1. Introduction
It goes without saying that we should counteract the changes to our planet’s climate. Nowadays, in many countries in the world, including Poland, intensive work is being undertaken to decrease the percentage of fossil fuels in their energy balances. One such action is the continuous development of electric energy sources based on renewable energy media, such as wind and solar energy. The power energy system within a town, commune, district, or voivodeship demonstrates variability in the electric energy demand. There are hours, or days, when such demand is very high, but, in the evening or in the night hours, as well as during holidays, electric energy consumption decreases. Periodicity in the production of electrical energy also occurs in the sources driven by wind power. Taking the above into consideration, the system, enabling energy storage in the form of chemical energy, is one among the many systems feasible from the technical and economical points of view, which will enable the long-term saving of renewable energy and the ability to meet the demand for energy [
1]. The electric energy originating from renewable sources can be converted into chemical energy, to the form of hydrogen, in which form it can be stored. This is why, in many countries in the world, including Poland, hydrogen is perceived of as the most promising renewable energy medium. This is supported by the fact that existing gas networks can serve as storage for the produced hydrogen. These networks may serve not only as energy storage, but also as a means of transporting the energy accumulated in the hydrogen, from the place of its production to the place of its most effective utilization [
2]. As stated in [
3,
4], in countries such as Poland, Germany, Belgium, France, and the Netherlands, apart from natural gas, nitrogen-rich natural gas is also delivered to end consumers. In Poland, according to the Ordinance [
5], requirements have been defined for four groups of nitrogen-rich natural gas: Lw, Ls, Ln, and Lm. From among them, the nitrogen-rich natural gas of the Lw group is the most widespread. Thus, not only the networks conveying the natural gas of the E group are available for energy storage and transport in the form of hydrogen, but also the networks for the nitrogen-rich natural gas of the Lw group.
According to [
6], pumping hydrogen into a gas network constitutes a serious challenge. Therefore, research on the susceptibility to the presence of hydrogen in natural gas is being undertaken for such gas system elements as: compressors, piping, gas fittings, gas pressure control equipment, and the instruments used to measure the gas fuel volume being delivered, or the gas-fired appliances.
The test results of the measuring apparatus employed to take measurements of the delivered gas fuel volume are presented in articles [
1,
6]. In [
1], the authors present the test results of the impact of the hydrogen addition to natural gas on bellows gas meters. As stated in [
1], these are the most frequently used instruments for the settlement of gas fuel for gas consumers. The most important conclusion formulated by the authors and resulting from the performed research indicates that, for the tested gas-meter samples, significant metrological differences between the obtained indication errors’ changes following tests of the gas-meter life, with gas featuring various hydrogen percentages (from 0% up to 15%), were not found.
In [
6], the influences of 2, 4, 5, 10, and 15% hydrogen additions to the natural gas on the thermal gas-meter indication errors are presented. Comparative tests of the various gas compressibility coefficients for mixtures of natural gas and hydrogen are shown in [
7]. The authors of [
8] present the test results of the hydrogen addition on the stability of tetrahydrothiophene (THT) mixtures in methane and natural gas. The results of the presented research enable the recognition of the potential safety hazards of using gas from a distribution network resulting from the change of its composition because of the hydrogen addition.
The authors of [
9] present the test results for a gas burner used in domestic gas hobs in the United States when supplied with a natural gas and hydrogen mixture. The authors focused on checking such parameters as: the flue gas composition, cooking effectiveness, ignition time, flame appearance, and burner temperature. Despite the burner being supplied with mixtures of natural gas and hydrogen containing in their composition 75% hydrogen as a maximum, the authors indicate that burners of such a design may operate safely and effectively at hydrogen contents up to 20% in a mixture with natural gas. The hydrogen contents in the mixture with natural gas [
10], which guarantees the stability of the tested appliance flame, is 25%. This is the conclusion reached by the authors based on the tests of the burner installed in a gas-fired oven. Apart from the flame stability, the authors also tested the flue gas emissions, ignition time, and combustion noise. The problem connected with the flame stability of domestic gas appliances is also described in [
11,
12]. A conclusion was put forward in [
11] that, in order to have the possibility of adding hydrogen to natural gas in an amount resulting in the lowering of the Wobbe index below a specific range, a revision of the standards used in the certification processes of gas appliances, as well as the regulations defining the requirements for distributed gases, is required. This will enable an assessment of the permissible maximum hydrogen volumes.
