Seasonal Variations in the Chemical Composition of Indoor and Outdoor PM10 in University Classrooms
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling Procedure
2.2. Analytical Procedure
3. Results and Discussion
3.1. Mass Concentration of PM10
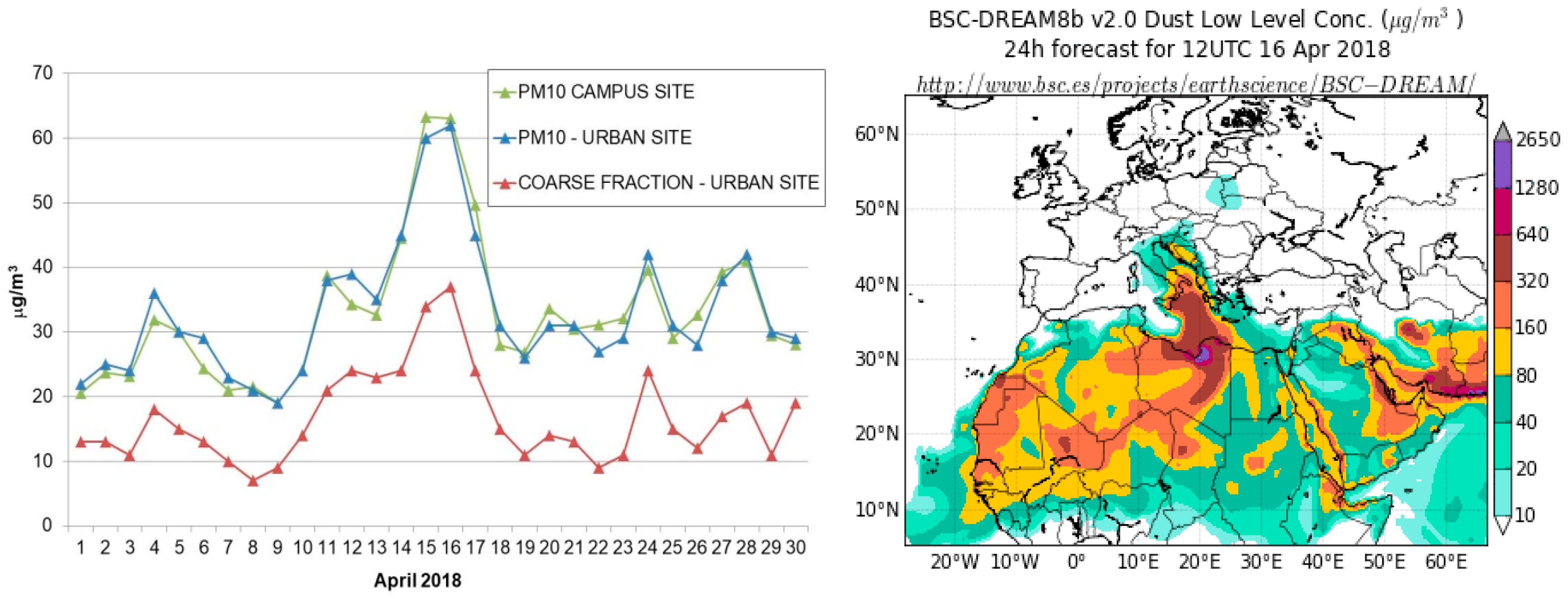
3.1.1. Outdoor PM10
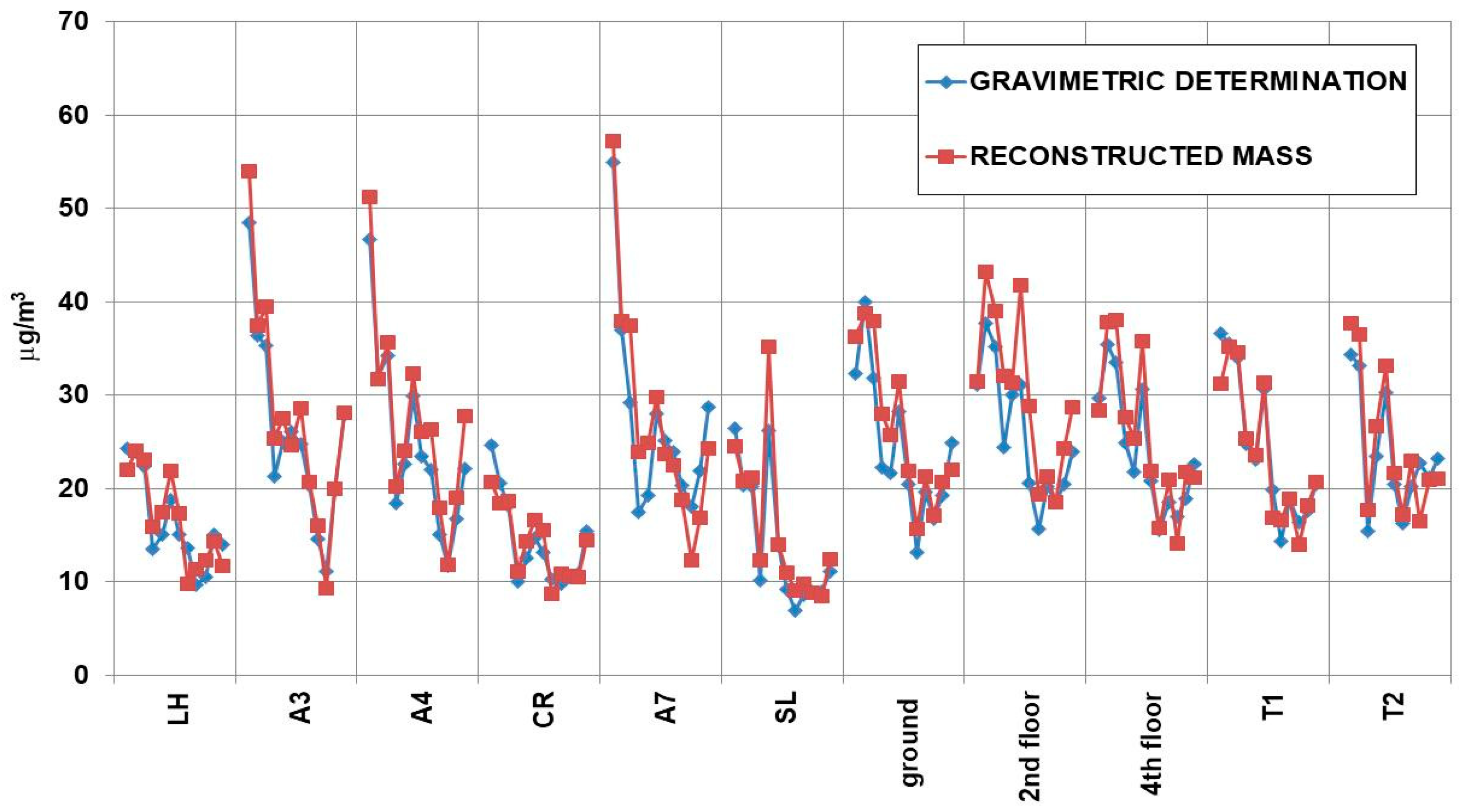
3.1.2. Indoor PM10
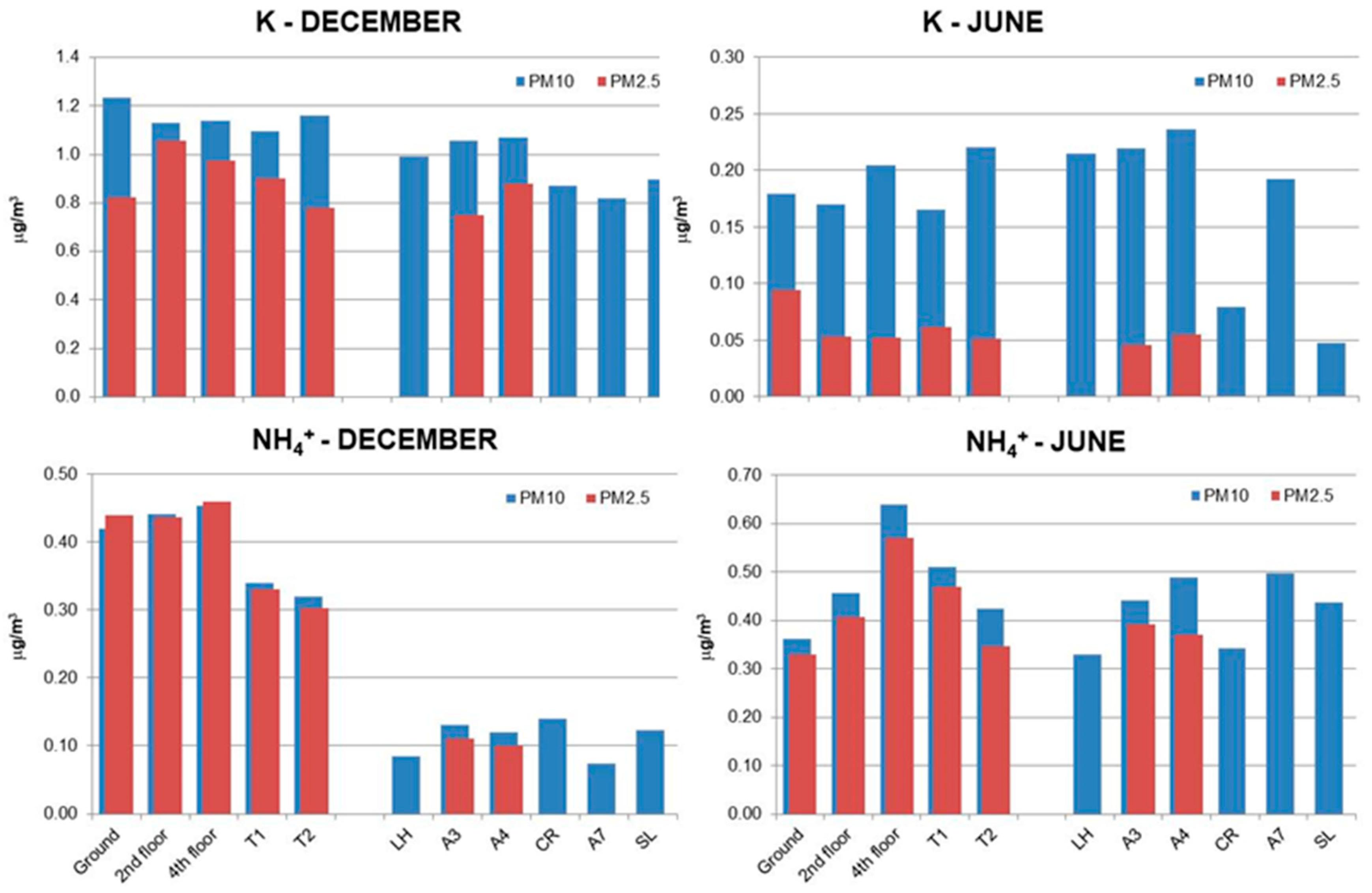
3.2. Chemical Composition of PM10
3.3. Chemical Composition of PM2.5
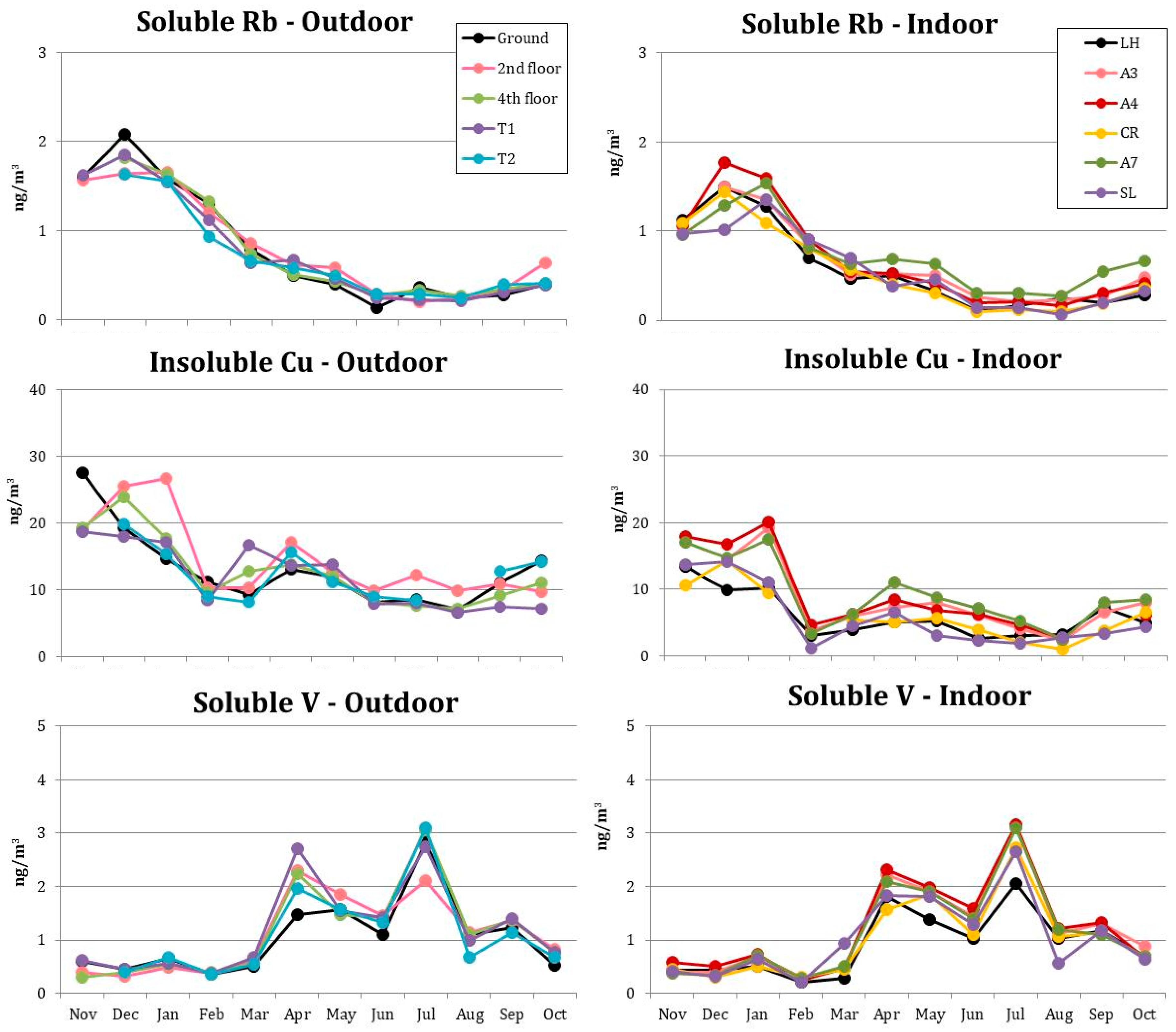
3.4. Micro-Elements
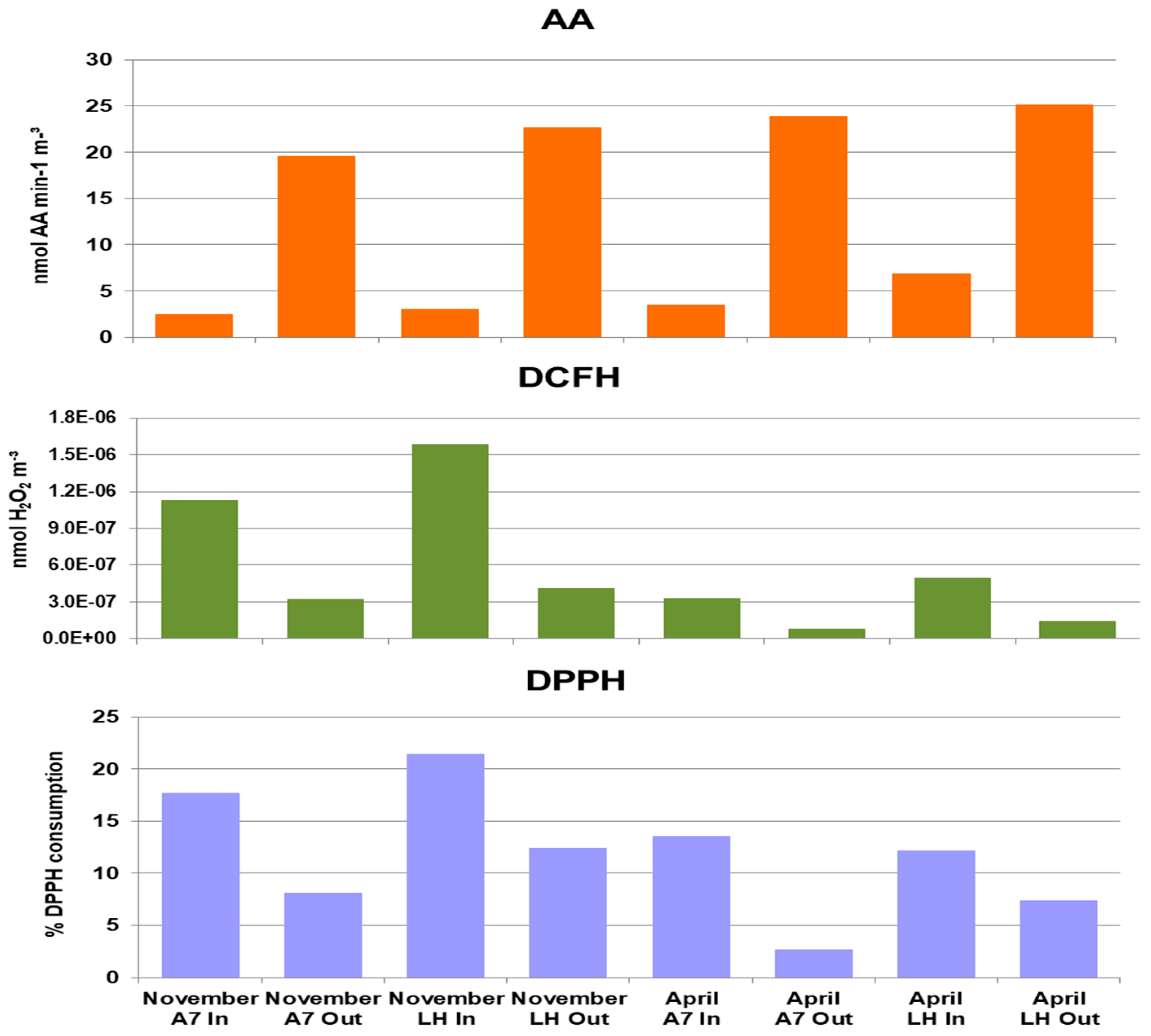
3.5. Redox Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cassee, F.R.; Héroux, M.-E.; Gerlofs-Nijland, M.E.; Kelly, F.J. Particulate matter beyond mass: Recent health evidence on the role of fractions, chemical constituents and sources of emission. Inhal. Toxicol. 2013, 25, 802–812. [Google Scholar] [CrossRef]
- Morawska, L.; Afshari, A.; Bae, G.N.; Buonanno, G.; Chao, C.Y.H.; Hänninen, O.; Hofmann, W.; Isaxon, C.; Jayaratne, E.R.; Pasanen, P.; et al. Indoor aerosols: From personal exposure to risk assessment. Indoor Air 2013, 23, 462–487. [Google Scholar] [CrossRef] [PubMed]
- Lai, H.; Kendall, M.; Ferrier, H.; Lindup, I.; Alm, S.; Hänninen, O.; Jantunen, M.; Mathys, P.; Colvile, R.; Ashmore, M.; et al. Personal exposures and microenvironment concentrations of PM2.5, VOC, NO2 and CO in Oxford, UK. Atmos. Environ. 2004, 38, 6399–6410. [Google Scholar] [CrossRef]
- Brasche, S.; Bischof, W. Daily time spent indoors in German homes e baseline data for the assessment of indoor exposure of German occupants. Int. J. Hyg. Environ. Health 2005, 208, 247–253. [Google Scholar] [CrossRef]
- Carrion-Matta, A.; Kang, C.-M.; Gaffin, J.M.; Hauptman, M.; Phipatanakul, W.; Koutrakis, P.; Gold, D.R. Classroom indoor PM2.5 sources and exposures in inner-city schools. Environ. Int. 2019, 131, 104968. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.; He, Z.; Li, C.; Chen, Z. Investigation of yearly indoor/outdoor PM2.5 levels in the perspectives of health impacts and air pollution control: Case study in Changchun, in the northeast of China. Sustain. Cities Soc. 2020, 53, 101871. [Google Scholar] [CrossRef]
- Chithra, V.S.; Shiva Nagendra, S.M. A review of scientific evidence on indoor air of school building: Pollutants, sources, health effects and management. Asian J. Atmos. Environ. 2018, 12, 87–108. [Google Scholar] [CrossRef]
- Nazaroff, W.W. Indoor particle dynamics. Indoor Air 2004, 14, 175–183. [Google Scholar] [CrossRef]
- Chen, C.; Zhao, B. Review of relationship between indoor and outdoor particles: I/O ratio, infiltration factor and penetration factor. Atmos. Environ. 2011, 45, 275–288. [Google Scholar] [CrossRef]
- Martins, V.; Faria, T.; Diapouli, E.; Manousakas, M.I.; Eleftheriadis, K.; Viana, M.; Almeida, S.M. Relationship between indoor and outdoor size-fractionated particulate matter in urban microenvironments: Levels, chemical composition and sources. Environ. Res. 2020, 183, 109203. [Google Scholar] [CrossRef]
