Sustainable Management of Peanut Shell through Biochar and Its Application as Soil Ameliorant
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Biochar
2.2. Characterization of Biochar
2.3. Pot Trials with PS Biochar
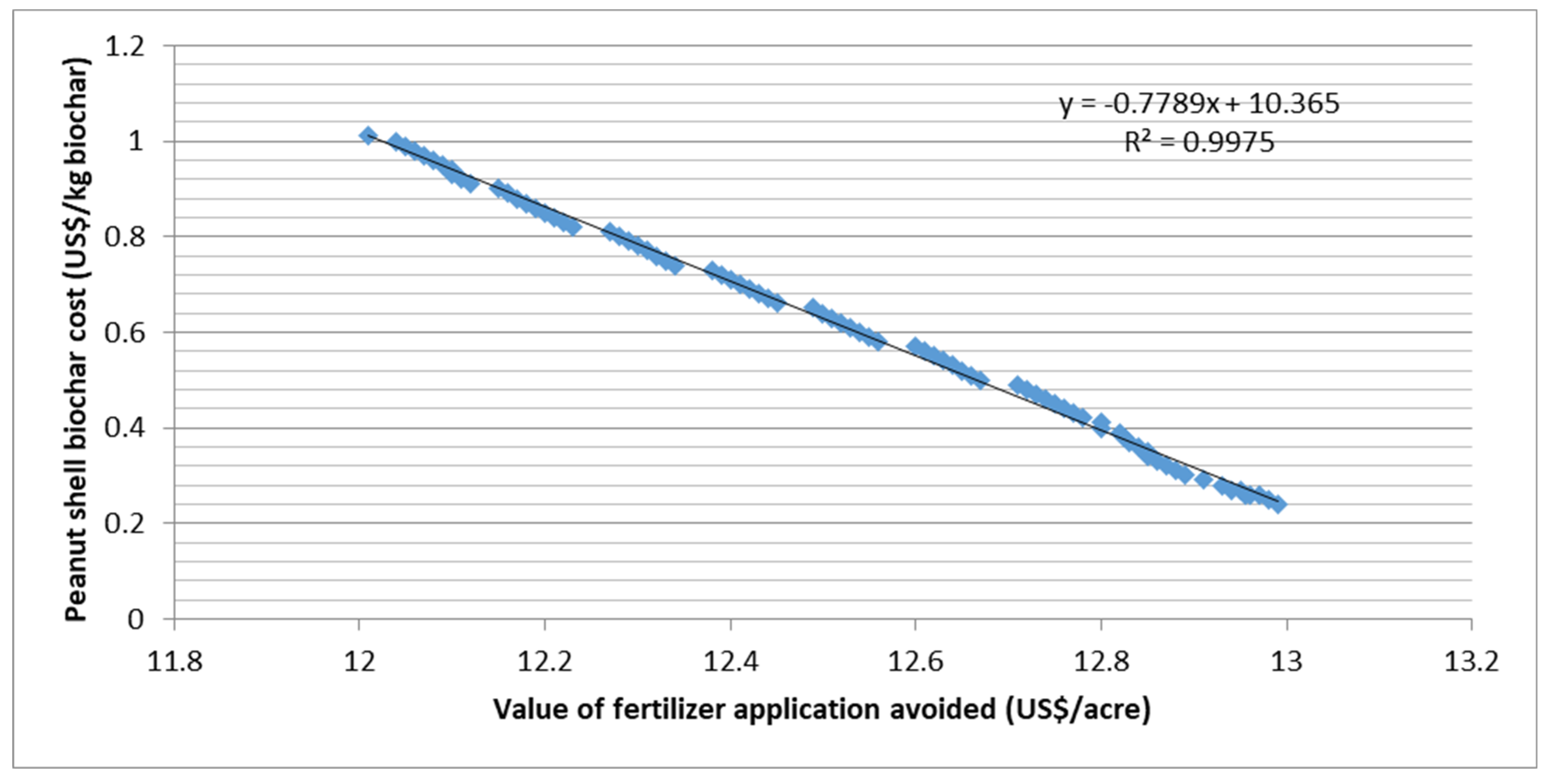
2.4. Prospecting Circular Economy Indicators of PS Biochar
2.5. Statistical Analysis
3. Results and Discussion
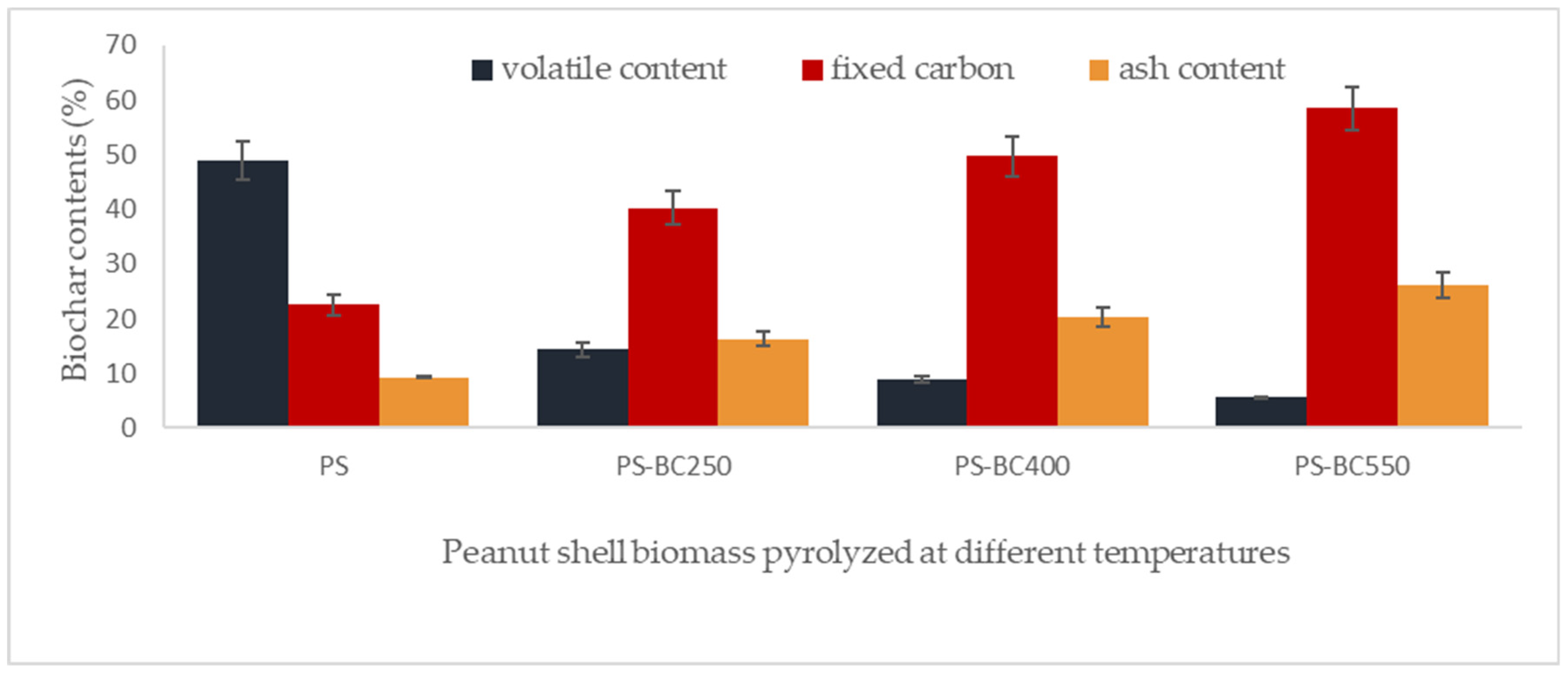
3.1. Proximate Analysis of Peanut Shell and Its Derived Biochars
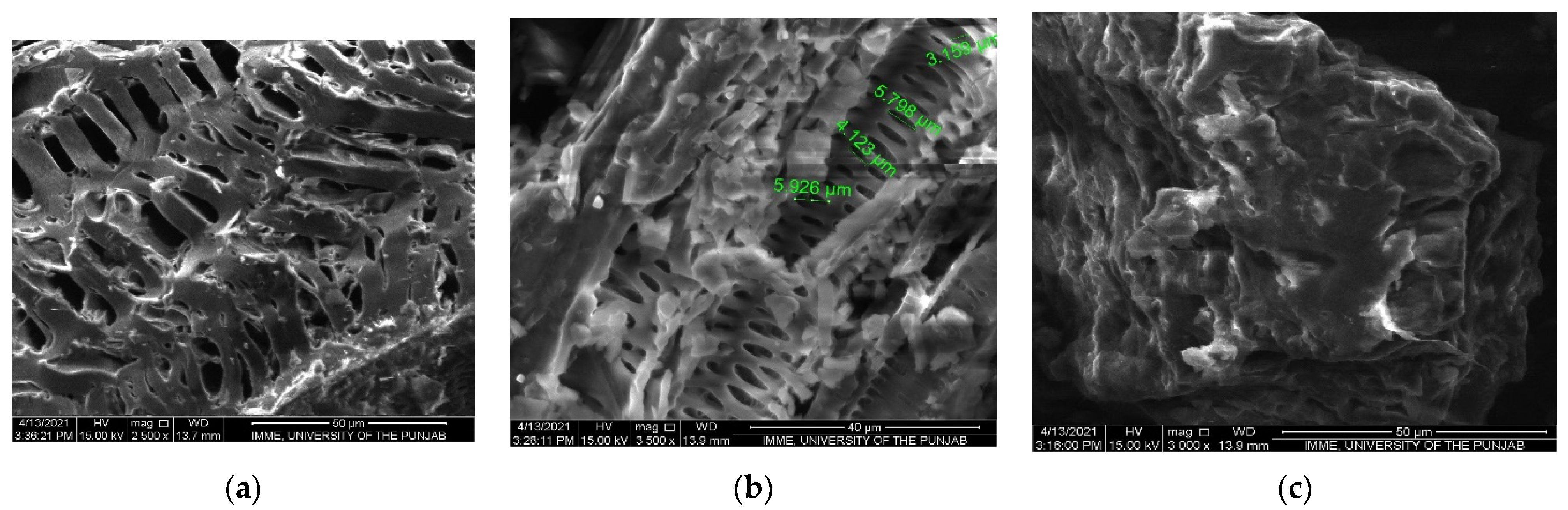
3.2. Scanning Electron Microscopy (SEM)
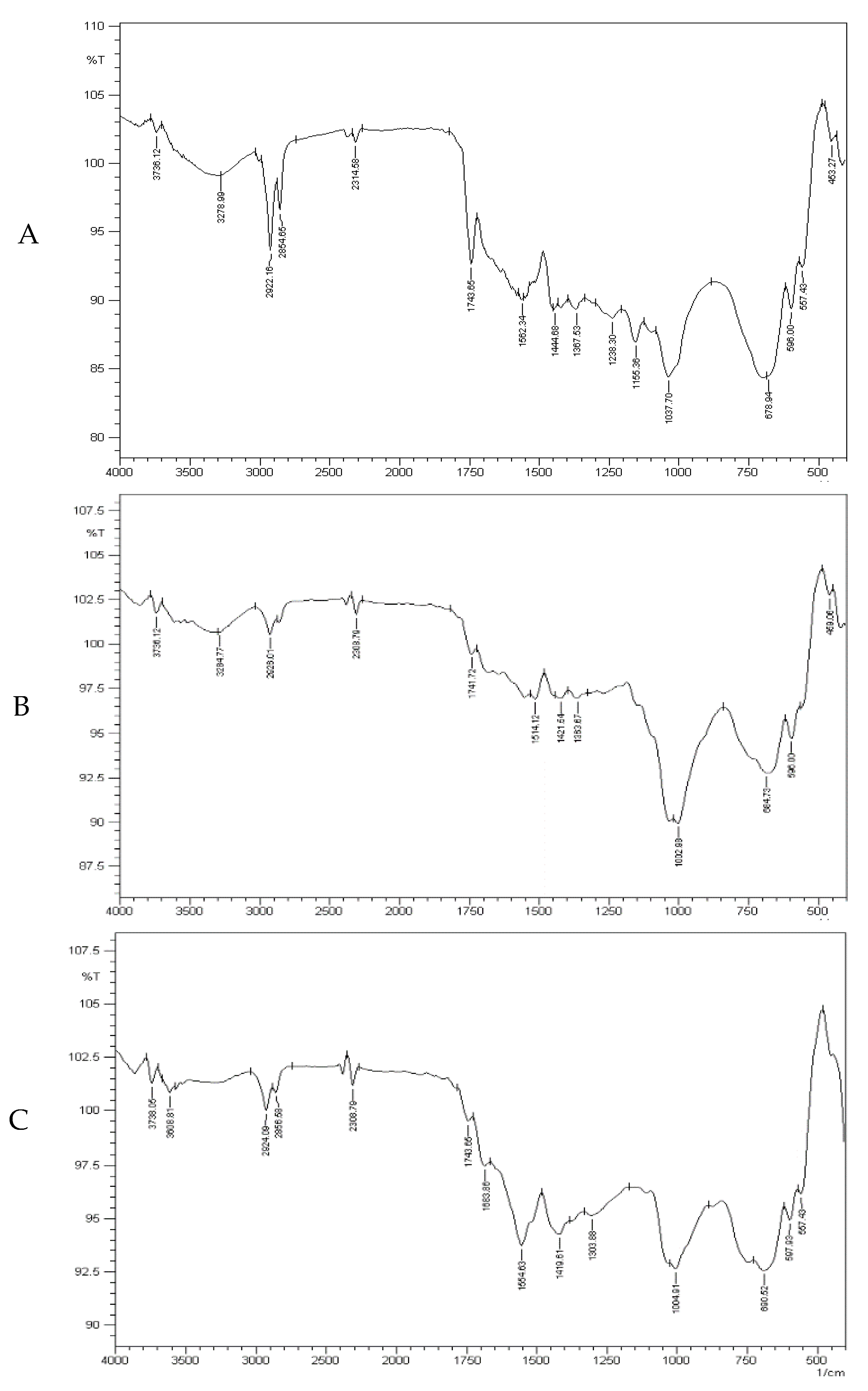
3.3. Spectral Analysis
3.4. Effect of Biochars Prepared at Various Pyrolysis Temperatures on Soil Physico-Chemical Properties
3.5. Effect of Biochar on Growth Parameters
3.6. Potential Role of Peanut Shell Biochar in Circular Economy of Agriculture
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Perea-Moreno, M.-A.; Manzano-Agugliaro, F.; Hernandez-Escobedo, Q.; Perea-Moreno, A.-J. Peanut Shell for Energy: Properties and Its Potential to Respect the Environment. Sustainability 2018, 10, 3254. [Google Scholar] [CrossRef] [Green Version]
- Kristoferson, L.A.; Bokalders, V. 3—Agricultural Residues and Organic Wastes. In Renewable Energy Technologies: Their Applications in Developing Countries; Kristoferson, L.A., Bokalders, V., Eds.; Elsevier: Amsterdam, The Netherlands, 1986. [Google Scholar]
