Computer Image Analysis as a Method of Evaluating the Quality of Selected Fine-Grained Food Mixtures
Abstract
1. Introduction
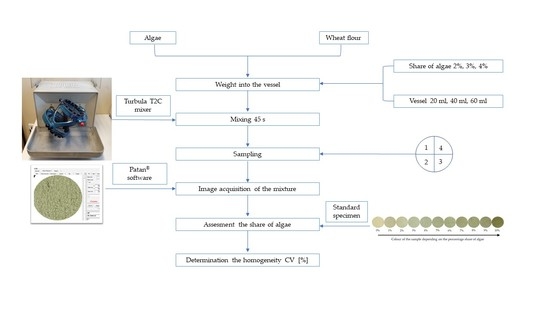
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
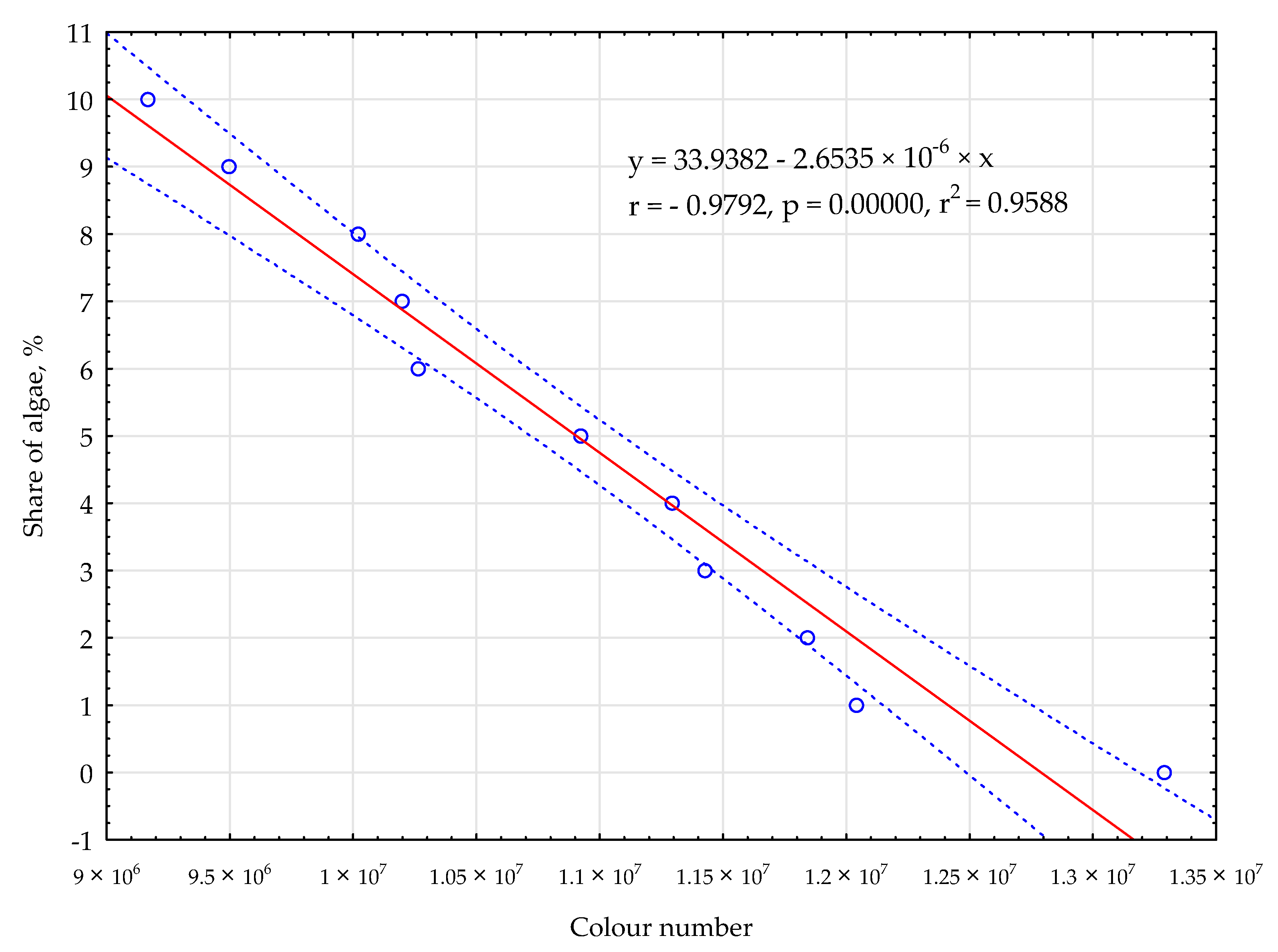
- The authors obtained a model (simple linear regression) to predict the concentration of algae in a mixture with wheat flour. This model is highly reliable, with an R2 value of 0.9588.
