Enhanced Optimization of Bioethanol Production from Palm Waste Using the Taguchi Method
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Palm Fiber and Frond Collection
2.1.2. Chemicals
2.1.3. Microorganisms and Enzymes
2.2. Methods
2.2.1. Physiochemical Analysis
2.2.2. Total Carbohydrates Estimation
2.2.3. Qualitative Examination
2.2.4. Quantitative Determination
2.2.5. Dewaxing PF and PFN
2.2.6. Alkaline Extraction Optimization
2.2.7. Taguchi Model Runs Analysis
2.2.8. Experimental Model Validation
2.2.9. FT-IR Spectroscopy
2.2.10. Enzymatic Hydrolysis Optimization
2.2.11. Glucose Concentration Determination
2.2.12. Bacterial Growth
2.2.13. Fermentation Optimization
2.2.14. Distillation and Bioethanol Determination
3. Results and Discussion
3.1. Chemical Composition and Total Carbohydrates
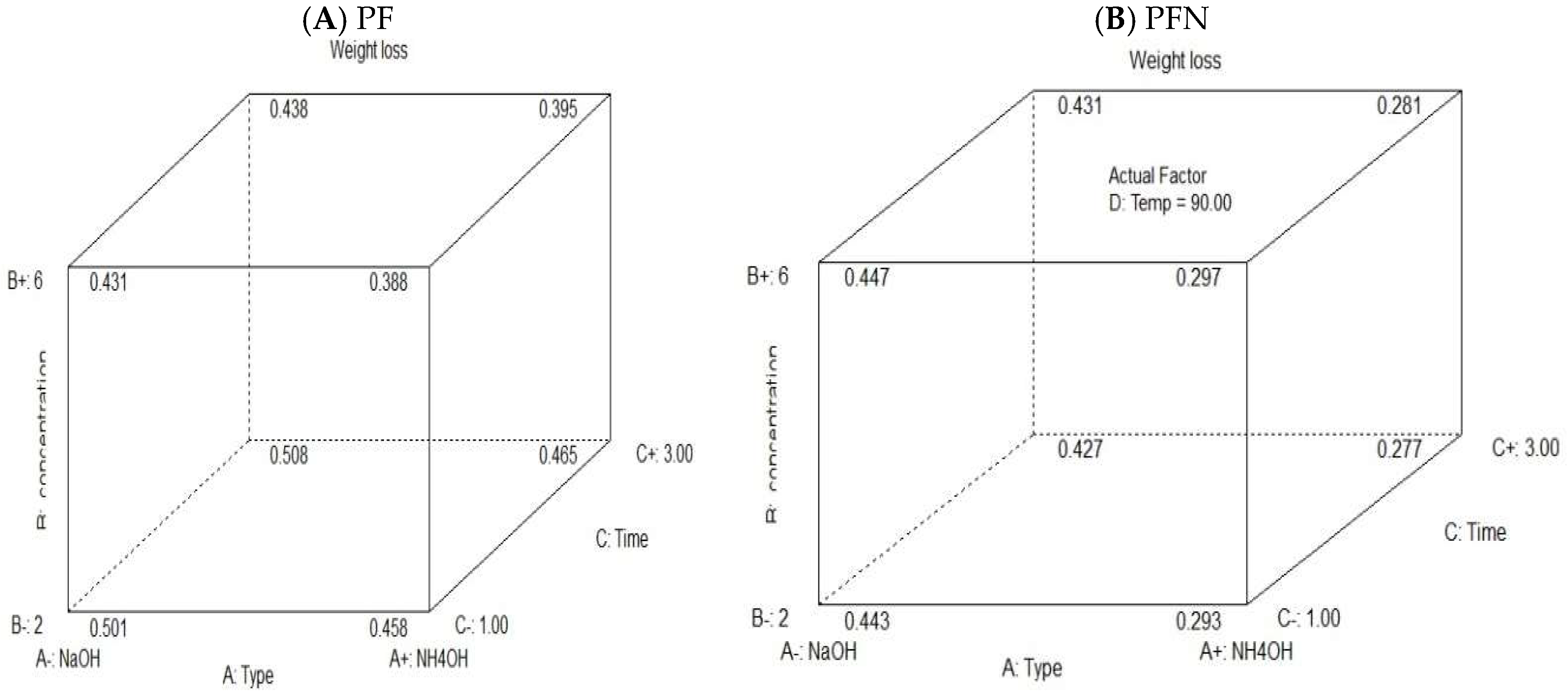
3.2. Cellulose Extraction Optimization by the Taguchi Method
3.3. ANOVA Analysis of the Taguchi Model Runs
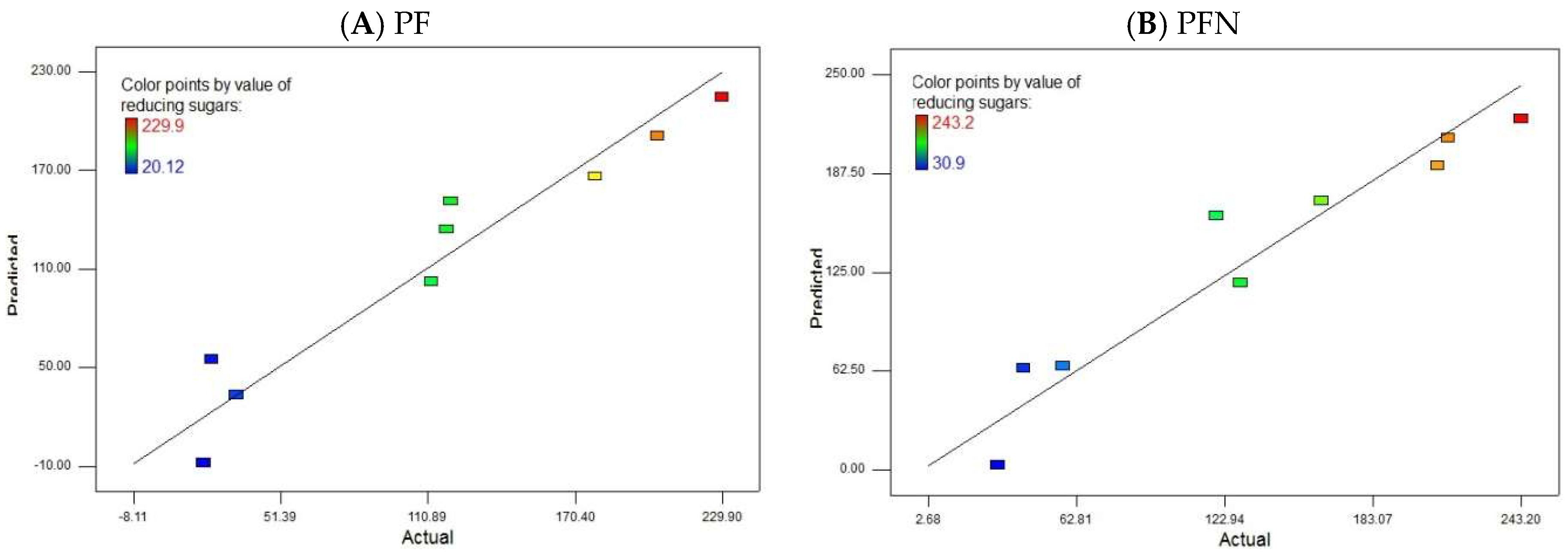
3.4. Model Evaluation
3.5. Response Surface Methodology (RSM)
3.6. Validation of the Experimental Model
3.7. Dewaxed PF and PFN Aqueous Extraction
3.8. Qualitative and QuantitativeDetermination of Carbohydrates
3.9. FT-IR Analysis
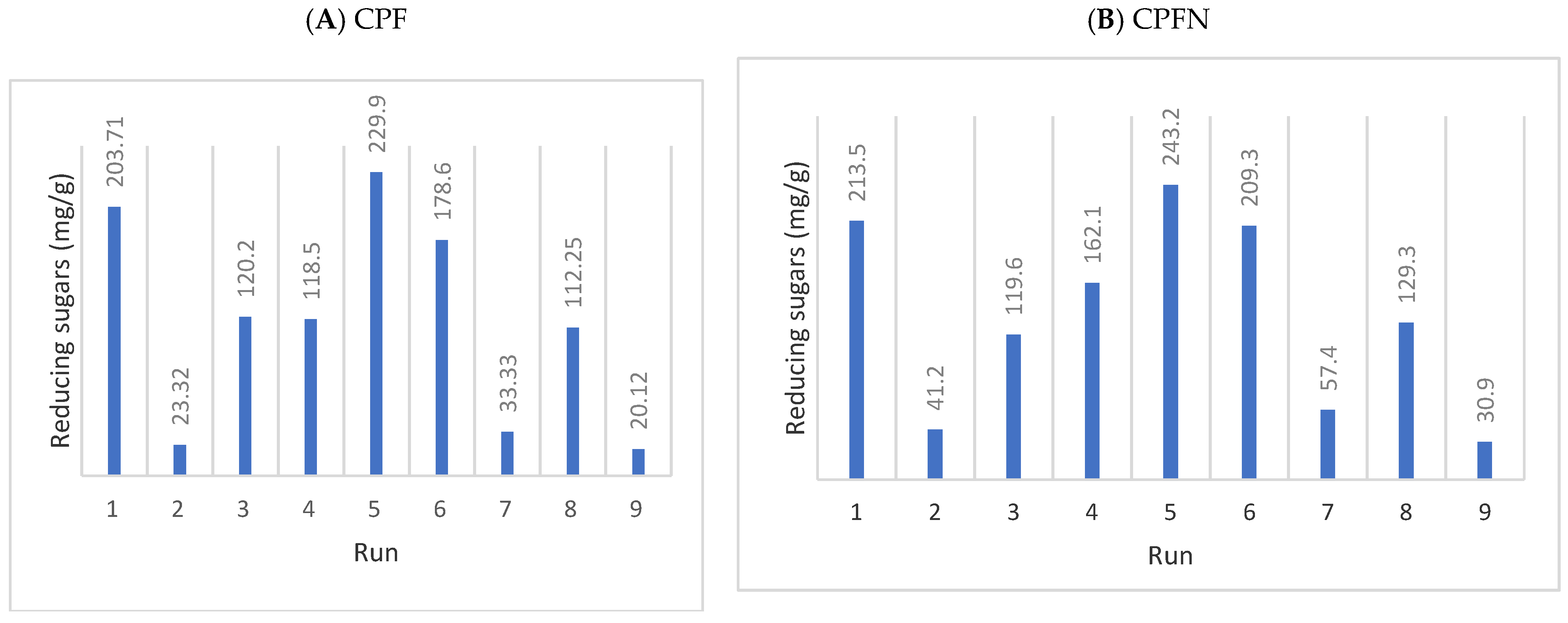
3.10. Crude Enzymatic Hydrolysis Optimization
3.11. ANOVA of Taguchi Model Runs
3.12. Model Evaluation
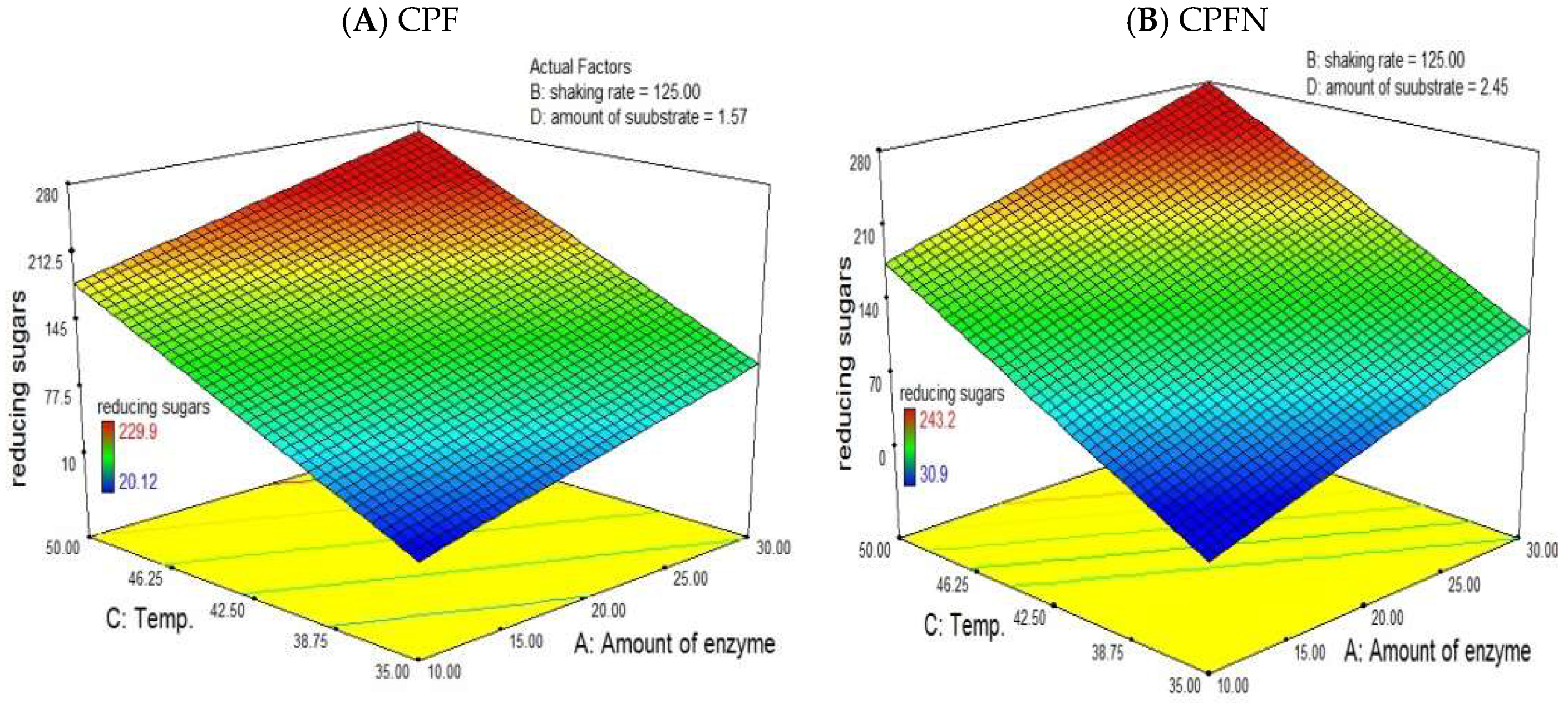
3.13. RSM
3.14. Validation of the Experimental Model
3.15. Commercial Enzymatic Hydrolysis
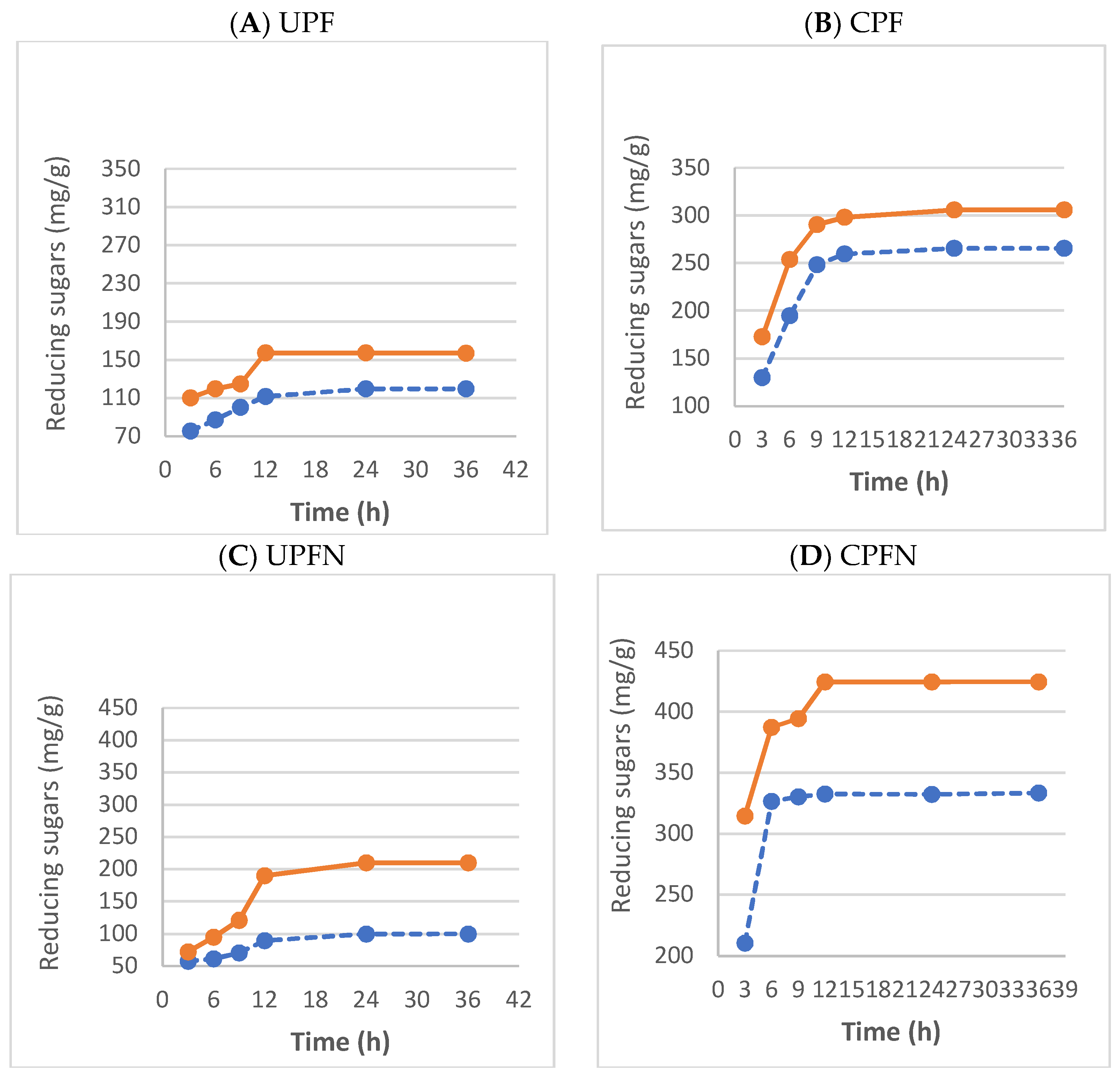
3.16. Estimation of Glucose in the Hydrolyzed Cellulose
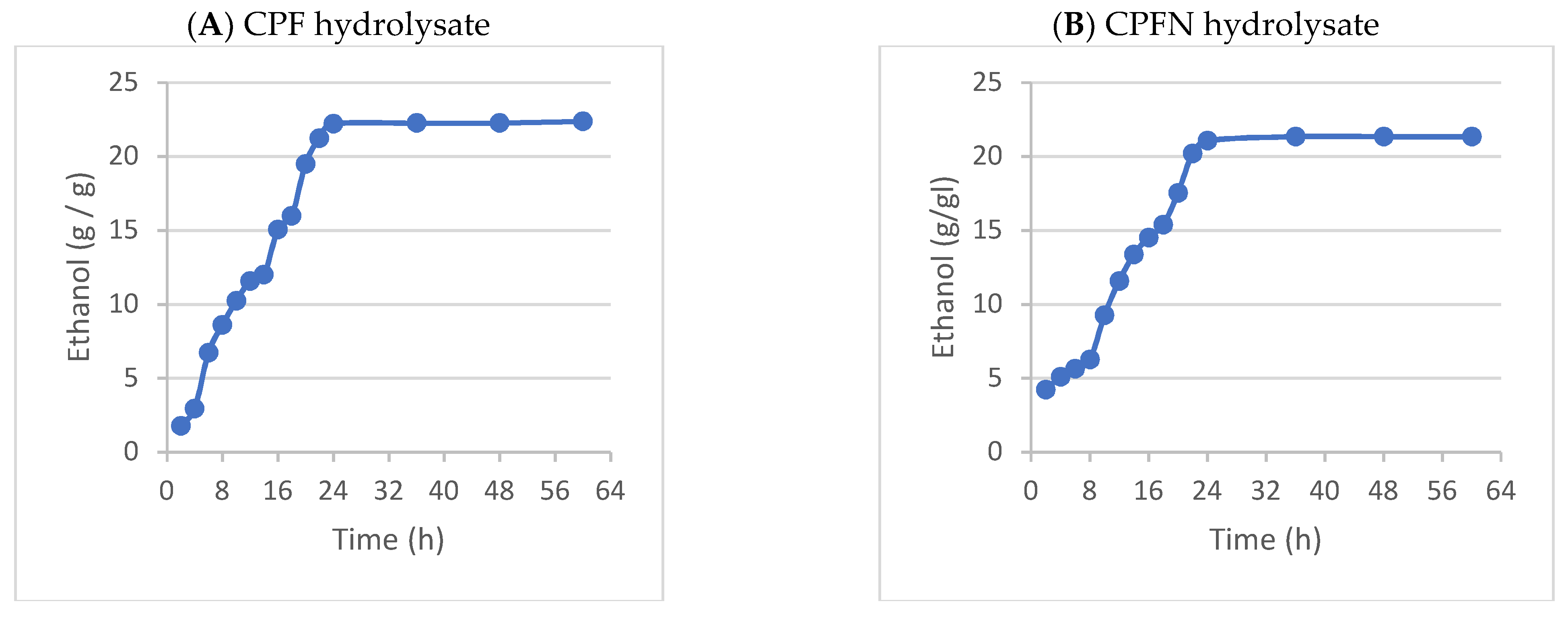
3.17. Bioethanol Production (Fermentation)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Statement of Novelty