- Hawash, S.; Farah, J.Y.; El-Diwani, G. Pyrolysis of agriculture wastes for bio-oil and char production. J. Anal. Appl. Pyrolysis 2017, 124, 369–372. [Google Scholar] [CrossRef]
- Spokas, K.A.; Cantrell, K.B.; Novak, J.M.; Archer, D.W.; Ippolito, J.A.; Collins, H.P.; Boateng, A.A.; Lima, I.M.; Lamb, M.C.; McAloon, A.J.; et al. Biochar: A Synthesis of Its Agronomic Impact beyond Carbon Sequestration. J. Environ. Qual. 2012, 41, 973–989. [Google Scholar] [CrossRef]
- Lahori, A.H.; Guo, Z.; Zhang, Z.; Li, R.; Mahar, A.; Awasthi, M.K.; Shen, F.; Sial, T.A.; Kumbhar, F.; Wang, P.; et al. Use of Biochar as an Amendment for Remediation of Heavy Metal-Contaminated Soils: Prospects and Challenges. Pedosphere 2017, 27, 991–1014. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, Y.; Zhang, S.; Wang, Y. What could promote farmers to replace chemical fertilizers with organic fertilizers? J. Clean. Prod. 2018, 199, 882–890. [Google Scholar] [CrossRef]
- Hossain, Z.; Bahar, M.; Sarkar, B.; Donne, S.W.; Ok, Y.S.; Palansooriya, K.N.; Kirkham, M.B.; Chowdhury, S.; Bolan, N. Biochar and its importance on nutrient dynamics in soil and plant. Biochar 2020, 2, 379–420. [Google Scholar] [CrossRef]
- Xu, G.; Zhang, Y.; Sun, J.; Shao, H. Science of the Total Environment Negative interactive effects between biochar and phosphorus fertilization on phosphorus availability and plant yield in saline sodic soil. Sci. Total Environ. 2016, 568, 910–915. [Google Scholar] [CrossRef] [PubMed]
