Abstract
Limiting the increase in CO2 concentrations in the atmosphere, and at the same time, meeting the increased energy demand can be achieved by applying carbon capture, utilization and storage (CCUS) technologies, which hold potential as the bridge for energy and emission-intensive industries to decarbonization goals. At the moment, the only profitable industrial large-scale carbon sequestration projects are large-scale carbon dioxide enhanced oil recovery (CO2-EOR) projects. This paper gives a general overview of the indirect and direct use of captured CO2 in CCUS with a special focus on worldwide large-scale CO2-EOR projects and their lifecycle emissions. On the basis of scientific papers and technical reports, data from 23 contemporary large-scale CO2-EOR projects in different project stages were aggregated, pointing out all the specificities of the projects. The specificities of individual projects, along with the lack of standardized methodologies specific for estimating the full lifecycle emissions resulting from CO2-EOR projects, pose a challenge and contribute to uncertainties and wide flexibilities when estimating emissions from CO2-EOR projects, making the cross-referencing of CO2-EOR projects and its comparison to other climate-mitigation strategies rather difficult. Pointing out the mentioned project’s differentiations and aggregating data on the basis of an overview of large-scale CO2-EOR projects gives useful information for future work on the topic of a CO2-EOR project’s lifecycle emissions.
1. Introduction
The Paris Agreement came into force in 2016 with the intention of mitigating global warming by keeping the global average temperature increase under 2 °C, and preferably even under 1.5 °C, when compared to pre-industrial levels. The only way to do this is through full harmonization with the energy and climate targets, which are comprised of a significant reduction of greenhouse gas emissions by 2030 (by 45%), as well as total decarbonization by 2050, based on the application of energy efficiency, renewable energy use and carbon capture and storage (CCS), or carbon capture, utilization and storage (CCUS). CCS technology implies avoiding CO2 emissions to the atmosphere by capturing and storing it in geological formations characterized with long-term containment capability [1,2]. As per the strategies submitted to the United Nations Framework Convention on Climate Change (UNFCCC), CCUS is often recognized as a favorable option to fight climate change due to the turning of unwanted greenhouse gas into valuable products [3]. In order to be reused for various purposes (used for yield boosting or for the production of fuels, chemicals, building materials, etc.) CO2 is captured from different sources, such as fossil fuel-based power plants, ammonia production plants, biomass fermentation facilities, natural gas processing plants, or it can be captured (removed) directly from the air. The commercial-industrial source of CO2 should be at least 0.01 to 0.5 Mt CO2/year [3,4].
At the moment, even though energy efficiency, use of renewable energy sources and fuel switching are often required as the exclusive priority in achieving climate goals, the world’s high dependency on fossil fuel is still very much present. Therefore, the fossil fuel production industry (oil, gas and coal industry) has been undertaking different carbon-reduction initiatives in order to retain market competitiveness by providing a constant energy supply with an ecological footprint that is as low as possible [5,6].
Significant experience and existing infrastructure for underground fluid injection represent an essential basis for the development of CO2 underground deposition technology. Additional oil production by CO2 injection and CO2 permanent storage within depleted oil and gas reservoirs or suitable geological formations seem to be sustainable options, which provide multiple benefits [7,8].
Keeping in mind that CO2 usage for different products should not necessarily result in overall emission reduction, the benefits of each utilization/storage project must be evaluated by performing a comprehensive lifecycle analysis. This requires clear methodological guidelines that are temporarily under development by several expert groups. Furthermore, the retention time for CO2 differs significantly, being in the range from one year, in the case of fuel generation, up to millions of years, in the case of carbonation [3]. Carbonation refers to a natural reaction of metal oxides, i.e., calcium (Ca) or magnesium (Mg) containing minerals (e.g., serpentine, olivine, wollastonite) with CO2, which results in the production of calcium or magnesium carbonates (CaCO3 or MgCO3). Such processes can be considered as a CO2 utilization or storage option. While the utilization refers to the recently developed, accelerated ex situ carbonation, able to produce valuable construction materials, a storage option refers to the last of the trapping mechanisms occurring within a geological formation (underground storage), which enables the permanent retention of CO2. Since the use of pure CO2 is not essential for mineralization (impurities simply do not interfere with the reaction), a purification step can be avoided, which results in lower costs [9,10].
Although, as stated before, emission-reduction results differentiate from project to-project, it is obvious that the best results, in terms of both sequestrated CO2 quantities and sequestration permanency, can be achieved by just performing CCS projects. Other CCUS options, in fact, delay emissions to a greater or lesser extent, but due to economical profitability (they produce valuable products), today, at a time of a relatively low CO2 market price, such projects are more preferable. However, due to residual oil production, currently, the only form of large-scale industrial carbon sequestration profitable projects are CO2-EOR projects. Although fossil fuel combustion and waste gas generated during CO2-EOR operations at an EOR site result in new emissions, substantial quantities of CO2 remain permanently stored within the depleted reservoirs. Since there are some disagreements over CO2-EOR emission assessment, a lack of standardized methods for measuring the full lifecycle emissions resulting from CO2-EOR projects (needed for crediting EOR’s carbon reductions) hinders CO2-EOR application as CCUS technology.
In this paper, captured CO2 utilization, an overview of the worldwide CO2-EOR projects and an analysis of CO2-EOR lifecycle emissions are presented.
2. Methods
As we noted an evident lack of systematic reviews of EOR projects and their emissions, the intention was to get a comprehensive picture of this topic. Therefore, we made a cross-section of the actual large-scale EOR projects. By presenting a qualitative analysis of the CO2-EOR site emission sources and related emissions, as well as by giving a literature review on large-scale CO2-EOR, we tried to consolidate and summarize the available data, which can serve as a comprehensive base for further research on the topic. The literature search was based on electronic resources available from the University of Zagreb system. The reviewed reports were limited to those from governmental agencies, companies or recognized professional associations. Due to turbulent climate-mitigation strategies and technology progress, we decided to focus only on recent publications to make sure that our search is limited only to relevant data. Searches focused on reports published from 2017 to 2020. These reports took on a variety of forms, from short pamphlets and factsheets to comprehensive book-like reports. However, when talking about future fossil fuel usage, we tried to avoid oil companies’ reports, which could be biased, and we rather used publicly available project databases to ensure that all datasets are verifiable to the readers. There are a number of online CCS project databases collected by different associations, e.g., Carbon Capture and Sequestration Technologies at the Massachusetts Institute of Technology (abbr. MIT) [11], Global CCS Institute [12], International Energy Agency (abbr. IEA) [13]. As per the KAPSARC data source [14], in 2018, there were 23 CO2-EOR large-scale projects, which are, after checking and comparing to other databases and science papers, briefly described in the paper.
Academic literature was searched much more broadly, covering peer-reviewed papers, theses, books, preprints, abstracts, technical reports, etc. In literature, search major databases and search engines were used, such as Web of Science, Conference Proceedings Citation Index—Science, etc.
Searches were refined to journal articles and titles, and abstracts were scanned for papers and articles discussing CCS/CCUS technologies and CO2-EOR projects. Additionally, a more systematic review of the literature was initiated to identify relevant research and data related to emissions from CCS/CO2-EOR projects. Searches focused on papers and articles published from 2015 to 2020, even though some of the earlier published papers covering the general characteristics of CCS/CCUS technologies were used. The literature search was limited to only Croatian and English-language documents. Trade publications were not considered.
3. Captured CO2 Utilization
Globally, about 230 Mt/y of CO2 is used for different purposes, covering both intermediate CO2 usage (CO2 is not chemically converted) and its conversion (into fuel, chemicals or building materials) (Table 1) [3].

Table 1.
Use of captured CO2. Modified according to [3,15].
3.1. CO2 Conversion
CO2 conversion includes different kinds or purposes of conversion, such as yield boosting, chemicals, fuels and building materials. They are briefly described below.
Yield Boosting. The largest usage of captured CO2 is by the fertilizer industry (about 130 Mt/y) for urea production, followed by the petroleum industry (about 80 Mt/y), where CO2 is used for EOR purposes. The rest of the captured CO2 serves as feedstock for different industries; for instance: food and drink industries use CO2 as a carbonating agent, preservative and solvent for flavor extraction, etc. Another option for CO2 use is CO2-based chemical production. Carboxylation reactions use CO2 (it replaces a part of the fossil fuel-based raw material) as a precursor for polymer-forming, while reduction reactions produce chemicals by breaking C = O bonds [3].
Chemicals. CO2-based synthetic fuels may refer to methane, methanol, gasoline, and aviation fuels. CO2 captured at power plant exhausts could be used directly in catalytic processes for the generation of synthetic gas. Syngas, as a mixture of CO and H2, is a crucial component in the production of hydrogen, ammonia, methanol, and synthetic hydrocarbon fuels. As an intermediate, it can be used in the production of synthetic petroleum by the Fischer–Tropsch process [1,16].
Fuels. The synthetic fuel generation process uses CO2 and hydrogen to produce a carbon-based fuel, which is, due to easier handling, used in aviation. CO2 conversion into methanol has a wide range of use (as a fuel, fuel additive or an intermediate for plastics, textiles, and other products) [10,17,18,19,20,21]. However, among all the above-mentioned CO2-based chemical production processes, only polymer production is market competitive due to its relatively low-energy intensity and high product market prices. On the other hand, the costs of synthetic fuel production are uncompetitively high, given that the chemical conversion of CO2 and pure hydrogen production are energy-intensive. Another disadvantage of CO2-based fuels is their short life span, which means that CO2 is reemitted very quickly into the atmosphere.
Building materials. Besides the options where CO2 can react with minerals to form carbonates for building materials, it can also be used in concrete production, as a part of cement, aggregate (sand, gravel or crushed stone), or instead of water for concrete curing. Nevertheless, the huge costs per ton of used CO2 are still not encouraging [3].
3.2. Direct Use of CO2
Significant direct use of CO2 refers to its underground injection, which may refer to (1) enhanced oil recovery (EOR), (2) enhanced coalbed methane recovery (ECBM), (3) enhanced gas recovery (EGR), (4) enhanced shale gas recovery (ESG), an enhanced geothermal system (EGS) and (6) a supercritical CO2 power cycle [21]. Injected CO2 serves as a solvent for residual oil production (EOR). In EGR projects, it pushes natural gas to the production wells, while in ECBM projects, desorption/adsorption processes are crucial for the displacement of methane with CO2 in the coalbed (Figure 1) [22,23]. When injected into reservoirs, CO2 participates in enhancing hydrocarbon recovery through different mechanisms, such as maintaining pressure, multi-contact miscible displacement, molecular diffusion, or the desorption of methane. The injection of CO2 in a supercritical state into the reservoirs decreases oil viscosity and improves its flow rate (a miscible CO2 process) or simply pushes the remaining oil (an immiscible CO2 process) [24,25].
