Impact of NaOH Concentration on Deweaving of Cotton Fabric in Aqueous Solutions
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
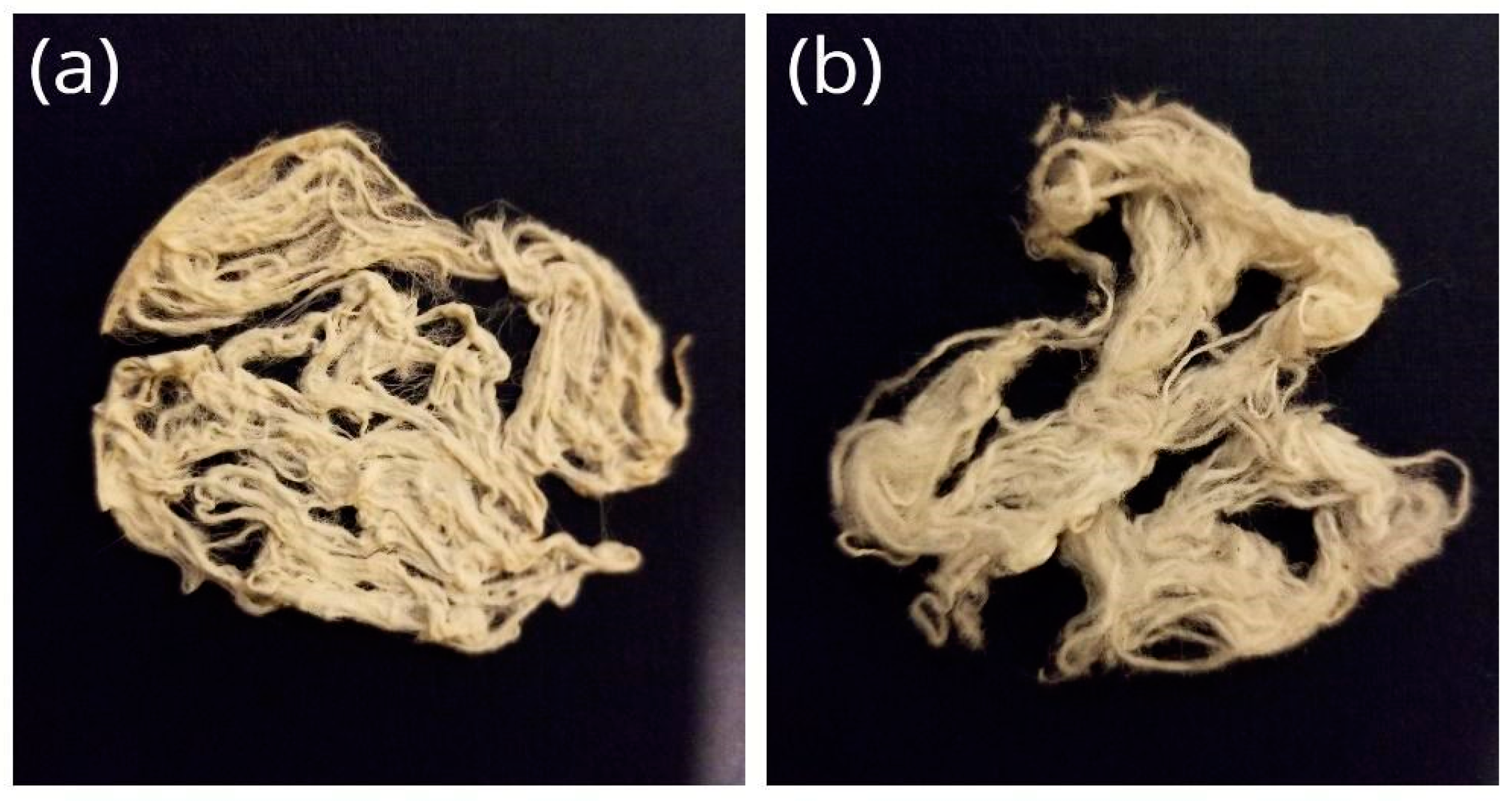
3.1. Cotton Fabric Treated under Various NaOH Concentrations