- Laghari, M.; Naidu, R.; Xiao, B.; Hu, Z.; Mirjat, M.S.; Hu, M.; Kandhro, M.N.; Chen, Z.; Guo, D.; Jogi, Q.; et al. Recent developments in biochar as an effective tool for agricultural soil management: A review. J. Sci. Food Agric. 2016, 96, 4840–4849. [Google Scholar] [CrossRef]
- Li, H.; Dong, X.; da Silva, E.B.; de Oliveira, L.M.; Chen, Y.; Ma, L.Q. Chemosphere Mechanisms of metal sorption by biochars: Biochar characteristics and modifications. Chemosphere 2017, 178, 466–478. [Google Scholar] [CrossRef]
- Quilliam, R.S.; Glanville, H.; Wade, S.C.; Jones, D.L. Life in the ‘charosphere’—Does biochar in agricultural soil provide a significant habitat for microorganisms? Soil Biol. Biochem. 2013, 65, 287–293. [Google Scholar] [CrossRef]
- Jaafar, N.M.; Clode, P.; Abbott, L. Microscopy Observations of Habitable Space in Biochar for Colonization by Fungal Hyphae from Soil. J. Integr. Agric. 2014, 13, 483–490. [Google Scholar] [CrossRef]
- Zheng, H.; Wang, X.; Chen, L.; Wang, Z.; Xia, Y.; Zhang, Y.; Wang, H.; Luo, X.; Xing, B. Enhanced growth of halophyte plants in biochar-amended coastal soil: Roles of nutrient availability and rhizosphere microbial modulation. Plant Cell Environ. 2017, 41, 517–532. [Google Scholar] [CrossRef]
- Ahmad, M.; Lee, S.S.; Dou, X.; Mohan, D.; Sung, J.-K.; Yang, J.E.; Ok, Y.S. Effects of pyrolysis temperature on soybean stover- and peanut shell-derived biochar properties and TCE adsorption in water. Bioresour. Technol. 2012, 118, 536–544. [Google Scholar] [CrossRef] [PubMed]
