Pilot Cultivation of the Vulnerable Cretan Endemic Verbascum arcturus L. (Scrophulariaceae): Effect of Fertilization on Growth and Quality Features
Abstract
:1. Introduction
2. Materials and Methods
2.1. Origin of Plant Material
2.2. Establishment of Field Experiment
2.3. Soil Analysis of the Experimental Field
2.4. Fertilization Treatments
2.5. Plant Measurements
2.5.1. Non-Invasive Evaluation of Leaf Coloration at Three Growth Stages
2.5.2. Non-Invasive Evaluation of Photosynthetic Performance at Three Growth Stages
2.5.3. Leaf Shape Indicators
2.5.4. Plant Growth, Morphology and Biomass Allocation
2.5.5. Leaf Photosynthetic Pigment Content
2.5.6. Leaf Total Phenolic and Total Flavonoid Contents
2.5.7. Leaf Soluble Sugar Content
2.5.8. Leaf, Stem and Floral Analysis
2.6. Statistical Analysis
3. Results
3.1. Soil Characteristics of the Experimental Field
3.2. Leaf Colour
3.3. Leaf Photosynthetic Performance
3.4. Leaf Shape
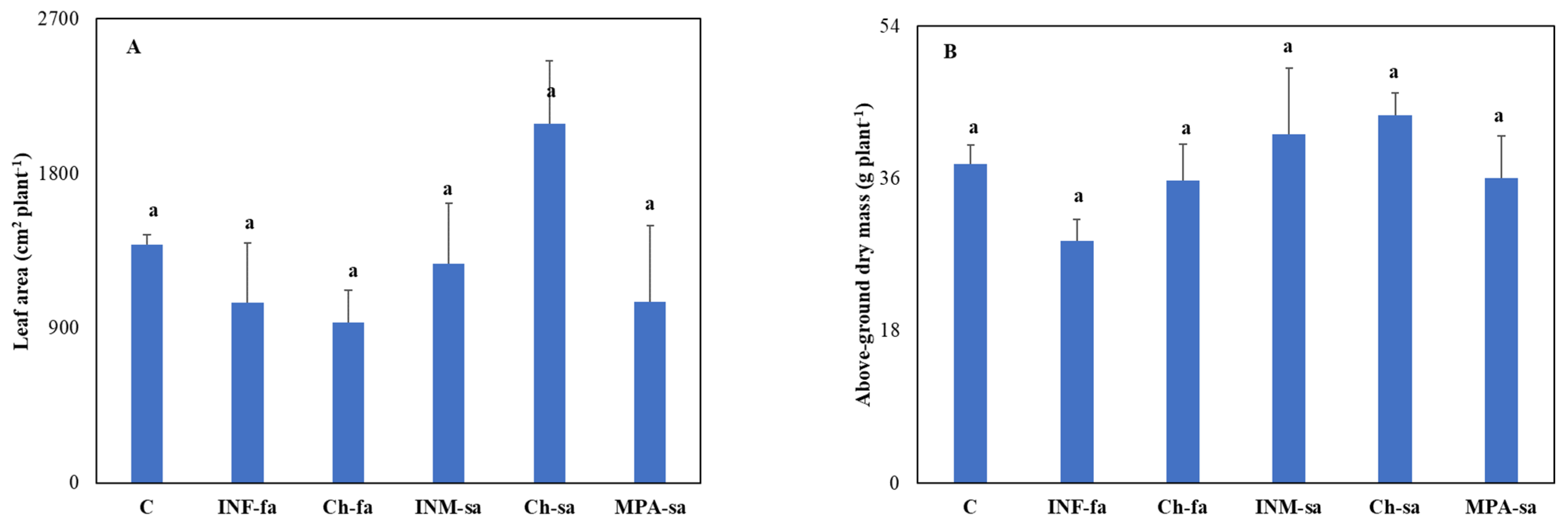
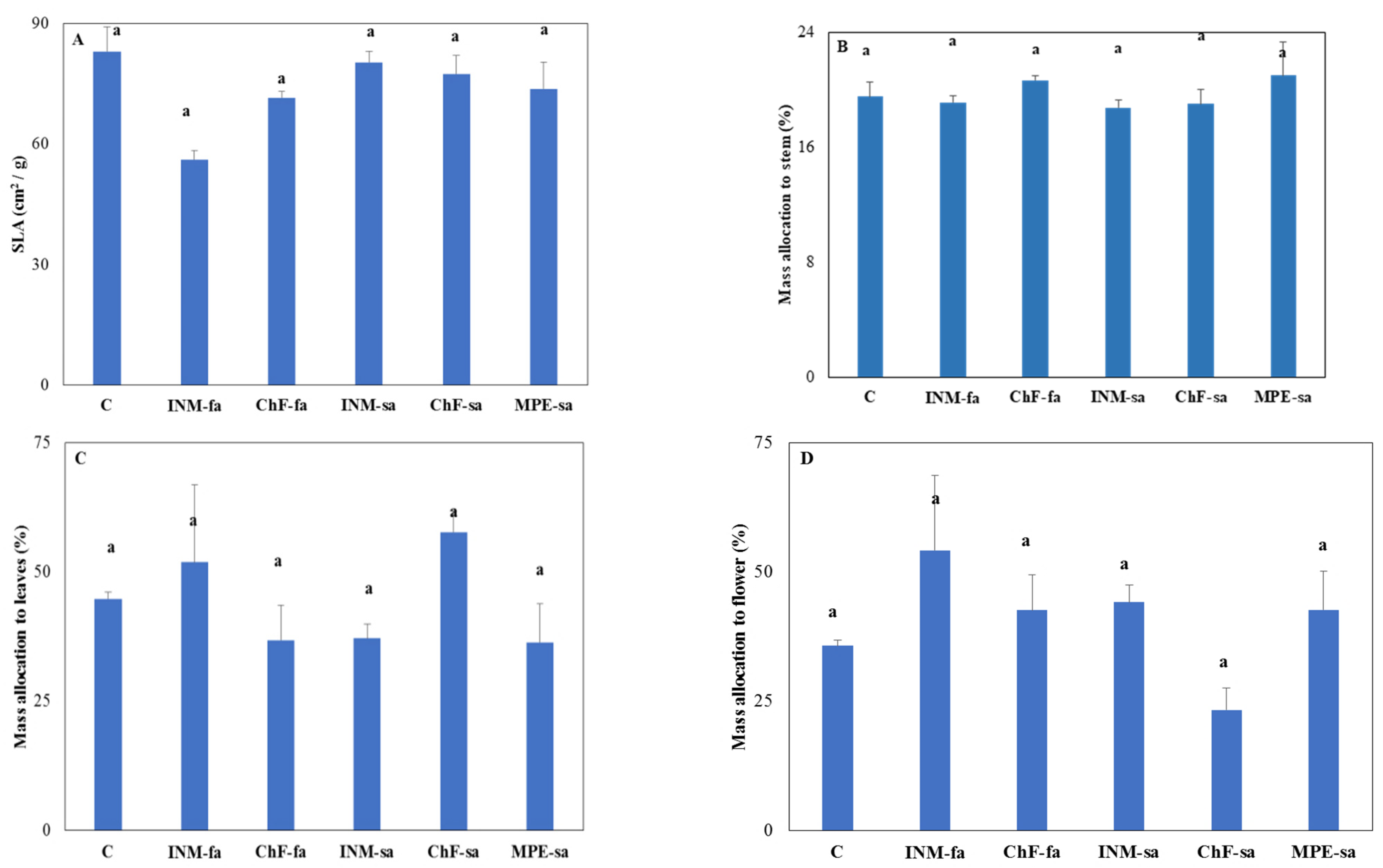
3.5. Plant Growth, Morphology and Biomass Allocation
3.6. Leaf Chlorophyll Content
3.7. Leaf Antioxidant Compound Content
3.8. Leaf Soluble Sugar Content
3.9. Leaf, Stem and Floral Mineral Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Fowler, M.W. Plants, medicines and man. J. Sci. Food Agric. 2006, 86, 1797–1804. [Google Scholar] [CrossRef]
- Sharmeen, J.B.; Mahomoodally, F.M.; Zengin, G.; Maggi, F. Essential oils as natural sources of fragrance compounds for cosmetics and cosmeceuticals. Molecules 2021, 26, 666. [Google Scholar] [CrossRef] [PubMed]
- Cheminal, A.; Kokkoris, I.P.; Strid, A.; Dimopoulos, P. Medicinal and aromatic Lamiaceae plants in Greece: Linking diversity and distribution patterns with ecosystem services. Forests 2020, 11, 661. [Google Scholar] [CrossRef]
- Krigas, N.; Tsoktouridis, G.; Anestis, I.; Khabbach, A.; Libiad, M.; Megdiche-Ksouri, W.; Ghrabi-Gammar, Z.; Lamchouri, F.; Tsiripidis, I.; Tsiafouli, M.A.; et al. Exploring the potential of neglected local endemic plants of three Mediterranean regions in the ornamental sector: Value chain feasibility and readiness timescale for their sustainable exploitation. Sustainability 2021, 13, 2539. [Google Scholar] [CrossRef]