- Palm frond and fiber agro-wastes can generate wealth by the production of bioethanol.
- Lignocellulosic biomass has many advantages, includingthat it is abundant, a renewable source of sugars, and inexpensive.
- Bioethanol production was increased under full optimization of the extraction, hydrolysis, and fermentation processes.
References
- Thiangtham, S.; Runt, J.; Manuspiya, H. Sulfonation of dialdehyde cellulose extracted from sugarcane bagasse for synergistically enhanced water solubility. Carbohydr. Polym. 2019, 208, 314–322. [Google Scholar] [CrossRef] [PubMed]
- Arab Organization for Agricultural Development (AOAD). Arab Agricultural Statistics Yearbook. Part III: Plant Production, Statistics Division; Arab Organization for Agricultural Development, ANS Publisher: Khartoum, Sudan, 2008; Volume 28. [Google Scholar]
- El-Juhany, L.I. Degradation of date palm trees and date production in Arab countries: Causes and potential rehabilitation. J. Basic Appl. Sci. 2010, 4, 3998–4010. [Google Scholar]
- Abdelwahab, O.; Nasr, S.M.; Thabet, W.M. Palm fibers and modified palm fibers adsorbents fordifferent oils. Alex. Eng. J. 2017, 56, 749–755. [Google Scholar] [CrossRef]
- AL-Oqla, F.M.; Alothman, O.Y.; Sapuan, M.J.S.; MandEs-Saheb, M.H. Processing and Properties of Date Palm Fibers and Its Composites. In Biomass and Bioenergy: Processing and Properties; Chapter one Springer International Publishing Switzerland; Hakeem, K.R., Jaeid, M., Rashid, U., Eds.; Springer Verbal: New York, NY, USA, 2014; pp. 1–25. [Google Scholar]