- Amato, F.; Rivas, I.; Viana, M.; Moreno, T.; Bouso, L.; Reche, C.; Alvarez-Pedrerol, M.; Alastuey, A.; Sunyer, J.; Querol, X. Sources of indoor and outdoor PM2.5 concentrations in primary schools. Sci. Total. Environ. 2014, 490, 757–765. [Google Scholar] [CrossRef]
- Tofful, L.; Perrino, C. Chemical composition of indoor and outdoor PM2.5 in three schools in the city of Rome. Atmosphere 2015, 6, 1422–1443. [Google Scholar] [CrossRef]
- Perrino, C.; Tofful, L.; Canepari, S. Chemical characterisation of indoor and outdoor fine particulate matter in an occupied apartment in Rome, Italy. Indoor Air 2016, 26, 558–570. [Google Scholar] [CrossRef]
- Saraga, D.; Maggos, T.; Sadoun, E.; Fthenou, E.; Hassan, H.; Tsiouri, V.; Karavoltsos, S.; Sakellari, A.; Vasilakos, C.; Kakosimos, K. Chemical characterisation of indoor and outdoor particulate matter (PM2.5, PM10) in Doha, Qatar. Aerosol Air Qual. Res. 2017, 17, 1156–1168. [Google Scholar] [CrossRef]
- Oliveira, M.; Slezakova, K.; Delerue-Matos, C.; Pereira, M.C.; Morais, S. Children environmental exposure to particulate matter and polycyclic aromatic hydrocarbons and biomonitoring in school environments: A review on indoor and outdoor exposure levels, major sources and health impacts. Environ. Int. 2019, 124, 180–204. [Google Scholar] [CrossRef] [PubMed]
- Mazaheri, M.; Reche, C.; Rivas, I.; Crilley, L.R.; ÁlvarezPedrerol, M.; Viana, M.; Tobias, A.; Alastuey, A.; Sunyer, J.; Querol, X.; et al. Variability in exposure to ambient ultrafine particles in urban schools: Comparative assessment between Australia and Spain. Environ. Int. 2016, 88, 142–149. [Google Scholar] [CrossRef]
- Fuoco, F.C.; Stabile, L.; Buonanno, G.; Trassiera, C.V.; Massimo, A.; Russi, A.; Mazaheri, M.; Morawska, L.; Andrade, A. Indoor air quality in naturally ventilated italian classrooms. Atmosphere 2015, 6, 1652–1675. [Google Scholar] [CrossRef]
- Isaxon, C.; Gudmundsson, A.; Nordin, E.; Lönnblad, L.; Dahl, A.; Wieslander, G.; Bohgard, M.; Wierzbicka, A. Contribution of indoor-generated particles to residential exposure. Atmos. Environ. 2015, 106, 458–466. [Google Scholar] [CrossRef]
- Meng, Q.Y.; Turpin, B.J.; Korn, L.; Weisel, C.P.; Morandi, M.; Colome, S.; Zhang, J.; Stock, T.; Spektor, D.; Winer, A.; et al. Influence of ambient (outdoor) sources on residential indoor and personal PM2.5 concentrations: Analyses of RIOPA data. J. Expo. Sci. Environ. Epidemiol. 2005, 15, 17–28. [Google Scholar] [CrossRef]
- Gaidajis, G.; Angelakoglou, K. Indoor air quality in university classrooms and relative environment in terms of mass concentrations of particulate matter. J. Environ. Sci. Health Part A 2009, 44, 1227–1232. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Zhang, Y. Relations between indoor and outdoor PM2.5 and constituent concentrations. Front. Environ. Sci. Eng. 2018, 13, 5. [Google Scholar] [CrossRef]
