1. Introduction
Chlorides have long been held as the main drivers of steel reinforcement corrosion in concrete structures in marine environments such as for coastal, harbor and offshore applications [
1]. The effects of such corrosion include the possibly serious impairment of structural capacity, unacceptable visual impact, high repair and maintenance costs and associated undesirable environmental impacts. Proposals to obviate these issues include using alternative materials such as glass-fiber or carbon-fiber instead of steel, additives to reduce concrete permeability and thus inward diffusion of chloride ions and also additives to aid concrete workability to reduce segregation. However, it seems to have been forgotten that there is a long history of very successful reinforced concrete (RC) structures made with conventional reinforcement steels and without additives of any kind and that these have performed, and in some cases still are performing, remarkably well after many decades of exposure in severe marine environments and elsewhere [
2,
3]. Some of these older concrete structures were made with seawater as mixing water [
4], and for many years, adding extra water to achieve workable concrete mixes was common practice [
5]. Since neither practice is now permitted, the question must be asked—what allowed many of the earlier reinforced concrete structures to survive so long? This question is addressed herein, referenced to an example structure some 80 years old and examined annually for some 30 years. As will become evident, the answers have implications for achieving long-term durability for steel-reinforced concrete structures. In turn, this has implications for structural replacement and effective use of materials and, thus, implications for the sustainability of RC structures.
The next section briefly overviews several important recent state-of-the-art research findings about the mechanisms for steel reinforcement corrosion initiation and long-term development. These findings depart considerably from the conventional wisdom but, importantly, are based on detailed observations and investigations for multiple actual RC structures, supplemented with careful observations of the extended performance (14 years) of a large set of laboratory model concretes. These observations reinforce the notion that the concept of a ‘critical chloride concentration’, so long ingrained in the conventional wisdom [
1], is flawed. The recent investigations have led to the development of a model for the long-term progression of reinforcement corrosion under different conditions. That model, described further below, departs considerably from the well-known Tuutti model [
1], mainly because the latter has been built on assumptions no longer justified and on results from electrochemical testing in fluids rather than in real or model concretes and also on results from short-term experiments (typically 3–12 months). The artificial test conditions do not mimic actual field conditions over the extended exposure periods relevant for real infrastructure.
It is recognized that observations on actual structures and the interpretation of results sometimes is problematic. However, extensive experience shows that field observations can provide useful information and insights when the observations are interpreted on the basis of corrosion fundamentals such as those reviewed herein and also on previous experience. An example of this is given below for the field observations of an 80-year-old RC structure observed annually for some 30 years. The observations support recent conclusions from laboratory observations that construction practices and quality of construction can have considerable influence on the rate of progression and severity of steel reinforcement corrosion [
6] and, hence, on durability and sustainability of RC structures in marine environments.
2. Background
The ‘concretes’ such as those used in reinforced concrete (RC) structures have a very long history and owe their durability to the types of aggregates and pozzolans used in the mix. Reinforced concrete structures are much more recent in origin but do have a remarkable history, often forgotten or ignored. Extensive technical documentation of the performance of early RC structures exposed to marine environments is available as summarized more than 60 years ago (e.g., [
4]) and again recently [
3]. Some of the RC structures showed significant reinforcement corrosion within a few years of exposure, yet others showed no such signs. More recently, other cases and examples have become available, including the more than 150 Phoenix caissons placed during WW2 on the coast of Normandy to form breakwaters for the Mulberry B harbor as part of the Allied invasion in 1944. These were intended as temporary structures, yet after more than 75 years of exposure to the harsh marine environment of the English Channel, they show remarkably little evidence of reinforcement corrosion [
7]. While some corrosion of reinforcement is visible, it is mainly at yield lines (plastic hinges) between concrete panels where the concrete had been severely damaged and concrete cover lost, and also at poorly made (‘cold’) construction joints. This is despite the very high chloride concentrations in the concretes next to the steel bars as measured by concrete cores taken at various locations. These also showed high concrete strengths and, by implication, low concrete permeability, despite the design of the caissons being very simple and the concrete specified without regard to durability and permitting the addition of water to the concrete mix [
5].