- Bhaduri, D.; Saha, A.; Desai, D.; Meena, H.N. Restoration of carbon and microbial activity in salt-induced soil by applica-tion of peanut shell biochar during short-term incubation study. Chemosphere 2016, 148, 86–98. [Google Scholar] [CrossRef]
- Bolognesi, S.; Bernardi, G.; Callegari, A.; Dondi, D.; Capodaglio, A.G. Biochar production from sewage sludge and microal-gae mixtures: Properties, sustainability and possible role in circular economy. Biomass Convers. Biorefin. 2021, 11, 289–299. [Google Scholar] [CrossRef]
- ASTM International. Standard Practice for Proximate Analysis of Coal and Coke; ASTM International: West Conshohocken, PA, USA, 2013. [Google Scholar]
- Baird, R.B.; Eaton, A.D.; Rice, E.W. Standard Methods for the Examination of Water and Wastewater; American Public Health Association: Washington, DC, USA, 2017; Volume 23, pp. 5–41. [Google Scholar]
- Bordoloi, N.; Narzari, R.; Chutia, R.S.; Bhaskar, T.; Kataki, R. Pyrolysis of Mesua ferrea and Pongamia glabra seed cover: Characterization of bio-oil and its sub-fractions. Bioresour. Technol. 2015, 178, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Brewer, C.E.; Levine, J. Weight or Volume for Handling Biochar and Biomss? In the Biochar Journal 2015, Arbaz, Switzerland. Available online: www.biochar-journal.org/en/Ct/71 (accessed on 15 September 2021).