- Chandrasekaran, M.; Bahkali, A.H. Valorization of Date palm (Phoenix dactylifera) fruit processing by-products and wastes using bioprocess technology-Review. Saudi J. Biol. Sci. 2013, 20, 105–120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Oqla, F.M.; Sapuan, S.M. Natural fiber reinforced polymer composites in industrial applications: Feasibility of date palm fibers for sustainable automotive industry. J. Clean. Prod. 2014, 66, 347–354. [Google Scholar] [CrossRef]
- Kim, S.; Dale, B.E. Global potential bioethanol production from wasted crops and crop residues. Biomass Bioenergy 2004, 26, 361–375. [Google Scholar] [CrossRef]
- Rosemarie, Y.; Jennifer, A.L. An Assessment of Biofuel Use and Burning of Agricultural Waste in the Developing World; Department of Earth and Planetary Sciences, Harvard University: Cambridge, MA, USA, 2003. [Google Scholar]
- Association of Official Agricultural Chemists (AOAC). Official Methods of Analysis. Washington, D.C., 15th ed.; Association of the Official Analytical Chemists, INC: Arlington, VA, USA, 1990; Volume 1. [Google Scholar]
- Jayme, G.; Knolle, H. Paper chromatography of sugar mixtures upon glass-fiber paper. Angew. Chem. 1956, 68, 243–246. [Google Scholar] [CrossRef]
- Partridge, S.M.; Beidler, L.M. Aniline hydrogen phthalate as a spraying reagent for chromatography of sugars. Nature 1949, 164, 443. [Google Scholar] [CrossRef] [PubMed]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric Method for Determination of Sugars and Related Substances. Anal. Chem. Am. Chem. Soc. (ACS) 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Ragab, T.I.M.; Wasfy, A.A.F.; Amer, H.; El-Gendi, A.; Abdel-Hady, M.S.; Liebner, F. Synthesis of cellulose acetate membrane from the Egyptian rice straws. J. Appl. Sci. 2014, 14, 3424–3435. [Google Scholar] [CrossRef] [Green Version]
- Wilson, C.M. Quantitative determination of sugars on paper chromatography. Analyt. Chem. 1959, 31, 1199–1201. [Google Scholar] [CrossRef]