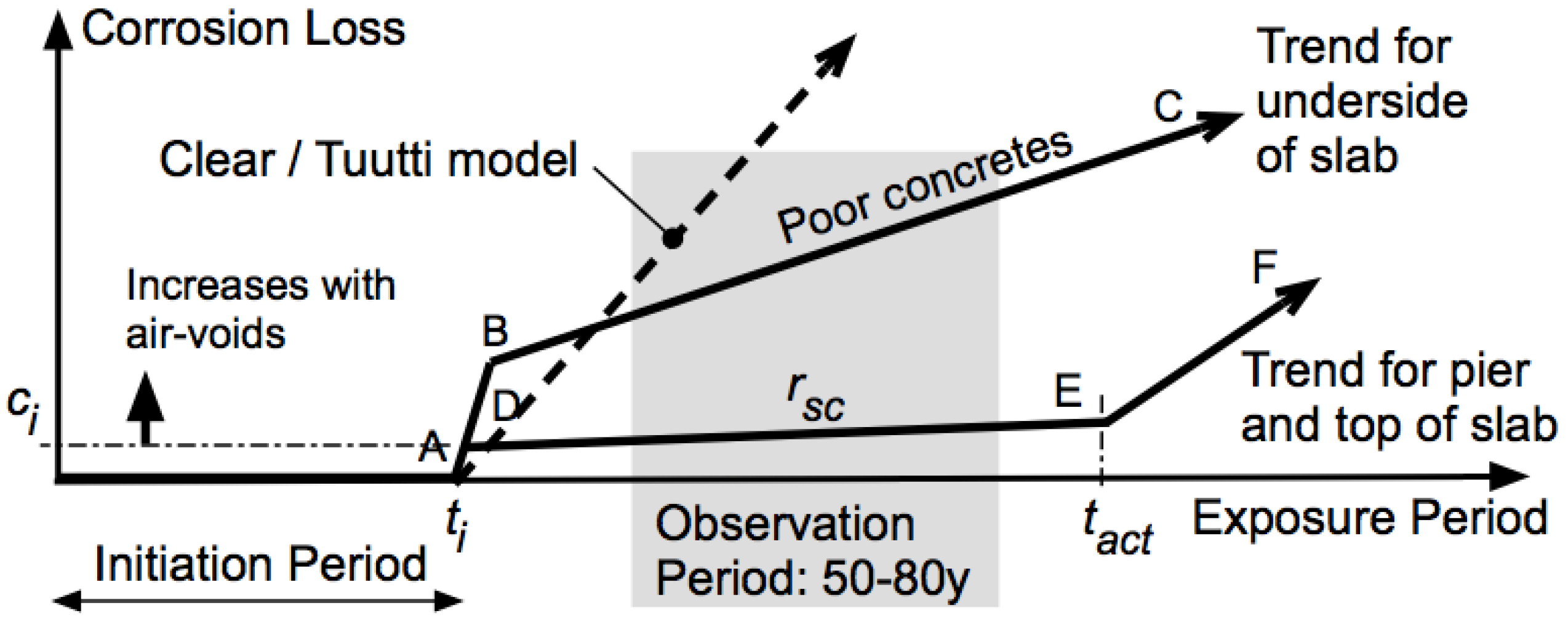
The initiation of reinforcement corrosion and its development leading to structural damage has for a long time been assumed to follow the simple bi-linear model attributed to Tuutti [
8] but earlier postulated by Clear [
9]. In this model, reinforcement corrosion commences at time
ti (
Figure 1), postulated to occur when a sufficiently high concentration of chlorides have reached the reinforcement bars [
1]. Although many experiments have been carried out to determine critical values for this concentration, an unsatisfactory high degree of scatter remains [
10]. Unfortunately, for testing the Tuutti model very few ‘longitudinal’ studies (i.e., studies of the progression of corrosion over the extended periods relevant for actual structures) are available. However, using multiple observations reported in the literature for actual RC structures, a different model is now available, originally proposed in a simpler form by Melchers and Li [
11]. That model has a more complex development of reinforcement corrosion loss after
ti. It provides for a degree of reinforcement corrosion loss
ci, after which reinforcement corrosion progresses at a much lower rate
rsc, more so for good quality concretes. Eventually, at time
tact, the loss of passivating alkalis, which also leads to higher permeability, permit longer-term corrosion, typically at a high rate.
Figure 1 shows the more recent, expanded version of that corrosion model [
12].