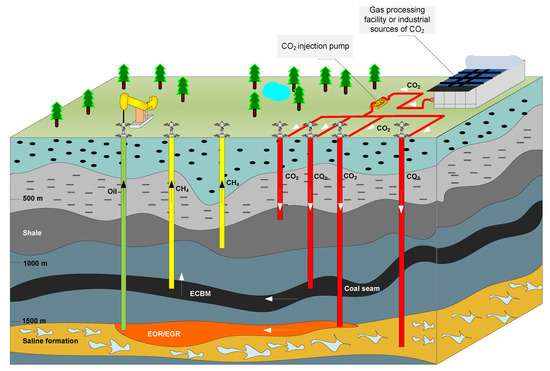
Figure 1.
Carbon capture, utilization and storage (CCUS) technology-underground injection of CO2 [26].
Conventional, water-based EGS requires huge water quantities for maintaining the reservoir’s pressure (870.64–15,898.68 L/MWh). Furthermore, about 10–20% of water is lost during EGS stimulation and operation, posing an issue, especially in water scarcity areas [27,28]. Since 2000, the use of SCCO2 as an alternative working fluid was proposed [29], numerous studies on the feasibility and extraction efficiency of the CO2-based systems have been conducted [27,30,31].
CO2 is non-toxic and noncombustible, and therefore convenient for use as a working fluid in enhanced geothermal systems (EGS). This geothermal energy concept uses supercritical CO2 instead of water to produce heat from deep, dry and impermeable rocks. Such a system is composed of a binary-cycle power plant, which operates based on heat exchange from the hot supercritical fluid (CO2) to a secondary working fluid used in a vapor cycle. There are many advantages of using CO2 as a working fluid in geothermal systems instead of water. Besides the benefits of water savings and CO2 sequestration, CO2 is less prone to dissolve minerals and other substances, having a positive impact on scaling and corrosion reduction. The high density of the supercritical CO2 allows for a reduction of most of the system’s components, resulting in a decrease of the environmental footprint and capital costs [32,33].
Power generation by the supercritical Brayton cycle (S-CO2 BC) is a promising alternative to the steam turbine process. Up to now, research on the integration of different heat sources (including fossil fuels, nuclear, waste heat, and renewables) has been performed, and there are some small-scale pilot units in operation. Although there have not been any commercial S-CO2 power plant installed up to now, low operational and maintenance costs, a small physical footprint, and higher thermal-to-electric energy conversion efficiency (density of a supercritical fluid is close to that of a liquid, and therefore allows for less pumping power) are listed as the main technological advantages [34,35].
There is a significant geothermal potential of hot dry rocks (HDR). Theoretical calculations showed that a 20 °C reduction of 1 km3 of HDR provides enough energy to operate a 10 MWe electric generator over a 20 year period, which is equivalent to 1.3 Mt of oil [27]. Although supercritical CO2 technology does not have a commercial application thus far, strong climate-energy goals and a strong commitment to renewables can be a trigger for its development and application. Cost–benefit analysis based on its CO2 storage capacity, power production and the costs of the heat extraction and energy conversion systems could give a solid base for further development phases.
Another use of CO2 is in preserving fruits, vegetables, meats, food grains, as well as inactivating microorganisms and extracting oils, flavors, colors, chemicals, etc. [36].
4. Enhanced Oil Recovery by Injecting CO2
The first step of CCS is to extract CO2 from other gaseous substances. CO2 can be captured from natural gas (if it comes from a CO2 rich oil or natural gas reservoir) using absorption, adsorption, chemical looping, or membrane gas separation, or it can be captured from flue gases at large CO2 point sources (power plants and industrial processes) by one of the following three methods: pre-combustion capture, post-combustion capture and oxyfuel combustion. Once captured, CO2 is dehydrated and prepared for transport. It is transported by pipelines, trucks, or ships in a supercritical state to the storage site. Currently, globally operating CCS projects and CCS projects under construction stand for around 40 × 106 tCO2/y [37].
Today’s main applications of CO2 usage refer to CCS and CO2-EOR projects [38]. Even though the two process types consist of the same phases (CO2 capture, transport, and injection; see Figure 2), they differentiate by injection purpose, storage preservation, injection depth and rate, injection–formation type, injection well completion and monitoring.
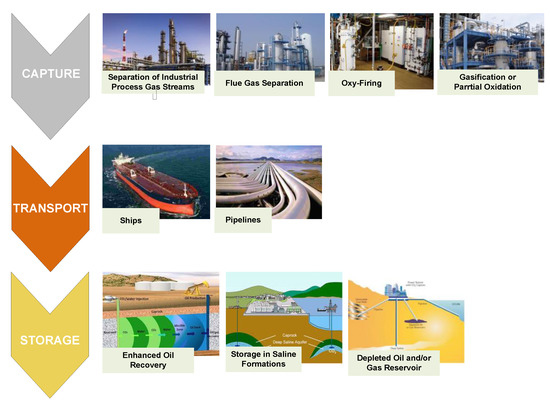
Figure 2.
Carbon capture and storage (CCS) value chain. Modified according to [39].
Permanent storage of the injected CO2 is provided by a carefully selected geological formation, which must meet certain criteria, among which the significant injection capacity and the presence of impermeable cap rock and bedrock (natural trap) are of the utmost importance [40,41,42,43,44,45,46].
In Croatia, the first application of CO2-EOR was started in October 2014 by the oil company INA–Oil Industry Ltd. The aim of the project was the enhancement of hydrocarbon production by alternating the injection of carbon dioxide and water (WAG) into the mature oil fields Žutica and Ivanić. During the estimated 25-year project’s lifetime, about 5 × 109 m3 of CO2 will be injected into the reservoirs of the mentioned fields, which will result in additional hydrocarbon production (3.4 × 106 t of oil and 599 × 106 m3 of natural gas). Due to geological and physical conditions, about 50% of the injected CO2 will remain permanently trapped in the reservoirs, while the rest will be produced along with associated gas [2]. CO2 injection into the Ivanić field during the period 2014–2019 has resulted in a total hydrocarbon production of 1,579,429 barrels of oil equivalent (boe), which represents a 35% recovery increase. The injection of CO2 into the Žutica field started in 2015. It has increased daily production by more than 7.5 times, resulting in a total hydrocarbon production of 390,136 boe. According to estimations, 77% of the production can be attributed to the EOR project. For EOR purposes on both fields, 1 billion m3 (1.98 Mt) of CO2 was injected within the five-year period. Permanently stored emission quantities are equivalent to 25% of annual emissions of road motor vehicles in Croatia [47,48,49].
An additional advantage of CO2-EOR projects is the fact that, once the project is completed, the site can be used for further injection for the purpose of permanent CO2 sequestration, without additional investment. A calculation of the CO2 volume that can be stored in the two selected reservoirs of the Ivanić field in Croatia was made within the MBAL (Material Balance) program module of the IPM (Integrated Production Modeling) petroleum engineering software package [50]. Such a model considered the injection of CO2 after the termination of the EOR project (predicted EOR project closure pressure is 138.5 bar) up to the level of the initial reservoir pressure (184 bar). The obtained capacity was at the level of 1.95 × 109 m3 (3.9 Mt) of CO2 (Figure 3). Although the estimated capacity is not big compared to the large world CCS demonstration projects capacities, considering the national emissions of the Republic of Croatia (as per the national Report on the projection of greenhouse gas emissions, CO2eq emissions in 2020 are 23.42 Mt), the obtained storage capacity is not negligible [51].
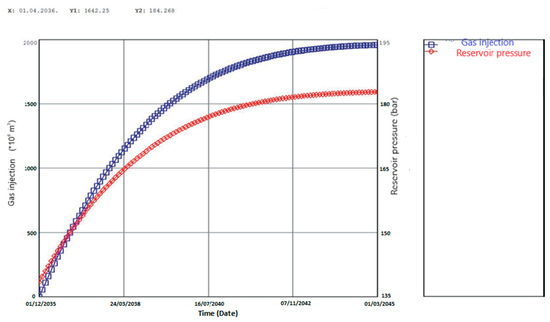
Figure 3.
MBAL (Material Balance) calculation of CO2 that can be injected into the selected reservoirs of Ivanić field after the termination of the carbon dioxide enhanced oil recovery (CO2-EOR) project [50,51].
An Overview of CO2-EOR Projects in the World
CO2-EOR has been applied successfully for almost fifty years, and nowadays, it is the most used EOR method. EOR-projects (CO2-EOR and other EOR methods) worldwide in the period 1971–2017 are shown in Figure 4.
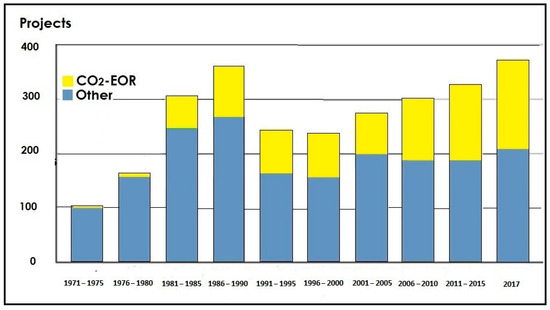
Figure 4.
EOR projects worldwide [52,53].
In 2020, more than 375 EOR projects were in operation (Figure 5), accounting for about 2% of the global oil production (more than 2 × 106 bbl/d). There are also good forecasts for the mentioned technology for the future since it is expected that by 2040, this share could double [23]. Although EOR application commenced in North America, recently, EOR technologies are being applied worldwide: in Malaysia, the United Arab Emirates, Kuwait, Saudi Arabia, India, Colombia, Ecuador, etc. While in 2013, almost 70% of the EOR projects were conducted in North America, today, this proportion has decreased to about 40% [53].
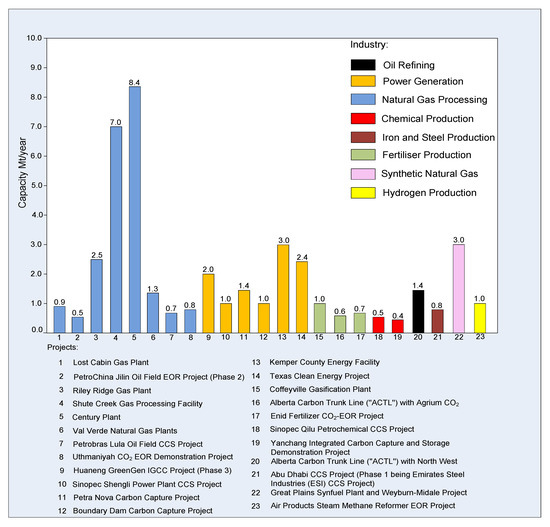
Figure 5.
Large CO2-EOR projects in different project stages by applied industries (according to [14]).
CO2-EOR and residual oil zone studies are under preparation by the US Geological Survey (USGS). Their purpose is the assessment of the national potential of hydrocarbons recovery after CO2 injection into conventional oil reservoirs in the USA [54].
Large-Scale CO2-EOR Case Studies
As per the KAPSARC data source, in 2018, there were 23 CO2-EOR large-scale projects in different project stages, having a CO2 capture capacity of approximately 42 Mt (Table 2 [14]). As per the Global CCS Institute, a large-scale project is defined as a project with a capture capacity of at least 0.8 Mt/y of CO2 for a coal-based power plant and 0.4 Mt/y for other industrial facilities [37].

Table 2.
CO2-EOR projects, status overview 2018. Modified according to [14].