- Marrez, D.A.; El Raey, M.A.; Ali, M.; Seif, M.M.; Ragab, T.I.M.; El Negoumy, S.I.; Emam, M. Phenolic profile and antimicrobial activity of green synthesized Acalypha wilkesiana seeds silver nanoparticles against some food borne pathogens. Biosci. Res. 2017, 14, 817–830. [Google Scholar]
- Ragab, T.I.M.; Amer, H.; Wasfy, A.A.F.; Abdel-Hady, M.S.A.; Mossa, A.T.H.; Liebner, F. Sulfated Cellulose from Agriculture Wastes, Anticoagulant, Fibrinolytic and Toxicological Studies. J. Environ. Sci. Technol. 2014, 7, 266–280. [Google Scholar] [CrossRef] [Green Version]
- Ohtani, K. Bootstrapping R2 and adjusted R2 in regression analysis. Econ. Model 2000, 17, 473–483. [Google Scholar] [CrossRef]
- Ragab, T.I.M.; Shalaby, A.G.; El Awdan, S.A.; Refaat, A.; Helmy, W.A. New applied pharmacological approach/trend on utilization of agro-industrial wastes. Environ. Sci. Pollut. Res. 2018, 25, 26446–26460. [Google Scholar] [CrossRef]
- Ragab, T.I.M.; Amer, H.; Mossa, A.T.; Emam, M.; Hasaballaha, A.A.; Helmy, W.A. Anticoagulation, fibrinolytic and the cytotoxic activities of sulfated hemicellulose extracted from rice straw and husk. Biocatal. Agric. Biotechnol. 2018, 15, 86–91. [Google Scholar] [CrossRef]
- Caputi, A.; Ueda, M.; Brown, T. Spectrophotometric determination of ethanol in wine. Am. J. Enol. Vitic. 1968, 19, 160–165. [Google Scholar]
- Mirmehdi, S.M.; Zeinaly, F.; Dabbagh, F. Date palm wood flour as filler of linear low-density polyethylene. Compos. Part B Eng. 2014, 56, 137–141. [Google Scholar] [CrossRef]