The notion that high concentrations of chlorides will ‘initiate’ reinforcement corrosion conflicts directly with practical experience, extending back to the early days of reinforced concrete (e.g., [
4]). There are many current examples in practice of excellent long-term behavior for reinforced concrete structures made with seawater [
3,
13]. There also are sound theoretical reasons why chlorides have little role in general corrosion, as noted already many decades ago [
14]. Additionally, classical results from controlled laboratory experiments for steels show that the main role of chlorides lies not in directly advancing general corrosion but in promoting pitting corrosion [
15,
16].
Recent detailed laboratory observations have shown that corrosion of steel embedded in concrete commences is pitting corrosion within wet air-voids in the concrete immediately adjacent to the steel [
17]. This is made possible as a result of reducing oxygen availability within the air-voids and subsequent pitting corrosion at low pH in the otherwise alkaline concrete matrix and pore waters. Consistent with fundamental corrosion mechanics [
15], the pitting corrosion is made more severe by the presence of chlorides [
17]. It directly controls the local corrosion, shown as
ci in
Figure 1. Since larger air-voids contain more oxygen, they are likely to produce deeper or more severe pitting, and with the size of the air-voids likely related to concrete permeability or porosity, concretes with low permeability or porosity are advantageous for controlling early corrosion of reinforcement. In most concretes, there is no spatial preference for the distribution of permeability or porosity voids, and thus, typically localized (pitting) corrosion occurs at multiple locations along a steel bar.
Pitting corrosion is associated with low solution pH and, therefore, tends to progress quickly, as seen in trend (A–B) [
15,
18]. As oxygen is drawn from the voids, corrosion slows (B–C) and, subsequently, may rely on oxygen diffusion inward from the external environment. For good-quality concretes, this is difficult, particularly through a wet concrete matrix, permitting only a low rate of corrosion (
rsc). Typically, under the resulting low oxygen availability, the main cathodic reaction for corrosion will become the (slow) hydrogen evolution reaction [
12]. Of course, depletion of water at the corrosion interface also slows the rate
rsc (
Figure 1). Both the declining rate of corrosion and the low long-term corrosion rate
rsc have been observed in practical concretes. One example concerns near-full-size RC beams exposed for 12 years in a simulated atmospheric corrosion environment [
19]. A declining corrosion rate has been observed also for model RC specimens made with seawater and continuously monitored during 14 years of exposure [
17].
Even for high-quality, low-permeability/porosity concretes,
Figure 1 shows that, after
tact is reached, the corrosion rate significantly increases (E–F). This is the result of a completely different corrosion mechanism. It occurs as a result of the loss of concrete alkalis that, for extended exposures, is mainly calcium hydroxide. Usually, it is only sparingly soluble in water and takes a long time for it to leach out of a concrete matrix and to lower the pore solution pH at the reinforcement. In the presence of chlorides, however, the dissolution rate of calcium hydroxide increases significantly [
20], and the pH drops relatively faster. The dissolution of calcium hydroxide also leaves an open and permeable structure suitable for entry of atmospheric or other oxygen [
21]. Together with the reduced pH, this then provides an environment in which corrosion can occur, as verified in controlled experiments. This situation corresponds to (E–F) in
Figure 1.
The rate of dissolution of calcium hydroxide depends much on the surface area available for dissolution within the concrete matrix. That surface area will be greater for more permeable, open concretes. Indeed, for the laboratory specimens, those having greater apparent permeability showed greater loss of alkali. This, then, is the third reason for the need for high-quality, low-permeability concretes—to delay
tact (
Figure 1) as long as possible.
3. Field Observations
Comparing the model in
Figure 1 with independent field observations made over an extended period of time usually is difficult because only a few ‘longitudinal’ records are available. However, occasionally there is an exception. One such is described below, inspected periodically over more than 30 years by one of the authors (R.E.M.).
The structural system consists of a number of cast-in-situ reinforced concrete piers along the upper part of the beach along Pacific Ocean at Bar Beach (NSW, Australia) supporting a 200 mm thick RC slab (
Figure 2) with a 40–50 mm concrete cover (top and bottom) to the reinforcement and located some 1–2 m above the beach sand. The site is about 5 km south of Newcastle harbor and subject to occasional very intense storm conditions. An example of this in May 1974 can be seen by comparing
Figure 2 to a historic photograph (
https://i0.wp.com/lostnewcastle.com.au/wp-content/uploads/2013/05/bar-beach-may-74-jeffrey-fergusson.jpg?ssi=1; last accessed 28 June 2021). This online photograph also shows the original 1930s pavilion in reinforced concrete construction, considered outdated and demolished in the mid-1980s and replaced with the current, more modest constructions (
Figure 2). Local surfers who had surfed at the beach for decades (i.e., since their pre-teenage years and then known colloquially as ‘grommets’) noted that, as best they could recall, there had been no renovation or maintenance of the piers or of the concrete slab at any time. Although the local council archives hold a few photographs of the original area around the pavilion, no records, such as maintenance records, could be located.