- Viana, M.; Rivas, I.; Querol, X.; Alastuey, A.; Alvarez-Pedrerol, M.; Bouso, L.; Sioutas, C.; Sunyer, J. Partitioning of trace elements and metals between quasi-ultrafine, accumulation and coarse aerosols in indoor and outdoor air in schools. Atmos. Environ. 2015, 106, 392–401. [Google Scholar] [CrossRef]
- Sulaiman, F.R.; Bakri, N.I.F.; Nazmi, N.; Latif, M.T. Assessment of heavy metals in indoor dust of a university in a tropical environment. Environ. Forensics 2017, 18, 74–82. [Google Scholar] [CrossRef]
- Hussein, T.; Glytsos, T.; Ondráček, J.; Dohányosová, P.; Ždímal, V.; Hämeri, K.; Mihalis, L.; Smolík, J.; Kulmala, M. Particle size characterization and emission rates during indoor activities in a house. Atmos. Environ. 2006, 40, 4285–4307. [Google Scholar] [CrossRef]
- Wallace, L. Indoor sources of ultrafine and accumulation mode particles: Size distributions, size-resolved concentrations, and source strengths. Aerosol Sci. Technol. 2006, 40, 348–360. [Google Scholar] [CrossRef]
- Turpin, B.J.; Lim, H. Species contribution to PM2.5 mass concentration: Revisiting common assumptions for estimating organic mass. Aerosol Sci Technol. 2001, 35, 602–610. [Google Scholar] [CrossRef]
- Wierzbicka, A.; Bohgard, M.; Pagels, J.; Dahl, A.; Londahl, J.; Hussein, T.; Swietlicki, E.; Gudmundsson, A. Quantification of differences between occupancy and total monitoring periods for better assessment of exposure to particles in indoor environments. Atmos. Environ. 2015, 106, 419–428. [Google Scholar] [CrossRef]
- Ali, M.Y.; Hanafiah, M.M.; Khan, F.; Latif, M.T. Quantitative source apportionment and human toxicity of indoor trace metals at university buildings. Build. Environ. 2017, 121, 238–246. [Google Scholar] [CrossRef]
- Alves, C.A.; Vicente, E.D.; Evtyugina, M.; Vicente, A.M.; Nunes, T.; Lucarelli, F.; Calzolai, G.; Nava, S.; Calvo, A.I.; del Blanco Alegre, C.; et al. Indoor and outdoor air quality: A university cafeteria as a case study. Atmos. Pollut. Res. 2020, 11, 531–544. [Google Scholar] [CrossRef]
- Braniš, M.; Řezáčová, P.; Domasová, M. The effect of outdoor air and indoor human activity on mass concentrations of PM10, PM2.5, and PM1 in a classroom. Environ. Res. 2005, 99, 143–149. [Google Scholar] [CrossRef]
- Pelliccioni, A.; Monti, P.; Cattani, G.; Boccuni, F.; Cacciani, M.; Canepari, S.; Capone, P.; Catrambone, M.; Cusano, M.; D’Ovidio, M.C.; et al. Integrated evaluation of indoor particulate exposure: The VIEPI project. Sustainability 2020, 12, 9758. [Google Scholar] [CrossRef]
- Bates, J.T.; Fang, T.; Verma, V.; Zeng, L.; Weber, R.J.; Tolbert, P.E.; Abrams, J.Y.; Sarnat, S.E.; Klein, M.; Mulholland, J.A.; et al. Review of acellular assays of ambient particulate matter oxidative potential: Methods and relationships with composition, sources, and health effects. Environ. Sci. Technol. 2019, 53, 4003–4019. [Google Scholar] [CrossRef]
