Influence Mechanism of Cyanobacterial Extracellular Polymeric Substances on the Water Quality in Dynamic Water Supply System
Abstract
:1. Introduction
2. Materials and Methods
2.1. Collection and Preparation of Water Samples
2.2. Reagents
2.3. Experimental Method
2.4. Detection Indicator
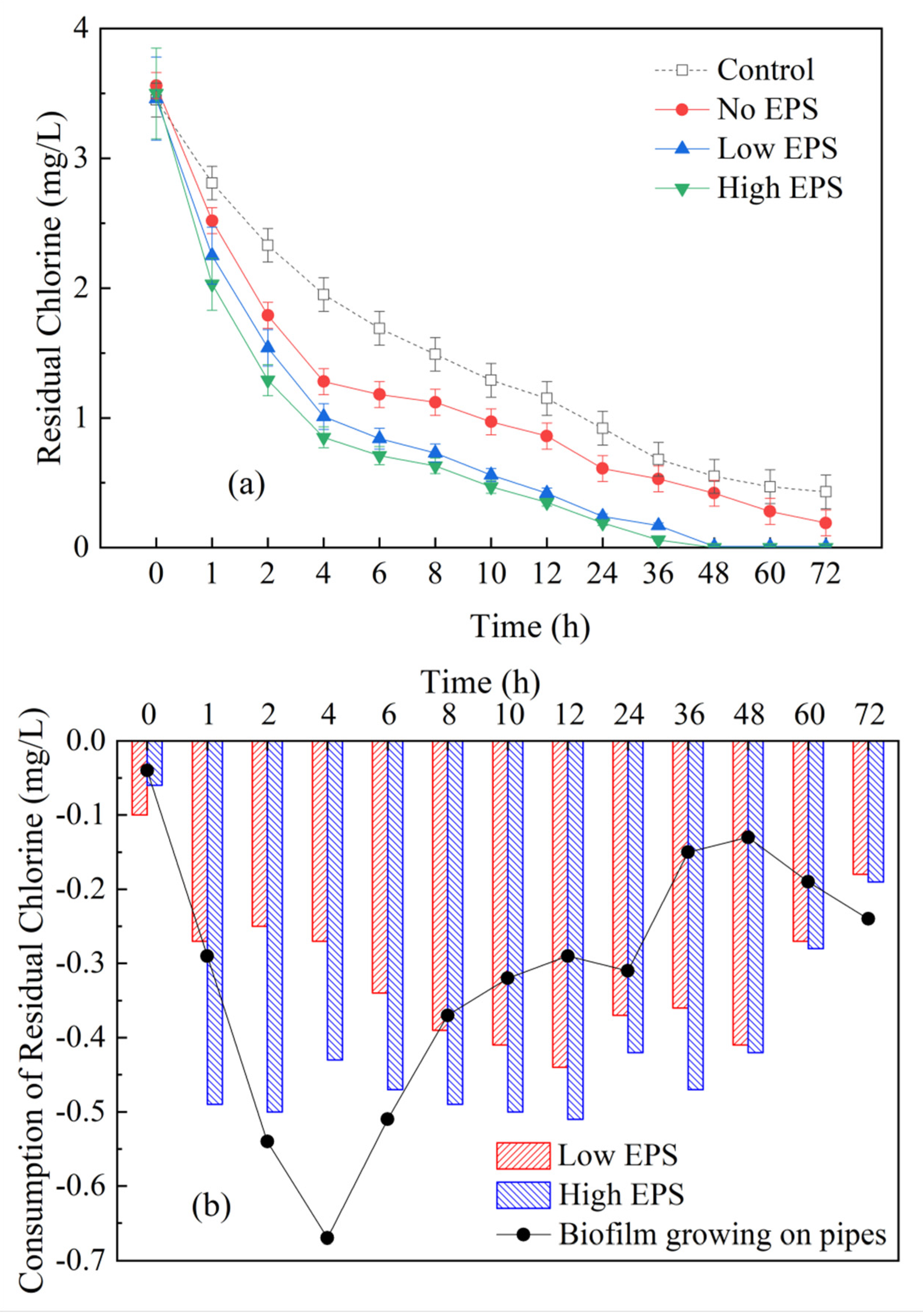
2.4.1. Residual Chlorine
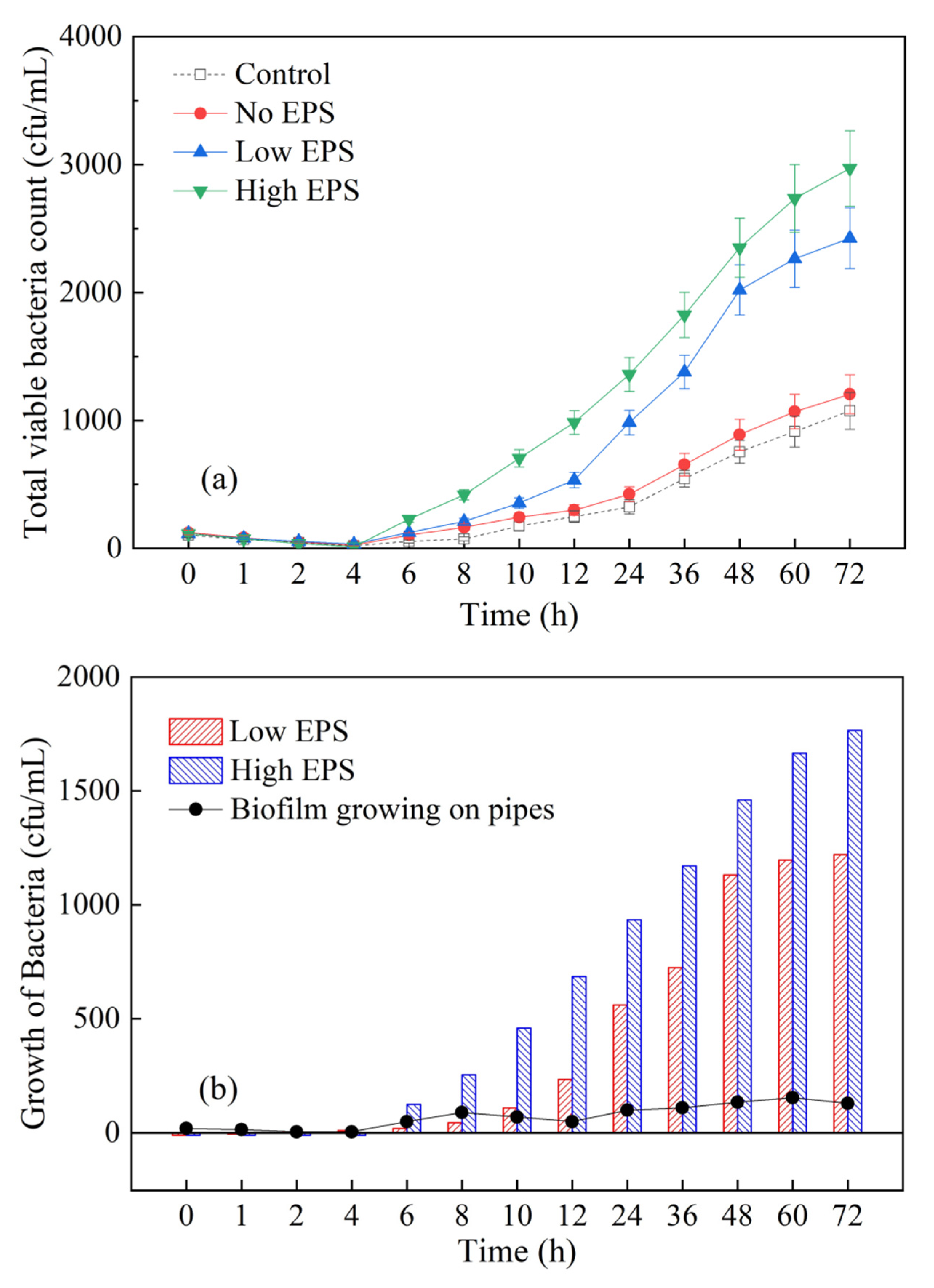
2.4.2. Total Viable Bacteria Count
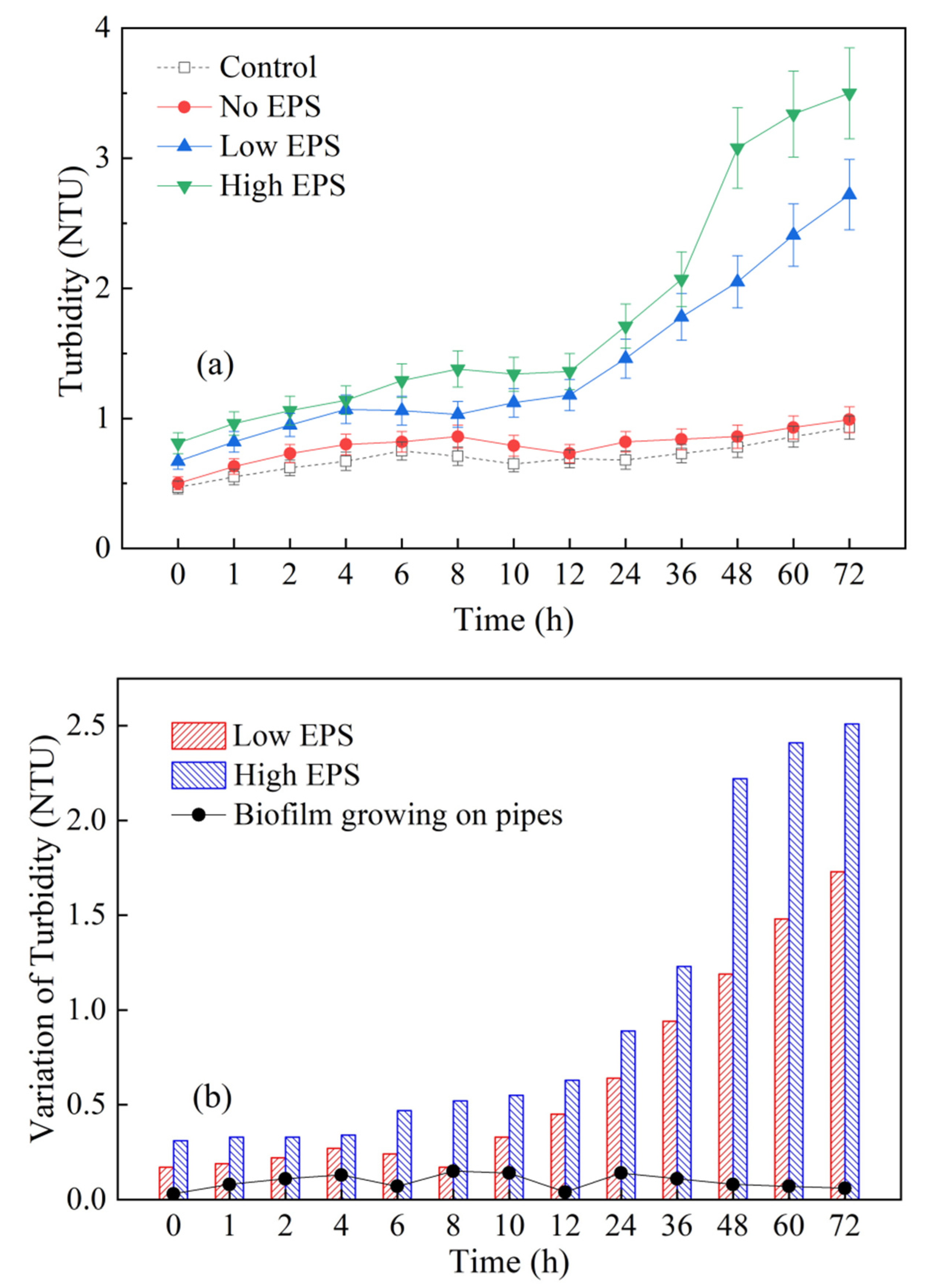
2.4.3. Turbidity
2.4.4. Total Organic Carbon (TOC)
2.4.5. Biodegradable Dissolved Organic Carbon (BDOC)
2.4.6. Assimilable Organic Carbon (AOC)
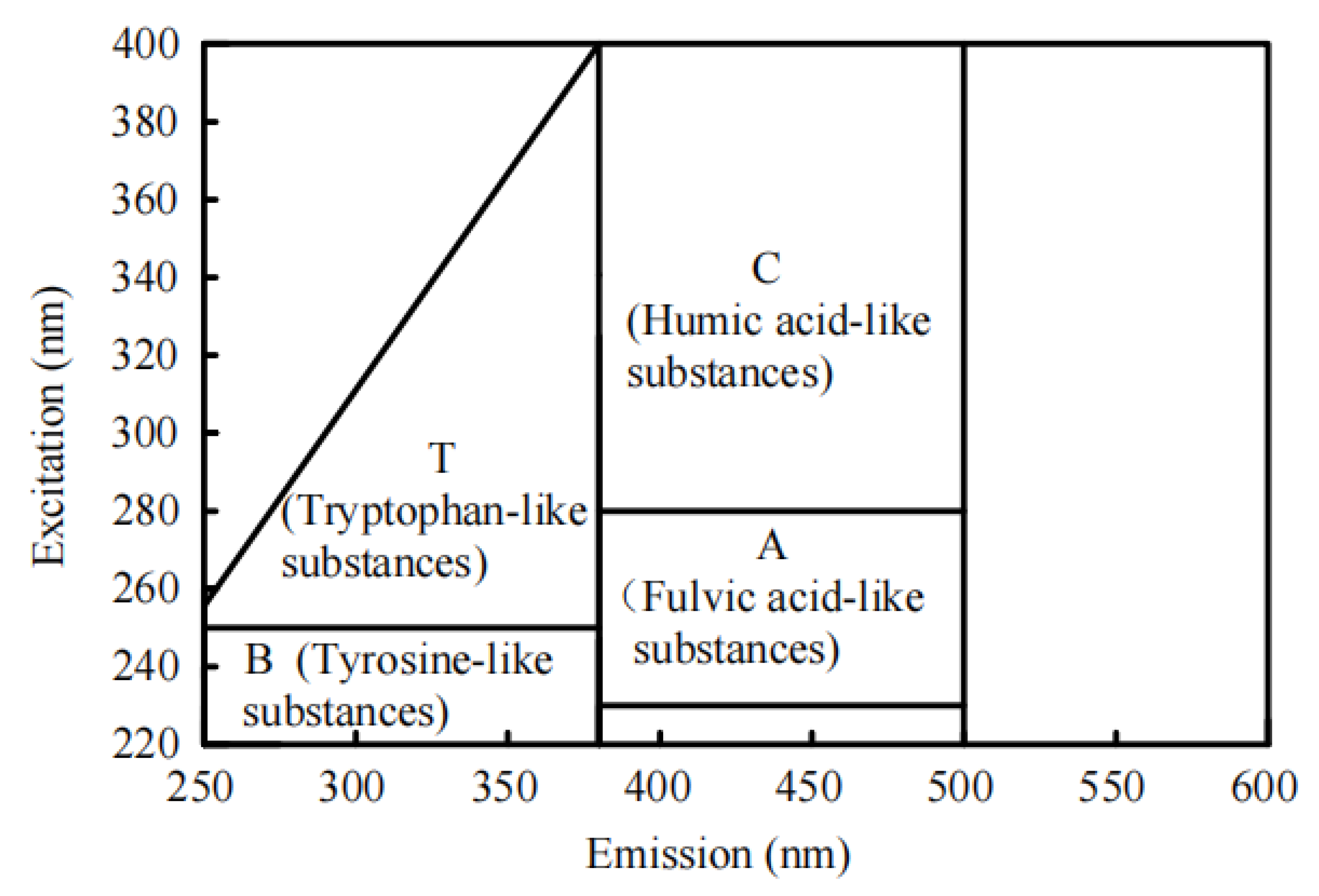
2.4.7. Fluorescent Organic Matters
3. Results and Discussion
3.1. Influence of Cyanobacterial EPS on Residual Chlorine in Dynamic Water Supply System
3.2. Influence of Cyanobacterial EPS on the Total Viable Bacteria Count in the Dynamic Water Supply System
3.3. Influence of Cyanobacterial EPS on the Turbidity in the Dynamic Water Supply System
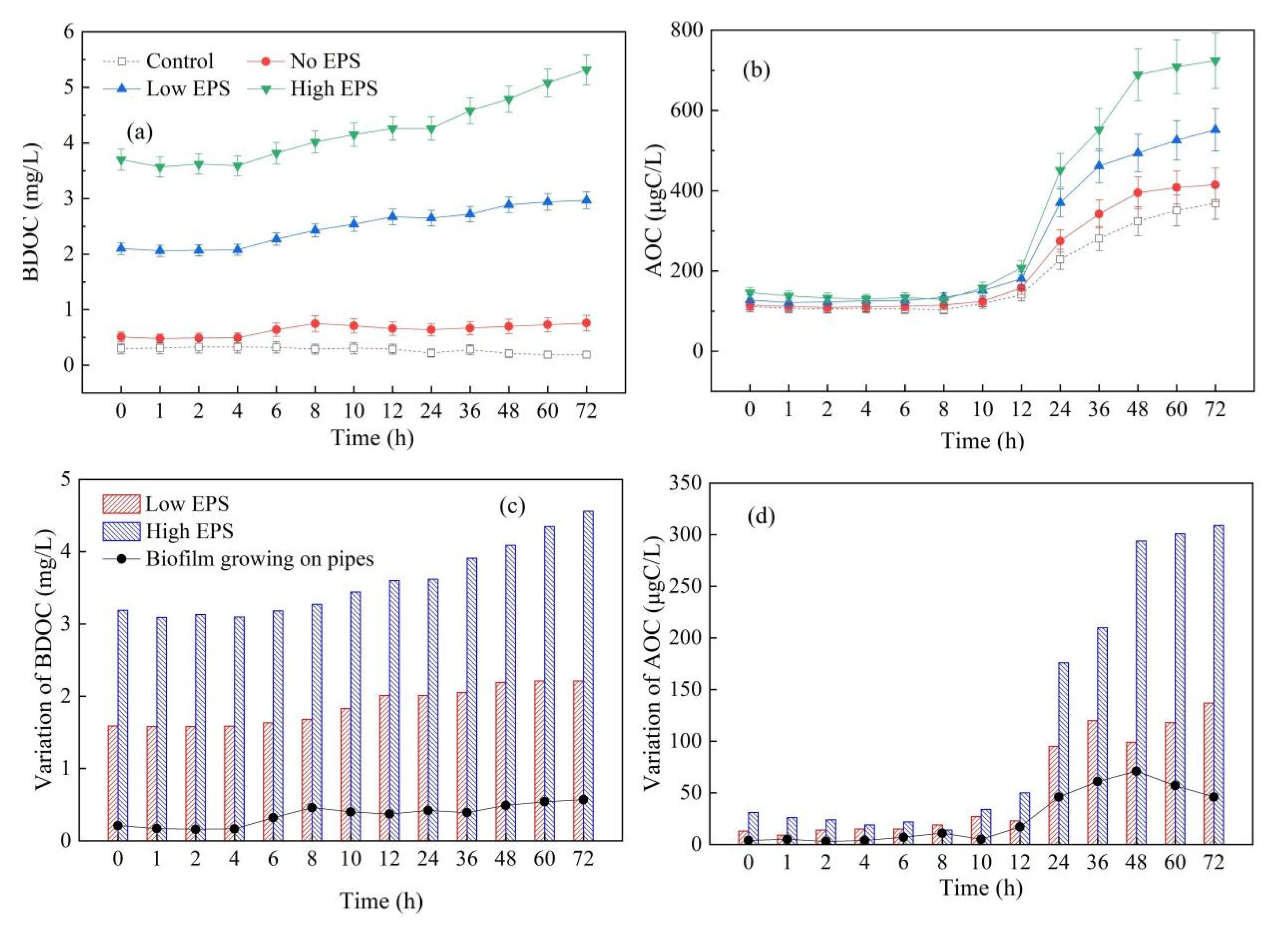