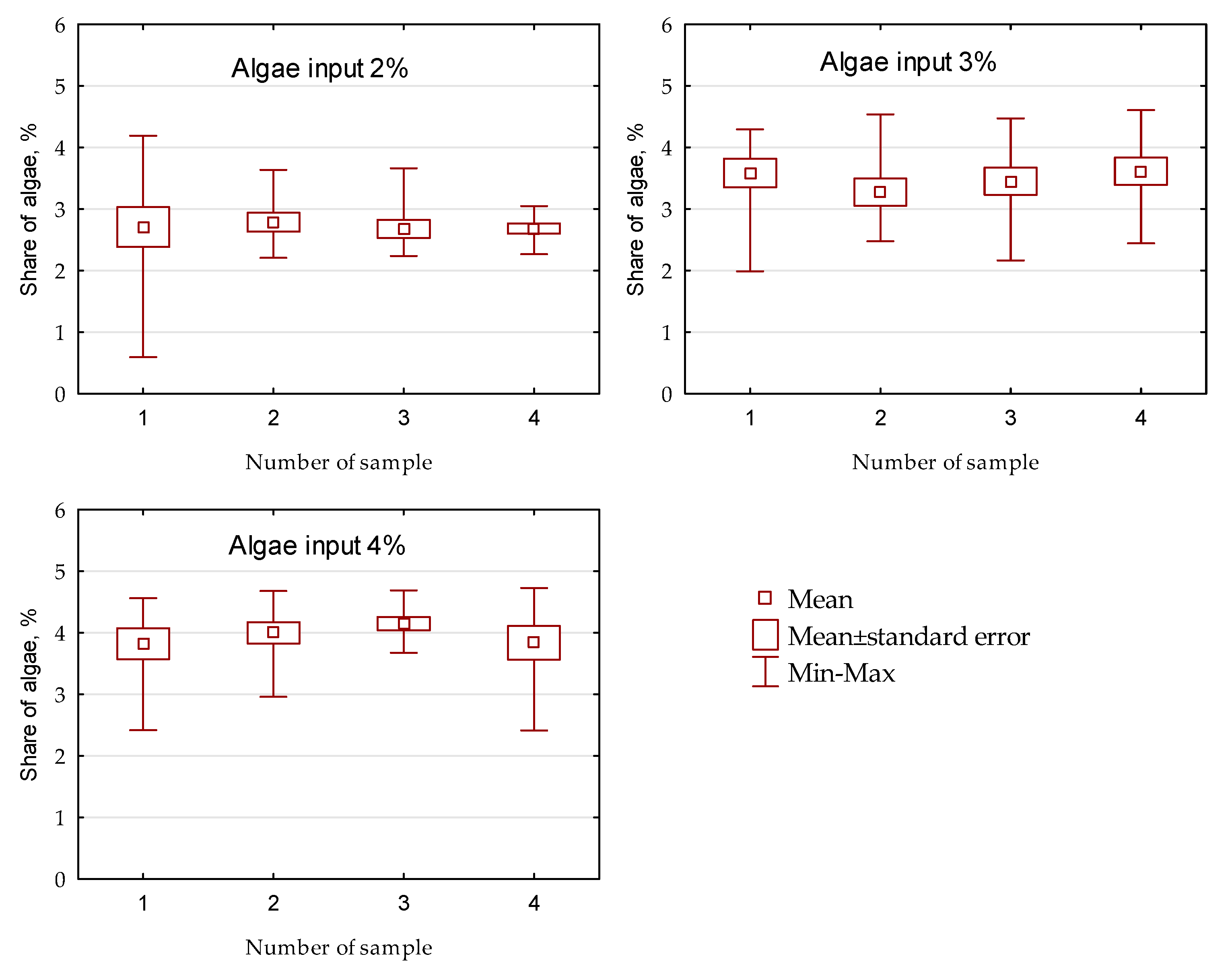
- The share of key components affects the accuracy of the presented method. Greater differentiation of the results and deviation from the settled amount of algae were noted for the 2% mixture than for the 3% and 4% mixtures.