- Molina, C.; Toro, A.R.; Manzano, C.A.; Canepari, S.; Massimi, L.; Leiva-Guzmán, M.A. Airborne aerosols and human health: Leapfrogging from mass concentration to oxidative potential. Atmosphere 2020, 11, 917. [Google Scholar] [CrossRef]
- Catrambone, M.; Canepari, S.; Cerasa, M.; Sargolini, T.; Perrino, C. Performance Evaluation of a Very-low-volume Sampler for Atmospheric Particulate Matter. Aerosol Air Qual. Res. 2019, 19, 2160–2172. [Google Scholar] [CrossRef]
- Perrino, C.; Pietrodangelo, A.; Febo, A. An atmospheric stability index based on radon progeny measurements for the evaluation of primary urban pollution. Atmos. Environ. 2001, 35, 5235–5244. [Google Scholar] [CrossRef]
- Perrino, C.; Catrambone, M.; Pietrodangelo, A. Influence of atmospheric stability on the mass concentration and chemical composition of atmospheric particles: A case study in Rome, Italy. Environ. Int. 2008, 34, 621–628. [Google Scholar] [CrossRef]
- Wang, F.; Chambers, S.D.; Zhang, Z.; Williams, A.G.; Deng, X.; Zhang, H.; Lonati, G.; Crawford, J.; Griffiths, A.D.; Ianniello, A.; et al. Quantifying stability influences on air pollution in Lanzhou, China, using a radon-based “stability monitor”: Seasonality and extreme events. Atmos. Environ. 2016, 145, 376–391. [Google Scholar] [CrossRef]
- Vecchi, R.; Piziali, F.; Valli, G.; Favaron, M.; Bernardoni, V. Radon-based estimates of equivalent mixing layer heights: A long-term assessment. Atmos. Environ. 2019, 197, 150–158. [Google Scholar] [CrossRef]
- Canepari, S.; Astolfi, M.L.; Farao, C.; Maretto, M.; Frasca, D.; Marcoccia, M.; Perrino, C. Seasonal variations in the chemical composition of particulate matter: A case study in the Po Valley. Part II: Concentration and solubility of micro- and trace-elements. Environ. Sci. Pollut. Res. 2014, 21, 4010–4022. [Google Scholar] [CrossRef] [PubMed]
- Perrino, C.; Canepari, S.; Cardarelli, E.; Catrambone, M.; Sargolini, T. Inorganic constituents of urban air pollution in the Lazio region (Central Italy). Environ. Monit. Assess. 2007, 136, 69–86. [Google Scholar] [CrossRef]
- Perrino, C.; Catrambone, M.; Torre, S.D.; Rantica, E.; Sargolini, T.; Canepari, S. Seasonal variations in the chemical composition of particulate matter: A case study in the Po Valley. Part I: Macro-components and mass closure. Environ. Sci. Pollut. Res. 2013, 21, 3999–4009. [Google Scholar] [CrossRef]
- Canepari, S.; Perrino, C.; Astolfi, M.L.; Catrambone, M.; Perret, D. Determination of soluble ions and elements in ambient air suspended particulate matter: Inter-technique comparison of XRF, IC and ICP for sample-by-sample quality control. Talanta 2009, 77, 1821–1829. [Google Scholar] [CrossRef] [PubMed]