Figure 5 shows project distribution according to related industries and capacity. Progress in project application can be tracked since the early 1970s (Figure 6).
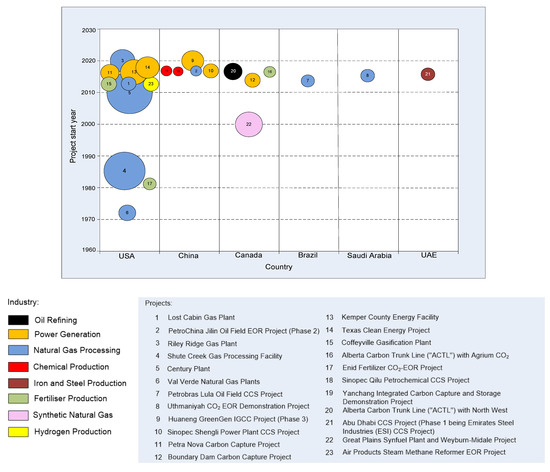
A great majority of the ongoing projects (81%) in 2018 were in the USA and Canada, mostly using CO2 from natural gas processing (Table 2; Figure 5 and Figure 6). Regarding CO2-EOR projects related to power generation, besides one operational project (Boundary Dam, Canada), there were two projects in the execution phase (Petra Nova and Kemper County, USA).
The USA is a good example of the positive effects of policy incentives on EOR projects. In the 1980s, a decrease in US domestic oil production led to the passing of the Windfall Profit Tax, which triggered the application of EOR by a significant reduction of its tax burden. Today, the US 45Q tax credit has been amended to provide a tax reduction of 35$/t of stored CO2 by EOR activities. The International Energy Agency (IEA) New Policies Scenario predicts a greater number of oil fields to become mature and therefore inclined to new EOR developments. According to the same scenario, the total EOR production will grow up to more than 4.5 × 106 bbl/d, accounting for approximately 4% of global oil production in 2040 [52].
The application of CCS technologies to a new conventional power plant can reduce CO2 emissions by up to 90%. However, the high costs of capturing and compressing CO2 is reflected in an energy price increase of 21–91%, especially in the case of distant transportation [42,55]. Although CCS was initially considered to be an acceptable solution for coal-based power facilities, low natural gas prices and renewable energy development are replacing coal-based power production in developed regions, which resulted in fewer CCUS application cases.
The CO2 -EOR case studies shown in Table 2 (in total 23) are briefly described below.
Val Verde Natural Gas Plants, TX, USA. Before the construction of the Pensoi pipeline in the mid-1970s, four natural gas processing plants in the Val Verde area was venting over 2 Mt/y of CO2. However, in 1996, this pipeline was converted into a natural gas transportation pipeline, again causing venting of significant quantities of CO2. Therefore, it was decided to redirect emitted CO2 by a new pipeline to the EOR projects in West Texas, located at a distance of several hundred kilometers. Nowadays, five separate gas-processing facilities in the Val Verde area are capturing around 1.3 Mt/y of CO2 for use in EOR operations at the Sharon Ridge oilfield. The CO2 content of the inlet gas stream at the Val Verde plant is in the range of 25 to 50% [56].
Enid Fertilizer CO2-EOR Project, OK, USA. This is one of the largest fertilizer production plants in North America, producing ammonia, liquid fertilizer and urea. The original plant was built in 1974, which was further upgraded in the 2000′s. Since 1982, about 0.7 Mt/y of CO2 has been transported by a 225 km long pipeline and injected into depleted oil fields in southern Oklahoma for the purpose of EOR [56].
Shute Creek Gas Processing facility Wyoming, USA. It has been processing natural gas from the LaBarge field since 1986. The raw gas is of the lowest hydrocarbon content commercially produced in the world (about 20%), containing CO2 in high concentration (65%). In order to separate sour gases, the Shute Creek Gas Processing facility was built. Before upgrading, H2S along with approximately 0.4 Mt/y of CO2 were disposed of. An expansion in plant capacity was completed in 2010, reaching a capturing capacity of 7 Mt/y of CO2. The separated CO2 is transported from the Shute Creek facility via the ExxonMobil, Chevron and Anadarko Petroleum pipeline systems to oil fields in Wyoming and Colorado for use in EOR activities. The pipeline distance from Shute Creek to the larger volume customers of Salt Creek and Rangely is approximately 460 km and 285 km, respectively [56].
Great Plains Synfuel Plant and Weyburn-Midale Project, Saskatchewan, Canada. The Great Plains Synfuel Plant, North Dakota, began with operation in 1984, representing the only commercial-scale coal gasification plant in the USA that produces synthetic natural gas. Since the waste stream contains a high content of CO2 (95%), no further processing is required. The CO2 is transported by a 329 km pipeline to the Weyburn and Midale oil fields Saskatchewan, Canada. About 2.4 Mt/y of CO2 is injected into the Weyburn field, and approximately 0.6 Mt/y of CO2 is injected into the Midale field. The main injection target zones are at a depth of about 1500 m. The Weyburn-Midale CO2 Monitoring and Storage Project was conducted in the period 2000–2011, supported by the International Energy Agency (IEA) Greenhouse Gas Research and Development Program, with a focus on monitoring behavior of the injected CO2 and permanent storage [56].
Century Plant, TX, USA. It is used for processing high CO2-content (more than 60%) gas streams produced from different reservoirs in West Texas. It began operating in 2010 with a smaller level of capturing capacity, but in 2012, the capacity extended to its full level of 8.4 Mt/y. After being compressed, the CO2 is transported to an industrial hub located in Denver City by a 160 km long pipeline and is finally injected into the Permian Basin for EOR activities. The Permian Basin of West Texas and southeast New Mexico is one of the largest and most active oil basins in the USA [56].
Air Products Steam Methane Reformer EOR Project, TX, USA. It has been operating since 2012. It captures approximately 1 Mt/y of CO2 at two steam methane reformers at the Port Arthur energy refinery. Captured CO2 is transported by 158 km of pipelines to an oil field for EOR. After 21 km, the pipeline is connected to a much larger diameter Green Pipeline, used for the collection and transportation of CO2 from different sources [56,57,58].
The Petrobras Santos Basin CO2-EOR project, Brazil. It is located offshore, approximately 300 km from the coast. EOR is applied to the Petrobras Lula oilfield, which is one of the largest oil fields in Brazil, positioned in the pre-salt carbonate reservoir, just below a thick, 2000 m salt column. After pilot injection of produced reservoir gas into the oil field, large-scale production began in 2013. Membrane processing units installed on-board of the floating production facility are used for the separation of the CO2 from the produced natural gas. While natural gas is transported to an onshore facility by pipeline, the CO2 is compressed and reinjected into the hydrocarbon’s producing reservoir. The produced oil is transported to shore by tankers. The project is known for the deepest CO2 injection well in operation. Since 2017, CO2 reinjection has been carried out by ten floating production storage and offloading (FPSO) units: seven at Lula Field, two at Sapinhoá Field and one at Lapa Field [59,60,61].
Coffeyville Gasification Plant, KS, USA. The project is an example of CCS applied to the fertilizer industry, which has been operational since 2013. The process of nitrogen fertilizer production involves petroleum coke gasification and synthetic natural gas creation. Although CO2 generated in the process is used for fertilizer manufacturing, a significant part of the CO2 is vented. Up to 1 Mt/y of the CO2 is captured by a carbon capture unit and is then delivered to the North Burbank Oil Unit in Oklahoma for EOR purposes [56].
The Lost Cabin Gas Plant CCS project, WY, USA. The project operates with a pre-combustion capture of 0.9 Mt/y of CO2. The feed gas, which has been purified at the Lost Cabin Gas Plant since 1996, contains around 20% of CO2. In 2013, the plant was connected with the EOR injection site at the Bell Creek oil field in MT, USA, by the 374 km long Greencore pipeline [56].
Boundary Dam 3, Saskatchewan, Canada. This project represents the world’s very first full-chain CCS applied on a coal-fired power plant. While producing 110 MW of electricity, it simultaneously enhances oil recovery and significantly reduces CO2 emissions by capturing and injecting up to 1 Mt/year of CO2 into 1.4 km deep Weyburn oilfield reservoirs and into a 3.4 km deep saline reservoir (Deadwood formation). Captured SO2 is used as feedstock for a sulfuric acid plant [58].
Uthmaniyah Carbon Dioxide Enhanced Oil Recovery (CO2-EOR) Demonstration Project, Saudi Arabia. The project captures approximately 0.8 Mt/y of CO2. The captured CO2 is compressed and transported via an 85 km pipeline to the injection site in Uthmaniyah field, which is a part of the giant Ghawar field (the largest oil field in the world). Besides the determination of additional oil recovery and sequestered CO2 quantities, the project goals are related to risk analyses and the identification of operational concerns. A comprehensive monitoring and surveillance plan, including advanced routine logging and use of new technologies for plume tracking and CO2 saturation modeling, follow the CO2-EOR operations [60].
Kemper County Energy Facility, MI, USA. It was a lignite based integrated gasification combined-cycle (IGCC) facility designed to convert locally mined lignite to synthesis gas. It was planned to be the first commercial application of air-blown transport integrated gasification (TRIG) technology. The peak capacity of 582 MW would occur when using both syngases in the combustion turbine and natural gas firing in the heat recovery steam generator duct burners. After carbon capture and removal of impurities, purified syngas would be used as fuel for combined–cycle power generating units. The project was expected to capture approximately 3 Mt/y of CO2. One part of it would be sent by a 98 km CO2 pipeline and used for EOR at Heidelberg oil field, replacing temporary EOR solution, which uses CO2 from natural CO2 reservoir. The rest of captured CO2 would be sent by an 87 km pipeline to another oil field in the vicinity of West King, Mississippi. The startup was originally projected for 2014, but due to a number of technical issues and huge costs, it was decided to operate using natural gas, without carbon capture and storage technology [56,62].
Petra Nova Carbon Capture facility, Huston, TX, USA. It was installed at the W. A. Parish power coal plant, and started with operations in 2017, as the world’s largest post-combustion CO2 capturing system, intended to capture 1.4 Mt/y of CO2. Captured CO2 was planned to be transported via a 130 km pipeline and injected into the West Ranch oil field. Nevertheless, very shortly after a successful start, a drop in oil prices in the first half of 2020 caused CO2 capture at Petra Nova to become uneconomical. Further project operation may be under the federal 45Q tax credit incentive, ensuring companies $35 per metric ton (1.102311 t) of geologically stored CO2 within the EOR and up to $50 for its storage in a saline formation [63,64].
Abu Dhabi CCS, Mussafah, United Arab Emirates. It is the first fully commercial large-scale CCS facility applied in the iron and steel industry, the Emirates Steel Industries factory. About 0.8 Mt/y of CO2, which is produced as a byproduct of the direct reduced ironmaking process, is captured and transported via pipeline to oil reservoirs for EOR purposes. Abu Dhabi National Oil Company is developing its second CCUS facility in the United Arab Emirates, which would capture 1.9 to 2.3 Mt/y of CO2 from its gas processing plant that will be used for EOR purposes in the same reservoir [56,60].