One RC pier is of particular interest. It is located along the ocean side of the 1930s reinforced concrete slab. For many years, about half of the upper-most part of the pier was visible above the sand (
Figure 3). It was the only one partly offset from the edge of the slab (
Figure 3a). Already during the late 1980s, it was noted that, when any sand on top of the out-stand was brushed away, the ends of vertical steel reinforcement bars could be seen from above (
Figure 3b). This showed that there was no concrete cover over the ends of the bars, and these themselves appeared to be quite smooth, partly corroded but without evidence of corrosion products.
Figure 4 shows a typical detail of the corrosion of the vertical steel bars in
Figure 3b.
The condition of the pier and the slab was examined irregularly over some 30 years from 1987 to 2017. Throughout that period, remarkably little changed in the visual appearance of the pier and of the concrete slab, including the part of its underside exposed on the occasions when beach sand had been washed away. For the pier, there was no evidence of concrete cracking, concrete spalling or visual evidence of corrosion products, despite the obviously high-chloride environment and the relatively high mean annual rainfall (1020 mm/year). This was also the case for other, similar piers located at the edge and also directly exposed to the atmosphere until 2017–2018, when there were significant construction works during which an additional RC slab with support structure was added (
Figure 5).
In 2017, the pier was partly demolished to make room for a wooden ramp for incapacitated surfers and others. This required demolition of the outstanding upper part of the pier (
Figure 3). Initial efforts with a standard-sized electric impact hammer proved very difficult, attributed by the demolition contactor to the ‘hardness’ of the concrete (
Figure 6). Heavier equipment eventually allowed demolition, and this revealed the excellent condition of the concrete, the main vertical steel reinforcement bars and the horizontal steel hoops. There was little evidence of reinforcement corrosion loss anywhere, except for very localized corrosion within the bends of the hoops (
Figure 7), corresponding to their ‘intersection’ with vertical reinforcement bars. Although there was no evidence of rust staining on the external surfaces (
Figure 3 and
Figure 5) at the intersection of the bars, rust staining was noted within the concrete (
Figure 6a). This is consistent with the localized corrosion of the hoop bars at this location in the concrete. Concrete cover was typically 50–60 mm, except for a short rebated section partway down the pier where the cover was only about 15 mm (
Figure 3a).
The demolition process and Occupational Health and Safety requirements did not permit the taking of concrete core samples from the pier. It also was not possible to take such cores from the existing, in-use, concrete slab. Even if they could have been taken, there are serious issues with interpreting test results for such older concretes [
7]. Some reliance, therefore, had to be placed on visible observations of the quality of the concrete and any revealed reinforcement surfaces, guided therein by many years of practical professional engineering and academic research experience. Spot checks on randomly selected pieces of concrete salvaged from the top of the pier at the time of demolition showed concrete pH values >9 (using MColorpHast
® strips, Merck, Darmstadt, Germany), indicating remaining presence of concrete alkalis. Observations made during the demolition process also confirmed the lack of evidence of spalling of concrete cover and lack of concrete cracking, including longitudinally along the vertical bars. It also showed very good interfacial contact between the concrete and the steel bars (cf.
Figure 6b). The concrete showed no evidence of voids, either next to the reinforcement bars overall or within the concrete matrix. This is entirely consistent with recent laboratory observations for 14-year-old concretes that showed only minor amounts of rust on the reinforcement bars despite very high chloride concentrations. However, the localized corrosion seen in
Figure 7 is consistent with the occurrence of very localized, severe corrosion at air-voids in the concrete immediately adjacent to the steel [
17]. In the case of
Figure 7, such air-voids almost certainly were present at the intersection between the hoop and the longitudinal reinforcement. The aggregate was seen to be slightly green in color, typical for crushed blast furnace slag, a waste product material from the (then) local iron and steel mills. It was at one time widely used as a coarse aggregate for concretes. It is a product known to be hard and brittle and high in calcium carbonate content. Over the 30 years of observation, the condition of the pier did not appear to change to any noticeable degree.