- Grigoriadou, K.; Sarropoulou, V.; Krigas, N.; Maloupa, E.; Tsoktouridis, G. GIS-facilitated effective propagation protocols of the endangered local endemic of Crete Carlina Diae (Rech. f.) Meusel and A. Kástner (Asteraceae): Serving ex situ conservation needs and its future sustainable exploitation as an ornamental. Plants 2020, 9, 1465. [Google Scholar] [CrossRef] [PubMed]
- Scariot, V.; Seglie, L.; Gaino, W.; Devecchi, M. Evaluation of European native bluebells for sustainable floriculture. Acta Hortic. 2012, 937, 273–279. [Google Scholar] [CrossRef]
- Libiad, M.; Khabbach, A.; El Haissoufi, M.; Anestis, I.; Lamchouri, F.; Bourgou, S.; Megdiche-Ksouri, W.; Ghrabi-Gammar, Z.; Greveniotis, V.; Tsiripidis, I.; et al. Agro-alimentary potential of the neglected and underutilized local endemic plants of Crete (Greece), Rif-Mediterranean coast of Morocco and Tunisia: Perspectives and challenges. Plants 2021, 10, 1770. [Google Scholar] [CrossRef]
- Ye, L.; Zhao, X.; Bao, E.; Li, J.; Zou, Z.; Cao, K. Bio-organic fertilizer with reduced rates of chemical fertilization improves soil fertility and enhances tomato yield and quality. Sci. Rep. 2020, 10, 177. [Google Scholar] [CrossRef] [Green Version]
- Ibrahim, M.; Jaafar, H.; Karimi, E.; Ghasemzadeh, A. Impact of organic and inorganic fertilizers application on the phytochemical and antioxidant activity of Kacip Fatimah (Labisia pumila Benth). Molecules 2013, 18, 10973–10988. [Google Scholar] [CrossRef] [Green Version]
- Rehim, A.; Amjad Bashir, M.; Raza, Q.-U.-A.; Gallagher, K.; Berlyn, G.P. Yield enhancement of biostimulants, Vitamin B12, and CoQ10 compared to inorganic fertilizer in radish. Agronomy 2021, 11, 697. [Google Scholar] [CrossRef]
- Selim, M.M. Introduction to the integrated nutrient management strategies and their contribution to yield and soil properties. Int. J. Agron. 2020, 2020, 2821678. [Google Scholar] [CrossRef]
- Gezahegn, A.M. Role of integrated nutrient management for sustainable maize production. Int. J. Agron. 2021, 2021, 9982884. [Google Scholar] [CrossRef]
- Khoshgoftarmanesh, A.H.; Schulin, R.; Chaney, R.L.; Daneshbakhsh, B.; Afyuni, M. Micronutrient-efficient genotypes for crop yield and nutritional quality in sustainable agriculture. A review. Agron. Sustain. Dev. 2010, 30, 83–107. [Google Scholar] [CrossRef] [Green Version]
- Rengel, Z.; Batten, G.D.; Crowley, D.E. Agronomic approaches for improving the micronutrient density in edible portions of field crops. Field Crops Res. 1999, 60, 27–40. [Google Scholar] [CrossRef]