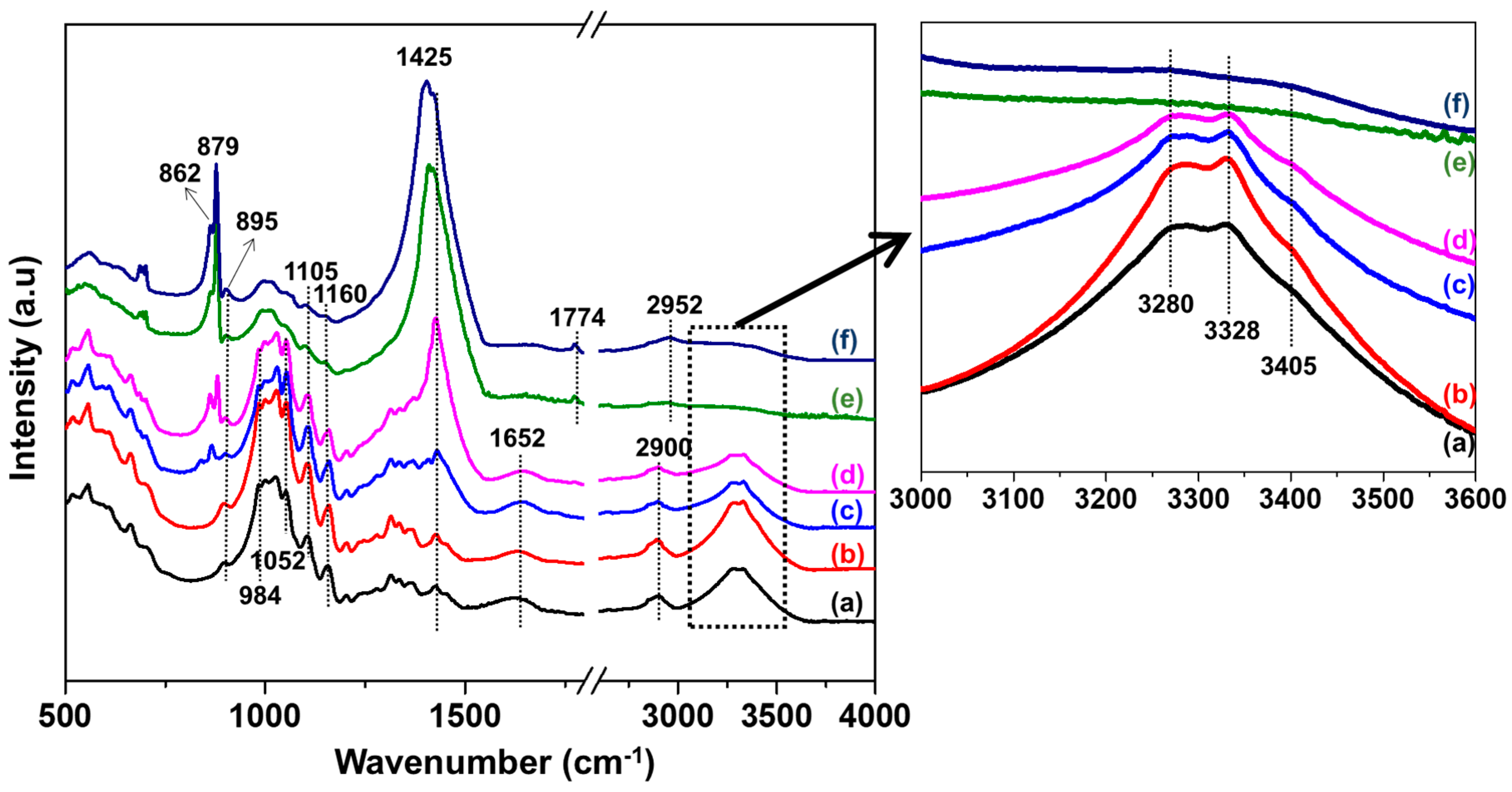
3.2. FTIR Analysis of Cotton Fabrics
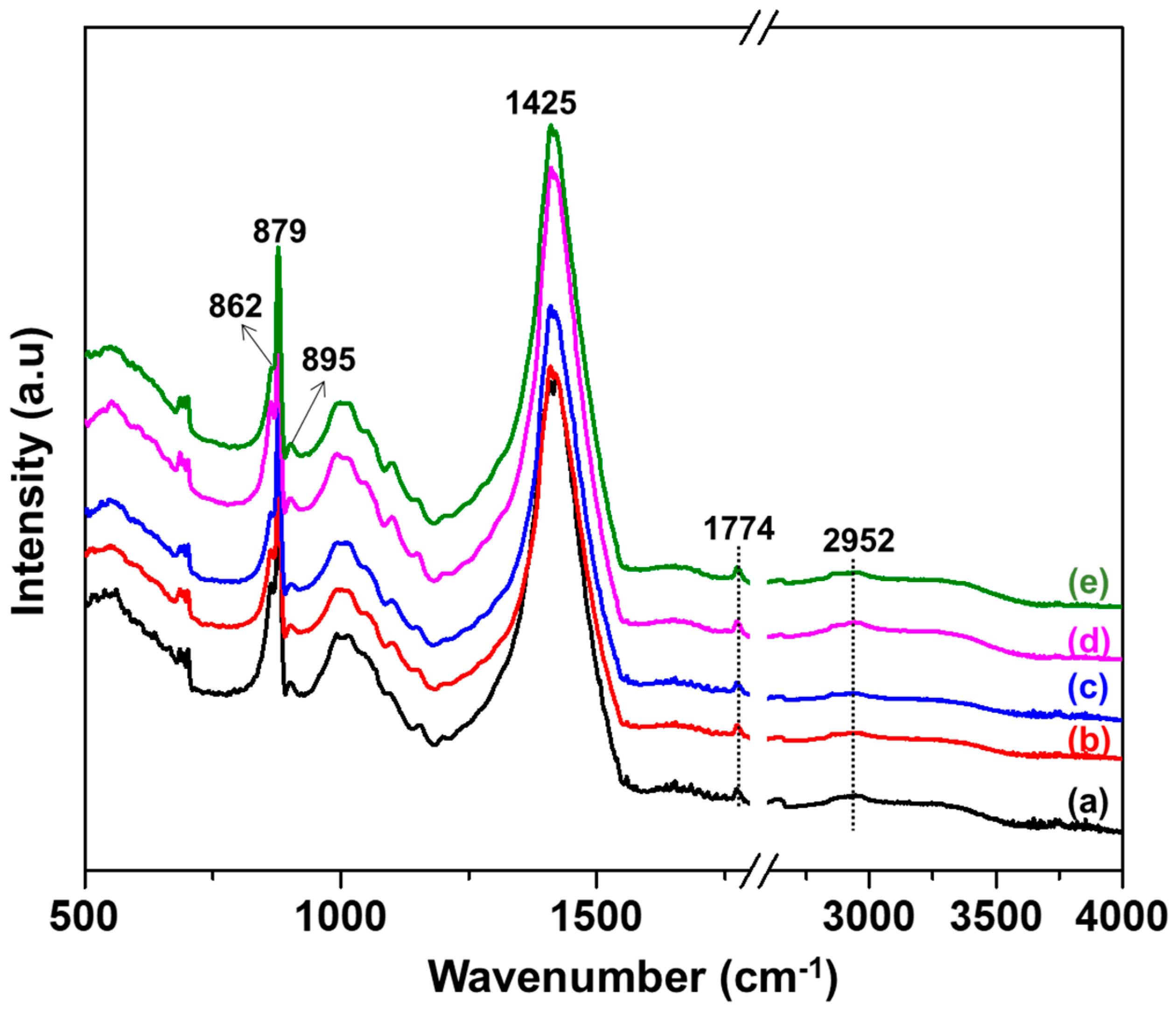
3.3. FTIR Analysis of Cotton Treated with Recycled NaOH
3.4. FTIR Analysis of Samples with and without Washing Using DI Water
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Advancing Sustainable Materials Management: 2018 Fact Sheet; United States Environmental Protection Agency, U.S. Government Printing Office: Washington, DC, USA, 2020; p. 3.