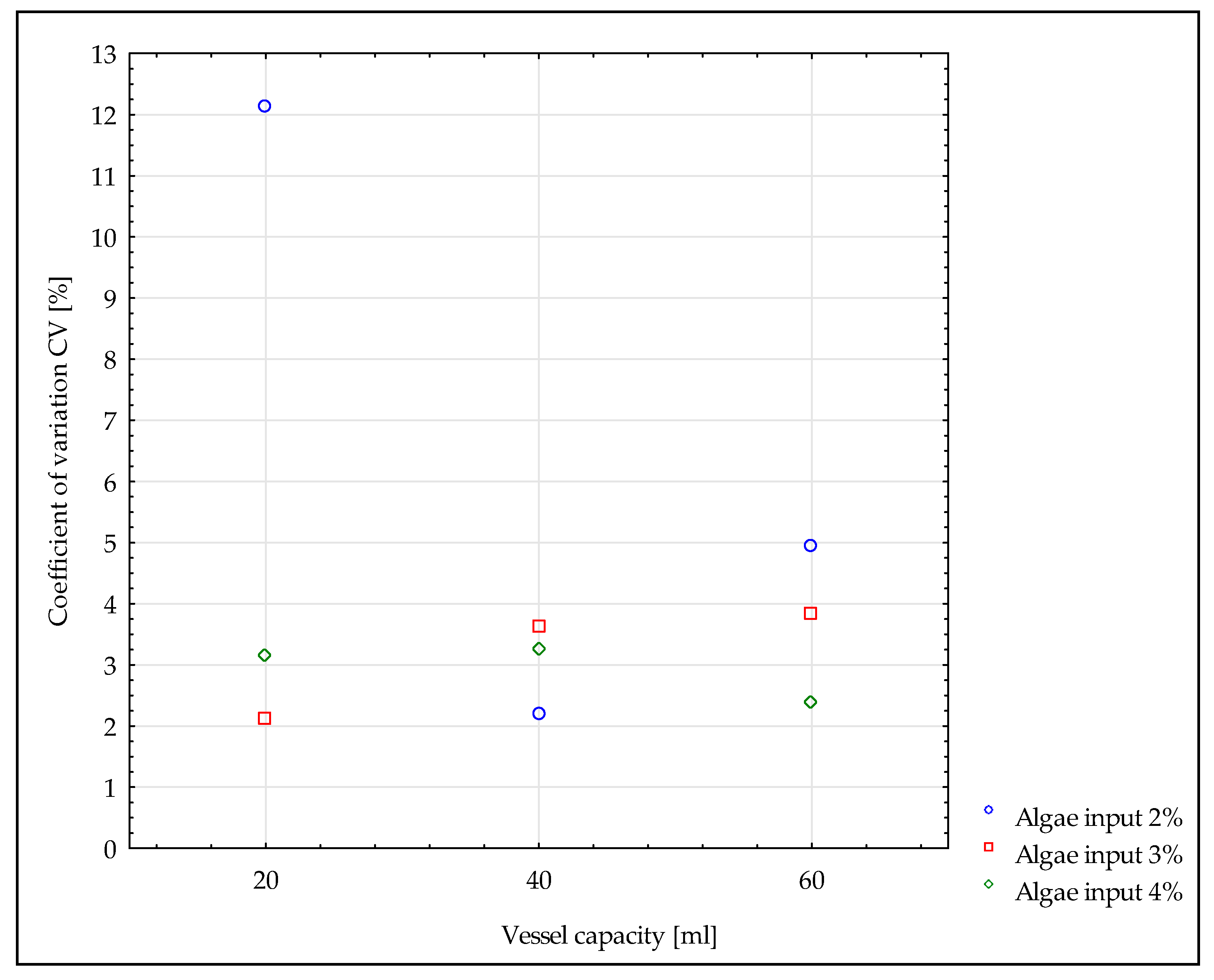
- Only in one case (mixture with 2% algae mixed in a 20 mL vessel) did the obtained mixture not have good homogeneity (CV = 12.13%). The coefficient of variation for the remaining mixtures showed good homogeneity (CV < 6%).
- The obtained results indicate the possibility of using the RGB scale for image analysis to assess the homogeneity of the two-component powder mixture (in this case, wheat flour–algae). However, the possibilities of the described method seem to be dependent on the share of the coloring component. A small amount of additives (like algae) can be a limitation of this method. Moreover, tests were carried out on two-component systems, so it is not known if (and how) they can be used in multicomponent mixtures.
- The presented results are a preliminary element of the planned work. The next step will require a closer look at the acquisition and analysis of the image. It is possible that there are available techniques to improve the accuracy of this method for mixtures with a small share of key components. Moreover, tests with other flours, such as maize and rice, or the starch of these flours with additivities, paprika, spinach, tomato and beetroot, are planned.
- The authors believe it is possible to develop a method to assess the homogeneity of fine-grained mixtures (for example, pastas fortified with algae, spinach, paprika and others) that can be used in industrial conditions (of course also in laboratory conditions).
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cuq, B.; Berthiaux, H.; Gatumel, C. Powder mixing in the production of food powders. In Handbook of Food Powders, Processes and Properties, 1st ed.; Bhandari, B., Bansal, N., Zhang, M., Schuck, P., Eds.; Woodhead Publishing Series in Food Science, Technology and Nutrition: Cambridge, UK, 2013; pp. 200–229. [Google Scholar]
- Fitzpatrick, J.J.; Ahrné, L. Food powder handling and processing: Industry problems, knowledge barriers and research opportunities. Chem. Eng. Proces. 2005, 44, 209–214. [Google Scholar] [CrossRef]
- Cuq, B.; Rondet, E.; Abecassis, J. Food powders engineering from knowhow to science: Industrial constraints, stakes and research opportunities. Powder Technol. 2011, 208, 244–251. [Google Scholar] [CrossRef]
- Barbosa-Canovas, G.V.; Ortega-Rivas, E.; Juliano, P.; Yan, H. Food Powders: Physical Properties, Processing, and Functionality; Kluwer Academic, Plenum Publishers: DorDrecht, The Netherlands, 2005. [Google Scholar]
- Pernenkil, L.; Cooney, C.L. A review on the continuous blending of powders. Chem. Eng. Sci. 2006, 61, 720–742. [Google Scholar] [CrossRef]
- Królczyk, J.B. The effect of mixing time on the homogeneity of multi-component granular systems. Trans. Famena 2016, XL-1, 45–56. [Google Scholar]