- Brodeur, G.; Yau, E.; Badal, K.; Collier, J.; Ramachandran, K.B.; Ramakrishnan, S. Chemical and physicochemical pretreatment of lignocellulosic biomass. Enzym. Res. Hindawi 2011, 2011, 787532. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.X.; Sun, F.X.; Sun, R.C.; Su, Y.Q. Fractional extraction and structural characterization of sugarcane bagasse hemicelluloses. Carbohydr. Polym. 2004, 56, 195–204. [Google Scholar] [CrossRef]
- Cai, Z.S.; Paszner, L. Salt catalyzed wood bonding with hemicellulose. Holzforschung 1988, 42, 11–12. [Google Scholar]
- Lupoi, J.S.; Singh, S.; Parthasarthi, R.; Simmons, B.A.; Henry, R.J. Recent innovations in analytical methods for the qualitative and quantitative assessment of lignin. Renew. Sustain. Energy Rev. 2015, 49, 871–906. [Google Scholar] [CrossRef] [Green Version]
- Rahman, M.M.; Maniruzzaman, M.; Islam, M.R.; Rahman, M.S. Synthesis of Nano-Cellulose from Okra Fibre and FTIR as Well as Morphological Studies on It. Am. J. Polym. Sci. Technol. 2018, 4, 42–52. [Google Scholar] [CrossRef] [Green Version]
- Ibrahim, N.A.; Azraaie, N.; Aimi, N.; Zainul, M.; Amira, N.; Razali, M.; Aziz, F.A.; Zakaria, S. XRD and FTIR Studies of Natural Cellulose Isolated from Pineapple (Ananas comosus) Leaf Fibres. Adv. Mater. Res. 2015, 1087, 197–201. [Google Scholar]
- Jeya, M.; Zhang, Y.W.; Kim, I.W.; Lee, J.K. Enhanced saccharification of alkali-treated palm fibres by cellulase from Trametes hirsuta and statistical optimization of hydrolysis conditions by RSM. Bioresour. Technol. Elsevier BV 100 2009, 21, 5155–5161. [Google Scholar] [CrossRef]



| Run | A Alkaline | B Concentration (%) | C Time (h) | D Temperature (°C) |
|---|---|---|---|---|
| 1 | NaOH | 2 | 1 | 90 |
| 2 | Ca(OH)2 | 2 | 2 | 30 |
| 3 | NH4OH | 6 | 2 | 90 |
| 4 | NaOH | 4 | 2 | 55 |
| 5 | Ca(OH)2 | 4 | 3 | 90 |
| 6 | NH4OH | 4 | 1 | 30 |
| 7 | Ca(OH)2 | 6 | 1 | 55 |
| 8 | NH4OH | 2 | 3 | 55 |