- Björquist, S. Separation for regeneration—Chemical recycling of cotton and polyester textiles. Master’ Thesis, The Swedish School of Textiles, Borås, Sweden, June 2017. [Google Scholar]
- Gustav Sandina, B.; Petersbc, G.M. Environmental impact of textile reuse and recycling—A review. J. Clean. Prod. 2018, 184, 353–365. [Google Scholar] [CrossRef]
- Wang, Y. Fiber and Textile Waste Utilization. Waste Biomass Valoriz. 2010, 1, 135–143. [Google Scholar] [CrossRef]
- Gordon, S.; Hsieh, Y.-L. 1—Chemical structure and properties of cotton. In Cotton: Science and Technology; Woodhead Publishing: Cambridge, UK, 2007; pp. 3–34. ISBN 978-1-84569-026-7. [Google Scholar]
- Zhou, J.; Zhang, L.; Cai, J. Behavior of Cellulose in NaOH/Urea Aqueous Solution Characterized by Light Scattering and Viscometry. J. Polym. Sci. Part B Polym. Phys. 2004, 42, 347–353. [Google Scholar] [CrossRef]
- Northolt, M.G.; Boerstel, H.; Maatman, H.; Huisman, R.; Veurink, J.; Elzerman, H. The structure and properties of cellulose fibers spun from an anisotropic phosphoric acid solution. Polymer 2001, 42, 8249–8264. [Google Scholar] [CrossRef]
- Kosan, B.; Michels, C.; Meister, F. Dissolution and forming of cellulose with ionic liquids. Cellulose 2008, 15, 59–66. [Google Scholar] [CrossRef]
- Zhang, H.; Wu, J.; Zhang, J.; He, J. 1-Allyl-3-methylimidazolium chloride room temperature ionic liquid: A new and powerful nonderivatizing solvent for cellulose. Macromolecules 2005, 38, 8272–8277. [Google Scholar] [CrossRef]
- Kamide, K.; Okajima, K.; Kowsaka, K. Dissolution of natural cellulose into aqueous alkali solution: Role of supermolecular structure of cellulose. Polym. J. 1992, 24, 71–86. [Google Scholar] [CrossRef]
- Davidson, G.F. The dissolution of chemically modified cotton cellulose in alkaline solutions. Part 1: In solutions of NaOH, particularly at T C below the normal. J. Text. Inst. 1934, 25, 174–196. [Google Scholar] [CrossRef]
- Davidson, G.F. The dissolution of chemically modified cotton cellulose in alkaline solutions. Part 2: A comparison of the solvent action of solutions of Lithium, Sodium, Potassium and tetramethylammonium hydroxides. J. Text. Inst. 1936, 27, 112–130. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, L. Solubility of cellulose in NaOH/Urea aqueous solution. Polym. J. 2000, 32–10, 866–870. [Google Scholar] [CrossRef]
- Ershova, O.; Costa, E.V.; Fernandes, A.J.S.; Domingues, M.R.; Evtuguin, D.V.; Sixta, H. Effect of urea on cellulose degradation under conditions of alkaline pulping. Cellulose 2012, 19, 2195–2204. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, W.; Li, F.; Yu, J. Swelling and dissolution of cellulose in NaOH aqueous solvent systems. Cellul. Chem. Technol. 2013, 47, 671–679. [Google Scholar]
- Santos, N.M.; Puls, J.; Saake, B.; Navard, P. Effects of nitren extraction on a dissolving pulp and influence on cellulose dissolution in NaOH–water. Cellulose 2013, 20, 2013–2026. [Google Scholar] [CrossRef]
- Ying, W. Cellulose Fiber Dissolution in Sodium Hydroxide Solution at Low Temperature: Dissolution Kinetics and Solubility Improvement. Ph.D. Thesis, Georgia Institute of Technology, Atlanta, GA, USA, December 2008. [Google Scholar]
- Sobue, H.K.H.; Hess, K. The cellulose-sodium hydroxide-water system as a function of the temperature. Z. Phys. Chem. B 1939, 43, 309–328. [Google Scholar]
- Budtova, T.; Navard, P. Cellulose in NaOH–water based solvents: A review. Cellulose 2016, 23, 5–55. [Google Scholar] [CrossRef]