- Boss, J. Time of Mixing Granular Materials; Studia i Monografie Wyższa Szkoła Inżynierska: Opole, Poland, 1991. [Google Scholar]
- Sobczak, P.; Zawiślak, K.; Panasiewicz, M.; Mazur, J.; Oniszczuk, T. The quality of the mixing process depending on selected technical parameters. TEKA Comm. Mot. Energetics Agric. 2012, 12, 199–202. [Google Scholar]
- Bhalode, P.; Ierapetritou, M. A review of existing mixing indices in solid-based continuous blending operations. Powder Technol. 2020, 373, 195–209. [Google Scholar] [CrossRef]
- Matuszek, D.; Królczyk, J. Aspects of safety in production of feeds—A review. Anim. Nutr. Feed Technol. 2017, 17, 367–385. [Google Scholar] [CrossRef]
- Bridgewater, J. Mixing of powders and granular materials by mechanical means. A perspective. Particuology 2012, 10, 397–427. [Google Scholar] [CrossRef]
- Çiftci, I.; Ercan, A. Effects of diets of different mixing homogeneity on performance and carcass traits of broilers. J. Anim. Feed Sci. 2003, 12, 163–171. [Google Scholar] [CrossRef]
- Clark, P.M.; Behnke, K.C.; Poole, D.R. Effects of indicator selection and mix time on the coefficient of variation (mix uniformity) of broiler feed. J. Appl. Poultry Res. 2007, 16, 464–470. [Google Scholar] [CrossRef]
- Rocha, A.G.; Montanhini, R.N.; Dilkin, P.; Tamiosso, C.D.; Mallmann, C.A. Comparison of different indicators for the evaluation of feed mixing efficiency. Anim. Feed Sci. Technol. 2015, 209, 249–256. [Google Scholar] [CrossRef]
- Eisenberg, D.A. Mixer performance, cross-contamination testing examined. Feedstuffs 2004, 76, 1–2. [Google Scholar]
- Krzaczek, P.; Sobczak, P.; Stamirowska-Krzaczek, E.; Zawiślak, K.; Maj, G.; Żukiewicz-Sobczak, W.; Piekarski, W.; Królczyk, J. Assessment of Reliability of Mixing Process in Diverse Mixers. Tehnički Vjesn. 2020, 27, 1181–1185. [Google Scholar]
- Matuszek, D.B. Ultraviolet fluorescence in the assessment of quality in the mixing of granular material. Sustainability 2020, 12, 1546. [Google Scholar] [CrossRef]
- Matuszek, D. Modelling selected parameters of granular elements mixing process. Int. Agrophys. 2015, 29, 75–81. [Google Scholar] [CrossRef]
- Królczyk, J.; Matuszek, D.; Tukiendorf, M. Modelling of quality changes in a multicomponent granular mixture during mixing Electon. J. Pol. Agric. Univ. 2010, 3, 11. [Google Scholar]
- Matuszek, D.B. The use of UV-induced fluorescence for the assessment of homogeneity of granular mixtures. Open Chem. 2019, 17, 485–491. [Google Scholar] [CrossRef]
- Matuszek, D.; Biłos, Ł. Use of fluorescent tracers for the assessment of the homogeneity of multicomponent granular feed mixtures. Przem Chem. 2017, 96, 2356–2359. [Google Scholar] [CrossRef]
