under Different Content of Chloride Ion
3.3. Corrosion Rate of Steel Bar under Different Potential Differences Between Cathode and Anode
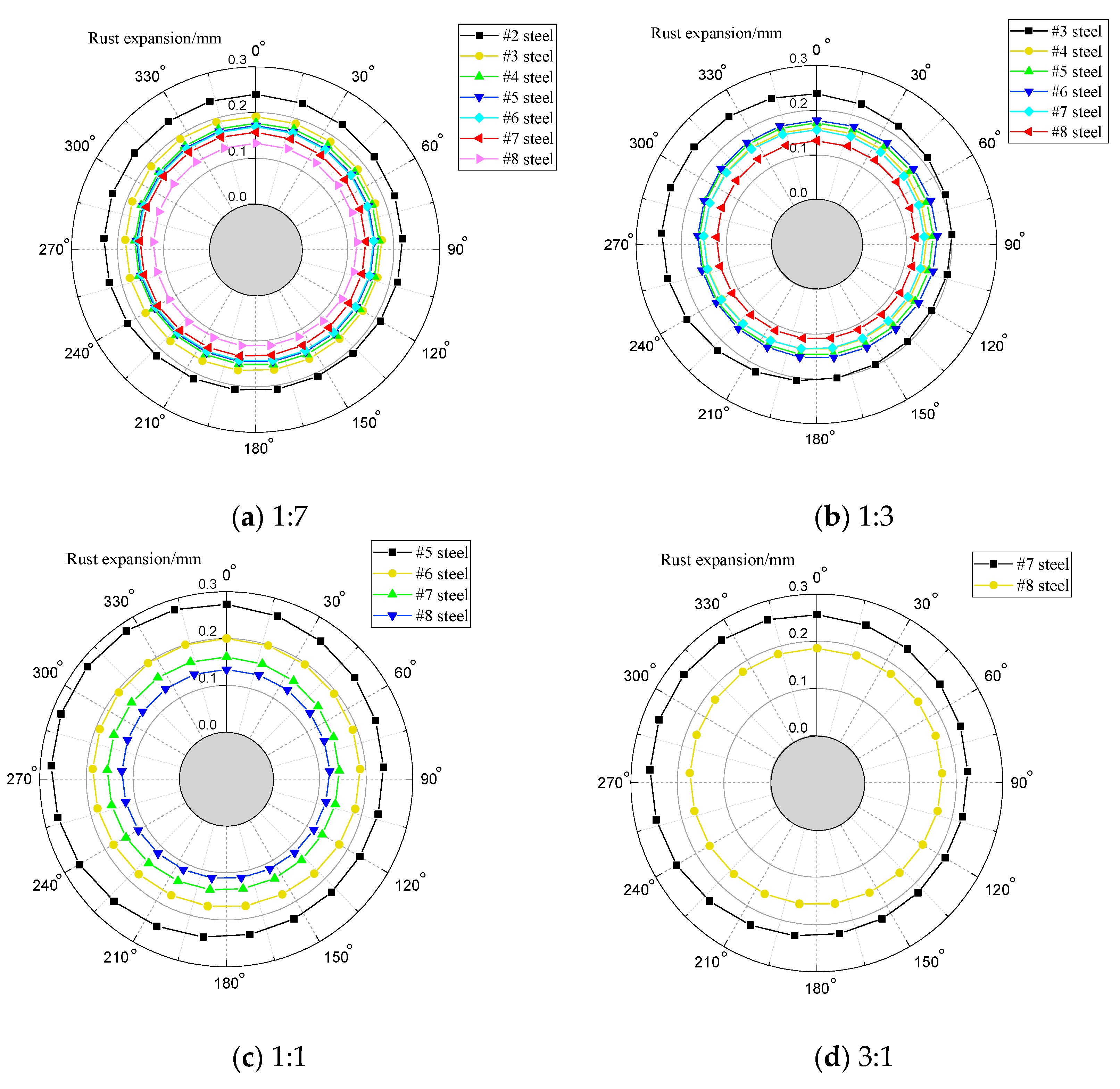
4. Rust Expansion Form of Segment Steel Bar
5. Conclusions
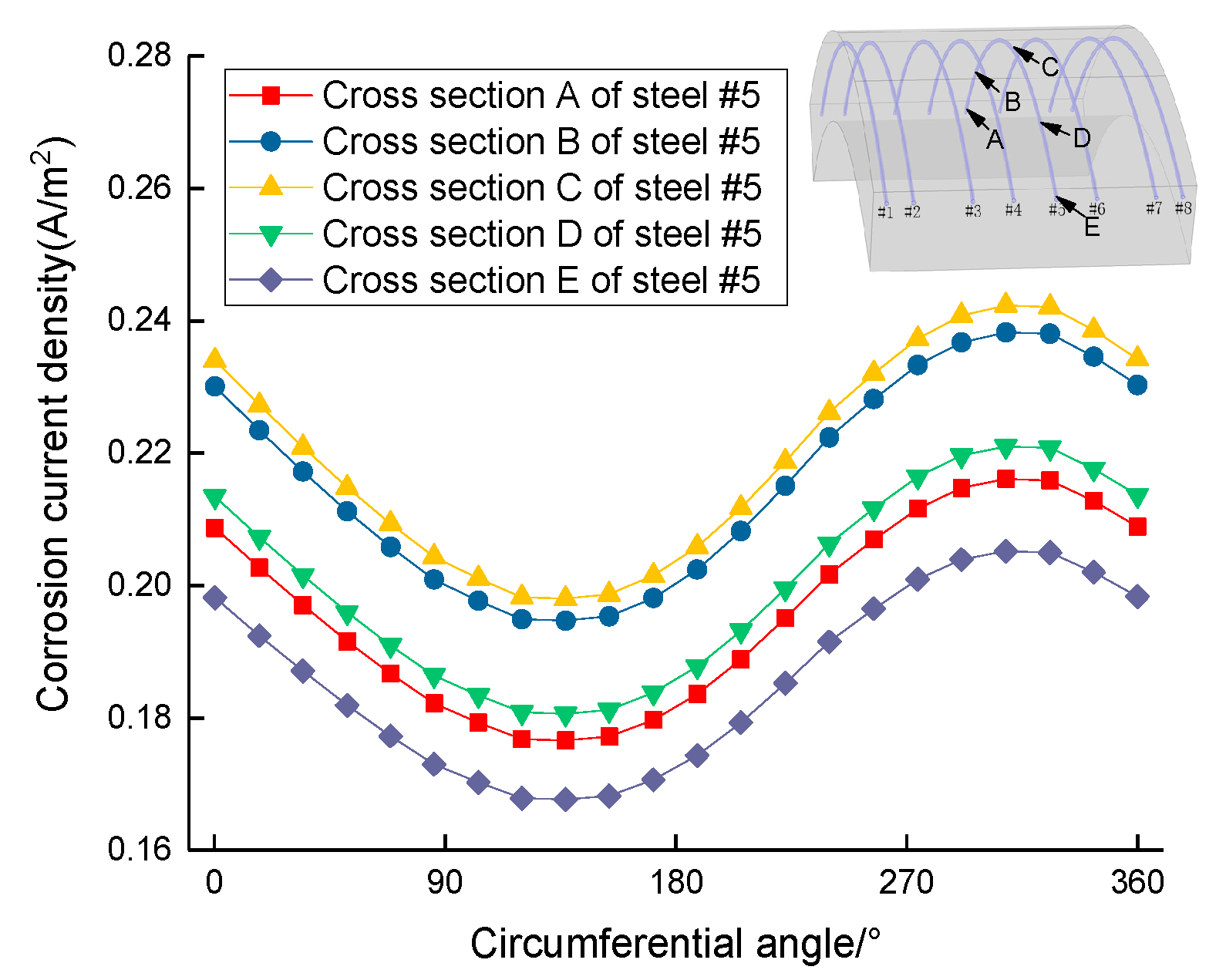
- The steel corrosion rate near the outside of the segment is larger than that inside; the deflection between the vertical line and slant line is approximately 0°~52°;
- The segment steel corrosion rate is related to volumetric strain under loading, and the segment steel corrosion rate in the middle is larger than that at the two ends;
- When the steel experiences depassivation, it increases logarithmically with the free chloride ion content, and the steel corrosion rate increases linearly with the potential difference between the cathode and anode;
- Under the joint action of three factors, the segment steel rust layer form appears as an eccentric circle.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ng, C.W.W.; Liu, G.B.; Li, Q. Investigation of the long-term tunnel settlement mechanisms of the first metro line in Shanghai. Can. Geotech. J. 2013, 50, 674–684. [Google Scholar] [CrossRef]
- Maeda, M.; Kushiyama, K. Use of compact shield tunneling method in urban underground construction. Tunn. Undergr. Space Technol. 2005, 20, 159–166. [Google Scholar] [CrossRef]
- Cooper, M.L.; Chapman, D.N.; Rogers, C.D.; Chan, A.H. Movements in the Piccadilly Line tunnels due to the Heathrow Express construction. Geotechnique 2002, 52, 243–257. [Google Scholar] [CrossRef]
- Jin, H.; Zhou, X.; Sun, X.; Li, Z. Decay rate of rail with egg fastening system using tuned rail damper. Appl. Acoust. 2021, 172, 107622. [Google Scholar] [CrossRef]
- Chen, J.S.; Mo, H.H. Numerical study on crack problems in segments of shield tunnel using finite element method. Tunn. Undergr. Space Technol. 2009, 24, 91–102. [Google Scholar] [CrossRef]
- Huang, Z.; Fu, H.; Chen, W.; Zhang, J.; Huang, H. Damage detection and quantitative analysis of shield tunnel structure. Autom. Constr. 2018, 94, 303–316. [Google Scholar] [CrossRef]
- Yan, Q.; Xu, Y.; Zhang, W.; Geng, P.; Yang, W. Numerical analysis of the cracking and failure behaviors of segmental lining structure of an underwater shield tunnel subjected to a derailed high-speed train impact. Tunn. Undergr. Space Technol. 2018, 72, 41–54. [Google Scholar] [CrossRef]