- Thamkaew, G.; Sjöholm, I.; Galindo, F.G. A review of drying methods for improving the quality of dried herbs. Crit. Rev. Food Sci. Nutr. 2021, 61, 1763–1786. [Google Scholar] [CrossRef]
- Asami, D.K.; Hong, Y.-J.; Barrett, D.M.; Mitchell, A.E. Comparison of the total phenolic and ascorbic acid content of freeze-dried and air-dried marionberry, strawberry, and corn using conventional, organic, and sustainable agricultural practices. J. Agric. Food Chem. 2003, 51, 1237–1241. [Google Scholar] [CrossRef]
- Amujoyegbe, B.J.; Opabode, J.T.; Olayinka, A. Effect of organic and inorganic fertilizer on yield and chlorophyll content of maize (Zea mays L.) and sorghum Sorghum bicolour (L.) Moench. Afr. J. Biotechnol. 2007, 6, 1869–1873. [Google Scholar] [CrossRef]
- Kougioumoutzis, K.; Kokkoris, I.P.; Panitsa, M.; Strid, A.; Dimopoulos, P. Extinction risk assessment of the Greek endemic flora. Biology 2021, 10, 195. [Google Scholar] [CrossRef] [PubMed]
- Sarropoulou, V.; Grigoriadou, K.; Tsoktouridis, G.; Krigas, N.; Maloupa, E. Sexual and Asexual Propagation of Verbascum arcturus, a Vulnerable Local Endemic of Crete, Greece; Institute of Plant Breeding and Genetic Resources, Hellenic Agricultural Organization Demeter: Thermi-Thessaloniki, Macedonia, Greece, 2021; manuscript in preparation. [Google Scholar]
- Bouyoucos, G.J. Hydrometer method improved for making particle size analysis of soils. Agron. J. 1962, 54, 464–465. [Google Scholar] [CrossRef]
- Walkley, A.; Black, I.A. An examination of the Degtjareff method for determining soil organic matter, and a proposed modification of the chromic acid titration method. Soil Sci. 1934, 37, 29–38. [Google Scholar] [CrossRef]
- Bremner, J.M. Nitrogen-total. In Methods of Soil Analysis, Part 3: Chemical Methods; Sparks, D.L., Ed.; SSSA Book Series 5; Soil Science Society of America: Madison, WI, USA; American Society of Agronomy: Madison, WI, USA, 1996; pp. 1085–1121. [Google Scholar]
- Rhoades, J.D. Salinity: Electrical conductivity and total dissolved solids. In Methods of Soil Analysis, Part 3: Chemical Methods; Sparks, D.L., Ed.; SSSA Book Series 5; Soil Science Society of America: Madison, WI, USA; American Society of Agronomy: Madison, WI, USA, 1996; pp. 417–435. [Google Scholar]