- Realpe, A.; Velảzquez, C. Image processing and analysis for determination of concentrations of powder mixtures. Powder Technol. 2003, 134, 193–200. [Google Scholar] [CrossRef]
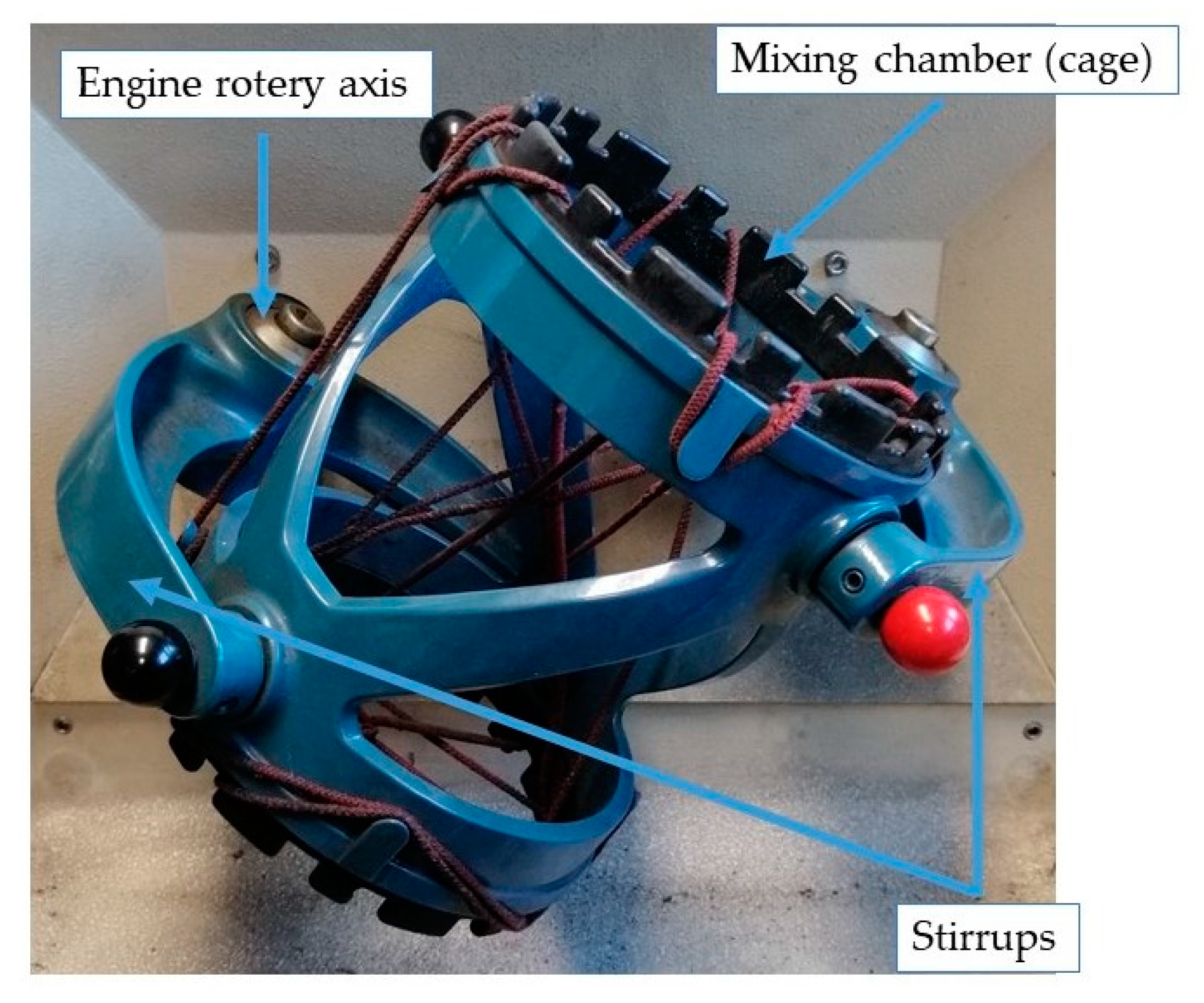
- Mayer, C.; Gatumel, C.; Berthiaux, H. Mixing dynamics for easy flowing powders in a lab scale Turbula® mixer. Chem. Eng. Res. Des. 2015, 95, 248–261. [Google Scholar] [CrossRef]
- Garth, A.L. Blend Uniformity and Vitamin Stability in Dairy-Based Foods Fortified with Lipid-Encapsulated Ferrous Sulfate. Master’s Thesis, Theses and Dissertations, 8138. Brigham Young University, Provo, UT, USA, 1 March 2020. [Google Scholar]
- Marigo, M.; Cairns, D.L.; Davies, M.; Ingram, A.; Stitt, E.H. Developing mechanistic understanding of granular behaviour in complex moving geometry using the Discrete Element Method: Part B: Investigation of flow and mixing in the Turbula® mixer. Powder Technol. 2011, 212, 17–24. [Google Scholar] [CrossRef]
- Marigo, M.; Cairns, D.L.; Davies, M.; Ingram, A.; Stitt, E.H. A numerical comparison of mixing efficiencies of solids in a cylindrical vessel subject to a range of motions. Powder Technol. 2012, 217, 540–547. [Google Scholar] [CrossRef]
- Sommier, N.; Porion, P.; Evesque, P.; Leclerc, B.; Tchoreloff, P.; Couarraze, G. Magnetic resonance imaging investigation of the mixing-segregation process in a pharmaceutical blender. Int. J. Pharm. 2001, 222, 243–258. [Google Scholar] [CrossRef]
- Bauman, I.; Duška, Ć.; Boban, M. Mixing of solids in different mixing devices. Sādhanā 2008, 33, 721–731. [Google Scholar] [CrossRef]
- Sundell-Bredenberg, S.; Nyström, C. The possibility of achieving an interactive mixture with high dose homogeneity containing an extremely low proportion of a micronised drug. Eur. J. Pharm. Sci. 2001, 12, 285–295. [Google Scholar] [CrossRef]
- Obadele, B.A.; Masuku, Z.H.; Olubambi, P.A. Turbula mixing characteristics of carbide powders and its influence on laser processing of stainless steel composite coatings. Powder Technol. 2012, 230, 169–182. [Google Scholar] [CrossRef]
- Jankowski, M. Elements of Computer Graphics; Wydawnictwa Naukowo-Techniczne: Warsaw, Poland, 2006. (In Polish) [Google Scholar]
- Zouari, N.; Abid, M.; Fakhfakh, N.; Ayadi, M.A.; Zorgui, L.; Ayadi, M.; Attia, H. Blue-green algae (Arthrospira platensis) as an ingredient in pasta: Free radical scavenging activity, sensory and cooking characteristics evaluation. Int. J. Food Sci. Nutr. 2011, 62, 811–813. [Google Scholar] [CrossRef]
- Willy, A.; Bachofen, A.G. Turbula® Shaker Mixer Type T2C Operating Instruction. Available online: https://www.novatechuk.com/content/products/f/turbula-t2c-manual.pdf (accessed on 20 December 2020).