| 9 | NaOH | 6 | 3 | 30 |
| Run | A Enzymeamount (IU) | B Shaking Rate (rpm) | C Temperature (°C) | D Substrateamount (% w/v) |
|---|---|---|---|---|
| 1 | 30.00 | 125 | 42.50 | 1.00 |
| 2 | 20.00 | 125 | 35.00 | 2.00 |
| 3 | 10.00 | 137.5 | 50.00 | 2.00 |
| 4 | 30.00 | 150 | 42.50 | 1.00 |
| 5 | 20.00 | 125 | 50.00 | 3.00 |
| 6 | 20.00 | 150 | 50.00 | 2.00 |
| 7 | 30.00 | 150 | 35.00 | 3.00 |
| 8 | 20.00 | 137.5 | 42.50 | 3.00 |
| 9 | 10.00 | 137 | 35.00 | 1.00 |
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value Prob > F | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| PF | PFN | PF | PFN | PF | PFN | PF | PFN | PF | PFN | |
| Model | 0.14 | 0.13 | 5 | 5 | 0.029 | 0.025 | 28.07 | 10.06 | 0.0101 | 0.0431 |
| A—Type | 0.064 | 0.070 | 2 | 2 | 0.032 | 0.035 | 31.11 | 14.04 | 0.0099 | 0.0300 |
| B—concentration | 7.35 × 10−3 | 1.667 × 10−5 | 1 | 1 | 7.350 × 10−3 | 1.667 × 10−5 | 7.14 | 6.701 × 10−3 | 0.0756 | 0.9399 |
| C—Time | 6.667 × 10−5 | 3.840 × 10−4 | 1 | 1 | 6.667 × 10−5 | 3.840 × 10−4 | 0.065 | 0.15 | 0.8156 | 0.7206 |
| D—Temp | 0.073 | 0.055 | 1 | 1 | 0.073 | 0.055 | 70.93 | 22.06 | 0.0035 | 0.0183 |
| Monosaccharide Constituents | Monosaccharide Constituents of Acid Hydrolysis (% w/w) | |||||
|---|---|---|---|---|---|---|
| CPF | CPFN | HPF | HPFN | LPF | LPFN | |
| D-glucuronic acid | 4 | 5 | 6 | 9 | t | t |
| D-glucose | 60 | 62 | 29 | 37 | t | t |
| L-arabinose | 10 | 11 | 30 | 22 | t | t |
| D-xylose | 26 | 22 | 35 | 32 | t | t |
| Peak Wave Number (cm−1) | Band Assignment | ||
|---|---|---|---|
| PF | PFN | ||
| Hemicellulose | 874 cm−1 | 895 cm−1 | C1 group frequency or ring frequency is characteristic of b-glycosidic linkages between the sugar units |