- Mulvaney, R.L. Nitrogen-Inorganic Forms. In Methods of Soil Analysis, Part 3: Chemical Methods; Sparks, D.L., Ed.; SSSA Book Series 5; Soil Science Society of America: Madison, WI, USA; American Society of Agronomy: Madison, WI, USA, 1996; pp. 1123–1184. [Google Scholar]
- Kuo, S. Phosphorus. In Methods of Soil Analysis, Part 3: Chemical Methods; Sparks, D.L., Ed.; SSSA Book Series 5; Soil Science Society of America: Madison, WI, USA; American Society of Agronomy: Madison, WI, USA, 1996; pp. 869–919. [Google Scholar]
- Thomas, G.W. Exchangeable Cations. In Agronomy Monographs; Page, A.L., Ed.; American Society of Agronomy: Madison, WI, USA; Soil Science Society of America: Madison, WI, USA, 2015; pp. 159–165. [Google Scholar]
- Lindsay, W.L.; Norvell, W.A. Development of a DTPA soil test for zinc, iron, manganese, and copper. Soil Sci. Soc. Am. J. 1978, 42, 421–428. [Google Scholar] [CrossRef]
- Keren, R. Boron. In Methods of Soil Analysis, Part 3: Chemical Methods; Sparks, D.L., Ed.; SSSA Book Series 5; Soil Science Society of America: Madison, WI, USA; American Society of Agronomy: Madison, WI, USA, 1996; pp. 603–626. [Google Scholar]
- Asayesh, E.J.; Aliniaeifard, S.; Askari, N.; Roozban, M.; Sobhani, M.; Tsaniklidis, G.; Woltering, E.J.; Fanourakis, D. Supplementary light with increased blue fraction accelerates emergence and improves development of the inflorescence in Aechmea, Guzmania and Vriesea. Agronomy 2021, 7, 485. [Google Scholar] [CrossRef]
- Costa, G.; Noferini, M.; Fiori, G.; Torrigiani, P. Use of vis/nir spectroscopy to assess fruit ripening stage and improve management in post-harvest chain. Fresh Prod. 2009, 3, 35–41. [Google Scholar]
- Taheri-Garavand, A.; Mumivand, H.; Fanourakis, D.; Fatahi, S.; Taghipour, S. An artificial neural network approach for non-invasive estimation of essential oil content and composition through considering drying processing factors: A case study in Mentha aquatica. Ind. Crops Prod. 2021, 171, 113985. [Google Scholar] [CrossRef]
- Sørensen, H.K.; Fanourakis, D.; Tsaniklidis, G.; Bouranis, D.; Nejad, A.R.; Ottosen, C.-O. Using artificial lighting based on electricity price without a negative impact on growth, visual quality or stomatal closing response in Passiflora. Sci. Hortic. 2020, 267, 109354. [Google Scholar] [CrossRef]
- Yang, L.; Fanourakis, D.; Tsaniklidis, G.; Li, K.; Yang, Q.; Li, T. Contrary to red, blue monochromatic light improves the bioactive compound content in broccoli sprouts. Agronomy 2021, 11, 2139. [Google Scholar] [CrossRef]