- Chapman, H. Cation-Exchange Capacity. Agron. Monogr. 2016, 9, 891–901. [Google Scholar]
- Gessert, G. Measuring a medium’s Airspace and Water Holding Capacity. Ornam. Northwest Arch. 1976, 1, 11–12. [Google Scholar]
- Mrówczyńska-Kamińska, A.; Bajan, B.; Pawłowski, K.P.; Genstwa, N.; Zmyślona, J. Greenhouse gas emissions intensity of food production systems and its determinants. PLoS ONE 2021, 16, e0250995. [Google Scholar] [CrossRef]
- Ain, Q.-U.; Shafiq, M.; Capareda, S.C.; Bareen, F.-E. Effect of different temperatures on the properties of pyrolysis products of Parthenium hysterophorus. J. Saudi Chem. Soc. 2021, 25, 101197. [Google Scholar] [CrossRef]
- Butnan, S.; Deenik, J.; Toomsan, B.; Vityakon, P. Biochar properties affecting carbon stability in soils contrasting in texture and mineralogy. Agric. Nat. Resour. 2017, 51, 492–498. [Google Scholar] [CrossRef]
- Cao, X.; Harris, W. Properties of dairy-manure-derived biochar pertinent to its potential use in remediation. Bioresour. Technol. 2010, 101, 5222–5228. [Google Scholar] [CrossRef]
- Chen, B.; Zhou, D.; Zhu, L. Transitional adsorption and partition of nonpolar and polar aromaric contaminants by biochars of pine needles with different pyrolytic temperatures. Environ. Sci. Technol. 2008, 42, 5137–5143. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Chen, C.; Gray, E.M.; Boyd, S.E. Effect of feedstock and pyrolysis temperature on properties of biochar governing end use efficacy. Biomass Bioenergy 2017, 105, 136–146. [Google Scholar] [CrossRef]
- Suliman, W.; Harsh, J.B.; Abu-Lail, N.I.; Fortuna, A.-M.; Dallmeyer, I.; Garcia-Perez, M. Influence of feedstock source and pyrolysis temperature on biochar bulk and surface properties. Biomass Bioenergy 2016, 84, 37–48. [Google Scholar] [CrossRef]
- Xu, C.-Y.; Bai, S.H.; Hao, Y.; Rachaputi, R.C.N.; Xu, Z.; Wallace, H. Peanut shell biochar improves soil properties and peanut kernel quality on a red Ferrosol. J. Soils Sediments 2015, 15, 2220–2231. [Google Scholar] [CrossRef]
- Sathe, P.S.; Adivarekar, R.V.; Pandit, A.B. Valorization of peanut shell biochar for soil amendment. J. Plant Nutr. 2021, 1–19. Available online: https://www.tandfonline.com/doi/pdf/10.1080/01904167.2021.1963771?casa_token=zb3FczP6xt4AAAAA:kf1eRalGYZEvswNSPuEYUFT8FAZOEsHWRiMc3MkxAajKfjZuLA0l9OTfJ8Ebr4vYUFsBWpi9vjrLPw (accessed on 12 November 2021). [CrossRef]
- Zhang, H.; Voroney, R.; Price, G. Effects of temperature and processing conditions on biochar chemical properties and their influence on soil C and N transformations. Soil Biol. Biochem. 2015, 83, 19–28. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, P.; Yuan, X.; Li, Y.; Han, L. Effect of pyrolysis temperature and correlation analysis on the yield and physicochemical properties of crop residue biochar. Bioresour. Technol. 2020, 296, 122318. [Google Scholar] [CrossRef]
- Novak, J.M.; Busscher, W.J.; Laird, D.L.; Ahmedna, M.; Watts, D.W.; Niandou, M.A.S. Impact of Biochar Amendment on Fertility of a Southeastern Coastal Plain Soil. Soil Sci. 2009, 174, 105–112. [Google Scholar] [CrossRef] [Green Version]