3.4. Influence of Cyanobacterial EPS on the Biological Stability in the Dynamic Water Supply System
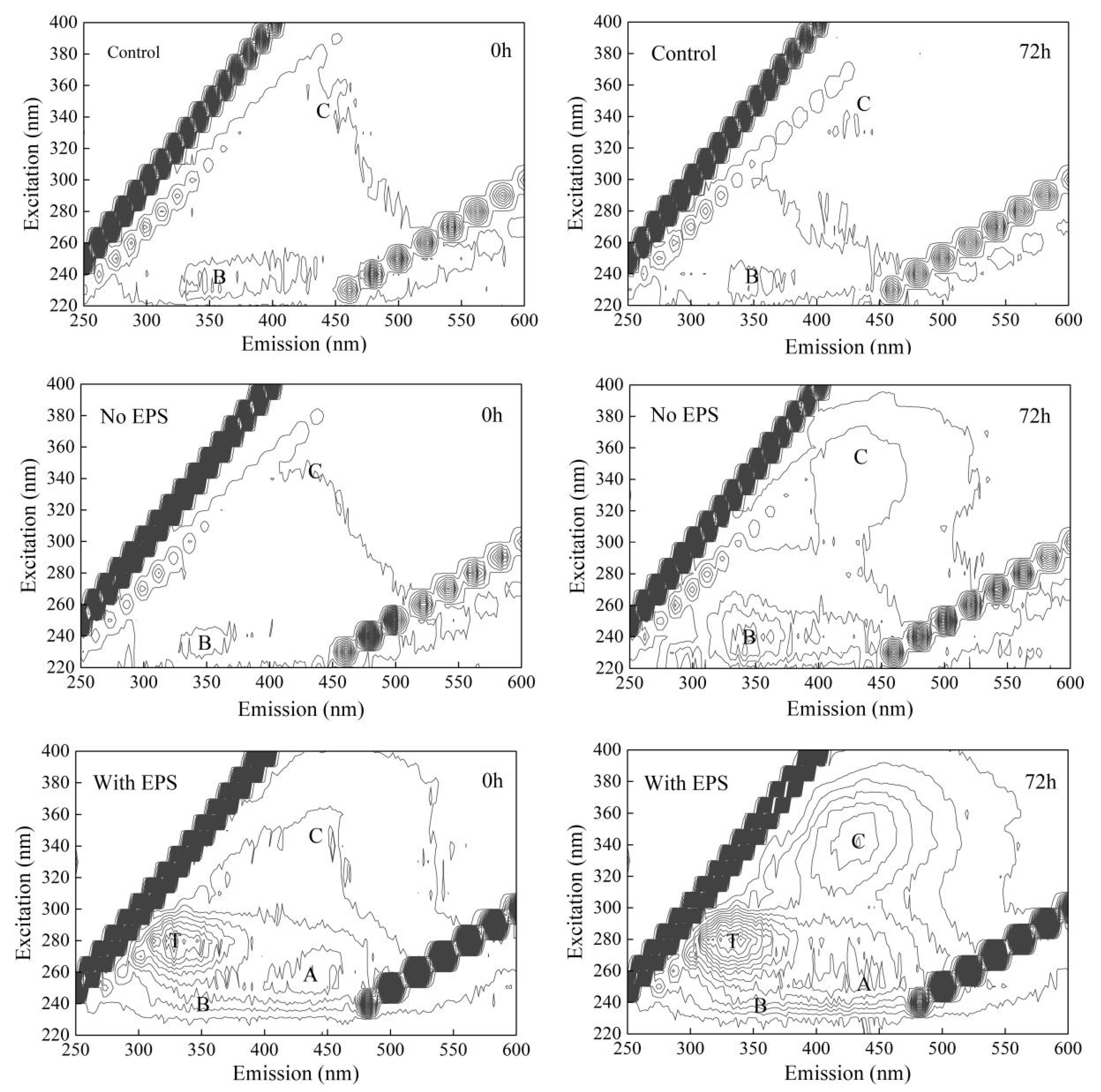
3.5. Analysis of Substance Transformation of Cyanobacteria EPS in the Process of Water Supply Network Transmission and Distribution
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fu, Y.L.; Peng, H.X.; Liu, J.Q.; Nguyen, T.H.; Hashmi, M.Z.; Shen, C.F. Occurrence and quantification of culturable and viable but non-culturable (VBNC) pathogens in biofilm on different pipes from a metropolitan drinking water distribution system. Sci. Total Environ. 2021, 764, 142851. [Google Scholar] [CrossRef]
- Moyo, W.; Chaukura, N.; Msagati, T.A.M.; Mamba, B.B.; Heijman, S.G.J.; Nkambule, T.T.I. The properties and removal efficacies of natural organic matter fractions by South African drinking water treatment plants. Environ. Chem. Eng. 2019, 7, 103101. [Google Scholar] [CrossRef]
- Zhang, C.Q.; Lu, J.G. Optimizing disinfectant residual dosage in engineered water systems to minimize the overall health risks of opportunistic pathogens and disinfection by-products. Sci. Total Environ. 2021, 770, 145356. [Google Scholar] [CrossRef]