- Koubouris, G.; Bouranis, D.; Vogiatzis, E.; Nejad, A.R.; Giday, H.; Tsaniklidis, G.; Ligoxigakis, E.K.; Blazakis, K.; Kalaitzis, P.; Fanourakis, D. Leaf area estimation by considering leaf dimensions in olive tree. Sci. Hortic. 2018, 240, 440–445. [Google Scholar] [CrossRef]
- Fanourakis, D.; Kazakos, F.; Nektarios, P.A. Allometric individual leaf area estimation in chrysanthemum. Agronomy 2021, 11, 795. [Google Scholar] [CrossRef]
- Gupta, S.; Rosenthal, D.M.; Stinchcombe, J.R.; Baucom, R.S. The remarkable morphological diversity of leaf shape in sweet potato (Ipomoea Batatas): The influence of genetics, environment, and G×E. New Phytol. 2020, 225, 2183–2195. [Google Scholar] [CrossRef]
- Ahmadi-Majd, M.; Rezaei Nejad, A.; Mousavi-Fard, S.; Fanourakis, D. Postharvest application of single, multi-walled carbon nanotubes and graphene oxide stimulates rose keeping quality. J. Hortic. Sci. Biotechnol. 2021, in press. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Wellburn, A.R. Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochem. Soc. Trans. 1983, 11, 591–592. [Google Scholar]
- Chen, Y.; Fanourakis, D.; Tsaniklidis, G.; Aliniaeifard, S.; Yang, Q.; Li, T. Low UVA intensity during cultivation improves the lettuce shelf-life, an effect that is not sustained at higher intensity. Postharvest Biol. Technol. 2021, 172, 111376. [Google Scholar] [CrossRef]
- DuBois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Mills, H.A.; Jones, J.B., Jr. Plant Analysis Handbook II. A Practical Sampling, Preparation, Analysis, and Interpretation Guide; Micro-Macro Publishing: Athens, GA, USA, 1996; ISBN 978-1-878148-05-6. [Google Scholar]
- Dahnke, W.C.; Johnson, G.V. Testing soils for available nitrogen. In Soil Testing and Plant Analysis, 3rd ed.; Westerman, R.L., Ed.; SSSA Book Series 3; SSSA: Madison, WI, USA, 1990; pp. 127–139. [Google Scholar]
- Thomas, G.W.; Peaslee, D.E. Testing soils for phosphorus. In Soil Testing and Plant Analysis; Walsh, L.M., Beaton, J.D., Eds.; SSSA: Madison, WI, USA, 1973; pp. 115–132. [Google Scholar]
- Doll, E.C.; Lucas, R.E. Testing soils for potassium, calcium, and magnesium. In Soil Testing and Plant Analysis; Walsh, L.M., Beaton, J.D., Eds.; SSSA: Madison, WI, USA, 1973; pp. 133–151. [Google Scholar]