- Usman, A.R.; Abduljabbar, A.; Vithanage, M.; Ok, Y.S.; Ahmad, M.; Ahmad, M.; Elfaki, J.; Abdulazeem, S.S.; Al-Wabel, M.I. Biochar production from date palm waste: Charring temperature induced changes in composition and surface chemistry. J. Anal. Appl. Pyrolysis 2015, 115, 392–400. [Google Scholar] [CrossRef]
- Irfan, M.; Chen, Q.; Yue, Y.; Pang, R.; Lin, Q.; Zhao, X.; Chen, H. Co-production of biochar, bio-oil and syngas from halo-phyte grass (Achnatherum splendens L.) under three different pyrolysis temperatures. Bioresour. Technol. 2016, 211, 457–463. [Google Scholar] [CrossRef]
- Chutia, R.S.; Kataki, R.; Bhaskar, T. Characterization of liquid and solid product from pyrolysis of Pongamia glabra deoiled cake. Bioresour. Technol. 2014, 165, 336–342. [Google Scholar] [CrossRef]
- Reddy, P.M.K.; Mahammadunnisa, S.; Ramaraju, B.; Sreedhar, B.; Subrahmanyam, C. Low-cost adsorbents from bio-waste for the removal of dyes from aqueous solution. Environ. Sci. Pollut. Res. 2013, 20, 4111–4124. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Zhang, Y.; Wang, H.; Lu, W.; Zhou, Z.; Zhang, Y.; Ren, L. Influence of pyrolysis temperature on characteristics and heavy metal adsorptive performance of biochar derived from municipal sewage sludge. Bioresour. Technol. 2014, 164, 47–54. [Google Scholar] [CrossRef]
- Guo, S.-H.; Niu, Y.-J.; Zhai, H.; Han, N.; Du, Y.-P. Effects of alkaline stress on organic acid metabolism in roots of grape hybrid rootstocks. Sci. Hortic. 2018, 227, 255–260. [Google Scholar] [CrossRef]
- Dai, Z.; Zhang, X.; Tang, C.; Muhammad, N.; Wu, J.; Brookes, P.C.; Xu, J. Potential role of biochars in decreasing soil acidification—A critical review. Sci. Total Environ. 2017, 581-582, 601–611. [Google Scholar] [CrossRef]
- Laila, U.-E.; Hussain, A.; Nazir, A.; Shafiq, M.; Bareen, F.-E. Potential Application of Biochar Composite Derived from Rice Straw and Animal Bones to Improve Plant Growth. Sustainability 2021, 13, 11104. [Google Scholar] [CrossRef]
- Du, Z.; Xiao, Y.; Qi, X.; Liu, Y.; Fan, X.; Li, Z. Peanut-Shell Biochar and Biogas Slurry Improve Soil Properties in the North China Plain: A Four-Year Field Study. Sci. Rep. 2018, 8, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Siebers, N.; Leinweber, P. Bone Char: A Clean and Renewable Phosphorus Fertilizer with Cadmium Immobilization Capability. J. Environ. Qual. 2013, 42, 405–411. [Google Scholar] [CrossRef] [Green Version]
- Luo, X.; Liu, G.; Xia, Y.; Chen, L.; Jiang, Z.; Zheng, H.; Wang, Z. Use of biocharcompost to improve properties and productivity of the degraded coastal soil in the Yellow River Delta, China. J. Soils Sediments 2017, 17, 780–789. [Google Scholar] [CrossRef]
- Liu, B.; Cai, Z.; Zhang, Y.; Liu, G.; Luo, X.; Zheng, H. Comparison of efficacies of peanut shell biochar and biochar-based compost on two leafy vegetable productivity in an infertile land. Chemosphere 2019, 224, 151–161. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zheng, H.; Jiang, Z.; Dai, Y.; Liu, G.; Chen, L.; Luo, X.; Liu, M.; Wang, Z. Efficacies of biochar and biochar-based amendment on vegetable yield and nitrogen utilization in four consecutive planting seasons. Sci. Total Environ. 2017, 593-594, 124–133. [Google Scholar] [CrossRef]