- Nishiyama, Y.; Kuga, S.; Okano, T. Mechanism of mercerization revealed by X-ray diffraction. J. Wood Sci. 2000, 46, 452–457. [Google Scholar] [CrossRef]
- Lewin, M. Oxidation and aging of cellulose. Macromol. Symp. 1997, 118, 715–724. [Google Scholar] [CrossRef]
- Saloheimo, M.; Paloheimo, M.; Hakola, S.; Pere, J.; Swanson, B.; Nyyssonen, E.; Bhatia, A.; Ward, M.; Penttila, M. Swollenin, a Trichoderma ressi protein with sequence similarity to the plant expansions, exhib-its disruption activity on cellulosic materials. Eur. J. Biochem. 2002, 269, 4202–4211. [Google Scholar] [CrossRef]
- Öztürk, H.; Bechtold, T. Effect of NaOH Treatment on the Interfibrillar Swelling and Dyeing Properties of Lyocell (TENCEL®) Fibres. Fibres Text. East. Eur. 2007, 15, 114–117. [Google Scholar]
- Cuissinat, C.; Navard, P. Swelling and Dissolution of Cellulose Part II: Free Floating Cotton and Wood Fibres in NaOH–Water–Additives Systems. Macromol. Symp. 2006, 244, 19–30. [Google Scholar] [CrossRef]
- Wang, Y.; Deng, Y. The kinetics of cellulose dissolution in sodium hydroxide solution at low temperatures. Biotechnol. Bioeng. 2008, 102, 1398–1405. [Google Scholar] [CrossRef] [PubMed]
- Haque, A.; Akhtar, M.; Halilu, A.; Yun, H. Validation and extended application of cellulose microfibril swelling enzyme assay method to alkali induced swelling of cellulose. J. Chem. Eng. Bioanal. Chem. 2017, 2, 62–69. [Google Scholar]
- Duchemin, B.J.C. Mercerisation of cellulose in aqueous NaOH at low concentrations. Green Chem. 2015, 17, 3941–3947. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Z.; Cho, H.; Kim, M.; Sham, T.; Sun, X. Layer speciation and electronic structure investigation of freestanding hexagonal boron nitride nanosheets. Nanoscale 2015, 7, 1718–1724. [Google Scholar] [CrossRef] [PubMed]
- Ghosal, S.; Chen, M.; Wagner, J.; Wang, Z.; Wall, S. Molecular identification of polymers and anthropogenic particles extracted from oceanic water and fish stomach—A Raman microspectroscopy study. Environ. Pollut. 2018, 233, 1113–1124. [Google Scholar] [CrossRef]
- Zhang, S.; Lee, J.; Kim, D.H.; Kim, T. Effects of Ni Loading on the Physicochemical Properties of NiOx/CeO2 Catalysts and Catalytic Activity for NO Reduction by CO. Catal. Sci. Technol. 2020, 10, 2359–2368. [Google Scholar] [CrossRef]
- Poletto, M.; Junior, H.; Zattera, A. Native cellulose: Structure, characterization and thermal properties. Materials 2014, 7, 6105–6119. [Google Scholar] [CrossRef]
- Sedighi, A.; Montazer, M.; Hemmatinejad, N. Copper nanoparticles on bleached cotton fabric: In situ synthesis and characterization. Cellulose 2014, 21, 2119–2132. [Google Scholar] [CrossRef]
- Topalovic, T.; Nierstrasz, V.A.; Bautista, L.; Jocic, D.; Navarro, A.; Warmoeskerken, M. XPS and contact angle study of cotton surface oxidation by catalytic bleaching. Colloids Surf. A Physicochem. Eng. Asp. 2007, 296, 76–85. [Google Scholar] [CrossRef]
- Edwards, H.G.M.; Farwell, D.W. FT-Raman spectrum of cotton: A polymeric biomolecular analysis. Spectrochim. Acta 1994, 50A, 807–811. [Google Scholar] [CrossRef]
- Was-Gubala, J.; Machnowski, W. Application of Raman Spectroscopy for Differentiation Among Cotton and Viscose Fibers Dyed with Several Dye Classes. Spectrosc. Lett. 2014, 47, 527–535. [Google Scholar] [CrossRef]
- Abidi, N.; Cabrales, L.; Haigler, C.H. Changes in the cell wall and cellulose content of developing cotton fibers investigated by FTIR spectroscopy. Carbohydr. Polym. 2014, 100, 9–16. [Google Scholar] [CrossRef]