The Alberta Carbon Trunk Line (ACTL) CCUS, Alberta, Canada. The system started with full operation in 2020. It captures about 1.3 Mt/y of CO2 from a bitumen refinery using gasification technology (North West Redwater Partnership Sturgeon Refinery), while an additional 0.3–0.6 Mt/y of CO2 is gained from the Agrium’s Redwater fertilizer plant. Captured CO2 serves for the production of 1 billion bbl of oil from the Clive oil reservoir. The ACTL (16” trunkline), constructed in the length of 240 km with a huge capacity of 14.6 Mt/y of CO2, is the largest capacity pipeline for the transportation of anthropogenic CO₂. It will enable the connection of different emission sources, including coal-fired power plants, upgrading/refining operations, petrochemicals, and a natural gas processing plant [56,60].
Under defining and evaluation phases, there are eight large-scale CO2-EOR projects. Most of them (63%) are planned in China despite the fact that tight continental geology and heavier oil pose significant issues [65]. With regard to the CO2 source industry, there are an equal share of power generation and chemical production facilities (40%). While emerging Asian economies are still leaning on coal-based energy, and carbon capture and storage is seen as an effective emission reduction solution, a comprehensive framework and policy support are still missing [14,65].
Sinopec Shengli Power Plant, Dongying, Shangdong province, China. The project considers a conventional amine-based CO2 capture facility installed at the 25 MW Unit 1 of the coal-fired power plant. The implemented post-combustion capture process enables CO2 delivery to the Shengli oil field for EOR purposes. In the project’s final stage, about 1 Mt/y of CO2 will be captured, transported, and injected to increase oil recovery by up to 15%. Shengli oil field is the second-largest oil field in China, producing around 200 million bbl/y of oil. More favorable economic conditions, i.e., higher oil prices, are required for startups [53].
Sinopec Qilu Petrochemical Project, Shangdong Province, China. The project considers CCS applied at the Shengli coal-fired power plant. It captures up to 0.5 Mt/y of CO2, which is transported via gas pipeline to Shengli oil field for EOR [66].
Yanchang Integrated CCS Demonstration Facility, China. It is designed to capture CO2 from a coal gasification unit. The 0.05 Mt/y of CO2 unit has been in operation since 2012, while the larger 0.36 Mt/y of CO2 capture is under construction. Captured CO2 would be used for EOR in oil fields in the Ordos Basin. Since they are facing a severe water shortage, CO2-EOR would be a great solution for Yanchang Oilfield [67,68].
PetroChina Jilin Oil Field EOR Project (Phase 2), Jilin Province, China. CO2-EOR operations have been performed on the Jilin oil field since 2006. The CO2 source is a natural gas processing facility that processes natural gas from the Changchun gas field that contains approximately 2.5% CO2. Phase 2 refers to the extension to a larger scale CO2-EOR, which would sequester 0.8–1 Mt/y of CO2 and increase oil production by 500,000 t/y [11].
Texas Clean Energy Project, TX, USA. It was a proposed 400 MW coal-fired power plant with installed capturing technology of 2.4 Mt/y. The project would combine Integrated gasification combined cycle (IGCC) technologies, carbon monoxide (CO) shift, and Linde Rectisol® wash unit (RWU) acid gas removal (AGR). A part of the captured CO2 would be used for EOR activities in the West Texas Permian Basin, while another part of the captured CO2, as well as a portion of the high-H2 syngas, would be used for urea fertilizer production. Captured sulfur-containing gases would be converted to marketable sulfuric acid [69]. Although an environmental impact statement was issued in 2011, an operation was planned by 2018; the bankruptcy of the company’s sponsor, Summit Power, occurred in 2017 after the US Department of Energy (DOE) decided to give up financial support due to improper spending of money.
Riley Ridge Sweetening Plant, WY, USA. A pre-combustion CO2 capture plant was designed to capture 2.5 Mt/y of CO2. Sour gas, produced from the Madison Formation, which contains CO2, N2, CH4, He, and H2S, is originally processed at the Riley Ridge Treatment plant, which has been in operation since 2013. Separated CH4 and He are sold, while non-gaseous H2S/CO2 mixture is planned to be transported via 16” pipeline to the Riley Ridge Sweetening Plant. After separation, H2S would be reinjected into deep injection wells, while the CO2 would be transported via a 24” CO2 pipeline (the Greencore Pipeline), planned to connect another source of CO2, which would be provided from the existing Shute Creek Gas Plant. The CO2 destination is Bell Creek Field and other oilfields in SE Montana, where it will be used for EOR purposes. Produced CO2, separated from the oil at the surface, would be reinjected into the oil recovery process, which means that after field decommissions, it would remain permanently geologically stored [70].
Huaneng GreenGen IGCC project, Tianjin, China. The project has been developing through three phases. The first two project phases refer to the construction of a 250 MW integrated gasification combined cycle (IGCC) facility (completed in 2012) and the construction of a pilot facility that produces electricity from hydrogen with a small size capturing of 0.2 Mt/y of CO2. The third phase refers to the construction of a 400 MW IGCC power plant with an installed unit of 2 Mt/y of CO2 capture capacity. The captured CO2 fate is still unknown, but one of the solutions considers EOR application at the Tianjin Dagang oil field [65].
5. CO2-EOR Site Emissions
Besides the fact that CO2 is the most abundant greenhouse gas, causing increased global warming and consequently climate changes, which result in a wide spectrum of consequences, CO2 is also directly adverse to human health and nature in high concentrations. Humans are immediately endangered if CO2 concentration in the air rises above 7–10%, while plants, insects and soil organisms show a higher tolerance [10]. Furthermore, a higher concentration of CO2 in a marine environment leads to seawater pH reduction, while partial pressure enhancement is responsible for physical stress [71]. Due to the previously mentioned facts, it is of high importance to manage CO2 in a closed system to prevent any leakage to the atmosphere during all phases of the CCS process. In order to achieve that, for leakage risk mitigation, special technical and non-technical measures during the processes of CO2 capture, transportation and injection into geological formations must be applied.
Although in the CO2-EOR production process, CO2 is the predominant hazardous compound, other harmful substances may also appear. Besides hazardous byproducts characteristic for hydrocarbon production (e.g., produced water contains traces of light hydrocarbons: BTEX, naphthalene, PAHs, alkylphenols, etc.), other CO2 related hazards (e.g., capture chemicals, or formation of strong acids from traces NOx and SOx gases) are possible. Trace elements (As, Cd, Hg, Pb, etc.) can appear as products from CO2-water-reservoir interactions, or some radioactive elements (e.g., Ra), which are prone to be incorporated in scaling minerals, can appear [71].
Different emission sources that could occur across CCS and/or CO2-EOR project activities are shown in Figure 7.
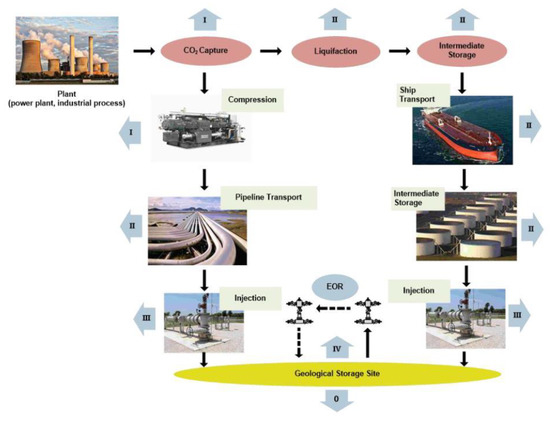
Figure 7.
Potential greenhouse gas emissions sources and types of emissions in CCS and/or CO2-EOR value chain. Modified according to [39].
CO2-EOR project emissions (combustion, vented and fugitive emission) can be observed as direct and indirect emissions. Engines, heaters, and flares (combustion emissions), venting points (intentional gas release from non-combustion sources) and joints (fugitive emissions) on processing vessels, tanks, pipelines, and other equipment refer to the direct CO2 emissions sources. Indirect emissions from the petroleum industry come from different powering equipment and devices producing power outside the petroleum industry (e.g., CO2, CO, N2O, CH4 are emitted at power plants during electricity generation), and therefore are also present in all phases of the EOR process (CO2 stream compression, water-alternating-gas (WAG) injection, reservoir fluid production, fluid processing, etc.). The intensity of indirect emissions is proportional to energy consumption and depends on the energy source. However, in the case of on-site power production (usually in the case of offshore production or on-site power production from an associated gas produced during oil production), emissions related to energy production are considered as a part of direct emissions. Fugitive emissions refer to unintentional gas releases (CH4, volatile organic compounds (VOCs), CO2, N2O) from pressurized equipment at a connection point (e.g., valves, flanges, pipe connections, mechanical seals, etc.) due to imperfect hermetical tightness. Even though compared to the combustion and vented emissions, fugitive emissions are relatively small (up to 5% of total emissions in upstream activities [72]), sealing device degradation, equipment failures and operating conditions (pressure, temperature, etc.) may lead to its increase. A simplified overview of the emissions occurring during the EOR process is given in Table 3 and Figure 8.

Table 3.
Simplified overview of CCS/CO2-EOR projects emissions. Modified according to [39].
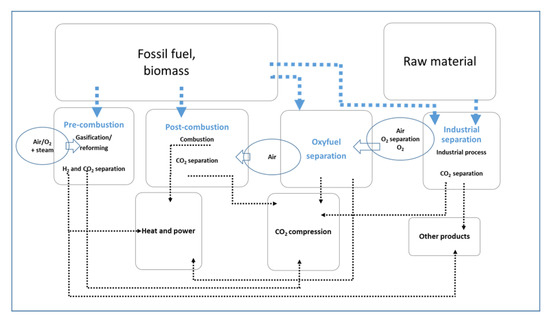
Figure 8.
CO2 capture processes. Modified according to [39,53].
Dilmore [73] assessed atmospheric emissions occurring during site evaluation and characterization, construction, closure, and post-closure monitoring to be less than 1% of total emissions resulting from CO2-EOR activity, which means that the remaining 99% is assigned to project operation.
CO2 capture processes (Figure 8) are, compared to other CCS/CO2-EOR phases, most energy-intensive (use of fossil fuels or electricity) and therefore most emission-intensive. In that phase of the CO2-EOR project, all types of petroleum industry emissions occur (combustion, vented and fugitive emissions). Captured gas processing activities (impurity extraction, dehydration, compression, etc.) are also sources of combustion, vented and fugitive emissions.
In this phase, different types and different concentrations of impurities may appear in the CO2 stream, depending on the CO2 emission source (gas or coal-based power plant, cement plant, natural gas processing plant, ammonia plant, etc.), and on the type of capture process. If the captured gas is not processed at the capture site (CO2 source site), the CO2 capturing process results in reduced emissions at the CO2 source site, but in that case, the environmental impact of the CO2-EOR project on the atmosphere is moved to the injection/storage site. While a post-combustion scrubbing process results in a low level of impurities, the pre-combustion process results in a CO2 stream with 1–2% impurities in the form of H2, CO, traces of H2S, and other sulfur compounds. An oxyfuel process CO2 stream contains O2, N2, Ar, SOx and NOx, which requires an impurity reduction in the subsequential cryogenic purification process [42].