- Zheng, H.; Wang, Z.; Deng, X.; Herbert, S.; Xing, B. Impacts of adding biochar on nitrogen retention and bioavailability in agricultural soil. Geoderma 2013, 206, 32–39. [Google Scholar] [CrossRef]
- de la Rosa, J.M.; Paneque, M.; Miller, A.Z.; Knicker, H. Relating physical and chemical properties of four different biochars and their application rate to biomass production of Lolium perenne on a Calcic Cambisol during a pot experiment of 79days. Sci. Total Environ. 2014, 499, 175–184. [Google Scholar] [CrossRef] [Green Version]
- Tubiello, F.N. Greenhouse Gas Emissions Due to Agriculture. In Encyclopedia of Food Security and Sustainability; Ferranti, P., Berry, E.M., Anderson, J.R., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; Volume 1, pp. 196–205. [Google Scholar] [CrossRef] [PubMed]
- Czyżewski, B.; Łukasz, K. Impact of different models of agriculture on greenhouse gases (GHG) emissions: A sectoral approach. Outlook Agric. 2018, 47, 68–76. [Google Scholar] [CrossRef] [Green Version]
- Popp, A.; Lotze-Campen, H.; Bodirsky, B.L. Food consumption, diet shifts and associated non-CO2 greenhouse gases from agricultural production. Glob. Environ. Chang. 2010, 20, 451–462. [Google Scholar] [CrossRef]


| PS | PS-BC250 | PS-BC400 | PB-BC550 | ||
|---|---|---|---|---|---|
| Biochar Yield (%) | - | 41.0 ± 2.28 | 36.0 ± 2.16 | 33.0 ± 2.03 | |
| Chemical Constituents (wt.%) | C | 28 ± 2.33 | 55 ± 4.02 | 59 ± 4.12 | 62 ± 4.66 |
| N | 6 ± 0.65 | 2 ± 0.05 | 1.60 ± 0.04 | 1.30 ± 0.03 | |
| H | 5.6 ± 0.08 | 2.8 ± 0.12 | 2.02 ± 0.08 | 1.8 ± 0.06 | |
| O | 36 ± 2.87 | 26 ± 2.88 | 18 ± 1.66 | 13 ± 1.02 | |
| C/N | 5 ± 0.08 | 27 ± 2.33 | 36 ± 2.68 | 47 ± 3.55 | |
| H/C | 0.19 ± 0.03 | 0.05 ± 0.02 | 0.04 ± 0.03 | 0.02 ± 0.02 | |
| HHV (MJ kg−1) | 11.851 ± 1.67 | 18.389 ± 2.06 | 19.903 ± 2.12 | 21.323 ± 2.44 | |
| Mineral Constituents (g kg−1) | K | 0.233 ± 0.04 | 0.81 ± 0.08 | 1.02 ± 0.10 | 1.66 ± 0.13 |
| Ca | 0.113 ± 0.06 | 0.241 ± 0.07 | 0.94 ± 0.13 | 1.226 ± 0.14 | |
| Mg | 0.101 ± 0.02 | 0.251 ± 0.04 | 0.421 ± 0.02 | 0.355 ± 0.04 | |
| P | 0.09 ± 0.01 | 0.109 ± 0.02 | 0.138 ± 0.06 | 0.153 ± 0.05 | |
| Cu | 0.010 ± 0.01 | 0.031 ± 0.02 | 0.060 ± 0.02 | 0.091 ± 0.03 | |
| Fe | 0.098 ± 0.02 | 0.198 ± 0.02 | 0.255 ± 0.03 | 0.368 ± 0.03 | |
| Zn | 0.002 ± 0.01 | 0.005 ± 0.01 | 0.008 ± 0.01 | 0.012 ± 0.02 |
| pH | ECe | CEC (cmolc kg−1) | SA (m2 g−1) | PV (cm3 g−1) | APS (µm) | BD (g cm−3) | |
|---|---|---|---|---|---|---|---|
| PS-BC250 | 8.4 b,c ± 0.04 | 0.29 b,c ± 0.02 | 11 c ± 0.32 | 44.20 c ± 3.32 | 0.1040 c ± 0.02 | ND | 0.54 a ± 0.06 |
| PS-BC400 | 8.8 b ± 0.03 | 0.38 b ± 0.02 | 13.2 b,c ± 1.44 | 88.34 b,c ± 4.28 | 0.1186 b,c ± 0.03 | 4.72 b ± 0.14 | 0.44 b,c ± 0.04 |
| PB-BC550 | 9.4 a ± 0.04 | 0.49 a ± 0.03 | 17.41 a ± 1.88 | 100.21 a ± 4.66 | 0.1378 a ± 0.02 | 7.06 a ± 0.23 | 0.28 c ± 0.03 |