- Feng, K.; He, C.; Zhou, J.; Zhang, Z. Model test on impact of surrounding rock deterioration on segmental lining structure for underwater shield tunnel with large cross-section. Procedia Environ. Sci. 2012, 12, 891–898. [Google Scholar] [CrossRef]
- Jaffer, S.J.; Hansson, C.M. The influence of cracks on chloride-induced corrosion of steel in ordinary Portland cement and high performance concretes subjected to different loading conditions. Corros. Sci. 2008, 50, 3343–3355. [Google Scholar] [CrossRef]
- Arya, C.; Ofori-Darko, F.K. Influence of crack frequency on reinforcement corrosion in concrete. Cem. Concr. Res. 1996, 26, 345–353. [Google Scholar] [CrossRef]
- Otieno, M.; Beushausen, H.; Alexander, M. Chloride-induced corrosion of steel in cracked concrete—Part I: Experimental studies under accelerated and natural marine environments. Cem. Concr. Res. 2016, 79, 373–385. [Google Scholar] [CrossRef]
- Tang, K. Stray current induced corrosion of steel fibre reinforced concrete. Cem. Concr. Res. 2017, 100, 445–456. [Google Scholar] [CrossRef]
- Bertolini, L.; Carsana, M.; Pedeferri, P. Corrosion behaviour of steel in concrete in the presence of stray current. Corros. Sci. 2007, 49, 1056–1068. [Google Scholar] [CrossRef]
- Ožbolt, J.; Balabanić, G.; Kuster, M.W. 3D Numerical modelling of steel corrosion in concrete structures. Corros. Sci. 2011, 53, 4166–4177. [Google Scholar] [CrossRef]
- Yuan, Y.; Bai, Y.; Liu, J. Assessment service state of tunnel structure. Tunn. Undergr. Space Technol. 2012, 27, 72–85. [Google Scholar] [CrossRef]
- Zhang, D.M.; Huang, Z.K.; Wang, R.L.; Yan, J.Y.; Zhang, J. Grouting-based treatment of tunnel settlement: Practice in Shanghai. Tunn. Undergr. Space Technol. 2018, 80, 181–196. [Google Scholar] [CrossRef]
- Yang, L.F.; Bin, H.; Bo, Y. Durability design of tunnel concrete lining under chloride attack. Adv. Mater. Res. 2012, 594, 1014–1017. [Google Scholar] [CrossRef]
- Lei, M.; Peng, L.; Shi, C. An experimental study on durability of shield segments under load and chloride environment coupling effect. Tunn. Undergr. Space Technol. 2014, 42, 15–24. [Google Scholar] [CrossRef]
- Li, Q.; Yu, H.; Ma, H.; Chen, S.; Liu, S. Test on durability of shield tunnel concrete segment under coupling multi-factors. Open Civ. Eng. J. 2014, 8, 451–457. [Google Scholar] [CrossRef][Green Version]