- Fang, T.; Verma, V.; Bates, J.T.; Abrams, J.; Klein, M.; Strickland, M.J.; Stefanie, E.; Sarnat, S.E.; Chang, H.H.; Mulholland, J.A.; et al. Oxidative potential of ambient water-soluble PM2.5 in the southeastern United States: Contrasts in sources and health associations between ascorbic acid (AA) and dithiothreitol (DTT) assays. Atmos. Chem. 2016, 16, 3865–3879. [Google Scholar] [CrossRef]
- Huang, W.; Zhang, Y.; Zhang, Y.; Fang, D.; Schauer, J.J. Optimisation of the measurement of particle-bound reactive oxygen species with 2’,7’-dichlorofluorescin (DCFH). Water Air Soil Pollut. 2016, 227, 164. [Google Scholar] [CrossRef]
- Simonetti, G.; Conte, E.; Perrino, C.; Canepari, S. Oxidative potential of size-segregated PM in an urban and an industrial area of Italy. Atmos. Environ. 2018, 187, 292–300. [Google Scholar] [CrossRef]
- Massimi, L.; Ristorini, M.; Simonetti, G.; Frezzini, M.A.; Astolfi, M.L.; Canepari, S. Spatial mapping and size distribution of oxidative potential of particulate matter released by spatially disaggregated sources. Environ. Pollut. 2020, 266, 115271. [Google Scholar] [CrossRef]
- Molyneux, P. The use of the stable free radical diphenylpicrylhydrazyl (DPPH) for estimating antioxidant activity. Songklanakarin J. Sci. Technol. 2004, 26, 211–219. [Google Scholar]
- Frezzini, M.A.; Castellani, F.; De Francesco, N.; Ristorini, M.; Canepari, S. Application of DPPH assay for assessment of particulate matter reducing properties. Atmosphere 2019, 10, 816. [Google Scholar] [CrossRef]
- BSC. Dust Regional Atmospheric Model (BSC-DREAM8b), operated by the Barcelona Supercomputing Center. Available online: http://www.bsc.es/ess/bsc-dust-daily-forecast/ (accessed on 6 August 2020).
- Chan, Y.; Simpson, R.; McTainsh, G.; Vowles, P.; Cohen, D.; Bailey, G. Characterisation of chemical species in PM2.5 and PM10 aerosols in Brisbane, Australia. Atmos. Environ. 1997, 31, 3773–3785. [Google Scholar] [CrossRef]
- Marcazzan, G.M.; Vaccaro, S.; Valli, G.; Vecchi, R. Characterisation of PM10 and PM2.5 particulate matter in the ambient air of Milan (Italy). Atmos. Environ. 2001, 35, 4639–4650. [Google Scholar] [CrossRef]
- Viidanoja, J.; Sillanpää, M.; Laakia, J.; Kerminen, V.M.; Hillamo, R.; Aarnio, P.; Koskentalo, T. Organic and black carbon in PM2.5 and PM10: 1 year of data from an urban site in Helsinki, Finland. Atmos. Environ. 2002, 36, 3183–3193. [Google Scholar] [CrossRef]
- Zhang, Q.; Jimenez, J.L.; Canagaratna, M.R.; Ulbrich, I.M.; Ng, N.L.; Worsnop, D.R.; Sun, Y. Understanding atmospheric organic aerosols via factor analysis of aerosol mass spectrometry: A review. Anal. Bioanal. Chem. 2011, 401, 3045–3067. [Google Scholar] [CrossRef]
- Farao, C.; Canepari, S.; Perrino, C.; Harrison, R.M. Sources of PM in an industrial area: Comparison between receptor model results and semiempirical calculations of source contributions. Aerosol Air Qual. Res. 2014, 14, 1558–1572. [Google Scholar] [CrossRef]
- Lunden, M.M.; Revzan, K.L.; Fischer, M.L.; Thatcher, T.L.; Littlejohn, D.; Hering, S.V.; Brown, N.J. The transformation of outdoor ammonium nitrate aerosols in the indoor environment. Atmos. Environ. 2003, 37, 5633–5644. [Google Scholar] [CrossRef]