The research presented in [
12] proves that the flame stability in typical gas-fired domestic appliances used in the United Kingdom is satisfactory, even at 30% hydrogen content in a mixture with natural gas. Nevertheless, the authors emphasize that adding 30% hydrogen to natural gas results in a lowering of the energy parameters of the resulting mixture below the values specified in the regulations. The authors of [
2] present the results of an examination of the influence of the hydrogen addition to natural gas on selected elements of a gas system that were performed at INiG-PIB. The authors of [
13] present the results of the theoretical analyses of the influence of the hydrogen addition to natural gas on the thermal efficiency of conventional and condensing gas boilers. On the grounds of the calculations, the authors prove the potential efficiency increases of using the energy contained in gas, which results from the higher contents of water in the flue gases originating from the combustion of a mixture of natural gas and hydrogen. The authors of [
14] present the results of research on the influence of a hydrogen addition to natural gas on the efficiency of domestic- and commercial-use gas appliances adapted for the combustion of natural gas. The appliances selected for the tests were supplied with natural gas and hydrogen mixtures that contained 10, 15, and 23% hydrogen in their composition. On the grounds of the performed tests, the conclusion was formulated that the safe amount of hydrogen that can be added to natural gas, while ensuring the safe and effective combustion of the mixture in domestic and commercial gas appliances without the need for changing their design, is 15%.
The performed analyses described in the articles indicate that research on domestic gas appliances adapted for the combustion of the nitrogen-rich natural gas of the Lw, group, when supplied with mixtures of this gas with hydrogen, has not been performed. The nitrogen-rich natural gas of the Lw group, with a Wobbe index ranging from 37.5 MJ/m
3 up to 45.0 MJ/m
3 (reference conditions: 298.15 K and 101.325 kPa for the combustion process; 273.15 K and 1013.25 mbar for the volume measurement) [
5], is distributed in Poland. In reference conditions of 288.15 K and 1013.25 mbar for the combustion process and the volume measurement, respectively, the variability range of the Wobbe index for group-Lw gas ranges from 35.6 MJ/m
3 up to 42.7 MJ/m
3. The gas of a similar Wobbe index is distributed in Belgium (variability range of the Wobbe index is 41.61 ÷ 44.45 MJ/m
3 [
3]); in Germany (variability range of the Wobbe index is 35.83 ÷ 44.36 MJ/m
3 [
3]); and in France (variability range of the Wobbe index is 40.29 ÷ 44.36 MJ/m
3 [
3]). The values of the Wobbe index for Belgium, Germany, and France were stated for reference conditions of 288.15 K and 1013.25 mbar for the combustion processes and the volume measurements, respectively. This is why it was decided at INiG-PIB to perform the research on a group of various types of gas appliances, while focusing particularly on analyses of the heat input and the thermal efficiency.
2. Materials and Methods
The most important area of the research pertained to checking the influence of the hydrogen addition to the nitrogen-rich natural gas of the Lw group on the operation of gas-fired domestic appliances. Because the assumptions of the research project covered the testing of gas appliances adapted for combusting nitrogen-rich group Lw natural gas without modifying them, it was agreed that the parameters that are most sensitive to changes in the gas composition are the energy parameters and the safety of operation. Prior to commencing the research realization, the composition of the nitrogen-rich natural gas of the Lw group mixture with hydrogen was determined, and was then used for supplying the tested appliances. When determining the percentage of the hydrogen contents in mixtures with the nitrogen-rich Lw-group natural gas, the average composition of this gas, in terms of the span of years [
15] and the requirements of law regulations, was taken into account.