- Jin, H.; Yu, S. Effect of DC stray current on rebar corrosion in cracked segment of shield tunnel. Constr. Build. Mater. 2021, 272, 121646. [Google Scholar] [CrossRef]
- Yoon, S.; Wang, K.; Weiss, W.J. Interaction between loading, corrosion, and serviceability of reinforced concrete. Mater. J. 2000, 97, 637–644. [Google Scholar]
- Ballim, Y.; Reid, J.C. Reinforcement corrosion and the deflection of RC beams—An experimental critique of current test methods. Cem. Concr. Compos. 2003, 25, 625–632. [Google Scholar] [CrossRef]
- Malumbela, G.; Moyo, P.; Alexander, M. Behaviour of RC beams corroded under sustained service loads. Constr. Build. Mater. 2009, 23, 3346–3351. [Google Scholar] [CrossRef]
- Malumbela, G.; Alexander, M.; Moyo, P. Variation of steel loss and its effect on the ultimate flexural capacity of RC beams corroded and repaired under load. Constr. Build. Mater. 2010, 24, 1051–1059. [Google Scholar] [CrossRef]
- Hariche, L.; Ballim, Y.; Bouhicha, M.; Kenai, S. Effects of reinforcement configuration and sustained load on the behaviour of reinforced concrete beams affected by reinforcing steel corrosion. Cem. Concr. Compos. 2012, 34, 1202–1209. [Google Scholar] [CrossRef]
- Ye, H.; Fu, C.; Jin, N.; Jin, X. Performance of reinforced concrete beams corroded under sustained service loads: A comparative study of two accelerated corrosion techniques. Constr. Build. Mater. 2018, 162, 286–297. [Google Scholar] [CrossRef]
- Dong, J.; Zhao, Y.; Wang, K.; Jin, W. Crack propagation and flexural behaviour of RC beams under simultaneous sustained loading and steel corrosion. Constr. Build. Mater. 2017, 151, 208–219. [Google Scholar] [CrossRef]
- Jin, H.; Yu, K.; Gong, Q.; Zhou, S. Load-carrying capability of shield tunnel damaged by shield shell squeezing action during construction. Thin Walled Struct. 2018, 132, 69–78. [Google Scholar] [CrossRef]
- Cao, C.; Cheung, M.M. Non-uniform rust expansion for chloride-induced pitting corrosion in RC structures. Constr. Build. Mater. 2014, 51, 75–81. [Google Scholar] [CrossRef]
- Ogunsola, A.; Mariscotti, A.; Sandrolini, L. Estimation of stray current from a DC-electrified railway and impressed potential on a buried pipe. IEEE Trans. Power Deliv. 2012, 27, 2238–2246. [Google Scholar] [CrossRef]
- Rodriguez, J.V.; Feito, J.S. Calculation of remote effects of stray currents on rail voltages in DC railways systems. IET Electr. Syst. Transp. 2013, 3, 31–40. [Google Scholar] [CrossRef]