- Sato, K.; Tamura, T.; Furuta, N. Partitioning between soluble and insoluble fractions of major and trace elements in size-classified airborne particulate matter collected in Tokyo. J. Environ. Monit. 2008, 10, 211–218. [Google Scholar] [CrossRef]
- Canepari, S.; Pietrodangelo, A.; Perrino, C.; Astolfi, M.L.; Marzo, M.L. Enhancement of source traceability of atmospheric PM by elemental chemical fractionation. Atmos. Environ. 2009, 43, 4754–4765. [Google Scholar] [CrossRef]
- Mazzei, F.; D’alessandro, A.; Lucarelli, F.; Nava, S.; Prati, P.; Valli, G.; Vecchi, R. Characterisation of particulate matter sources in an urban environment. Sci. Total Environ. 2008, 401, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Canepari, S.; Perrino, C.; Olivieri, F.; Astolfi, M.L. Characterisation of the traffic sources of PM through size-segregated sampling, sequential leaching and ICP analysis. Atmos. Environ. 2008, 42, 8161–8175. [Google Scholar] [CrossRef]
- Sarti, E.; Pasti, L.; Rossi, M.; Ascanelli, M.; Pagnoni, A.; Trombini, M.; Remelli, M. The composition of PM 1 and PM 2.5 samples, metals and their water soluble fractions in the Bologna area (Italy). Atmos. Pollut. Res. 2015, 6, 708–718. [Google Scholar] [CrossRef]
- Perrino, C.; Tofful, L.; Dalla Torre, S.; Sargolini, T.; Canepari, S. Biomass burning contribution to PM10 concentration in Rome (Italy): Seasonal, daily and two-hourly variations. Chemosphere 2019, 222, 839–848. [Google Scholar] [CrossRef]
- Massimi, L.; Simonetti, G.; Buiarelli, F.; Di Filippo, P.; Pomata, D.; Riccardi, C.; Ristorini, M.; Astolfi, M.L.; Canepari, S. Spatial distribution of levoglucosan and alternative biomass burning tracers in atmospheric aerosols, in an urban and industrial hot-spot of Central Italy. Atmos. Res. 2020, 239, 104904. [Google Scholar] [CrossRef]
- Szigeti, T.; Dunster, C.; Cattaneo, A.; Cavallo, D.; Spinazzè, A.; Saraga, D.E.; Sakellaris, I.A.; de Kluizenaar, Y.; Cornelissen, E.J.M.; Hanninen, O.; et al. Oxidative potential and chemical composition of PM2. 5 in office buildings across Europe–The OFFICAIR study. Environ. Int. 2016, 92, 324–333. [Google Scholar] [CrossRef] [PubMed]
- Hirvonen, M.-R.; Ruotsalainen, M.; Nevalainen, A. Toxicity and inflammatory mechanisms of bioaerosols. Pathophysiology 1998, 5, 107. [Google Scholar] [CrossRef]
- Marcovecchio, F.; Perrino, C. Bioaerosol contribution to atmospheric particulate matter in indoor university environments. Sustainability 2021, 13, 1149. [Google Scholar] [CrossRef]
- Øvrevik, J. Oxidative potential versus biological effects: A review on the relevance of cell-free/abiotic assays as predictors of toxicity from airborne particulate matter. Int. J. Mol. Sci. 2019, 20, 4772. [Google Scholar] [CrossRef] [PubMed]