The variabilities of the Wobbe index and the gross calorific value of the nitrogen-rich Lw-group natural gas, from January 2013 until January 2020, are presented in
Figure 1 and
Figure 2.
It was computed, while taking into account the above data, i.e., the variability of the nitrogen-rich Lw-group natural gas energy parameters and the requirements for the minimum gross calorific value and the Wobbe index of this gas, that the maximum hydrogen amount that can be added to the Lw gas amounts to approximately 13.2%. It was assumed, in connection with the above, that the mixture containing approximately 13.2% hydrogen would be used for testing the influence of the hydrogen addition to nitrogen-rich natural gas on the operation of domestic gas appliances.
The following gases were used in the tests: nitrogen-rich natural gas Lw as a reference gas for checking the rated parameters of the tested appliances and the possible corrections of the set values so that the gas change would always occur at the rated set values, which were not changed during the course of further tests; a mixture of hydrogen with Lw nitrogen-rich natural gas of a composition containing 86.8% Lw gas + 13.2% hydrogen (gas designation: GLwH13).
The compositions, densities, and energy parameters of the gas mixtures used in the tests are shown in
Table 1. The Lw gas and the mixture of Lw gas with hydrogen were produced in the Department of Fuels Usage gas-mixing plants on the basis of mass flowmeters and flow regulators. The abovementioned mixtures were composed with the use of E natural gas as the base gas.
It was assumed, on the grounds of the gas appliance operation analysis and the requirements that have to be met in order to guarantee safety for users, that the following parameters would be examined during the tests:
Figure 3 presents a general diagram of testing stand for gas-supplied appliances at INiG-PIB.
The measurements of the above-listed parameters were conducted on the basis of the test procedures described in the standards related to the equipment under testing. The following standards were used: EN 30-1-1 + A3 [
17]; EN 30-2-1 [
18]; EN 26 [
19]; and EN 15502-1 + A1 [
20]. The Oil and Gas Institute-National Research Institute (INiG-PIB) is the entity accredited by the Polish Accreditation Centre (PAC) for performing tests on the basis of the standards listed above. The tests were performed with the use of a measuring apparatus, with a measurement uncertainty that met the requirements of the above-listed standards. The specifications of the most important measuring instruments used in the tests, the measured parameters and their measuring accuracies, which are worse than those given in the parentheses, are shown below:
AS1: RBM 2000 gas analyzer: Wobbe index, net calorific value, density (accuracy < 1% of full scale);
AS2: gas analyzer, model Horiba VA/VS-3000: NOx: 0–200 ppm; CO: 0–500 ppm; CO2: 0–10% (zero and span drift ± 2.0% of full scale by week, repeatability ± 0.5% of full scale); O2: 0–25% (zero and span drift ± 1.0% of full scale by week, repeatability ± 0.5% of full scale);
AS3: gas analyzer, model Thermo-FID TG: THC 0–3000 ppm (zero and span drift ± 2% of full scale by week, repeatability ± 2.0% of full scale).
For composing the gas mixtures necessary for the tests, the gas-mixing plant located at the Department of Fuels Usage, INiG-PIB, was used. The gas-mixing plant operates on the basis of flowmeters and mass regulators. Its construction and software provide for the possibility of composing multicomponent gas mixtures, including hydrogen. The maximum capacity of the gas-mixing plant reaches 7 m3/h. The energy parameters and the densities of the mixtures being formulated were controlled with the use of a gas analyzer, model RBM 2000, calibrated with standard gas.