- Chen, H.Y.; L, J.Q.; W, Z.Y.; Wei, Z.Y.; Zhang, H.H.; He, X.F.; Zhou, X.Y.; Qiu, S.D.; Hu, B.L.; Lou, L.P. Radial differences in contaminant distribution in large-diameter pipe scales of main pipelines in drinking water distribution system. China Environ. Sci. 2015, 35, 2706–2712. [Google Scholar]
- Pan, R.J.; Zhang, K.J.; Cen, C.; Zhou, X.Y.; Xu, J.; Wu, J.J.; Wu, X.G. Characteristics of biostability of drinking water in aged pipes after water source switching: ATP evaluation, biofilms niches and microbial community transition. Environ. Pollut. 2021, 271, 116293. [Google Scholar] [CrossRef]
- Skjevrak, I.; Lund, V.; Ormerod, K.; Due, A.; Herikstad, H. Biofilm in water pipelines: A potential source for off-flavours in the drinking water. Water Sci. Technol. 2004, 49, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, S.; Gomez-Smith, C.K.; Jeon, Y.; LaPara, T.M.; Waak, M.B.; Hozalski, R.M. Effects of chloramine and coupon material on biofilm abundance and community composition in bench-scale simulated water distribution systems and comparison with full-scale water mains. Environ. Sci. Technol. 2018, 52, 13077–13088. [Google Scholar] [CrossRef]
- Tsvetanova, Z.G.; Dimitrov, D.N. Biofilms and bacteriological water quality in a domestic installation model simulating daily drinking water consumption. Water Sci. Technol.-Water Supply 2012, 12, 720–726. [Google Scholar] [CrossRef]
- Lehtola, M.J.; Juhna, T.; Miettinen, I.T.; Vartiainen, T.; Martikainen, P.J. Formation of biofilms in drinking water distribution networks, a case study in two cities in Finland and Latvia. J. Ind. Microbiol. Biot. 2004, 31, 489–494. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.S.; Chu, C.W. Effects of acetic acid on the regrowth of heterotrophic bacteria in the drinking water distribution system. World J. Microb. Biot. 2005, 21, 989–998. [Google Scholar] [CrossRef]
- Hem, L.J.; Efraimsen, H. Isolation of natural organic matter—The influence on the assimilable organic carbon. Environ. Int. 1999, 25, 367–371. [Google Scholar] [CrossRef]