| 1032 cm−1 | 1039 cm−1 | C–O and C–C stretching or C–OH bending in hemicelluloses | |
| 1456 cm−1 | 1420 cm−1 | C–H vibrations of a polysaccharide or OH or CH2 bending | |
| 1633 cm−1 | 1634 cm−1 | Absorption ofwater | |
| 2925 cm−1 | 2924 cm−1 | C-H symmetric vibrations | |
| 3696 cm−1 | 3434 cm−1 | Hydrogen bond in the –OH group | |
| Lignin | 1025 cm−1 | 1025 cm−1 | Skeletal vibrations of C-O |
| 1122 cm−1 | 1122 cm−1 | Associated with cyclic ether | |
| 1390 cm−1 | 1383 cm−1 | Bending vibrations of phenolic OH | |
| 1456 cm−1 | 1456 cm−1 | C–H vibrations of a polysaccharide or OH or CH2 bending | |
| 1790 cm−1 | 1720 cm−1 | Carbonyl group C=Ocharacteristic of lignins | |
| 2925 cm−1 | 2925 cm−1 | C-H symmetric vibrations | |
| 3696 cm−1 | 3696 cm−1 | Hydrogen bond in the –OH group | |
| 3778, 3911 cm−1 | 3780, 3937 cm−1 | C-H stretching vibrations of methoxy groups. | |
| Cellulose | 875 cm−1 | 875 cm−1 | Refers to -CH rocking vibrations characteristic of a cellulose backbone and the β-glycosidic linkages between glucose units. |
| 1032 cm−1 | 1032 cm−1 | C–O and C–C stretching or C–OH bending in hemicelluloses | |
| 1044 cm−1 | 1037 cm−1 | C-O-C stretching vibrations of the polysaccharide or ring stretching | |
| - | 1382 cm−1 | C-H stretching | |
| 1456 cm−1 | 1430 cm−1 | C–H vibrations of a polysaccharide or OH or CH2 bending | |
| 1632cm−1 | 1632 cm−1 | Absorption ofwater | |
| 2925 cm−1 | 2925 cm−1 | C-H symmetric vibrations, asymmetric stretching of methyl and methylene groups | |
| 3434 cm−1 | 3429 cm−1 | Hydrogen bond in the –OH group | |
| Source | Sum of Squares | df | Mean Square | F Value | p-Value Prob > F | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| CPF | CPFN | CPF | CPFN | CPF | CPFN | CPF | CPFN | CPF | CPFN | |