- Matuszek, D.B.; Królczyk, J.B. Fluorescence optical analysis method for assessing homogeneity of granular mixtures. Metrol. Meas. Syst. 2021, 28. (In press)
- Tukiendorf, M. Characteristics of mixing granular materials achieved by using methods of variance analysis and geostatistical functions. J. Pol. Agric. Univ. 2003, 6, 1. Available online: http://www.ejpau.media.pl/volume6/issue1/engineering/art-03.html (accessed on 20 December 2020).
- Berthiaux, H.; Marikh, K.; Gatumel, C. Continuous mixing of powder mixtures with pharmaceutical process constraints. Chem. Eng. Process Process Intensifi Cation 2008, 47, 2315–2322. [Google Scholar] [CrossRef]
- Shenoy, P.; Innings, F.; Tammel, K.; Fitzpatrick, J.; Ahrné, L. Evaluation of a digital colour imaging system for assessing the mixture quality of spice powder mixes by comparison with a salt conductivity method. Powder Technol. 2015, 286, 48–54. [Google Scholar] [CrossRef]
- Kwiatek, K.; Przeniosło-Siwczyńska, M. Instructions for Testing the Homogeneity of Medicated Feeds and Intermediate Products on the Basis of Testing the Degree of Mixing of the Active Substance; Państwowy Instytut Weterynaryjny: Puławy, Poland, 2007. (In Polish) [Google Scholar]
- Marigo, M.; Davies, M.; Leadbeater, T.; Cairns, D.L.; Ingram, A.; Stitt, E.H. Application of positron emission particle tracking (PEPT) to validate a Discrete Element Method (DEM) model of granular flow and mixing in the Turbula mixer. Int. J. Pharm. 2013, 446, 46–58. [Google Scholar] [CrossRef] [PubMed]
- Mihailova, O.; Lim, V.; McCarthy, M.J.; McCarthy, K.L.; Bakalis, S. Laminar mixing in a SMX static mixer evaluated by positron emission particle tracking (PEPT) and magnetic resonance imaging (MRI). Chem. Eng. Sci. 2015, 137, 1014–1023. [Google Scholar] [CrossRef]
- Asachi, M.; Nourafkan, E.; Hassanpour, A. A review of current techniques for the evaluation of powder mixing. Adv. Powder Technol. 2018, 29, 1525–1549. [Google Scholar] [CrossRef]
- Wahl, P.R.; Fruhmann, G.; Sacher, S.; Straka, G.; Sowinski, S.; Khinast, J.G. PAT for tableting: Inline monitoring of API and excipients via NIR Spectroscopy. Eur. J. Pharm. Biopharm. 2014, 87, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Osorio, J.G.; Stuessy, G.; Kemeny, G.J.; Muzzio, F.J. Characterization of pharmaceutical powder blends using in situ near-infrared chemical imaging. Chem. Eng. Sci. 2014, 108, 244–257. [Google Scholar] [CrossRef]