- Pang, C.M.; Liu, W.T. Biological filtration limits carbon availability and affects downstream biofilm formation and community structure. Appl. Environ. Microbiol. 2006, 72, 5702–5712. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bai, X.H.; Zhang, L.; Zhu, B.; Jiang, C.T. Relationship between water biological stability and the bacteria growth in drinking water distribution system. China Environ. Sci. 2006, 26, 180–182. [Google Scholar]
- Niquette, P.; Servais, P.; Savoir, R. Bacterial Dynamics in the drinking water distribution system of Brussels. Water Res. 2001, 35, 675–682. [Google Scholar] [CrossRef]
- Qian, A.J.; Pan, R.; Sun, F.; Cong, H.B. Contamination and regulation trends of cyanobacterial extracellular polymeric substance. Environ. Pollut. Prev. 2017, 8, 916–919. [Google Scholar]
- Xiao, R.; Yang, X.; Li, M.; Li, X.; Wei, Y.Z.; Cao, M.; Ragauskas, A.; Thies, M.; Ding, J.H.; Zheng, Y. Investigation of composition, structure and bioactivity of extracellular polymeric substances from original and stress-induced strains of Thraustochytrium striatum. Carbohyd. Polym. 2018, 195, 515–524. [Google Scholar] [CrossRef]