Even though there are some other transport options (ship or truck transport), CO2 is usually transported by pipelines. Dominant emissions during the transport of pressurized CO2 are fugitive emissions. Fugitive emissions from CO2 pipeline transport depend on the CO2 stream composition as well as the type, number and size of the equipment installed in the pipeline systems. Compressors, as the most important part of a CO2 transmission system, are the main sources of combustion emissions during CO2 transport. To ensure the supercritical state of CO2, the high injection pressure is needed, which makes CO2 compression/pumping to be highly energy-intensive, and therefore the most emission-intensive process. If the compressors are powered by an electric drive, the compression process only results in indirect CO2 emission (generated at a power plant). Direct CO2 emissions may occur in the case of gas-engine-driven compressors. However, emission quantities are determined by the pressure of delivered CO2 and the required injection pressure (depends on the reservoir pressure). Pipelines or tanks (in case of transport by ships, trucks, or intermediate storage) are also potential sources of vented emissions (maintenance, emergency releases, etc.).
Emissions associated with CO2 injection activities include all types of emissions (combustion, vented and fugitive) from surface equipment as the emission sources. A common surface CO2-EOR operation site is comprised of an injection system (distribution manifold at the end of the transport pipeline, distribution pipelines to wells, additional compression facilities, measurement and control systems, and the injection wells), produced fluid (gas and liquid) separation and processing units, brine management units, CO2 recompression or recycle gas unit and optionally artificial lift equipment (Figure 9).
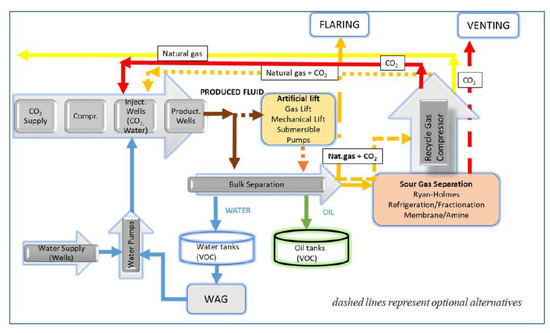
Figure 9.
CO2-EOR site operation-elements emission sources. Modified according to [75].
Direct emissions at the injection site are related to CO2 and CH4 and, to a lesser extent, to nonmethane volatile organic compounds (NMVOCs), N2O, CO, SO2 and NOx releases. VOC are released due to pressure and/or temperature changes in the tank (surface storage of the produced oil prior to transport to the refinery) caused by surface conditions and loading and unloading activities. Released from oil into the space between the tank content level and the tank roof, volatiles are usually vented directly from the tank into the atmosphere [72].
When a reservoir’s natural drive is not sufficient for bringing the fluid up to the surface, a method of artificial lift must be applied to increase the oil production rate. Artificial lift methods (selection primarily depending on the well depth, hydrocarbon composition and production rate) vary in energy consumption; thus, the indirect emissions vary through this phase of the project. The lowest energy consuming method, if any, is applied when a low viscosity fluid, e.g., oil with dissolved CO2, is produced [74].
During CO2-EOR, a certain amount of the injected CO2 is produced along with oil. The injected CO2, produced along with associated gas, is recaptured and reinjected into the geological formation. The gathering of EOR produced oil is carried out by pipelines. At this stage, potential emissions are related to fugitive emissions from the equipment, gas-operated pneumatic devices and valves.
The first step in the produced hydrocarbon processing is the separation of the produced fluids (oil, gas (in the case of CO2-EOR natural gas and CO2) and water). A bulk separator is a three-phase vessel. Oil and gas separation commonly results in vented CH4 emissions (separators and tanks), as well as CH4 fugitive emission. In the case of CO2-EOR, since CO2 is a part of the gaseous phase of the production fluid, the vented and fugitive emissions, except CH4, also contain a significant portion of CO2. If the heat from fossil fuel combustion is used during the separation process, additional emissions of CO2 and N2O are present [72]. Natural gas processing is done in order to comply with the gas quality specifications. It can be achieved by chemical adsorption (an amine process), by cryogenic fractionation, or by adsorption using molecular sieves [65]. If the associated gas is produced at a high enough pressure, and if there is adequate infrastructure for its gathering and processing, it can be used on-site as a powering fuel. In some cases, the high cost of CO2 separation from the produced associated gas justifies reinjection of the entire reproduced gas stream [76,77,78]. Nowadays, with high environmental standards and the knowledge of the significant global warming potential (GWP) of CH4, the release of the associated gas into the atmosphere is not considered to be a viable solution, so flaring and venting are considered solely as safety actions, used for emergency shutdown, injectivity issues or maintenance activities.
As mentioned before, fugitive emissions are related to all process units operating at the CO2-EOR injection site. Although they are still not well investigated, it is assumed that the amount up to a 2% loss of purchased CO2 [73].
CO2 can escape from a geological formation if its pressure, when injected into the formation, exceeds formation capillary and fracture pressure, resulting in its passing through the caprock. Other leakage risks refer to CO2 escape through poorly plugged abandoned wells, faults, or fractures. The low leakage probability of a geological storage site is assured by the selection of the injection formation that meets all the needed prerequisites, such as impermeable cap and bedrocks, geological stability, the absence of leakage paths and effective trapping mechanisms [79,80].
6. CO2-EOR Lifecycle Emissions
It is well-known that during EOR processes, a certain amount of CO2 is permanently retained and stored in the injection formation, but up to which level can a real emission reduction during the CO2-EOR process be achieved? First, to achieve emission reductions, CO2 anthropogenic sources are required. When CO2 comes from its natural underground reservoirs, a net negative emission is impossible to achieve. Even though in CO2-EOR projects, CO2 is injected with the function of a solvent and a portion of it is produced with a mixture of brine and hydrocarbons, ultimately about 60% of the injected CO2 remains trapped in the oil reservoir pore space, while around 40% of it is reproduced with the reservoir fluids (hydrocarbons and brine). However, as per Melzer [81], up to 95% of the EOR injected CO2 can be permanently retained within the reservoir if the closed-loop injection system is applied, which implies produced fluid separation and reinjection of the reproduced CO2.
According to International Energy Agency (IEA), EOR activities in the USA use 0.3–0.6 t CO2/bbl of produced oil [82]. However, the primary goal of the EOR project is additional oil production that generates additional emissions, but besides the already mentioned emissions resulting from production processes, there is a whole range of production-related activities that generate emissions, as well as the emissions generated by the final product usage (fossil fuel burning). Therefore, the environmental performance of CO2-EOR projects must be assessed, which is possible by the lifecycle assessment technique.
Lifecycle analyses for the CO2-EOR technological systems appear in different literature [83,84,85,86,87,88,89], where the benefit of stored CO2 is put against the environmental impact of the required additional process. All the studies concur on the substantial benefits of CO2 emission reduction.
Azzolina et al. [90] made a lifecycle analysis of incremental oil produced by CO2-EOR by developing an integrated model with a coal-based power plant and quantitative analysis of GHG emissions in comprehensive system boundaries. The system boundaries were the same as those used by Cooney et al. [91].
Cooney et al. differed emissions by upstream (coal mining, processing and transport; coal-burning with CO2 capture, and CO2 transport), “gate-to-gate” (emissions associated with site operation), and downstream (crude oil transport, refining, fuel transport, fuel combustion). As a result of the analysis, the incremental oil was lower-carbon fuel that resulted in a lower emission factor. Coal mining, processing and transport have an average emission factor of 60.7 kg CO2 eq/MWh. The emission factor of a coal-based power plant with 85% CO2 capture is about 146 kg CO2 eq/MWh, while the emission factor for CO2 pipeline transport from the capture unit to the CO2 injection site amounts to 9.93 × 104 kg emitted/kg of CO2 transport. Downstream emissions are at the level of 485 kg CO2 eq/bbl [91].
As per the Clean Air Task Force (CATF) [92] (Figure 10), lifecycle emissions for conventional oil production (well-to-wheel boundaries) are 0.51 t CO2/bbl. Average use of 0.3 t CO2/bbl in EOR would decrease conventional lifecycle emissions to 0.21 t CO2/bbl. However, given that EOR site operations increase process emissions by 0.03 t CO2/bbl, that the additional emissions from incremental oil consumption amount to 0.04 t CO2/bbl, and the emissions from conventional oil production not displaced by EOR also amount to 0.04 t CO2/bbl, it can be concluded that every CO2-EOR produced barrel emits 0.32 t of CO2. Compared to conventional oil production, CO2 emissions are decreased by about 37% [92].
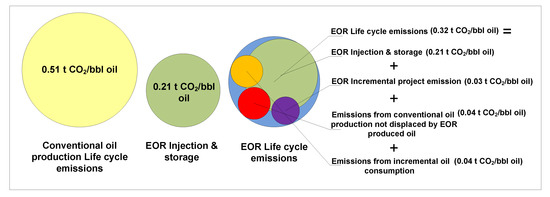
Figure 10.
Net CO2 emission reduction from a barrel of oil produced through CO2-EOR. Modified according to [92].
Thorne et al. [93] developed a conceptual EOR system with an oxyfuel power plant in Poland, as the CO2 source, and an oil reservoir on the Norwegian continental shelf, as the EOR operational site. The model used ship transport of CO2 based on two ships transporting CO2 over a distance of 1253.3 km (677 nautical miles) and resulted in 71% emission reduction compared to a non-CCUS system (a case when oil and electricity are conventionally produced).
Shminchak et al. [94] documented a GHG lifecycle analysis of the CO2-EOR site in the Northern Michigan Basin, USA. In the period 1996–2017, about 2.29 × 106 bbl of oil was produced by cycling a total of 2.09 × 106 t of CO2 into 1500–2000 m deep reservoirs. The lifecycle analysis, based on site-specific operational records, included the processes shown in Figure 11. All of them had total emissions of 1.93 × 106 t of CO2 eq. Thus, the lifecycle analysis showed negative net emissions of −0.16 × 106 t of CO2 eq. However, CO2 process emissions highly depend on the equipment used., e.g., a CO2 capture process applied at a natural gas processing facility has lower emissions than CO2 captured from coal-fired power plants, the CO2 compressors which use natural gas have fewer emissions than electrical compressors which use electricity generated by coal-fired power plants, and the oil–CO2 separation method which includes high- and low-pressure separators, is less energy-intensive than other gas separation methods.
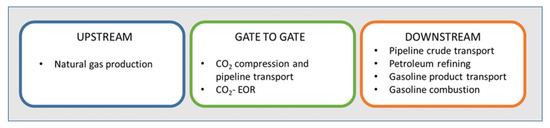
Figure 11.
Cradle-to-grave CO2-EOR emission processes. Modified according to [94].