| SOIL | SEA | SEC. INORGANICS | ORGANICS | TRAFFIC | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| INDOOR g/m3 | OUTDOOR g/m3 | I/O | INDOOR g/m3 | OUTDOOR g/m3 | I/O | INDOOR g/m3 | OUTDOOR g/m3 | I/O | INDOOR g/m3 | OUTDOOR g/m3 | I/O | INDOOR g/m3 | OUTDOOR g/m3 | I/O | |
| Nov | 18.3 ± 12.5 | 10.4 ± 2.0 | 1.8 | 0.8 ± 0.2 | 1.8 ± 0.6 | 0.5 | 2.2 ± 0.4 | 4.2 ± 0.8 | 0.5 | 8.3 ± 2.2 | 11.2 ± 1.4 | 0.7 | 3.9 ± 0.6 | 3.8 ± 0.4 | 1.0 |
| Dec | 11.7 ± 6.5 | 12.4 ± 1.4 | 0.9 | 1.3 ± 0. 2 | 3.8 ± 0.1 | 0.3 | 2.0 ± 0.5 | 4.5 ± 0.5 | 0.5 | 8.0 ± 0.8 | 11.8 ± 2.4 | 0.7 | 3.5 ± 0.6 | 5.9 ± 1.0 | 0.6 |
| Jan | 13.7 ± 8.0 | 12.4 ± 1.5 | 1.1 | 1.0 ± 0.2 | 2.9 ± 0.2 | 0.3 | 2.4 ± 0.4 | 5.3 ± 0.3 | 0.4 | 9.1 ± 2.2 | 14.0 ± 2.0 | 0.6 | 2.3 ± 0.7 | 3.5 ± 1.3 | 0.6 |
| Feb | 7.0 ± 4.8 | 7.9 ± 1.6 | 0.9 | 0.3 ± 0.1 | 1.2 ± 0.1 | 0.3 | 1.7 ± 0.3 | 4.6 ± 0.7 | 0.4 | 7.3 ± 0.4 | 10.9 ± 1.2 | 0.7 | 2.0 ± 0.3 | 2.4 ± 0.4 | 0.8 |
| Mar | 12.6 ± 6.8 | 10.5 ± 1.5 | 1.2 | 1.1 ± 0.2 | 2.8 ± 0.5 | 0.4 | 1.7 ± 0.3 | 3.4 ± 0.4 | 0.5 | 6.6 ± 0.6 | 7.3 ± 1.1 | 0.9 | 1.8 ± 0.5 | 2.6 ± 0.6 | 0.7 |
| Apr | 11.0 ± 5.1 | 17.8 ± 3.1 | 0.6 | 0.8 ± 0.3 | 1.6 ± 0.3 | 0.5 | 2.7 ± 0.6 | 4.8 ± 0.9 | 0.6 | 6.4 ± 1.1 | 7.4 ± 0.7 | 0.9 | 2.0 ± 0.5 | 3.0 ± 1.0 | 0.7 |
| May | 9.7 ± 5 0 | 8.6 ± 1.2 | 1.1 | 0.3 ± 0.2 | 0.6 ± 0.2 | 0.5 | 2.9 ± 0.8 | 3.2 ± 0.7 | 0.9 | 5.5 ± 0.9 | 7.0 ± 1.7 | 0.8 | 1.6 ± 0.4 | 2.2 ± 0.3 | 0.7 |
| Jun | 5.3 ± 5.4 | 4.7 ± 1.0 | 1.1 | 0.3 ± 0.2 | 0.5 ± 0.1 | 0.5 | 2.4 ± 0.8 | 3.3 ± 0.5 | 0.7 | 6.6 ± 1.6 | 7.2 ± 0.9 | 0.9 | 0.9 ± 0.3 | 1.1 ± 0.3 | 0.8 |
| Jul | 3.4 ± 2.1 | 7.9 ± 1.0 | 0.4 | 0.3 ± 0.2 | 0.4 ± 0.1 | 0.6 | 3.7 ± 1.2 | 4.4 ± 0.7 | 0.8 | 5.7 ± 0.5 | 6.8 ± 1.0 | 0.8 | 1.0 ± 0.2 | 1.8 ± 0.6 | 0.6 |
| Aug | 1.6 ± 0.5 | 4.5 ± 0.9 | 0.3 | 0.2 ± 0.2 | 0.4 ± 0.1 | 0.6 | 2.6 ± 0.7 | 2.1 ± 0.7 | 1.3 | 5.4 ± 0.6 | 7.8 ± 0.6 | 0.7 | 1.0 ± 0.2 | 1.2 ± 0.3 | 0.8 |
| Sep | 5.1 ± 3.3 | 6.5 ± 1.9 | 0.8 | 0.3 ± 0.2 | 0.8 ± 0.1 | 0.4 | 2.2 ± 0.8 | 3.5 ± 0.4 | 0.6 | 5.1 ± 0.5 | 7.8 ± 1.2 | 0.7 | 1.8 ± 0.3 | 2.5 ± 0.4 | 0.7 |
| Oct | 8.0 ± 5.4 | 6.7 ± 1.8 | 1.2 | 0.5 ± 0.4 | 1.1 ± 0.2 | 0.4 | 2.3 ± 1.1 | 3.3 ± 0.6 | 0.7 | 6.6 ± 1.4 | 8.4 ± 1.0 | 0.8 | 2.1 ± 0.4 | 3.0 ± 0.7 | 0.7 |
| SOLUBLE FRACTION | INSOLUBLE FRACTION | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LOD | INDOOR | OUTDOOR | I/O | LOD | INDOOR | OUTDOOR | I/O | |||||
| ng/m3 | N | ng/m3 | N | ng/m3 | ng/m3 | N | ng/m3 | N | ng/m3 | |||
| As | 0.2 | 61 | 0.40 ±0.25 | 50 | 0.41 ± 0.18 | 1.0 | 0.3 | - | <LOD | - | <LOD | - |
| Cd | 0.07 | 49 | 0.10 ± 0.05 | 47 | 0.15 ± 0.05 | 0.7 | 0.1 | - | <LOD | - | <LOD | - |
| Ce | 0.01 | 25 | 0.02 ± 0.01 | 28 | 0.03 ± 0.02 | 0.6 | 0.2 | 64 | 0.9 ± 0.6 | 47 | 0.6 ± 0.2 | 1.3 |
| Co | 0.05 | 30 | 0.07 ± 0.05 | 27 | 0.05 ± 0.03 | 1.1 | 0.05 | 52 | 0.15 ± 0.12 | 42 | 0.13 ± 0.05 | 1.3 |
| Cr | 0.1 | 59 | 0.2 ± 0.1 | 54 | 0.3 ± 0.1 | 1.0 | - | - | - | - | - | |
| Cs | 0.01 | 53 | 0.02 ± 0.01 | 43 | 0.03 ± 0.02 | 0.8 | 0.01 | 70 | 0.03 ± 0.02 | 58 | 0.05 ± 0.01 | 0.6 |