- He, X.; Han, X.; Ladyzhynsky, N.; Deanne, R. Assessing powder segregation potential by near infrared (NIR) spectroscopy and correlating segregation tendency to tabletting performance. Powder Technol. 2013, 236, 85–99. [Google Scholar] [CrossRef]
- Nawara, P.; Jakubowski, T.; Sobol, Z. Application of the CIE L*a*b* method for the evaluation of the colour of fried products from potato tubers exposed to C band ultraviolet light. E3S Web Conf. Polsita. 2019, 132, 1–6. [Google Scholar] [CrossRef]
- Sobol, Z.; Jakubowski, T.; Nawara, P.R. The effect of UV-C stimulation of potato tubers and soaking of potato strips in water on color and analyzed color by CIE L*a*b*. Sustainability 2020, 12, 3487. [Google Scholar] [CrossRef]
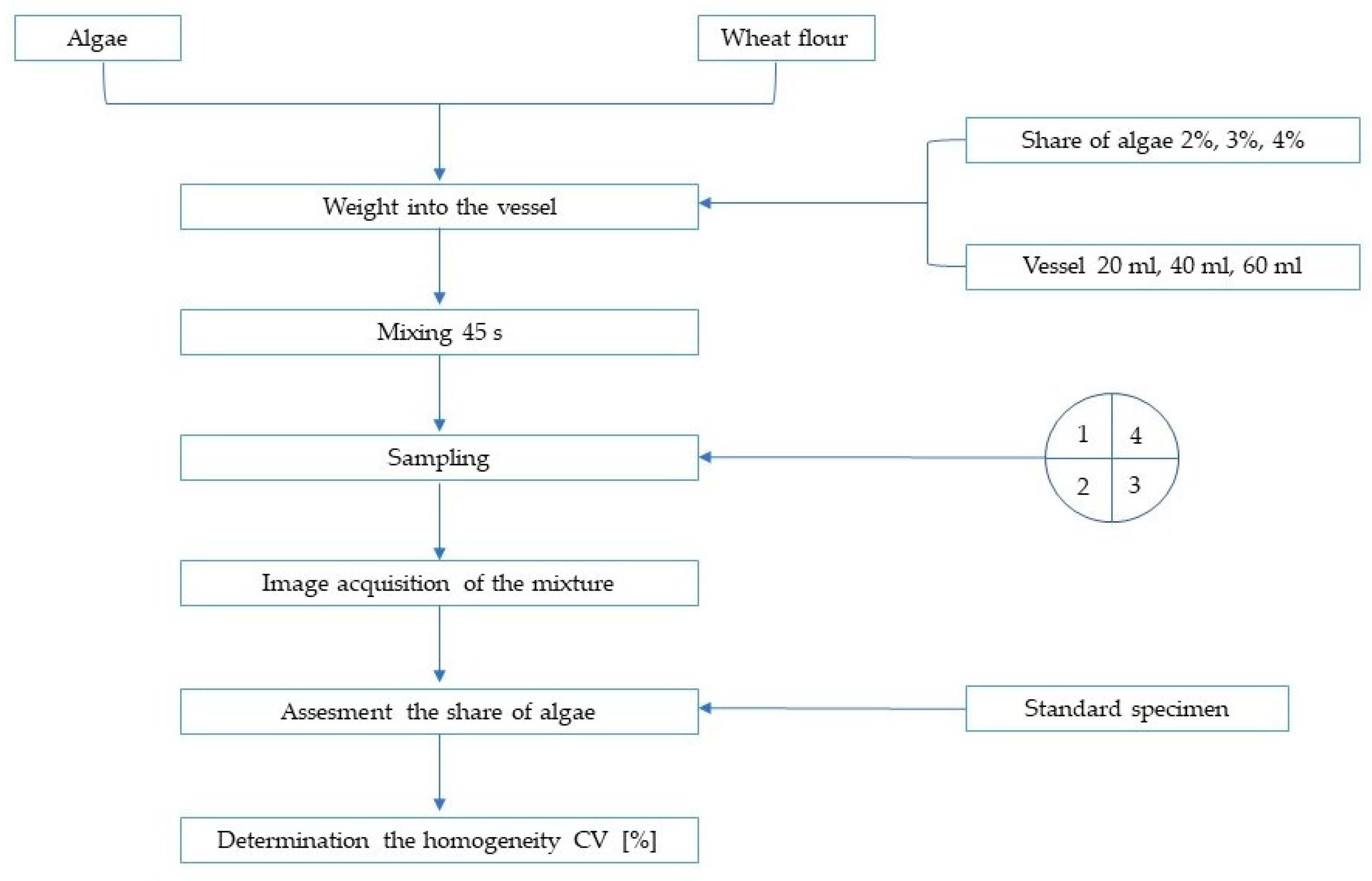


| Parameter | Algae | Wheat Flour |
|---|---|---|
| Geometric weighted mean of particle size dg (mm) | 0.16 | 0.24 |
| Humidity (%) | 4.61 | 7.98 |
| Parameter | Algae | Wheat Flour | Mixture |
|---|---|---|---|
| Mixture 1 | |||
| Mixing ratio (%) | 2 | 98 | 100 |
| Mass of constituents a (mg) | 0.26/0.52/0.78 | 12.74/25.48/38.22 | 13/26/39 |
| Mixture 2 | |||
| Mixing ratio (%) | 3 | 97 | 100 |
| Mass of constituents a (mg) | 0.39/0.78/1.17 | 12.61/25.22/37.83 | 13/26/39 |
| Mixture 3 | |||
| Mixing ratio (%) | 4 | 96 | 100 |
| Mass of constituents a (mg) | 0.52/1.04/1.56 | 12.48/24.96/37.44 | 13/26/39 |
| Parameter | Turbula Mixer |
|---|---|
| Volume of the vessel (mL) | 20/40/60 |
| Diameter of the vessel (mm) | 37 |
| Length of the vessel (mm) | 19/38/57 |
| Rotational speed of mixer (rpm) | 67 |
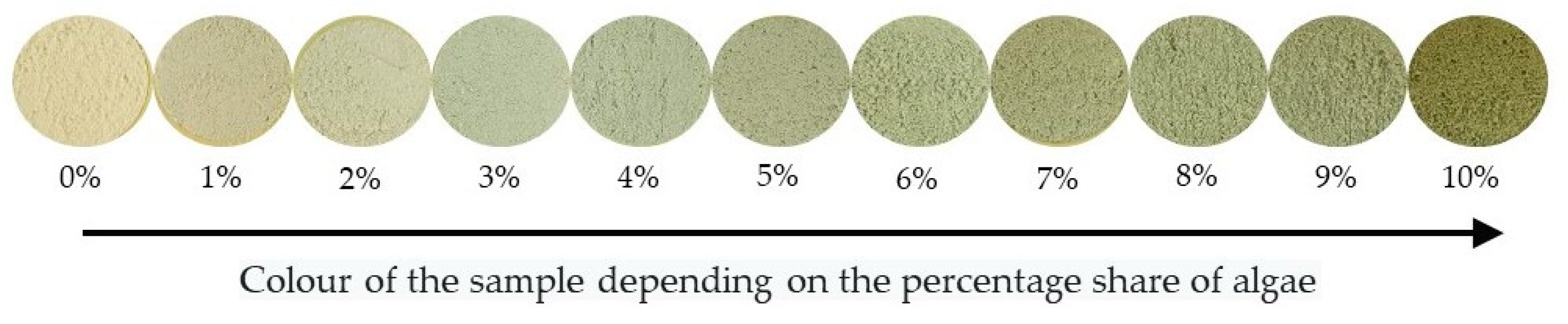
| Vessel Capacity (mL) | Assigned Share of Algae (%) | Number of Sample | Color Number a | Share of Algae (%) a,b | CV (%) |
|---|---|---|---|---|---|
| 20 | 2 | 1 | 11,858,063 ± 427,954 | 2.47 ± 1.14 | 12.13 |
| 2 | 11,617,468 ± 55,078 | 3.11 ± 0.15 | |||
| 3 | 11,889,742 ± 106,825 | 2.39 ± 0.28 | |||
| 4 | 11,627,086 ± 160,509 | 3.09 ± 0.43 | |||
| 3 | 1 | 11,481,165 ± 198,211 | 3.47 ± 0.53 | 2.13 | |
| 2 | 11,508,667 ± 145,946 | 3.40 ± 0.39 | |||
| 3 | 11,495,189 ± 83,518 | 3.44 ± 0.22 | |||