7. Discussion
Climate issues related to increasing concentrations of CO2, mainly released during fossil fuel combustion during power production, put strong initiatives to limit the use of fossil fuels and to increase the employment of alternative power production solutions like renewable energy sources. On the other hand, due to variability in the availability of renewable energy sources, the cost of energy production from it, along with energy storage issues and the constantly increasing global energy demand, especially in developing countries, the world still strongly depends on fossil fuels, and the transition to a carbon-free society will take place over several decades. A possible solution for the transition is seen in CCS and CCUS technologies, which allow the use of fossil fuels while eliminating the adverse climate change impacts associated with greenhouse gas emissions. Both technologies eliminate a facility’s direct CO2 emissions. Although the primary goal of CCS and CCUS technologies is CO2 sequestration, both technologies result in a certain amount of emissions. Even though CCUS, along with CO2 sequestration, creates additional benefits (production of new products), sometimes, depending on the type of project, it is a less favorable solution compared to CCS (in cases when CO2 retention time is relatively short).
CCS comprises various technical and technological solutions depending on the size and type of CO2 source, capture technology, transportation mean, and the final storage destination (distance from the CO2 source, depth and characteristics of the geological formation, etc.). Currently, the only type of large-scale CCUS projects are CO2-EOR projects (see Table 2, Figure 5 and Figure 6), which, along with CO2 sequestration, also result in residual oil production. According to the KAPSARC database [42], in 2018, 11 of 23 large-scale CO2-EOR projects were in operation (48%) (see Table 2), grouped, by related industries where applied, into natural gas processing (6 projects, or 55%), fertilizer production (2 projects, or 18%), power generation (1 project, or 9%), synthetic natural gas (1 project, or 9%), and hydrogen production (1 project, or 9%). The rest of the projects were in the execution stage (5 projects, or 22%), definition stage (5 projects, or 22%) or evaluation stage (2 projects, or 9%). With regard to the large-scale CO2-EOR projects in the execution phases, there is a visible shift towards smaller capacity projects and other industry applications, such as iron and steel production, fertilizer production and oil refining (Figure 6).
Regarding the capture capacity of these projects, projects related to natural gas processing (52%) have the largest share of capture capacity. This is expected since, as mentioned before, most of the large-scale CO2-EOR projects are related to natural gas processing, which is not surprising since CO2 injection technology was developed by the petroleum industry. The capture capacity of large-scale CO2-EOR projects related to power production is 26%, fertilizer production 5%, synthetic natural gas 7%, oil refining 3%, iron and steel production 2% and hydrogen production 2%.
Most of the projects, at different project stages, are conducted in the USA (48%) and China (22%), followed by Canada (17%), Brazil (4%), Saudi Arabia (4%) and the United Arab Emirates (4%) (Table 2, Figure 7).
As can be seen, all of the mentioned projects differ by CO2 source type and size (and if the same, they differ by fuel type, net output, efficiency, capture technology, capture capacity, captured CO2 purity, etc.), CO2 transport (choice of the transportation system and used fuel, distance from the CO2 capture point to injection/storage point, etc.), injection/storage site characteristics, time horizon, the geographical location of CO2-EOR value chain elements (thus different environmental impact due to different ecological sensitivity) and different market conditions (cost of CO2, oil price). All of these differences pose a challenge when estimating emissions from CO2-EOR projects. The mentioned varieties between CO2-EOR projects (and generally CCS/CCUS projects), but also the lack of standardized methods for estimating the full lifecycle emissions resulting from CO2-EOR projects, result in various uncertainties and wide flexibilities on how to estimate emissions from these kinds of projects.
Narrow-analysis of case-specific data could be done, but generally, there is a lack of appropriate lifecycle emission estimation methodologies specific to CO2-EOR projects (CCS/CCUS projects). In addition, due to the mentioned specifics, in order to estimate the full lifecycle emissions resulting from CO2-EOR projects, normalization and a set of benchmark information should be done, which will allow the cross-referencing of CO2-EOR projects and its comparison to other climate-mitigation strategies. Pointing out the mentioned differentiation and giving an overview of large-scale CO2-EOR projects gives useful information for the future development of standardized methods for estimating the full lifecycle emissions resulting from CO2-EOR projects.
8. Conclusions
CCUS will have an important role in achieving the Paris Amendment goal, as it has proven the potential to deliver significant emission reductions across the energy sector. Even though there are many technologies considered as CCUS, at the moment, the only profitable CCUS projects are large-scale CO2-EOR projects, which along with the sequestration of greenhouse gas, resulting in the production of additional value, i.e., incremental oil.
Due to the increasing global crude oil demand caused by economic growth in developing countries and rising needs in the transport sector, especially in market segments with poor or no fuel alternatives (such as aviation), the petroleum industry through the EOR projects could be an option which gives both, energy security and lower emissions.
According to the KAPSARC data source, in 2018, there were 23 large-scale CO2-EOR projects in different implementation phases. Most of the projects were in North America utilizing CO2 from natural gas processing. Considering the number of projects in the definition/evaluation phase and the CO2 capture capacity of these projects, CO2-EOR projects have a significant potential to play an important role in mitigating climate change in China.
However, besides using CO2 for commercial activity, the main aim of the CCUS projects is CO2 sequestration. They do not necessarily result in overall net negative emissions due to the fact that the CO2 retention time significantly differs among the projects ranging from one year, in case of fuel generation, to up to millions of years, in case of carbonation.
With regard to CO2-EOR projects, all the conducted studies on lifecycle emissions have shown substantial benefits in CO2 emission reduction. Studies have shown that CO2-EOR lifecycle emissions for every barrel of incremental oil produced are 37% less than in the case of conventional oil production methods. The practice has shown that about 60% of the CO2, injected with the purpose of a solvent used for driving the production of residual oil, remains trapped in the reservoir pore space, while 40% of it is reproduced with oil production. Ultimately, if a closed-loop injection system is applied, which is a common case when CO2 is a commodity that must be purchased or when it is generated as waste during natural gas processing, up to 95% of the cumulatively injected CO2 within the CO2-EOR project remains permanently sequestrated in the oil reservoir.
When considering lifecycle emissions of CO2-EOR projects, within the “gate-to-gate” (only CO2-EOR activities), the most carbon (and energy) intensive component is gas compression. When conducting “gate-to-grave”, and especially when conducting “cradle-to-grave” lifecycle emission analysis, due to various possible variants of all the processes involved within all the segments covered by the analysis (upstream-CO2 generation, CO2-EOR activities and downstream-utilization of the produced oil), and the lack of LCA methodologies specific for CO2-EOR projects (and CCUS/CCS projects in general), emission assessment is quite complex resulting in various uncertainties and wide flexibilities, which impedes the cross-referencing and comparison of CO2-EOR projects to other climate-mitigation strategies.
Author Contributions
L.H.: methodology, writing—original draft preparation, K.N.M. and L.H.; writing—review and editing, K.N.M., N.G.-M. and L.H.; supervision, N.G.-M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the institutional project CO2-EOR project emissions, EMICO2, Faculty of Mining, Geology and Petroleum Engineering, University of Zagreb, Croatia.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Fawzy, S.; Osman, A.I.; Doran, J.; Rooney, D.W. Strategies for mitigation of climate change: A review. Environ. Chem. Lett. 2020, 18, 2069–2094. [Google Scholar] [CrossRef]
- Gaurina-Međimurec, N.; Novak Mavar, K. Carbon Capture and Storage (CCS): Geological Sequestration of CO2. In CO2 Sequestration; Frazão, L.A., Ed.; IntechOpen: London, UK, 2019; pp. 1–21. [Google Scholar]
- IEA (International Energy Agency). Putting CO2 to Use. 2019. Available online: https://www.iea.org/topics/carbon-capture-and-storage/ (accessed on 5 January 2021).
- USEPA (United States Environmental Protection Agency). Inventory of U.S. Greenhouse Gas, Emissions and Sinks: 1990–2017. Available online: https://epa.gov/sites/production/files/2019-04/documents/us-ghg-inventory-2019-main-text.pdf (accessed on 5 January 2021).
- Martins, F.; Felgueiras, C.; Smitkova, M.; Caetano, N. Analysis of fossil fuel energy consumption and environmental impacts in European countries. Energies 2019, 12, 964. [Google Scholar] [CrossRef]
- Heuberger, C.F.; Staffell, I.; Shah, N.; Mac Dowell, N. What is the value of CCS in the future energy system? Energy Procedia 2017, 114, 7564–7757. [Google Scholar] [CrossRef]
- Budinis, S.; Krevor, S.; Mac Dowell, N.; Brandon, N.; Hawkes, A. An assessment of CCS costs, barriers and potential. Energy Strategy Rev. 2018, 22, 61–81. [Google Scholar] [CrossRef]
- Allinson, K.; Burt, D.; Campbell, L.; Constable, L.; Crombie, M.; Lee, A.; Lima, V.; Lloyd, T.; Solsbey, L. Best practice for transitioning from carbon dioxide (CO2) enhanced oil recovery EOR to CO2 storage. Energy Procedia 2017, 114, 6950–6956. [Google Scholar] [CrossRef]
- Husanović, E.; Novak, K.; Malvić, T.; Novak Zelenika, K.; Velić, J. Prospects for CO2 carbonation and storage in Upper Miocene sandstone of Sava Depression in Croatia. Geol. Q 2015, 59, 91–104. [Google Scholar] [CrossRef]
- Cuéllar-Franca, R.M.; Azapagic, F.A. Carbon capture, storage and utilisation technologies: A critical analysis and comparison of their life cycle environmental impacts. J. CO2 Util. 2015, 9, 82–102. [Google Scholar] [CrossRef]
- MIT (Technologies at the Massachusetts Institute of Technology). Available online: https://sequestration.mit.edu/tools/projects/ (accessed on 30 January 2021).
- Global CCS Institute. Available online: https://co2re.co/FacilityData (accessed on 30 January 2021).
- IEA (International Energy Agency). Available online: https://www.iea.org/fuels-and-technologies/carbon-capture-utilisation-and-storage (accessed on 30 January 2021).
- KAPSARC (King Abdullah Petroleum Studies and Research Center). Available online: https://datasource.kapsarc.org/explore/dataset/ (accessed on 30 January 2021).
- Hendriks, C.; Noothout, P.; Zakkour, P.; Cook, G. Implications of the reuse of captured CO2 for European climate action policies, Report to DG Climate Action. Ecofys and Carbon Count. 2013. Available online: http://www.scotproject.org/sites/default/files/Carbon%20Count,%20Ecofys%20%282013%29%20Implications%20of%20the%20reuse%20of%20captured%20CO2%20-%20report.pdf (accessed on 5 January 2021).
- IHS Markit. Chemical Economics Handbook—Carbon Dioxide. 2018. Available online: https://ihsmarkit.com/products/carbon-dioxide-chemical-economics-handbook.html (accessed on 5 January 2021).
- Olah, G.A. Beyond oil and gas: The methanol economy. Angew. Chem. Int. Ed. 2005, 44, 2636–2639. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Xiao, T.; Kuznetsov, V.L.; Edwards, P.P. Turning carbon dioxide into fuel. Phil. Trans. R. Soc. A 2010, 368, 3343–3364. [Google Scholar] [CrossRef] [PubMed]
- Gnanamani, M.K.; Jacobs, G.; Pendyala, V.R.R.; Ma, W.; Davis, B.H. Chapter 4: Hydrogenation of carbon dioxide to liquid fuels. In Green Carbon Dioxide; Advances in CO2 Utilization; Centi, G., Perathoner, S., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2014; pp. 99–115. [Google Scholar]
- Marlin, D.S.; Sarron, E.; Sigurbjörnsson, Ó. Process advantages of direct CO2 to methanol synthesis. Front. Chem. 2018, 6. [Google Scholar] [CrossRef] [PubMed]
- IEA (International Energy Agency). Exploring Clean Energy Pathways: The Role of CO2 Storage. 2019. Available online: https://www.iea.org/publications/reports/TheroleofCO2storage/ (accessed on 5 January 2021).