| Model | 45,564.45 | 46,032.29 | 4 | 4 | 11,391.11 | 11,508.07 | 12.40 | 11.48 | 0.0159 | 0.0182 |
| A—amount of enzyme | 8552.41 | 12,152.48 | 1 | 1 | 8552.41 | 12,152.48 | 9.31 | 12.12 | 0.0380 | 0.0253 |
| B—shaking rate | 4638.36 | 2345.25 | 1 | 1 | 4638.36 | 2345.25 | 5.05 | 2.34 | 0.0879 | 0.2009 |
| C—Temp. | 39,626.32 | 39,726.69 | 1 | 1 | 39,626.32 | 39,726.69 | 43.15 | 39.62 | 0.0028 | 0.0033 |
| D—amount of substrate | 417.86 | 595.70 | 1 | 1 | 417.86 | 595.70 | 0.45 | 0.59 | 0.5370 | 0.4838 |
| Residual | 3673.57 | 4010.73 | 4 | 4 | 918.39 | 1002.68 | -- | -- | -- | -- |
| Cor total | 49,238.01 | 50,043.02 | 8 | 8 | -- | -- | -- | -- | -- | -- |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ragab, T.I.M.; Alminderej, F.M.; El-Sayed, W.A.; Saleh, S.M.; Shalaby, A.S.G. Enhanced Optimization of Bioethanol Production from Palm Waste Using the Taguchi Method. Sustainability 2021, 13, 13660. https://doi.org/10.3390/su132413660
Ragab TIM, Alminderej FM, El-Sayed WA, Saleh SM, Shalaby ASG. Enhanced Optimization of Bioethanol Production from Palm Waste Using the Taguchi Method. Sustainability. 2021; 13(24):13660. https://doi.org/10.3390/su132413660
Chicago/Turabian StyleRagab, Tamer I. M., Fahad M. Alminderej, Wael A. El-Sayed, Sayed M. Saleh, and Al Shimaa Gamal Shalaby. 2021. "Enhanced Optimization of Bioethanol Production from Palm Waste Using the Taguchi Method" Sustainability 13, no. 24: 13660. https://doi.org/10.3390/su132413660
APA StyleRagab, T. I. M., Alminderej, F. M., El-Sayed, W. A., Saleh, S. M., & Shalaby, A. S. G. (2021). Enhanced Optimization of Bioethanol Production from Palm Waste Using the Taguchi Method. Sustainability, 13(24), 13660. https://doi.org/10.3390/su132413660