- Hospodarova, V.; Singovszka, E.; Stevulova, N. Characterization of Cellulosic Fibers by FTIR Spectroscopy for Their Further Implementation to Building Materials. Am. J. Anal. Chem. 2018, 9, 303–310. [Google Scholar] [CrossRef]
- Moses, J.J.; Venkataraman, V. Study of Mechanical and Surface Properties on Some Chemical Treated Cotton Fabric By KES-F, SEM and FTIR Analysis. Int. J. Eng. Res. Technol. 2014, 3, 2396–2411. [Google Scholar]
- Allen, A.; Foulk, J.; Gamble, G. Preliminary Fourier-Transform Infrared Spectroscopy Analysis of Cotton Trash. J. Cotton Sci. 2007, 11, 68–74. [Google Scholar]
- Colom, X.; Carrillo, F.; Nogues, F.; Garriga, P. Stuructural analysis of photodegraded wood by means of FTIR spectroscopy. Polym. Degrad. Stab. 2003, 80, 543–549. [Google Scholar] [CrossRef]
- Moigne, N.; Navard, P. Dissolution mechanisms of wood cellulose fibres in NaOH-water. Cellulose 2010, 17, 31–45. [Google Scholar] [CrossRef]
- Baldinger, T.; Moosbauer, J.; Sixta, H. Supermolecular structure of cellulosic materials by Fourier transform infrared spectroscopy (FT-IR) calibrated by WAXS and 13C NMR. Lenzing. Ber. 2000, 79, 15–17. [Google Scholar]
- Oh, S.Y.; Yoo, D.I.; Shin, Y.; Seo, G. FTIR analysis of cellulose treated with sodium hydroxide and carbon dioxide. Carbohydr. Res. 2005, 340, 417–428. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.Y.; You, D.I.; Shin, Y.; Kim, H.C.; Kim, H.Y.; Chung, Y.S.; Park, W.H.; You, J.H. Crystalline structure analysis of cellulose treated with sodium hydroxide and carbon dioxide by means of X-Ray diffraction and FTIR spectroscopy. Carbohydr. Res. 2005, 340, 2376–2391. [Google Scholar] [CrossRef] [PubMed]
- Schwanninger, M.; Rodrigues, J.C.; Pereira, H.; Hinterstoisser, B. Effects of short-time vibratory ball milling on the shape of FT-IR spectra of wood and cellulose. Vib. Spectrosc. 2004, 36, 23–40. [Google Scholar] [CrossRef]
- Chunga, C.; Leeb, M.; Choeb, E.K. Characterization of cotton fabric scouring by FT-IR ATR spectroscopy. Carbohydr. Polym. 2004, 58, 417–420. [Google Scholar] [CrossRef]
- Fengel, D.; Strobel, C. FTIR spectroscopic studies on the heterogeneous transformation of cellulose I into cellulose II. Acta Polym. 1994, 45, 319–324. [Google Scholar] [CrossRef]
- Yue, Y.; Han, G.; Wu, Q. Transitional properties of cotton fibers from cellulose I to cellulose II structure. BioResources 2013, 8, 6460–6471. [Google Scholar] [CrossRef]
- Liu, Y.; Hu, H. X-ray diffraction study of bamboo fibers treated with NaOH. Fibers Polym. 2008, 9, 735–739. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, H.Z.; Yao, Y.; Halada, G.; Jung, H.J.; Kim, T. Impact of NaOH Concentration on Deweaving of Cotton Fabric in Aqueous Solutions. Sustainability 2021, 13, 2015. https://doi.org/10.3390/su13042015
Cao HZ, Yao Y, Halada G, Jung HJ, Kim T. Impact of NaOH Concentration on Deweaving of Cotton Fabric in Aqueous Solutions. Sustainability. 2021; 13(4):2015. https://doi.org/10.3390/su13042015
Chicago/Turabian StyleCao, Hang Zhang, Yiqian Yao, Gary Halada, Hye Jung Jung, and Taejin Kim. 2021. "Impact of NaOH Concentration on Deweaving of Cotton Fabric in Aqueous Solutions" Sustainability 13, no. 4: 2015. https://doi.org/10.3390/su13042015
APA StyleCao, H. Z., Yao, Y., Halada, G., Jung, H. J., & Kim, T. (2021). Impact of NaOH Concentration on Deweaving of Cotton Fabric in Aqueous Solutions. Sustainability, 13(4), 2015. https://doi.org/10.3390/su13042015