| Cu | 0.5 | 71 | 4.2 ± 1.4 | 59 | 8.1 ± 4 | 0.5 | 1 | 70 | 8 ± 5 | 59 | 13 ± 5 | 0.6 |
| Fe | 5 | 66 | 13 ± 7 | 58 | 17 ± 6 | 0.8 | 100 | 67 | 360 ± 237 | 59 | 574 ± 227 | 0.6 |
| Li | 0.02 | 52 | 0.05 ± 0.02 | 44 | 0.05 ± 0.02 | 0.8 | 0.05 | 66 | 0.13 ± 0.08 | 58 | 0.18 ± 0.10 | 0.8 |
| Mn | 0.5 | 72 | 3.0 ± 1.7 | 59 | 3.4 ± 1.6 | 0.8 | 0.5 | 55 | 3.6 ± 1.7 | 59 | 6.1 ± 3.0 | 0.5 |
| Mo | 0.05 | 68 | 0.33 ± 0.15 | 58 | 0.37±0.12 | 0.9 | 0.1 | 64 | 0.4 ± 0.2 | 60 | 1.0 ± 0.5 | 0.5 |
| Ni | 0.2 | 41 | 0.7 ± 0.4 | 46 | 0.7 ± 0.4 | 0.9 | 0.5 | 67 | 1.4 ± 0.7 | 56 | 1.7 ± 1.0 | 0.9 |
| Pb | 0.1 | 72 | 0.4 ± 0.2 | 59 | 0.5 ± 0.3 | 0.9 | 0.5 | 69 | 3.7 ± 1.8 | 59 | 4.0 ± 1.4 | 0.9 |
| Rb | 0.1 | 71 | 0.6 ± 0.5 | 58 | 0.8 ± 0.6 | 0.8 | 0.1 | 45 | 0.4 ± 0.2 | 40 | 0.6 ± 0.3 | 0.6 |
| Sb | 0.1 | 72 | 0.8 ± 0.3 | 58 | 1.0 ± 0.3 | 0.8 | 0.2 | 71 | 1.2 ± 0.5 | 59 | 1.5 ± 0.6 | 0.8 |
| Sn | 0.02 | 69 | 0.17 ± 0.10 | 53 | 0.15 ± 0.06 | 1.1 | 0.1 | 70 | 1.7 ± 0.5 | 60 | 2.8 ± 1.2 | 0.6 |
| Sr | 0.5 | 67 | 2.8 ± 1.9 | 58 | 2.7 ± 1.7 | 1.0 | 0.5 | 50 | 2.6 ± 1.9 | 40 | 2.6 ± 1.3 | 1.1 |
| Ti | 0.1 | 32 | 0.2 ± 0.2 | 35 | 0.2 ± 0.1 | 1.2 | 0.5 | 72 | 8.2 ± 6.1 | 60 | 8.9 ± 4.0 | 1.0 |
| Tl | 0.01 | 61 | 0.04 ± 0.02 | 51 | 0.06 ± 0.03 | 0.8 | 0.01 | - | <LOD | <LOD | - | |
| U | 0.001 | - | <LOD | - | <LOD | - | 0.001 | 48 | 0.011 ± 0.007 | 43 | 0.014 ± 0.008 | 0.7 |
| V | 0.2 | 71 | 1.2 ± 0.9 | 58 | 1.1 ± 0.8 | 1.0 | 0.2 | 71 | 0.8 ± 0.4 | 60 | 1.1 ± 0.6 | 0.7 |
| Zn | 2 | 61 | 14 ± 12 | 58 | 16 ± 8 | 0.9 | - | - | - | - | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tofful, L.; Catrambone, M.; Giusto, M.; Pareti, S.; Rantica, E.; Sargolini, T.; Canepari, S.; Frezzini, M.A.; Massimi, L.; Ristorini, M.; et al. Seasonal Variations in the Chemical Composition of Indoor and Outdoor PM10 in University Classrooms. Sustainability 2021, 13, 2263. https://doi.org/10.3390/su13042263
Tofful L, Catrambone M, Giusto M, Pareti S, Rantica E, Sargolini T, Canepari S, Frezzini MA, Massimi L, Ristorini M, et al. Seasonal Variations in the Chemical Composition of Indoor and Outdoor PM10 in University Classrooms. Sustainability. 2021; 13(4):2263. https://doi.org/10.3390/su13042263
Chicago/Turabian StyleTofful, Luca, Maria Catrambone, Marco Giusto, Salvatore Pareti, Elena Rantica, Tiziana Sargolini, Silvia Canepari, Maria Agostina Frezzini, Lorenzo Massimi, Martina Ristorini, and et al. 2021. "Seasonal Variations in the Chemical Composition of Indoor and Outdoor PM10 in University Classrooms" Sustainability 13, no. 4: 2263. https://doi.org/10.3390/su13042263
APA StyleTofful, L., Catrambone, M., Giusto, M., Pareti, S., Rantica, E., Sargolini, T., Canepari, S., Frezzini, M. A., Massimi, L., Ristorini, M., Pelliccioni, A., & Perrino, C. (2021). Seasonal Variations in the Chemical Composition of Indoor and Outdoor PM10 in University Classrooms. Sustainability, 13(4), 2263. https://doi.org/10.3390/su13042263