- Papadakis, V.G.; Vayenas, C.G.; Fardis, M.N. Fundamental modeling and experimental investigation of concrete carbonation. Mater. J. 1991, 88, 363–373. [Google Scholar]
- Du, X.L.; Jin, L.; Zhang, R.B. Chloride diffusivity in saturated cement paste subjected to external mechanical loadings. Ocean Eng. 2015, 95, 1–10. [Google Scholar] [CrossRef]
- Hussain, R.R. Enhanced classical tafel diagram model for corrosion of steel in chloride contaminated concrete and the experimental non-linear effect of temperature. Int. J. Concr. Struct. Mater. 2010, 4, 71–75. [Google Scholar]
- Isgor, O.B.; Razaqpur, A.G. Modelling steel corrosion in concrete structures. Mater. Struct. 2006, 39, 291–302. [Google Scholar] [CrossRef]
- Muehlenkamp, E.B.; Koretsky, M.D.; Westall, J.C. Effect of moisture on the spatial uniformity of cathodic protection of steel in reinforced concrete. Corros. Sci. 2012, 61, 519–533. [Google Scholar] [CrossRef]
- Ahmad, S. Reinforcement corrosion in concrete structures, its monitoring and service life prediction—A review. Cem. Concr. Compos. 2003, 25, 459–471. [Google Scholar] [CrossRef]
- Cao, C.; Cheung, M.M.S.; Chan, B.Y.B. Modelling of interaction between corrosion-induced concrete cover crack and steel corrosion rate. Corros. Sci. 2013, 69, 97–109. [Google Scholar] [CrossRef]
- Suda, K.; Misra, S.; Motohashi, K. Corrosion products of reinforcing bars embedded in concrete. Corros. Sci. 1993, 35, 1543–1549. [Google Scholar] [CrossRef]
- Bhargava, K.; Ghosh, A.; Mori, Y.; Ramanujam, S. Model for cover cracking due to rebar corrosion in RC structures. Eng. Struct. 2006, 28, 1093–1109. [Google Scholar] [CrossRef]
- Zhao, Y.; Hu, B.; Yu, J.; Jin, W. Non-uniform distribution of rust layer around steel bar in concrete. Corros. Sci. 2011, 53, 4300–4308. [Google Scholar] [CrossRef]
- Thybo, A.E.A.; Michel, A.; Stang, H. Smeared crack modelling approach for corrosion-induced concrete damage. Mater. Struct. 2018, 50, 1–4. [Google Scholar] [CrossRef]





| Layer Number | Soil Name | Density (kg/m3) | Layer Thickness /m | Lateral Pressure Coefficient |
|---|---|---|---|---|
| 1 | Miscellaneous fill | 18.1 | 5.54 | 0.59 |
| 2 | mucky silty clay | 17.5 | 6.46 | 0.65 |
| 3 | silty clay with fine sand interbed | 18.2 | 8.56 | 0.71 |
| Load Type | Load Value/kN |
|---|---|
| q1 | 148.15 |
| q2 | 223.23 |
| G | 8.75 |
| q3 | 24.81 |
| p | 0 |
| W1 | 211.64 |
| Condition | Cathode Steel | Anode Steel | Area Ratio |