| 4 | 11,434,678 ± 90,323 | 3.60 ± 0.24 | |||
| 4 | 1 | 11,387,343 ± 35,841 | 3.72 ± 0.10 | 3.15 | |
| 2 | 11,304,299 ± 80,170 | 3.94 ± 0.21 | |||
| 3 | 11,415,133 ± 68,708 | 3.65 ± 0.18 | |||
| 4 | 11,324,020 ± 91,742 | 3.89 ± 0.24 | |||
| 40 | 2 | 1 | 11,802,365 ± 183,960 | 2.62 ± 0.49 | 2.21 |
| 2 | 11,740,319 ± 65,599 | 2.79 ± 0.17 | |||
| 3 | 11,781,122 ± 71,260 | 2.68 ± 0.19 | |||
| 4 | 11,779,214 ± 63,645 | 2.68 ± 0.17 | |||
| 3 | 1 | 11,460,124 ± 63,715 | 3.53 ± 0.17 | 3.61 | |
| 2 | 11,556,436 ± 110,043 | 3.27 ± 0.29 | |||
| 3 | 11,490,209 ± 49,729 | 3.45 ± 0.13 | |||
| 4 | 11,428,864 ± 84,112 | 3.61 ± 0.22 | |||
| 4 | 1 | 11,344,044 ± 45,510 | 3.84 ± 0.12 | 3.26 | |
| 2 | 11,283,480 ± 50,881 | 4.00 ± 0.14 | |||
| 3 | 11,227,395 ± 33,613 | 4.15 ± 0.09 | |||
| 4 | 11,344,248 ± 12,074 | 3.84 ± 0.03 | |||
| 60 | 2 | 1 | 11,162,919 ± 105,121 | 4.32 ± 0.28 | 4.94 |
| 2 | 11,361,941 ± 79,123 | 3.79 ± 0.21 | |||
| 3 | 11,196,296 ± 149,129 | 4.23 ± 0.40 | |||
| 4 | 11,267,031 ± 102,896 | 4.04 ± 0.27 | |||
| 3 | 1 | 11,313,492 ± 62,554 | 3.92 ± 0.17 | 3.84 | |
| 2 | 11,223,985 ± 163,167 | 4.16 ± 0.43 | |||
| 3 | 11,338,202 ± 19,465 | 3.85 ± 0.05 | |||
| 4 | 11,199,466 ± 114,112 | 4.22 ± 0.30 | |||
| 4 | 1 | 11,285,418 ± 8900 | 3.99 ± 0.02 | 2.38 | |
| 2 | 11,331,834 ± 35,978 | 3.87 ± 0.10 | |||
| 3 | 11,233,732 ± 141,806 | 4.13 ± 0.38 | |||
| 4 | 11,302,818 ± 49,965 | 3.95 ± 0.13 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matuszek, D.B.; Biłos, Ł.A. Computer Image Analysis as a Method of Evaluating the Quality of Selected Fine-Grained Food Mixtures. Sustainability 2021, 13, 3018. https://doi.org/10.3390/su13063018
Matuszek DB, Biłos ŁA. Computer Image Analysis as a Method of Evaluating the Quality of Selected Fine-Grained Food Mixtures. Sustainability. 2021; 13(6):3018. https://doi.org/10.3390/su13063018
Chicago/Turabian StyleMatuszek, Dominika Barbara, and Łukasz Andrzej Biłos. 2021. "Computer Image Analysis as a Method of Evaluating the Quality of Selected Fine-Grained Food Mixtures" Sustainability 13, no. 6: 3018. https://doi.org/10.3390/su13063018
APA StyleMatuszek, D. B., & Biłos, Ł. A. (2021). Computer Image Analysis as a Method of Evaluating the Quality of Selected Fine-Grained Food Mixtures. Sustainability, 13(6), 3018. https://doi.org/10.3390/su13063018