- Sims, J.T.; Johnson, G.V. Micronutrient soil tests. In Micronutrients in Agriculture, 2nd ed.; Mortvedt, J.J., Ed.; SSSA: Madison, WI, USA, 1991; pp. 427–476. [Google Scholar]
- Strid, A. Atlas of the Aegean Flora. Part 1: Text & Plates. Part 2: Maps; Englera 33 (1 & 2); Botanic Garden and Botanical Museum Berlin, Freie Universität Berlin: Berlin, Germany, 2016. [Google Scholar]
- Bourgou, S.; Jilani, I.B.H.; Karous, O.; Megdiche-Ksouri, W.; Ghrabi-Gammar, Z.; Khabbach, A.; Libiad, M.; El Haissoufi, M.; Lamchouri, F.; Greveniotis, V.; et al. Medicinal-cosmetic potential of the local endemic plants from of Crete (Greece), Rif-Mediterranean Coast of Morocco and Tunisia: Priorities for conservation and sustainable exploitation of neglected and underutilized phytogenetic resources. Biology 2021, 10, 1344. [Google Scholar] [CrossRef]
- Malterud, K.; Moradi, A. Assessment Report on Verbascum thapsus L., V. densiflorum Bertol. (V. thapsiforme Schrad) and V. phlomoides L., flos. European Medicines Agency, Committee on Herbal Medicinal Products (HMPC), EMA/HMPC/611531/2016. 2018. Available online: https://www.ema.europa.eu/en/documents/herbal-report/final-assessment-report-verbascum-thapsus-l-v-densiflorum-bertol-v-thapsiforme-schrad-v-phlomoides-l_en.pdf (accessed on 28 October 2021).
- Brown, P.H.; Cakmak, I.; Zhang, Q. Form and function of Zn in plants, Chapter 7. In Proceedings of the International Symposium on Zinc in Soils and Plants; Robson, A.D., Ed.; Springer Science and Business Media: Dordrecht, The Netherlands, 1993; pp. 93–106. [Google Scholar]
- Dotaniya, M.L.; Meena, V.D. Rhizosphere effect on nutrient availability in soil and its uptake by plants: A review. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2015, 85, 1–12. [Google Scholar] [CrossRef]
- Sharma, H.S.S.; Fleming, C.; Selby, C.; Rao, J.R.; Martin, T. Plant Biostimulants: A review on the processing of macroalgae and use of extracts for crop management to reduce abiotic and biotic stresses. J. Appl. Phycol. 2014, 26, 465–490. [Google Scholar] [CrossRef]
- Halpern, M.; Bar-Tal, A.; Ofek, M.; Minz, D.; Muller, T.; Yermiyahu, U. The use of biostimulants for enhancing nutrient uptake. In Advances in Agronomy; Elsevier: Amsterdam, The Netherlands, 2015; Volume 130, pp. 141–174. [Google Scholar]
- European Biostimulant Industry Council. 2012. Available online: http://www.biostimulants.eu (accessed on 28 October 2021).