|---|---|---|---|
| 1 | #1 | #2, #3, #4, #5, #6, #7, #8 | 1:7 |
| 2 | #1, #2 | #3, #4, #5, #6, #7, #8 | 1:3 |
| 3 | #1, #2, #3, #4 | #5, #6, #7, #8 | 1:1 |
| 4 | #1, #2, #3, #4, #5, #6 | #7, #8 | 3:1 |
| Parameters Symbol | Units | Value |
|---|---|---|
| VCSE | −0.76 | |
| VCSE | 0.189 | |
| A/m2 | 7.1 × 10−5 | |
| A/m2 | 7.7 × 10−7 | |
| V/decade | 0.41 | |
| V/decade | −0.18 |
| Area Ratios | Steel Bar Number | ||||||
|---|---|---|---|---|---|---|---|
| #2 | #3 | #4 | #5 | #6 | #7 | #8 | |
| 1:7 | 0.81 | 0.86 | 0.87 | 0.86 | 0.86 | 0.85 | 0.84 |
| 1:3 | / | 0.82 | 0.83 | 0.86 | 0.87 | 0.84 | 0.84 |
| 1:1 | / | / | / | 0.82 | 0.87 | 0.85 | 0.84 |
| 3:1 | / | / | / | / | / | 0.82 | 0.86 |
| Area Ratios | Steel Bar Number | ||||||
|---|---|---|---|---|---|---|---|
| #2 | #3 | #4 | #5 | #6 | #7 | #8 | |
| 1:7 | 0.17 | 0.14 | 0.13 | 0.12 | 0.12 | 0.11 | 0.10 |
| 1:3 | / | 0.18 | 0.12 | 0.12 | 0.13 | 0.11 | 0.10 |
| 1:1 | / | / | / | 0.21 | 0.15 | 0.12 | 0.10 |
| 3:1 | / | / | / | / | / | 0.19 | 0.13 |
| Area Ratios | Steel Bar Number | ||||||
|---|---|---|---|---|---|---|---|
| #2 | #3 | #4 | #5 | #6 | #7 | #8 | |
| 1:7 | 0 | 30 | 0 | 0 | 0 | 30 | 0 |
| 1:3 | / | / | 52 | 0 | 0 | 30 | 0 |
| 1:1 | / | / | / | 52 | 0 | 30 | 0 |
| 3:1 | / | / | / | / | / | 52 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Jin, H.; Yu, S. Investigation of Corrosion Rate and Rust Expansion Form of Segment Reinforcement for Shield Tunnel by Combined Action of Soil Loading, Chloride Ion and Stray Current. Sustainability 2021, 13, 3444. https://doi.org/10.3390/su13063444
Li Z, Jin H, Yu S. Investigation of Corrosion Rate and Rust Expansion Form of Segment Reinforcement for Shield Tunnel by Combined Action of Soil Loading, Chloride Ion and Stray Current. Sustainability. 2021; 13(6):3444. https://doi.org/10.3390/su13063444
Chicago/Turabian StyleLi, Zheng, Hao Jin, and Shuo Yu. 2021. "Investigation of Corrosion Rate and Rust Expansion Form of Segment Reinforcement for Shield Tunnel by Combined Action of Soil Loading, Chloride Ion and Stray Current" Sustainability 13, no. 6: 3444. https://doi.org/10.3390/su13063444
APA StyleLi, Z., Jin, H., & Yu, S. (2021). Investigation of Corrosion Rate and Rust Expansion Form of Segment Reinforcement for Shield Tunnel by Combined Action of Soil Loading, Chloride Ion and Stray Current. Sustainability, 13(6), 3444. https://doi.org/10.3390/su13063444