When selecting appliances for the tests, priority was given to those that are most frequently used by domestic households. Considering that, in households, gas is used mostly for preparing meals and for providing hot water for heating and sanitary needs, the following appliances were appointed for the tests:
A gas cooker equipped with so-called “bucket-type” burners;
A gas hob equipped with so-called “pipe-type” burners;
A gas-fired instantaneous water heater with an open combustion chamber, equipped with a kinetic-diffusion-type burner;
A gas-fired instantaneous water heater with a closed combustion chamber, equipped with a kinetic-diffusion-type burner;
A two-function gas-fired heating boiler, hanging, with a closed combustion chamber, equipped with a premix-type burner.
On the basis of several years of experience gained during the type-testing of domestic gas appliances in the Accredited Laboratory (Accreditation No. AB041), it can be stated that the designs of the burners (including the sizes of the fire holes) in the tested household appliances are representative of these appliances. In the age of globalization, gas burners for domestic appliances are produced by only a few companies, and their designs are very similar to each other. All burners installed, e.g., in a gas cooker, have the same diameter of the fire holes; only the diameter of the burner changes and, thus, the number of these holes. In the case of burners installed in a gas hob, the situation is similar. Because of the fact that the burners in the cooker and the gas hob differ in design, the tests were carried out on both devices. The burners used in gas-fired instantaneous water heaters are also representative for this group of devices. Kinetic-diffusion-type burners are used in both water heaters and gas boilers. These burners have a modular structure. Each module has the same fire holes. The increase in the thermal power of the device is achieved by increasing the number of modules in the device and enlarging the heat exchanger. The burner mounted in the tested gas boiler can also be considered representative for this group of devices available on the market. All devices available on the market are based on the same principle of operation because the process of creating the fuel-air mixture is based on the Venturi tube, as in the tested boiler.
The tests were first performed while supplying the appliances with the nitrogen-rich Lw-group natural gas. Then, without making any changes to the appliance settings, and while maintaining an unchanged supply pressure, as for the Lw gas, they were supplied with the GLwH13 mixture. The statement “without changes of appliances’ settings” should be understood as follows: the appliance under testing was not subjected to any modifications; the tests were performed with the use of the same gas nozzles, at the same settings as the gas cocks, and with the same upstream pressure of the gas nozzle, and at the same settings of the combustion air controllers. A brief description of the characteristics of the appliances used in the tests is given below.
2.1. The Gas Hob
For testing the influence of the hydrogen addition to the nitrogen-rich subgroup-Lw natural gas, a gas hob was selected (
Figure 4), in which so-called “pipe-type burners” are installed. They are characteristic in that the better mixing of the gas with primary air progresses in the result of the elongated injector. The mixture of primary air and gas, and not gas alone, flows into the burner nozzle, as with typical bucket-type burners. The burners suck more primary air for combustion than do typical bucket-type burners and, hence, they are more susceptible to unstable operation; however, simultaneously, they are characterized by better combustion quality. This translates into lower contents of carbon monoxide in the flue gases. The tests were performed on the following burners: the auxiliary burner (ABp),
Qnom = 1050 W; the semi-rapid burner (SRBp),
Qnom = 1600 W; and the large burner (URBp),
Qnom = 3300 W.
2.2. Gas Cooker
In the gas cooker selected for the tests (
Figure 5), so-called “bucket-type” burners were installed. The primary air for combustion in these burners is drawn in from above the burner plates, and this is why they cannot be mounted below the plate (“sunk” in the plate). The regulation of the primary air inflow, considering the gas type, is performed by selecting the appropriate nozzle. The gas nozzles for certain groups of gasses have special designs (with double, or even triple, drilling, and adequately dimensioned nozzle heights). The tests were performed on the following burners: the auxiliary burner (ABc),
Qnom = 1000 W; the semi-rapid burner (SRBc),
Qnom = 1800 W; and the large burner (RBc),
Qnom = 3000 W.