- Marschner, H. Mineral Nutrition of Higher Plants, 2nd ed.; Academic Press: Cambridge, MA, USA, 1995. [Google Scholar]
- Bergstrand, K.-J.I. Methods for for growth regulation of greenhouse produced ornamental pot- and bedding plants—A current review. Folia Hortic. 2017, 29, 63–74. [Google Scholar] [CrossRef] [Green Version]
- Sevik, H.; Karakas, H.; Karaca, U. Color—Chlorophyll relationship of some indoor ornamental plantsity. Int. J. Eng. Sci. Res. Technol. 2013, 2, 1706–1712. [Google Scholar]
- Sklirou, A.D.; Angelopoulou, M.T.; Argyropoulou, A.; Chaita, E.; Boka, V.I.; Cheimonidi, C.; Niforou, K.; Mavrogonatou, E.; Pratsinis, H.; Kalpoutzakis, E.; et al. Phytochemical study and in vitro screening focusing on the anti-aging features of various plants of the Greek flora. Antioxidants 2021, 10, 1206. [Google Scholar] [CrossRef] [PubMed]
- Ramaiya, S.D.; Lee, H.H.; Xiao, Y.J.; Shahbani, N.S.; Zakaria, M.H.; Bujang, J.S. Organic cultivation practices enhanced antioxidant activities and secondary metabolites in giant granadilla (Passiflora quadrangularis L.). PLoS ONE 2021, 16, e0255059. [Google Scholar] [CrossRef]
- Naguib, A.E.M.M.; El-Baz, F.K.; Salama, Z.A.; Hanaa, H.A.E.B.; Ali, H.F.; Gaafar, A.A. Enhancement of phenolics, favonoids and glucosinolates of Broccoli (Brassica olaracea var. Italica) as antioxidants in response to organic and bio-organic fertilizers. J. Saudi Soc. Agric. Sci. 2012, 11, 135–142. [Google Scholar] [CrossRef] [Green Version]





| Treatment | N | P | K | Ca | Mg |
|---|---|---|---|---|---|
| (g kg−1) | |||||
| C | 14.3 ± 0.3 a | 1.6 ± 0.0 a | 14.9 ± 0.1 b | 15.6 ± 0.1 b | 2.5 ± 0.1 a |
| INΜ-fa | 5.6 ± 0.0 d | 1.7 ± 0.0 a | 12.4 ± 0.2 c | 17.6 ± 0.9 b | 2.2 ± 0.1 a |
| ChF-fa | 9.2 ± 0.7 cd | 1.9 ± 0.0 a | 18.0 ± 0.5 a | 17.9 ± 0.4 b | 2.3 ± 0.0 a |
| INM-sa | 14.0 ± 0.3 a | 1.7 ± 0.0 a | 16.5 ± 0.2 ab | 18.0 ± 0.2 b | 2.4 ± 0.1 a |
| ChF-sa | 13.5 ± 0.6 ab | 1.7 ± 0.0 a | 16.0 ± 0.3 ab | 15.9 ± 0.4 b | 2.3 ± 0.1 a |
| MPE-sa | 9.7 ± 0.6 bc | 1.7 ± 0.0 a | 17.2 ± 0.3 ab | 23.4 ± 0.2 a | 2.5 ± 0.1 a |
| p F-test | 0.034 | NS | 0.049 | 0.042 | NS |
| Treatment | Cu | Zn | Fe | Mn | B |
|---|---|---|---|---|---|
| (mg kg−1) | |||||
| C | 15.9 ± 0.6 a | 21.2 ± 0.6 b | 1085 ± 53 a | 48.7 ± 2.5 bc | 35.3 ± 0.5 bc |
| INΜ-fa | 14.5 ± 0.9 a | 33.2 ± 2.0 a | 1041 ± 62 a | 60.9 ± 4.0 ab | 41.1 ± 0.9 a |
| ChF-fa | 16.5 ± 0.8 a | 28.9 ± 0.5 a | 1158 ± 67 a | 73.4 ± 3.6 a | 40.4 ± 0.4 a |
| INM-sa | 15.1 ± 0.9 a | 19.2 ± 0.9 b | 999 ± 30 a | 45.6 ± 0.5 bc | 34.1 ± 0.2 c |
| ChF-sa | 13.7 ± 0.5 a | 18.5 ± 0.6 b | 1064 ± 45 a | 39.2 ± 1.1 c | 35.4 ± 0.6 bc |