- Sun, F.; Zhang, H.Y.; Qian, A.J.; Yu, H.F.; Xu, C.H.; Pan, R.; Shi, Y.J. The influence of extracellular polymeric substances on the coagulation process of cyanobacteria. Sci. Total Environ. 2020, 720, 137573. [Google Scholar] [CrossRef]
- Sun, W.H. Study on pollution characteristics of water soure and safety guarantee technologies of drinking water during cyanobacteria blooming. Master’s Thesis, Shanghai Normal University, Shanghai, China, April 2009. [Google Scholar]
- Peng, F. Experiment research on algae monitoring and removal in drinking water supply system. Master’s Thesis, Xi’an University of Architecture and Technology, Xi’an, China, June 2008. [Google Scholar]
- Pan, R. Research on the Secretion and Release of Free Cyanobacterial Extracellular Polymeric Substances and Its Migration during Water Treatment. Master’s Thesis, Yangzhou University, Yangzhou, China, June 2019. [Google Scholar]
- Liu, X.T.; Wang, M.S.; Zhang, S.J. Application potential of carbon nanotubes in water treatment: A review. Environ. Sci. 2013, 25, 1263–1280. [Google Scholar] [CrossRef]
- Qi, Z.H.; Zhao, Y.; Chen, H.T.; Wu, Z.Y.; Yu, X. Restrictive nutrients in pipe network and their impacts on biofilm formation. Environ. Sci. Manag. 2012, 37, 109–112. [Google Scholar]
- Xu, H.C.; Cai, H.Y.; Yu, G.H.; Jiang, H.L. Insights into extracellular polymeric substances of cyanobacterium Microcystis aeruginosa using fractionation procedure and parallel factor analysis. Water Res. 2013, 47, 2005–2014. [Google Scholar] [CrossRef]
- National Standards of the People’s Republic of China. GB 4789.2-2016 National Food Safety Standard, Food Microbiology Test, Detection of Aerobic Bacterial Count. China, National Health and Family Planning Commission of the People’s Republic of China, State Food and Drug Administration of the People’s Republic of China. 2016. Available online: http://www.360doc.com/document/18/0831/19/10819955_782766052.shtml (accessed on 8 December 2021).