The concrete slab (
Figure 2), when viewed from above, showed little or no evidence of surface cracking (other than some hairline temperature and shrinkage cracks) or of reinforcement corrosion. However, the underside of the slab, where it could be inspected during low tide and when much of the sand had been washed away by storm conditions, showed, along the ocean-side edge region, a quite different state of affairs (
Figure 5). There was much loss of under-slab concrete cover to the reinforcement, evidence of voids, typically around 6 mm in size, in the remaining concrete, including around the reinforcement bars. Exposed reinforcement showed clear evidence of corrosion and corrosion products. As for the pier, over the 30 years of observation the degree of deterioration of the slab did not appear to change to any noticeable degree.
4. Analysis of Observations
The observations in
Figure 3,
Figure 4,
Figure 5,
Figure 6 and
Figure 7 essentially are ‘snap-shots’ at the time of demolition (in 2017) but, as can be seen by comparing to the literature referred to in the Introduction (and summarized in Melchers and Chaves [
3]), are not atypical of many well-made RC structures exposed to marine environments for extended periods of time. The important observation for the present structure is that the condition of the concrete and of the exposed visible reinforcement changed remarkably little over the 30 years of exposure to 2017 and, where still visible, have changed little to date (2021). This includes the upper part of the RC slab and the exposed upper part of the top of the RC pier. Importantly, the reinforcement corrosion and the associated spalling damage along the underside of the slab were evident already in 1987. Thus, in terms of the corrosion loss trends in
Figure 1, both are likely to have suffered most of the damage now still visible relatively early in the life of the structural system. This interpretation is consistent with a period for chloride diffusion (0 −
ti in
Figure 1), followed by A–D and then the subsequent slow rate of corrosion
rsc during phase D–E, covering the period of observation approximately 50–80 years after construction. The concrete for these parts appeared, as noted, to be very well compacted, showing high strength and low porosity (and likely low permeability) and little evidence of voids. Furthermore, the concrete pH > 9 in the immediate vicinity of the reinforcement implies a low loss of concrete alkali and that
tact had not been reached, even after some 80 years exposure.
The only exception to this generally favorable situation was the hoop reinforcement in the pier (
Figure 3) that showed, during demolition, highly localized corrosion in the bend region (
Figure 7), where it had been in contact with vertical reinforcement. As noted, it is likely that concrete compaction in this small region was not sufficient to completely eliminate one or more local air-voids. Their presence would have permitted highly localized pitting and differential aeration of the steels but only under chloride-rich conditions. Consistent with laboratory observations [
17], this undoubtedly is the reason for the observed deep, localized corrosion. Similarly, along the bottom of the slab, the concrete visible after the loss of the concrete cover showed clear evidence of the presence of larger air-voids, undoubtedly due to poor concrete compaction along the bottom of the bars. For beams and slabs, this is common problem in practice (e.g., [
22]).
At least from about 1987, and probably from much earlier, at the top of the RC pier (
Figure 3 and
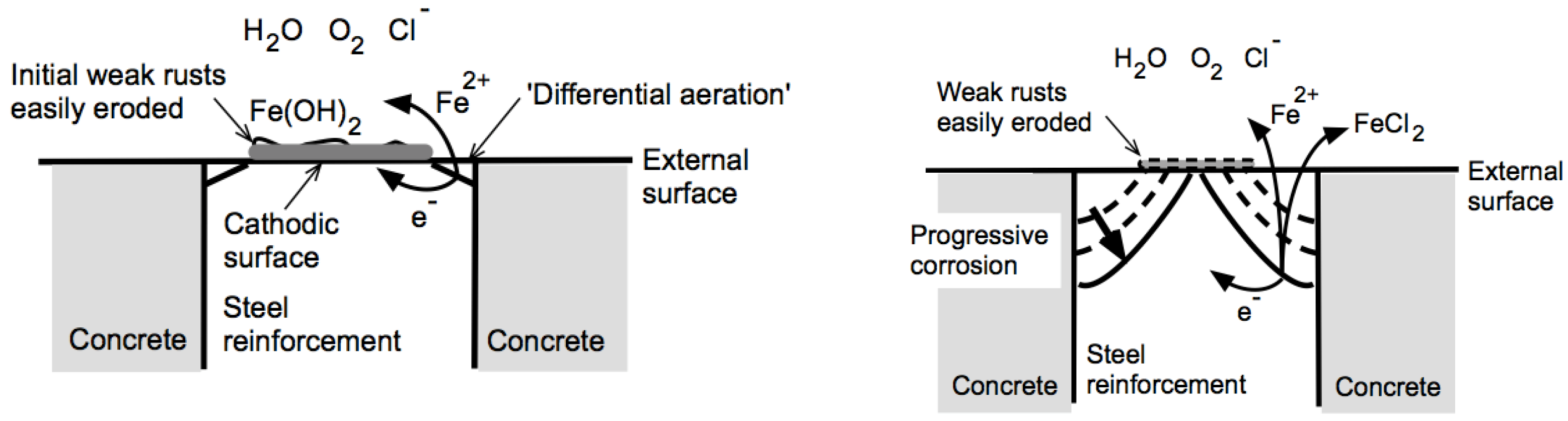