| MPE-sa | 14.0 ± 0.3 a | 20.6 ± 0.2 b | 1017 ± 41 a | 50.5 ± 1.9 bc | 38.6 ± 0.7 ab |
| p F-test | NS | <0.001 | NS | 0.016 | 0.027 |
| Inflorescence | Stem | |||||
|---|---|---|---|---|---|---|
| Treatment | N | P | K | N | P | K |
| (g kg−1) | ||||||
| C | 16.3 ± 1.6 a | 2.4 ± 0.1 ab | 11.9 ± 0.8 a | 9.1 ± 0.1 a | 3.7 ± 0.3 a | 17.6 ± 2.0 a |
| INΜ-fa | 14.8 ± 2.7 a | 1.2 ± 0.5 b | 9.4 ± 0.8 a | 8.9 ± 0.1 a | 2.2 ± 0.9 ab | 20.5 ± 2.1 a |
| ChF-fa | 13.4 ± 1.0 a | 2.4 ± 0.4 ab | 11.8 ± 0.7 a | 8.3 ± 0.5 a | 1.7 ± 0.2 b | 21.5 ± 3.6 a |
| INM-sa | 15.7 ± 0.8 a | 3.0 ± 0.4 a | 13.1 ± 0.4 a | 10.3 ± 1.2 a | 2.3 ± 0.6 ab | 22.3 ± 2.2 a |
| ChF-sa | 16.9 ± 2.2 a | 1.6 ± 0.3 b | 7.5 ± 3.0 a | 9.3 ± 0.9 a | 1.7 ± 0.3 b | 19.2 ± 3.0 |
| MPE-sa | 13.6 ± 0.7 a | 2.1 ± 0.1 ab | 12.9 ± 0.9 a | 9.0 ± 1.8 a | 2.2 ± 0.5 ab | 20.2 ± 4.5 |
| p F-test | NS | 0.031 | NS | NS | NS | NS |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paschalidis, K.; Fanourakis, D.; Tsaniklidis, G.; Tzanakakis, V.A.; Bilias, F.; Samara, E.; Kalogiannakis, K.; Debouba, F.J.; Ipsilantis, I.; Tsoktouridis, G.; et al. Pilot Cultivation of the Vulnerable Cretan Endemic Verbascum arcturus L. (Scrophulariaceae): Effect of Fertilization on Growth and Quality Features. Sustainability 2021, 13, 14030. https://doi.org/10.3390/su132414030
Paschalidis K, Fanourakis D, Tsaniklidis G, Tzanakakis VA, Bilias F, Samara E, Kalogiannakis K, Debouba FJ, Ipsilantis I, Tsoktouridis G, et al. Pilot Cultivation of the Vulnerable Cretan Endemic Verbascum arcturus L. (Scrophulariaceae): Effect of Fertilization on Growth and Quality Features. Sustainability. 2021; 13(24):14030. https://doi.org/10.3390/su132414030
Chicago/Turabian StylePaschalidis, Konstantinos, Dimitrios Fanourakis, Georgios Tsaniklidis, Vasileios A. Tzanakakis, Fotis Bilias, Eftihia Samara, Konstantinos Kalogiannakis, Faten Jamel Debouba, Ioannis Ipsilantis, Georgios Tsoktouridis, and et al. 2021. "Pilot Cultivation of the Vulnerable Cretan Endemic Verbascum arcturus L. (Scrophulariaceae): Effect of Fertilization on Growth and Quality Features" Sustainability 13, no. 24: 14030. https://doi.org/10.3390/su132414030
APA StylePaschalidis, K., Fanourakis, D., Tsaniklidis, G., Tzanakakis, V. A., Bilias, F., Samara, E., Kalogiannakis, K., Debouba, F. J., Ipsilantis, I., Tsoktouridis, G., Matsi, T., & Krigas, N. (2021). Pilot Cultivation of the Vulnerable Cretan Endemic Verbascum arcturus L. (Scrophulariaceae): Effect of Fertilization on Growth and Quality Features. Sustainability, 13(24), 14030. https://doi.org/10.3390/su132414030