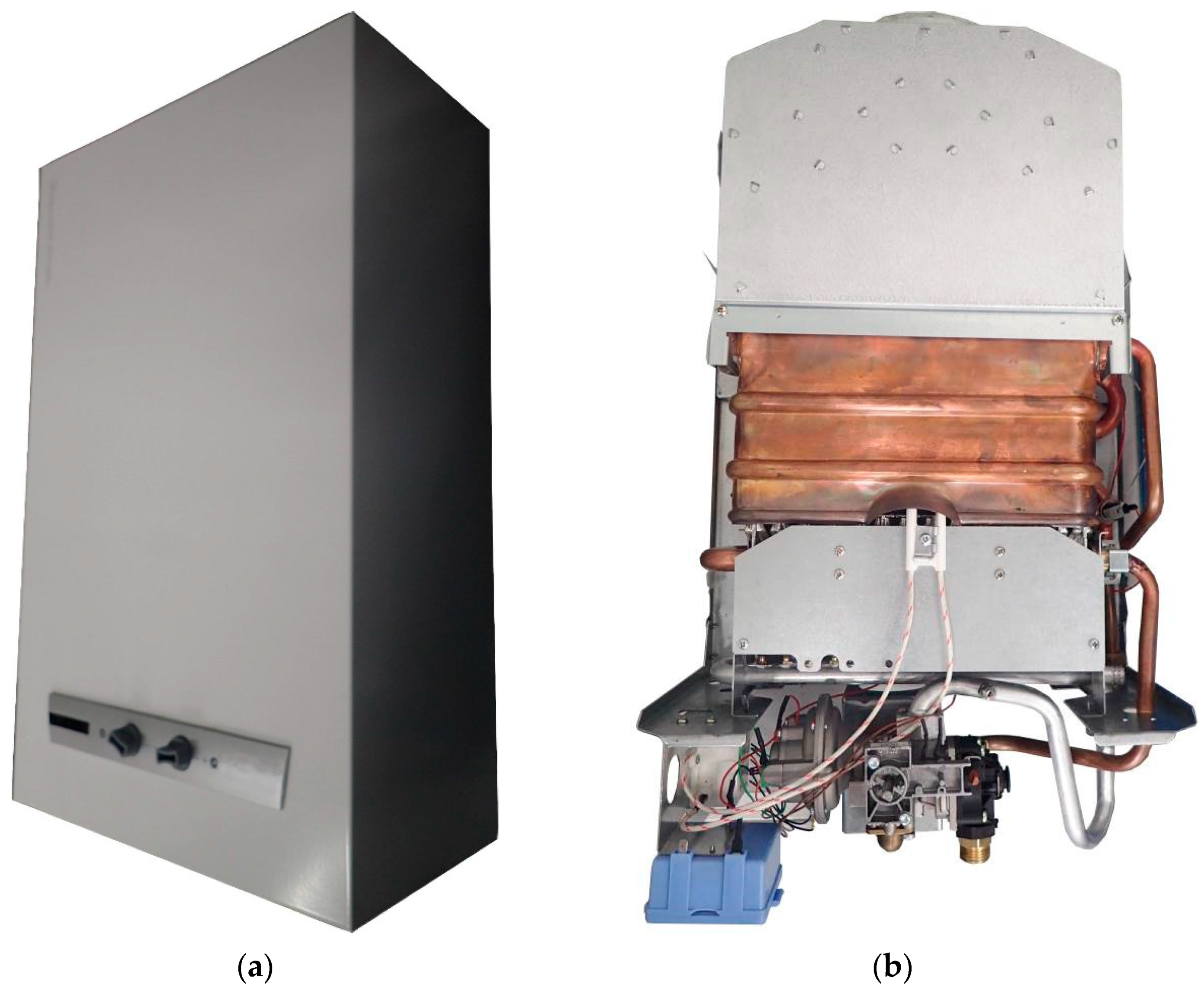
2.3. Gas-Fired Heating Boiler
The device under testing is a gas-fired heating boiler of the premix type (
Figure 6a,b). For several years, gas-fired heating boiler burners have been used, in which the gas stream delivered to the burner head is drawn in by the air stream. The process is performed on Venturi. Such solutions result in inflows of the ready fuel-air mixture to the burner head. The rated heat input of the boiler for CH circulation is 3.0 ÷ 24.0 kW.