- Liu, H.J.; Were, P.; Li, Q.; Gou, Y.; Hou, Z. Worldwide status of CCUS technologies and their development and challenges in China. Geofluids 2017. [Google Scholar] [CrossRef]
- Mazzotti, M.; Pini, R.; Storti, G. Enhanced coalbed methane recovery. J. Supercrit. Fluids 2009, 47, 619–627. [Google Scholar] [CrossRef]
- Du, F.; Nojabaei, B. A review of gas injection in shale reservoirs: Enhanced oil/gas recovery approaches and greenhouse gas control. Energies 2019, 12, 2355. [Google Scholar] [CrossRef]
- Du, X.; Gu, M.; Liu, Z.; Zhao, Y.; Sun, F.; Wu, T. Enhanced shale gas recovery by the injections of CO2, N2, and CO2/N2 mixture gases. Energy Fuels 2019, 33, 5091–5101. [Google Scholar] [CrossRef]
- Novak Mavar, K.; Gaurina-Međimurec, N.; Hrnčević, L. Carbon Capture, Utilization and Storage (CCUS)—The Environmental Impact Rom Project Start to Closure. In Proceedings of the 7th International Symposium Mining and Environmental Protection, Vrdnik, Serbia, 25–28 September 2019; Ristović, I., Ed.; Faculty of Mining and Geology: Belgrade, Serbia, 2019; pp. 63–68. [Google Scholar]
- Wu, Y.; Li, P. The potential of coupled carbon storage and geothermal extraction in a CO2-enhanced geothermal system: A review. Geotherm. Energy 2020, 8, 19. [Google Scholar] [CrossRef]
- Harto, C.B.; Schroeder, J.N.; Horner, R.M.; Patton, T.L.; Durham, L.A.; Murphy, D.J.; Clark, C.E. Water Use in Enhanced Geothermal Systems (EGS): Geology of U.S. Stimulation Projects, Water Costs, and Alternative Water Source Policies; Argonne National Lab.(ANL): Argonne, IL, USA, 2014. [CrossRef]
- Brown, D.W. A Hot Dry Rock Geothermal Energy Concept Utilizing Supercritical CO2 Instead of Water. In Proceedings of the 25th Workshop on Geothermal Reservoir Engineering, Stanford, CA, USA, 24–26 January 2000; Stanford University: Stanford, CA, USA, 2000. [Google Scholar]
- Pan, F.; McPherson, B.J.; Kaszuba, J. Evaluation of CO2-fluid-rock interaction in enhanced geothermal systems: Field-scale geochemical simulations. Geofluids 2017. [Google Scholar] [CrossRef]
- Wang, C.L.; Cheng, W.L.; Nian, Y.L.; Yang, L.; Han, B.B.; Liu, M.H. Simulation of heat extraction from CO2-based enhanced geothermal systems considering CO2 sequestration. Energy 2018, 142, 157–167. [Google Scholar] [CrossRef]
- Olasolo, P.; Juárez, M.C.; Morales, M.P.; D’Amico, S.; Liarte, I.A. Enhanced geothermal systems (EGS): A review. Renew. Sustain. Energy Rev. 2016, 56, 133–144. [Google Scholar] [CrossRef]
- U.S. Department of Energy’s Office of Energy Efficiency and Renewable Energy. Enhanced Geothermal Systems Demonstration Projects. Available online: https://www.energy.gov/eere/geothermal/enhanced-geothermal-systems-demonstration-projects (accessed on 10 December 2020).
- Conboy, T.; Pasch, J.; Fleming, D. Control of a Supercritical CO2 Recompression Brayton Cycle Demonstration Loop. J. Eng. Gas. Turbines Power 2013, 135, 11. [Google Scholar] [CrossRef]
- Siddiqui, M.E.; Almitani, K.H. Energy analysis of the S-CO2 Brayton cycle with improved heat regeneration. Processes 2013, 7, 3. [Google Scholar] [CrossRef]
- Kaliyan, N.; Morey, R.; Wilcke, W.; Alagusundaram, K.; Gayathri, P. Applications of carbon dioxide in food and processing industries: Current status and future thrusts. In Proceedings of the ASABE Annual International Meeting, Conference Paper Number 076113, Minneapolis, MN, USA, 17–20 June 2007. [Google Scholar] [CrossRef]
- Global CCS Institute. Global Status of CCS. 2019. Available online: https://www.globalccsinstitute.com/resources/global-status-report/ (accessed on 10 December 2020).
- Tcvetkov, P.; Cherepovitsyn, A.; Fedoseev, S. The changing role of CO2 in the transition to a circular economy: Review of carbon sequestration projects. Sustainability 2019, 11, 5834. [Google Scholar] [CrossRef]
- IPIECA (International Petroleum Industry Environmental Conservation Association) & API (American Petroleum Institute). Oil and Natural Gas Industry Guidelines for Greenhouse Gas Reduction Projects, Part II: Carbon Capture and Geological Storage Emission Reduction Family. 2007. Available online: https://www.api.org/~/media/Files/EHS/climate-change/Carbon-Capture-Geological-Storage-Emission-Reduction-Family.pdf (accessed on 10 December 2020).
- Zevenhoven, R.; Kohlmann, J. Direct Dry Mineral Carbonation for CO2 Emissions Reduction in Finland. In Proceedings of the 27th International Technical Conference on Coal Utilization & Fuel Systems, Clearwater, FL, USA, 4–7 March 2002; pp. 743–754. [Google Scholar]
- Zevenhoven, R.; Kavaliauskaite, I. Mineral carbonation for long-term CO2 storage: An exergy analysis. Int. J. Appl. Thermodyn. 2004, 7, 23–31. [Google Scholar]
- IPCC (Intergovernmental Panel on Climate Changes). Special Report on Carbon Dioxide Capture and Storage. 2005. Available online: https://www.ipcc.ch/report/carbon-dioxide-capture-and-storage/ (accessed on 7 January 2021).
- Zevenhoven, R.; Teir, S.; Eloneva, S.; Aatos, S.; Sorjonen-Ward, P. CO2 Sequestration by Carbonation of Minerals and Industrial by-Products in Finland. In Proceedings of the R’07, Davos, Switzerland, 3–5 September 2007. [Google Scholar]
- Sipila, J.; Teir, S.; Zevenhoven, R. Carbon Dioxide Sequestration by Mineral Carbonation—Literature Review Update 2005–2007. Heat Engineering Lab. In Report VT 2008-1; Abo Akademi University: Espoo, Finland, 2008. [Google Scholar]
- Lu, J.; Wilkinson, M.; Haszeldine, R.S. Carbonate cements in Miller field of the UK North Sea: A natural analog for mineral trapping in CO2 geological storage. Environ. Earth Sci. 2011, 62, 507–517. [Google Scholar] [CrossRef]
- Bauer, S.; Class, H.; Ebert, M.; Gotze, H.; Holzheid, A.; Kolditz, O.; Rosenbaum, S.; Rabbel, W.; Schäfer, D.; Dahmke, A. Modelling, parameterization, and evaluation of monitoring methods for CO2 storage in deep saline formations the CO2 MoPa project. Environ. Earth Sci. 2012, 67, 351–367. [Google Scholar] [CrossRef]
- Novosel, D.; Leonard, N.; Mikulić, S.; Mudrić, D. Početni rezultati primjene utiskivanja ugljičnog dioksida za povećanje iscrpka nafte na proizvodnom polju Ivanić i Žutica (Initial results of CO2 injection for enhanced oil recovery from the Ivanić and Žutica Oil fields—In Croatian). Nafta i plin 2018, 38, 57–66. [Google Scholar]
- INA Group Annual Report. 2019. Available online: https://www.ina.hr/home/press-centar/publikacije/godisnja-izvjesca/ (accessed on 7 January 2021).
- Novosel, D. Pet godina utiskivanja CO2 za Povećanje Iscrpka Nafte na Polju Ivanić i Žutica—iskustva i rezultati (Five years of CO2 Injection for Enhanced oil Recovery from the Ivanić and Žutica fields—Experience and Results-In Croatian). Nafta i plin 2020, 40, 33–47. [Google Scholar]
- Novak Mavar, K. Modeliranje površinskoga transporta i geološki aspekti skladištenja ugljikova dioksida u neogenska pješčenjačka ležišta Sjeverne Hrvatske na primjeru polja Ivanić. (Surface Transportation Modelling and Geological Aspects of Carbon-Dioxide Storage into Northern Croatian Neogene Sandstone Reservoirs, Case Study Ivanić Field). Ph.D. Thesis, University of Zagreb, Zagreb, Croatia, 13 April 2015. [Google Scholar]
- Novak, K.; Zelenika, I. Carbon Capture and Storage Possibility, Case Study Ivanić Field. In Geomathematics—From Theory to Practice, Proceedings of the 6th Croatian-Hungarian and 17th Hungarian Geomathematical Congress, Opatija, Croatia, 21–23 May 2014; Cvetković, M., Novak Zelenika, K., Geiger, J., Eds.; Croatian Geological Society: Zagreb, Croatia, 2014; pp. 151–158. [Google Scholar]
- Interational Energy Agency (IEA). 2020. Available online: https://www.iea.org/commentaries/whatever-happened-to-enhanced-oil-recovery (accessed on 7 January 2021).
- Gaurina-Međimurec, N.; Novak Mavar, K.; Majić, M. Carbon Capture and Storage (CCS): Technology, projects and monitoring review. Geol. Min. Pet. Eng. Bull. 2018, 33, 1–15. [Google Scholar]
- Warwick, P.D.; Attanasi, E.D.; Blondes, M.S.; Brennan, S.T.; Buursink, M.L.; Doolan, C.A.; Freeman, P.A.; Jahediesfanjani, H.; Özgen Karacan, H.C.; Lohr, C.D.; et al. Carbon dioxide-enhanced oil recovery and residual oil zone studies at the U.S. Geological Survey. In Proceedings of the 14th International Conference on Greenhouse Gas Control Technologies, GHGT-14, Melbourne, Australia, 21–25 October 2018. [Google Scholar]
- Leung, D.Y.; Caramanna, G.; Maroto-Valer, M.M. An overview of current status of carbon dioxide capture and storage technologies. Renew. Sust. Energ. Rev. 2014, 39, 426–443. [Google Scholar] [CrossRef]
- Global CCS Institute. Introduction to industrial carbon capture and storage, Special Report. 2016. Available online: https://www.globalccsinstitute.com/wp-content/uploads/2019/08/Introduction-to-Industrial-CCS.pdf/ (accessed on 8 January 2021).