Figure 4), the ends of the vertical reinforcement bars were not protected with cover concrete. The ends were exposed directly to the local high-chloride marine atmosphere and, as noted, often covered with (chloride-rich) beach sand and also periodically wet from sea-spray and occasionally from extreme high tide and storm waters and wet from rain. This is an aggressive environment and makes the pattern of corrosion (
Figure 4) particularly interesting. It very clearly is not more or less uniform corrosion over the ends of the bars. Rather, all the bars show, to varying degrees, a truncated conical pattern of corrosion, with most metal loss at the outer periphery and least metal loss at the center of the bars. This pattern almost certainly is the result of corrosion, despite the lack of evidence of rust staining.
The corrosion pattern in
Figure 3 and
Figure 4 has been seen before. It was observed at the intersection of a deep, fine (hairline) crack intersecting the reinforcement for 80-year-old, void-free, highly impermeable, high-strength concrete columns exposed in warmer temperature Pacific Ocean seawater [
23]. That corrosion was attributed eventually to the aggressive action of ferrous chloride, the only marine corrosion product that is highly soluble in water and that has a low solution pH. It can form only under very low oxygen and anoxic conditions [
24]. Each of the reinforcement bars that outcrop at the concrete surface would have been subject to ‘differential aeration’ [
25] around the periphery of the bar where it meets the concrete. It is a phenomenon often noted at sealed edges around a steel element or for sealants around the circumference of a steel pipe [
26]. Similarly, for the steel bar surrounded by concrete, likely at elevated pH, at least in the earlier stages, a ring of more severe corrosion can be expected (
Figure 8). Under initial corrosion conditions, the cathode is in the central zone of the end of the bar and, thus, not subject to significant corrosion. As the corrosion increases around the peripheral zone, the central zone remains the cathode, although as corrosion increases, the cathodic corrosion process moves from oxygen reduction in Mode 1 to the cathodic hydrogen evolution reaction in Mode 2 (
Figure 1). Any rust depositing on the cathodic region initially will be weak and easily eroded by occasional, discontinuous atmospheric wind and seawater wave action. The relatively severe corrosion in the periphery region (
Figure 8 right) occurs under essentially anaerobic conditions. Further, the presence of chlorides from the external environment, together with the pitting involved, allows generation of soluble ferrous chloride [
18]. This can leach out and, under the action of seawater and rainfall, wash away, leaving little or no trace. Similar scenarios have been reported for deep cracks in concrete columns and beams that, after many years of exposure in wet chloride-rich marine conditions, leave reinforcement corrosion loss patterns very similar to that in
Figure 4 [
12,
24].
5. Discussion
Before proceeding, it is relevant to put the pier–slab structure in context. It is almost certain that the slab and the piers supporting it were cast at about the same time, in the 1930s, using the same concrete mixing and batching equipment and placement technology and presumably the same standard concrete mix [
5], similar aggregates and cement and using the same compaction technique(s). The latter most likely was hand-rodding. Mechanical vibrators and similar did not appear until the 1940s [
27]. Hence, apart from the fact that part of the system being considered was a pier and part a slab, they should be comparable, particularly in terms of their long-term performance in essentially the same marine atmospheric environment.