2.4. Gas-Fired Instantaneous Water Heater with Open Combustion Chamber
The appliance under testing is a gas-fired instantaneous water heater with an open combustion chamber, equipped with a kinetic-diffusion-type burner (
Figure 7a,b). In appliances of this type, the volume of primary air sucked into the combustion process depends on the gas pressure set on the burner nozzle, whereas the secondary air is drawn in from the space above the burner. Both primary and secondary air are fed to the combustion chamber by leakages in the appliance structure, and their volume depends on negative pressure in the flue duct. The rated heat input of the instantaneous heater is 10.5 ÷ 27.0 kW.
2.5. Gas-Fired Instantaneous Water Heater with Closed Combustion Chamber
The appliance under testing is a gas-fired instantaneous water heater with a closed combustion chamber (
Figure 8a,b), equipped with kinetic-diffusion-type burner and a fan downstream of the combustion chamber, sucking the combustion air in and assisting the flue gas evacuation. In an appliance of this type, the volume of primary air sucked into the combustion process depends on the gas pressure set on the burner nozzle, whereas the secondary air is drawn in from the space above the burner. Both primary and secondary air are fed to the combustion chamber by the fan, and their volume depends on the RPM set on the fan. Controlling the fan rotational speed enables the delivery of an adequate volume of combustion air, guaranteeing the proper course of this process, depending on the length of the flue air duct used. The rated heat input of the instantaneous heater is 10.5 ÷ 24.0 kW.
4. Discussion
The results of the flue gas composition testing obtained for combusting a Lw nitrogen-rich natural gas mixture with a hydrogen addition and Lw gas are shown in
Table 2. The tests showed that, for carbon monoxide, nitrogen oxides, and unburned hydrocarbons, a significant increase in their concentrations in the flue gas was not observed.
In the tests of the gas-fuel-fired appliances, conducted on the grounds of the requirements and testing methods contained in the harmonized standards, the admissible level of carbon monoxide emissions converted on dry nondiluted flue gas is 1000 ppm (0.1%). This value was not exceeded in any instance during the tests, and, with respect to the gas-fired heating boiler and the gas-fired instantaneous water heater with an open combustion chamber, the concentration of carbon monoxide in the flue gas decreased approximately 2 and 2.5 times, respectively. In the standards related to the domestic gas hob and gas hooker, the requirements in terms of the NOx emissions and the unburned hydrocarbons have not been defined. Nevertheless, during the tests, the contents of these components in the combustion products were also checked, assuming the values obtained for the Lw nitrogen-rich natural gas as a reference level for the GLwH13 mixture. In the standards regarding the gas-fired heating boilers and the instantaneous water heaters, there are requirements as to the admissible level of NOx contents in the flue gas, but their measurement is performed at various levels of the heat input, and the final result is computed with a weighted method. Taking into account that all of the appliances selected for testing within the framework of this study possessed CE marking, the level of NOx contents in the flue gas emitted by them had to be compliant with the requirements of the relevant standards. From the research-led point of view, the most interesting question was whether a hydrogen addition to the Lw-group nitrogen-rich natural gas would not result in NOx propagation, as compared to the results for the Lw group natural gas itself. As was mentioned earlier, this phenomenon was not observed.