- Liu, B.S.; Yu, Z.Q. A Modified plate count for a strain of highly sticky Bacillus subtilis. Heilongjiang Anim. Sci. Vet. Med. 2011, 9, 19–21. [Google Scholar]
- Li, X.; Ma, J.W. Dynamic method for determination of the biodegradable dissolved organic carban (BDOC) in drinking water. Harbin Inst. Technol. 2005, 37, 1183–1184. [Google Scholar]
- Hammers, F.A.; Egli, T. New method for assimilable organic carbon determination using flow-cytometric enumeration and a natural microbial consortium as inoculum. Environ. Sci. Technol. 2005, 35, 3289–3294. [Google Scholar] [CrossRef]
- Zhao, Z.Y.; Wen, G.; Huang, T.L. Variation of AOC during pre-chlorination of algae-containing water and its mechanism. China Water Wastewater 2016, 32, 46–51. [Google Scholar]
- Song, X.N.; Yu, T.; Zhang, Y.; Zhang, Y.; Yin, X.Y. Distribution characterization and source analysis of dissolved organic matter in Taihu Lake using a three dimensional fluorescence excitation-emission matrix. Acta Scien. Circum. 2010, 30, 2321–2331. [Google Scholar]
- Wei, Y.D.; Ma, J.; Wen, G. Characteristics of algal organics. Water Technol. 2015, 9, 9–14. [Google Scholar]
- Chen, J.X.; Gao, N.Y.; Li, L.; Zhu, M.Q.; Yang, J.; Lu, X.; Zhang, Y.S. Disinfection by-product formation during chlor(am)ination of algal organic matters (AOM) extracted from Microcystis aeruginosa: Effect of growth phases, AOM and bromide concentration. Environ. Sci. Pollut. Res. 2017, 24, 8469–8478. [Google Scholar] [CrossRef]
- National Standards of the People’s Republic of China. GB5749-2006 Standards for Drinking Water Quality. China, Minister of Health of the People’s Republic of China, Standardization Administration of the People’s Republic of China. 2006. Available online: http://c.gb688.cn/bzgk/gb/showGb?type=online&hcno=73D81F4F3615DDB2C5B1DD6BFC9DEC86 (accessed on 8 December 2021).
- Yang, X.P. Effect of turbidity in drinking water on disinfection efficiency and bacterial survival. Foreign Med. (Hyg.) 1983, 3, 184–185. [Google Scholar]
- Escobar, I.C.; Randall, A.A. Assimilable organic carbon (AOC) and biodegradable dissolved organic carbon (BDOC): Complementary measurements. Water Res. 2001, 35, 4444–4454. [Google Scholar] [CrossRef]
- Ren, X.L.; Chen, H.B. Effect of residual chlorine on the interaction between bacterial growth and assimilable organic carbon and biodegradable organic carbon in reclaimed water. Sci. Total Environ. 2021, 752, 141223. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.P.; Li, W.Y.; Wang, F.; Qian, L.; Xu, C.; Liu, Y.; Qi, W.Q. Exploring the biological stability situation of a full scale water distribution system in south China by three biological stability evaluation methods. Chemosphere 2016, 161, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Li, B.L. Community diversity and resistance mechanism of the chlorine-resistant bacteria in drinking water distribution system. Master’s Thesis, Harbin Institute of Technology, Harbin, China, June 2015. [Google Scholar]
- Wang, Y.H.; Wu, Y.H.; Tong, X.; Yu, T.; Peng, L.; Bai, Y.; Zhao, X.H.; Huo, Z.Y.; Ikuno, N.; Hu, H.Y. Chlorine disinfection significantly aggravated the biofouling of reverse osmosis membrane used for municipal wastewater reclamation. Water Res. 2019, 154, 246–257. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Wang, Y.H.; Wu, Y.H.; Wu, Y.H.; Hu, H.Y. Effect of ultraviolet disinfection on growth and secretion characteristics of microorganism in reclaimed water. Environ. Eng. 2020, 38, 28–34. [Google Scholar]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, F.; Yu, P.; Xu, C.; Yu, H.; Wang, F.; Zhou, Y.; Cong, H. Influence Mechanism of Cyanobacterial Extracellular Polymeric Substances on the Water Quality in Dynamic Water Supply System. Sustainability 2021, 13, 13913. https://doi.org/10.3390/su132413913
Sun F, Yu P, Xu C, Yu H, Wang F, Zhou Y, Cong H. Influence Mechanism of Cyanobacterial Extracellular Polymeric Substances on the Water Quality in Dynamic Water Supply System. Sustainability. 2021; 13(24):13913. https://doi.org/10.3390/su132413913
Chicago/Turabian StyleSun, Feng, Peng Yu, Chenhui Xu, Hongfei Yu, Fengyi Wang, Yang Zhou, and Haibing Cong. 2021. "Influence Mechanism of Cyanobacterial Extracellular Polymeric Substances on the Water Quality in Dynamic Water Supply System" Sustainability 13, no. 24: 13913. https://doi.org/10.3390/su132413913
APA StyleSun, F., Yu, P., Xu, C., Yu, H., Wang, F., Zhou, Y., & Cong, H. (2021). Influence Mechanism of Cyanobacterial Extracellular Polymeric Substances on the Water Quality in Dynamic Water Supply System. Sustainability, 13(24), 13913. https://doi.org/10.3390/su132413913