That RC structures can remain viable over many decades and not be subject to reinforcement corrosion, even in aggressive marine environments, has been noted before [
3,
4,
7] and is supported by the present observations for an 80-year-old RC pier and slab system observed in some detail for about 30 years. Both the observations reported herein and those for earlier structures show the opposite of the conventional wisdom that RC structures do not last long in marine environments. Specifically, the increasingly available evidence indicates that, despite very high concentrations of chlorides in the concretes for long periods (decades), reinforcement corrosion either does not initiate or progress for high-quality concretes with low permeability. This is entirely consistent with the detailed observations from 14-year exposures of a variety of model concretes and with the interpretations that have been made from those observations [
17,
22]. These have demonstrated that, contrary to the conventional wisdom, chlorides are not the main drivers of the initiation or the progression of reinforcement corrosion in concrete. Instead, the long-term (30 year) observations of the concrete at Bar Beach and the fortuitous opportunity to inspect the concrete after some 80 years of exposure provides yet another set of observations that support the notion, derived from laboratory observations [
17,
22], that low concrete permeability and, by implication, a high degree of concrete compaction all around the reinforcement bars are crucial to the important role of delaying the onset and progression of reinforcement corrosion. As noted in the earlier experimental work, one aspect of good compaction is that it governs the size and number of (wet) air-voids in the concrete and, importantly, also at the interface between concrete and steel. The air-voids and the (lack of adequate) compaction are directly responsible for corrosion initiation, a process only made possible in high-pH concrete by the presence of chlorides [
17]. The chlorides are necessary to achieve the thermodynamic conditions that permit commencement of corrosion [
16]. Without voids and the air and water they contain, corrosion initiation without chlorides to cause local pitting would not be possible. The Bar Beach RC structural system with its high-strength, very dense, low-permeability/porosity concrete provides direct field evidence of this.
The other aspect for long-term durability also relates to compaction of the concrete. The porosity/permeability achieved through concrete compaction governs the internal surface area available for the dissolution of calcium hydroxide. As is well-known, the maintenance of the presence of calcium hydroxide in the interfacial zone is a necessary condition for maintenance of passivity, that is, through keeping the interfacial pH sufficient high for corrosion inhibition. Once the passivity is lost, through leaching of calcium hydroxide, perhaps accelerated by the presence of chlorides that also increase concrete permeability [
22], damaging reinforcement corrosion can occur (i.e., at
tact in
Figure 1). It follows directly that compaction, and specifically very good compaction, around the reinforcement bars is crucial for achieving long-term durability of reinforced concrete. Again, the observations at Bar Beach support this concept.
Compaction is a workmanship matter and certainly is recognized as important in practical concrete construction practice, although it is normally associated with reducing the ingress by diffusion of atmospheric or seawater chlorides. A number of requirements for achieving good compaction of concrete around reinforcement bars exist, including the need for adequate bar spacing relative to maximum concrete aggregate size and limiting the ratio between minimum concrete cover and maximum aggregate size. However, the effectiveness of conventional equipment for compaction, such as poker vibrators, appears to have received little or no research investigation, particularly in terms of achieving small air-voids at the concrete–steel interface. In the light of the recent findings considered above, effective means for achieving low to negligible air-voids at the concrete–steel interface would benefit from further investigation.
One other aspect important for long-term durability of reinforced concrete also needs to be considered—the type of minerals in the aggregates in the concrete. An earlier investigation, covering a review of some 300 reinforced concrete structures, mainly highway bridges, indicated that both
ti and
tact (
Figure 1) appear to be extended for concretes made with calcareous (coarse) aggregate, such as limestone or non-reactive dolomite [
2]. That investigation followed an earlier one of 60-year-old concretes that contained seashells and were also likely made with seawater. For the more than 1000 cast-in-situ reinforced concrete elements, all subject to coastal atmospheric exposure, there was very little evidence of reinforcement corrosion [
28]. The effectiveness of (coarse) calcareous aggregates in inhibiting reinforcement corrosion was demonstrated recently in a controlled, 14-year-long laboratory study of model reinforced concrete elements that, despite being made with seawater as mixing water, showed very little reinforcement corrosion, even when broken open to reveal the steel bars [
29]. When examined under the microscope (optical and SEM) the concretes made with calcareous aggregates showed a much less open concrete matrix structure than the concretes made with igneous or siliceous aggregates, indicating greater permeability or porosity or both. Further, the concretes made with the calcareous aggregate also showed a quite homogeneous, apparently void-free, almost featureless internal structure, indicating a small internal surface area available for alkali dissolution. This is consistent with the slower rate of loss of calcium hydroxide.