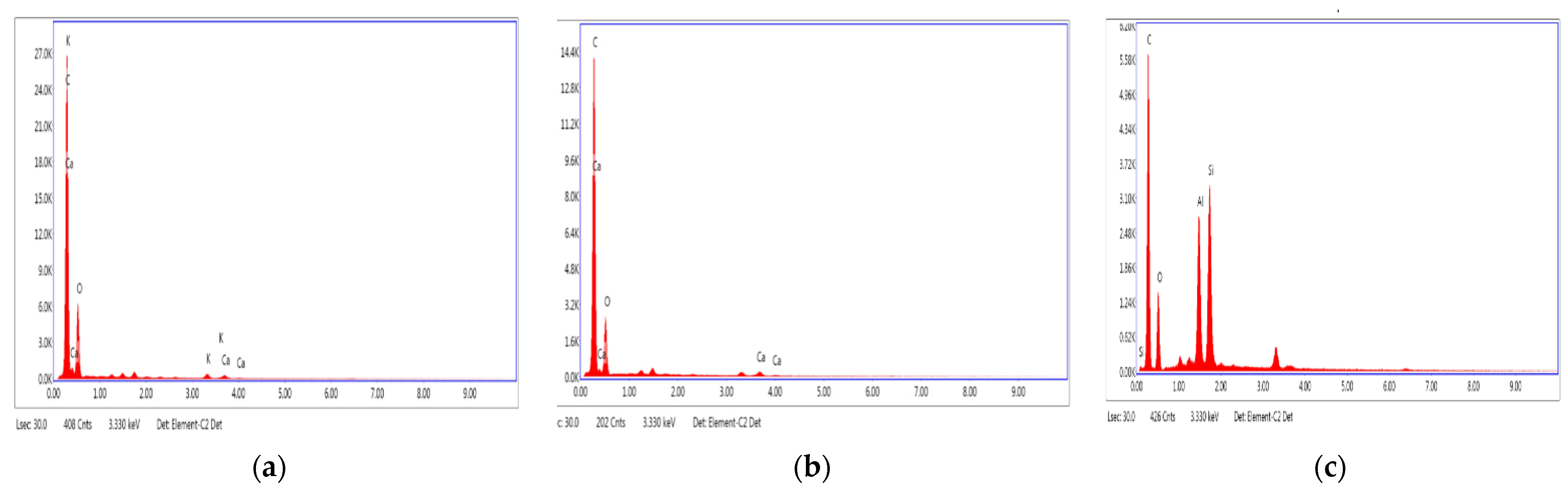
| Samples | C | O | K | Ca | ||||
|---|---|---|---|---|---|---|---|---|
| PS-BC250 | Weight (%) | Atomic (%) | Weight (%) | Atomic (%) | Weight (%) | Atomic (%) | Weight (%) | Atomic (%) |
| 62.42 ± 4.44 | 69.94 ± 5.12 | 34.50 ± 2.54 | 29.02 ± 2.23 | 1.54 ± 0.06 | 0.53 ± 0.18 | 1.54 ± 0.49 | 0.52 ± 0.12 | |
| PS-BC400 | C | O | Ca | |||||
| Weight (%) | Atomic (%) | Weight (%) | Atomic (%) | Weight (%) | Atomic (%) | |||
| 67.12 ± 6.13 | 73.91 ± 5.23 | 30.66 ± 3.23 | 25.35 ± 2.31 | 10.25 ± 2.02 | 5.50 ± 0.24 | |||
| PS-BC550 | C | O | Al | Si | ||||
| Weight (%) | Atomic (%) | Weight (%) | Atomic (%) | Weight (%) | Atomic (%) | Weight (%) | Atomic (%) | |
| 62.50 ±4.33 | 75.28 ± 5.12 | 13.31 ±1.23 | 12.04 ± 1.02 | 10.25 ± 1.05 | 5.50 ± 0.66 | 13.94 ±1.82 | 7.18 ± 0.23 | |
| Treatments | Conc. | Growth Performance | ||||
|---|---|---|---|---|---|---|
| Plant Height (cm) | Plant Dry Biomass (g) | Fruit Yield (No. of Fruit pot−1) | Fruit Weight (g pot−1) | Chlorophyll Contents (SPAD Values) | ||
| Control | 68 G ± 4.56 | 2.83 G ± 0.21 | 4 E ± 0.12 | 280 F ± 4.45 | 32.2 G ± 2.23 | |
| PS-BC250 | 2% | 70 b,E,F ± 4.05 | 2.8 b,E,F ± 0.23 | 6 b,D ± 0.22 | 420 b,D ± 3.88 | 34.2 b,E ± 3.44 |
| 4% | 74 a,E ± 4.88 | 2.90 a,E ± 0.11 | 7 a,C ± 0.02 | 546 a,C,D ± 5.33 | 36.1 a,C ± 3.82 | |
| 6% | 65 c,F ± 5.66 | 2.66 c,F ± 0.32 | 4 c,E ± 0.23 | 282 c,E,F ± 6.06 | 30.8 c,H ± 2.88 | |
| PS-BC400 | 2% | 74 b,E ± 5.23 | 3.13 b,D,E ± 0.44 | 7 b,C ± 0.24 | 560 b,C ± 5.88 | 33.4 b,E,F ± 2.79 |
| 4% | 84 a,D ± 4.66 | 4.31 a,C ± 0.12 | 9 a,B ± 0.06 | 729 a,B ± 6.82 | 35.6 a,D ± 3.02 | |
| 6% | 72 c,D,E ± 5.02 | 3.12 b,D,E ± 0.14 | 4c,D ± 0.04 | 289 b,B ± 4.26 | 32.0 c,G ± 3.04 | |
| PS-BC550 | 2% | 98 b,B ± 4.99 | 4.20 b,B ± 0.43 | 9 b,B ± 0.22 | 710 b,B,C ± 5.44 | 37.2 b,B ± 3.66 |
| 4% | 110 a,A ± 6.88 | 5.52 a,A ± 0.58 | 12 a,A ± 0.23 | 880 a,A ± 6.54 | 39.8 a,A ± 3.64 | |
| 6% | 95 c,C ± 5.72 | 3.28 c,D ± 0.66 | 5 c,D,E ± 0.02 | 302 c,E ± 4.88 | 32.4 c,F ± 3.08 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nazir, A.; Laila, U.-e.-; Bareen, F.-e.-; Hameed, E.; Shafiq, M. Sustainable Management of Peanut Shell through Biochar and Its Application as Soil Ameliorant. Sustainability 2021, 13, 13796. https://doi.org/10.3390/su132413796
Nazir A, Laila U-e-, Bareen F-e-, Hameed E, Shafiq M. Sustainable Management of Peanut Shell through Biochar and Its Application as Soil Ameliorant. Sustainability. 2021; 13(24):13796. https://doi.org/10.3390/su132413796
Chicago/Turabian StyleNazir, Aisha, Um-e- Laila, Firdaus-e- Bareen, Erum Hameed, and Muhammad Shafiq. 2021. "Sustainable Management of Peanut Shell through Biochar and Its Application as Soil Ameliorant" Sustainability 13, no. 24: 13796. https://doi.org/10.3390/su132413796
APA StyleNazir, A., Laila, U.-e.-, Bareen, F.-e.-, Hameed, E., & Shafiq, M. (2021). Sustainable Management of Peanut Shell through Biochar and Its Application as Soil Ameliorant. Sustainability, 13(24), 13796. https://doi.org/10.3390/su132413796