- National Energy Technology Laboratory (NETL). Air Products and Chemicals, Inc.: Demonstration of CO2 Capture and Sequestration of Steam Methane Reforming Process Gas used for Large Scale Hydrogen Production. 2017. Available online: https://www.netl.doe.gov/sites/default/files/netl-file/FE0002381.pdf (accessed on 9 January 2021).
- Preston, C. The CCS Project at Air Products’ Port. Arthur Hydrogen Production Facility; IEA Greenhouse Gas R&D Programme: Cheltenham, UK, 2018. [Google Scholar]
- U.S. Department of Energy (DOE) & National Energy Technology Laboratory (NETL). CO2-EOR Offshore Resource Assessment. 2014. Available online: https://netl.doe.gov/projects/files/FY14_CO2-EOROffshoreResourceAssessment_060114.pdf (accessed on 9 January 2021).
- Liu, H.; Consoli, C.; Zapantis, A. Overview of Carbon Capture and Storage (CCS) facilities globally. In Proceedings of the 14th Greenhouse Gas Control Technologies Conference (GHGT-14), Melbourne, Australia, 21–26 October 2018; 10pAvailable online: https://ssrn.com/abstract=3366353 or http://dx.doi.org/10.2139/ssrn.3366353 (accessed on 9 January 2021).
- Eide, L.I.; Batum, M.; Dixon, T.; Elamin, Z.; Graue, A.; Hagen, S.; Hovorka, S.; Nazarian, B.; Nøkleby, P.; Olsen, G.I.; et al. Enabling large-scale carboon capture, utilisation, and storage (CCUS) using offshore carbon dioxide (CO2) infrastructure developments—A review. Energies 2019, 12, 1945. [Google Scholar] [CrossRef]
- Madden, D.R. Case study: Kemper County IGCC project, USA. In Integrated Gasification Combined Cycle (IGCC) Technologies; Wang, T., Stiegel, G., Eds.; Woodhead Publishing: Sawston, UK, 2017; pp. 817–832. [Google Scholar]
- Paulson Institute. Financing Mega-Scale Energy Projects: A Case Study of the Petra Nova Carbon Capture Project. 2015. Available online: http://www.paulsoninstitute.org/wp-content/uploads/2015/10/CS-Petra-Nova-EN.pdf (accessed on 9 January 2021).
- Clean Air Task Force (CATF). Petra Nova: De-Risking Carbon Capture Business Models with Saline Storage. 2020. Available online: https://www.catf.us/2020/08/petra-nova-de-risking-carbon-capture-business-models-with-saline-storage/ (accessed on 9 January 2021).
- Hill, L.B.; Li, X.; Wei, N. CO2-EOR in China: A comparative review. Int. J. Greenh. Gas. Control. 2020, 103, 103173. [Google Scholar] [CrossRef]
- Cornot-Gandolphe, S. Carbon Capture, Storage and Utilization to the Rescue of Coal? Global Perspective with Focus on China and SAD; French Institute of International Relations IFRI: Paris, France, 2019. Available online: https://www.ifri.org/en/publications/etudes-de-lifri/carbon-capture-storage-and-utilization-rescue-coal-global-perspectives (accessed on 9 January 2021).
- Li, Q.; Ma, J.; Li, X.; Xu, L.; Niu, Z.; Lu, X. Integrated monitoring of China’s Yanchang CO2-EOR demonstration project in Ordos Basin. Energy Procedia 2018, 154, 112–117. [Google Scholar] [CrossRef]
- Dong, G.J.; Wang, Z.; Liu, H.; Ma, R.; Kong, D.; Wang, F.; Xin, X.; Li, L.; She, H. EOR survey in China-Part 1. In Proceedings of the SPE Improved Oil Recovery Conference, Society of Petroleum Engineers, Tulsa, OK, USA, 14–18 April 2018. [Google Scholar] [CrossRef]
- MIT. Carbon Capture and Sequestration Technologies. 2016. Available online: https://sequestration.mit.edu/tools/projects/jilin.html (accessed on 9 January 2021).
- National Energy Technology Laboratory (NETL). Summit Texas Clean Energy, LLC: Texas Clean Energy Project (TCEP). 2017. Available online: https://www.netl.doe.gov/sites/default/files/netl-file/FE0002650.pdf (accessed on 10 January 2021).
- U.S. Department of the Interior Bureau of Land Management. Draft Environmental Impact Statement for the Riley Ridge to Natrona Project. Volume 1, 2018. Available online: https://eplanning.blm.gov/public_projects/nepa/64342/138479/170483/01_Volume_I_of_II.pdf (accessed on 10 January 2021).
- Carruthers, K. Environmental Impact of CO2-EOR; Scottish Carbon Capture and Storage: Edingurgh, Scottland, 2014. [Google Scholar]
- Hrnčević, L. Analysis of the Impact of Implementation of the Kyoto Protocol on the Oil Industry and the Operations of the Oil Company (Analiza Utjecaja Provedbe Kyoto Protokola na Naftnu Industriju i Poslovanje Naftne Tvrtke-in Croatian). Ph.D. Thesis, University of Zagreb, Zagreb, Croatia, 2008. [Google Scholar]
- Dilmore, R. An Assessment of Gate-To-Gate Environmental Life Cycle Performance of Water-Alternating-gas CO2-Enhanced oil Recovery in the Permian Basin; Department of Energy (DOE)/National Energy Technology Laboratory (NETL): Pittsburgh, PA, USA, 2010.
- Department of Energy (DOE)/National Energy Technology Laboratory (NETL). Electricity Use of Enhanced oil Recovery with Carbon Dioxide (CO2-EOR); National Energy Technology Laboratory: Pittsburgh, PA, USA, 2009.
- Núñez-López, V.; Ramon, G.; Seyyed, H. Environmental and operational performance of CO2-EOR as a CCUS Technology: A Cranfield Example with Dynamic LCA Considerations. Energies 2019, 12, 448. [Google Scholar] [CrossRef]
- Finn, A.J.; O’Brien, J.V. Processing of Carbon Dioxide Rich Gas. In Proceedings of the GPA Conference, Madrid, Spain, 17–19 September 2014; Available online: https://www.costain.com/media/13020/gpa-conference-sept-2014-1mb.pdf (accessed on 10 January 2021).
- Faltison, J.; Gunter, B. Net CO2 Stored in North American EOR projects. Society of Petroleum Engineers. J. Can. Pet. Technol. 2010, 50, 7. [Google Scholar] [CrossRef]
- Hansen, H.; Eiken, O.; Aasum, T. Tracing the Path of CO2 from a Gas/Condensate Reservoir through an Amine Plant and Back into a Subsurface Aquifer-Case Study: The Sleipner Area, Norwegian North Sea. In Proceedings of the Offshore Europe oil and Gas Exhibition and Conference: Inform, Innovate, Inspire Conference, Aberdeen, UK, 6–9 September 2005. [Google Scholar] [CrossRef]
- Gaurina-Međimurec, N.; Pašić, B. CO2 underground storage and wellbore integrity. In Risk Analysis for Prevention of Hazardous Situations in Petroleum and Natural Gas Engineering; Matanović, D., Gaurina-Međimurec, N., Simon, K., Eds.; IGI Global: Hershey, PA, USA, 2013; pp. 169–217. [Google Scholar]
- Gaurina-Međimurec, N.; Novak Mavar, K. Depleted hydrocarbon reservoirs and CO2 injection wells—CO2 leakage assessment. Min. Geol. Pet. Eng. Bull. 2017, 32, 15–26. [Google Scholar] [CrossRef]
- Melzer, L.S. Carbon Dioxide Enhanced Oil Recovery (CO2 EOR): Factors Involved in Adding Carbon Capture, Utilization and Storage (CCUS) to Enhanced oil Recovery. Midland Texas: Melzer Consulting. National Enhanced Oil Recovery Initiative Resource. 2012. Available online: http://neori.org/Melzer_CO2EOR_CCUS_Feb2012.pdf (accessed on 10 January 2021).
- International Energy Agency (IEA). Can CO2-EOR Really Provide Carbon-Negative Oil? 2019. Available online: https://www.iea.org/commentaries/can-co2-eor-really-provide-carbon-negative-oil (accessed on 10 January 2021).
- Pehnt, M.; Henkel, J. Lifecycle assessment of carbon dioxide and storage from lignite power plant. Int. J. Greenh. Gas. Control 2009, 3, 49–66. [Google Scholar] [CrossRef]
- Marx, J.; Schreiber, A.; Zapp, P.; Haines, M.; Hake, J.-F.; Gale, J. Environmental evaluation of CCS using lifecycle assessment (LCA)—A synthesis report. Energy Procedia 2011, 4, 2448–2456. [Google Scholar] [CrossRef]
- Singh, B.; Stromman, A.H.; Hertwich, E.G. Environmental damage assessment of carbon capture and storage application of end point indicators. J. Ind. Ecol. 2012, 16, 407–419. [Google Scholar] [CrossRef]
- Turconi, R.; Boldrin, A.; Astrup, T. Lifecycle assessment of electricity generation technologies: Overview, comparably and limitations. Renevable Sustain. Energy Rev. 2013, 28, 555–565. [Google Scholar] [CrossRef]
- Singh, B.; Bouman, E.A.; Stromman, A.H.; Hertwich, E.G. Material use for electricity generation with carbon dioxide capture and storage: Extending life cycle analysis indices for material accounting. Res. Conserv. Recycle 2015, 100, 49–57. [Google Scholar] [CrossRef]
- Petrescu, I.; Cormos, C.C. Environmental assessment of IGCC power plants with pre-combustion CO2 capture by chemical and calcium looping methods. J. Clean. Prod. 2017, 158, 233–244. [Google Scholar] [CrossRef]
- Azzolina, N.A.; Hamling, J.A.; Peck, W.D.; Gorecki, C.D.; Nakles, D.V.; Melzer, L.S. A life cycle analysis of incremental oil produce via CO2 EOR. Energy Procedia 2017, 114, 6588–6596. [Google Scholar] [CrossRef]
- Cooney, G.; Littlefield, J.; Mariott, J.; Skone, T.J. Evaluatinng the climate benefits of CO2-enhanced oil recovery using life cycle analysis. Environ. Sci. Technol. 2015, 49, 7491–7500. [Google Scholar] [CrossRef] [PubMed]
- Clean Air Task Force (CATF). Net CO₂ Emission Reductions from Anthropogenic CO₂-EOR. 2019. Available online: https://www.catf.us/resource/co2-eor-emission-reduction/ (accessed on 10 January 2021).
- Thorne, R.J.; Sundseth, K.; Bouman, E.; Czarnowska, L.; Mathisen, A.; Skagestad, R.; Stanek, W.; Pacyna, J.M.; Pacyna, E.G. Technical and environmental viability of a European CO2 EOR system. Int. J. Greenh. Gas. Control. 2020, 92, 102857. [Google Scholar] [CrossRef]
- Sminchak, J.R.; Mawalkar, S.; Gupta, N. Large CO2 Storage volumes result in net negative emissions for greenhouse gas life cycle analysis based on records from 22 Years of CO2-Enhanced Oil Recovery operations. Energy Fuels 2020, 34, 3566–3577. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