The reason for the above observations can be found in the little-known work of Farran [
30], who observed, using an optical microscope, that bonding between calcareous aggregates and calcium hydroxide was much greater than for concretes made with igneous or siliceous aggregates. Farran interpreted this as chemical bonding and noted the much lower porosity (and possibly permeability) for the calcareous concretes. Graves [
31] provided independent verification of these observations using concretes made with Florida limestones and others made with Georgia granites. He also noted that factors such as surface roughness and the shape of the aggregates could also be involved [
32].
The concretes at Bar Beach (
Figure 2) were noted to contain larger aggregates of a greenish color (
Figure 6b), known locally as being crushed blast furnace slag. It is a highly calcareous by-product of iron production and was for many years widely used in local concretes. Given the above comments about the interaction of calcareous aggregates and calcium hydroxide, it follows that, apart from the compaction of the concretes, the extended life of the RC piers and the upper part of the concrete slab may have been influenced by the presence of calcareous aggregate. Irrespective of their contribution to the life of the Bar Beach concretes, it is clear, more generally, that the use of calcareous aggregates is likely to enhance long-term reinforcement corrosion durability.
The RC pier and slab at Bar Beach showed no evidence of concrete cracking and associated rust staining and, importantly, no evidence of hairline concrete cracking. Almost certainly, this accounts for the lack of localized, very aggressive reinforcement corrosion inside the concrete (
Figure 6). It contrasts with observations for some other RC structures for which very severe reinforcement corrosion was discovered, where deep hairline cracks intersected with the reinforcement bars [
24,
29]. Likely, the hairline cracks permitted diffusion of chlorides inward to the steel reinforcement where, under the low oxygen conditions and associated low pH conditions, ferrous chloride was formed [
25]. Unlike other rust products, ferrous chloride is highly soluble and, thus, can leach out easily through the hairline cracks. Once exposed to the atmosphere or to oxygenated seawater, it either oxidizes to the usual FeOOH-type rust products, settling on local external surfaces as rust staining, or it may be transported away in seawater or rainwaters. Corrosion of this type has been estimated to progress at around 0.2 mm/y in 20 °C seawaters and around 0.12 mm/y in 10 °C seawaters [
12]. While limitations on maximum crack width are given in most specifications, these cannot be translated to limits on maximum crack depth, particularly under variable loadings. This matter requires urgent research attention.
No evidence was observed for the Bar Beach concretes of deterioration of the concrete itself (apart from minor surface discoloration and slight increased roughness). In this context, it is noted that, over the last few years, increasing evidence has been found that some aggregates used in marine concretes may react chemically with the alkali generated by the cement setting process. This is the so-called alkali–aggregate reactivity, in which the aggregate expands and thereby weakens the concrete matrix and the concrete cover and potentially exposes reinforcement to the environment [
33]. In marine environments, the resulting corrosion is easily attributed to ‘chloride-induced’ corrosion, in part because, for concrete with reinforcement close to the surface, the surface expression of the corrosion damage is similar to that for conventional reinforcement corrosion [
34]. For this reason, it appears alkali reactivity of the concrete as a driver for reinforcement corrosion has been largely overlooked until recently [
35].
The Bar Beach example given herein and the many cases summarized earlier [
2,
3] are clear evidence that excellent, long-lasting RC structures can be achieved with basic concrete and steel as materials, provided excellent workmanship and good construction practices are followed. It is now clear that these should aim to produce concretes of very low permeability, thereby ensuring zero or very small voids at the concrete–steel interface and minimal internal surface areas for alkali leaching from the concrete matrix. These cases of excellent long-term durability should not be seen as ‘isolated’ or ‘unusual’ or ‘special’ cases as sometimes claimed. Rather, they should be seen as examples of what can be, and has been, achieved with cement-based concretes with conventional reinforcement, without additives, and even without limitations on added water, and without undue concern about chloride concentrations within the concrete. The field evidence given herein and in earlier examples is overwhelming—it is entirely feasible to produce long-lasting RC structures without reinforcement corrosion, even when exposed to marine environments. It is important to understand, as is now possible, the factors that result in such excellent performance. Producing such structures is important not only for asset owners in reduced maintenance and possibly rebuilding costs but also in the more effective use of materials and obviating the need for alternative materials. There are also obvious implications for sustainability.