The quality of combustion, especially in kinetic-diffusion-type burners, is influenced by the primary air suction coefficient. While maintaining the same settings of the tested devices for Lw gas and supplying them with gas with the addition of hydrogen (GLwH13), the amount of intake primary air is affected by the gas density and the speed of the gas mixture at the nozzle outlet. In order to determine the value of the primary air coefficient for the Lw and GLwH13 gases, numerical calculations were performed in the Fluent software. The simulation was carried out on a large burner of the tested gas cooker. The primary air suction coefficient for the Lw gas was 0.33, while, for the GLwH13 gas, it was 0.34. As can be seen, the addition of 13.2% hydrogen to the mixture of Lw gas does not have a significant effect at the value of the primary air suction coefficient and, thus, the quality of combustion in kinetic-diffusion-type burners (
Table 2).
The performed tests of the flame stability, the results of which are presented in
Table 3, showed that the hydrogen addition to the Lw nitrogen-rich natural gas did not result in a worsening of the flame stability of the burners of the tested appliances. The phenomena of flame lifting and light back were not observed, and the flame transfer was correct.
The analysis of the obtained heat inputs for burners of the gas hob and gas cooker indicates, unambiguously, that the Lw nitrogen-rich natural gas mixture with hydrogen had a lower Wobbe index and gross calorific value than the Lw nitrogen-rich natural gas itself. The heat inputs of the burners and their efficiencies were lower.
Figure 9 and
Figure 10 present the percentage decrease of the gross calorific value and gross Wobbe index of the Lw nitrogen-rich natural gas mixture with hydrogen in relation to Lw natural gas itself, and how it influenced the heat inputs and efficiencies obtained by the appliances. It should be noted that the value of the gross Wobbe index decreased by 2.1% following the hydrogen addition to the Lw gas, while the gross calorific value of the LGwH13 mixture decreased by 8.1%
In the case of the gas hob, the highest decrease in the heat input occurred for the large burner and amounted to 3.9%, while, for the auxiliary and semi-rapid burners, the decrease amounted to 3.3%. With respect to thermal efficiency, the parameter for the large burner decreased by 3.3%, and by 4.6% for the semi-rapid burner. The thermal efficiency of the auxiliary burner was not tested because, according to the standard [
18], the parameter is determined for burners of a gross calorific value higher than 1.16 kW (the auxiliary burner of the gas hob features a declared gross calorific value equal to 1.0 kW).
In the case of the gas cooker, the highest decrease in the heat input occurred for the rapid burner and amounted to 2.3%, while it amounted to 1.7% for the auxiliary burner, and 1.6% for the semi-rapid burner. With respect to the thermal efficiency, the parameter for the rapid burner decreased by 1.8%, and by 1.3% for the semi-rapid burner.
Efficiency lowering, in the case of the GLwH13 mixture, should be mainly attributed to the lower energy parameters of this gas. The efficiency decrease can, to a lesser degree, be related to the shortening of the length of the burners’ flames, as occurred for the mixtures of natural gas with hydrogen. Nevertheless, considering its much higher combustion speed as compared to methane, hydrogen should cause flame shortening. Yet, in the nitrogen-containing mixture, this effect is less noticeable. The most noticeable flame shortening of the burning GLwH13 mixture, in relation to the flame obtained in the case of Lw gas combustion, was observed for the auxiliary burner of the gas hob (
Figure 11 and
Figure 12). Nitrogen, as a nonflammable gas component, causes flames to exhibit a tendency to lift. It can therefore be stated that adding hydrogen to subgroup-Lw nitrogen-rich natural gas stabilizes the operation of the burners adapted for combusting this gas.
Steam, considering its higher thermal capacity, accumulates more thermal energy. Therefore, the increase in its contents in flue gas should, in actuality, increase the efficiency. In the open burners, it is not possible to make use of the fact that the hydrogen addition to Lw natural gas results in a higher content of steam, as compared to flue gas from the Lw gas itself. This effect is noticeable in appliances equipped with a heat exchanger (
Figure 13,
Figure 14 and
Figure 15). The lack of an enclosed space, formed by the combustion chamber and the developed heat exchanger, produces lower thermal energy, resulting from the heat-input decrease, and cannot be compensated by taking away the heat contained in the steam. However, it should be pointed out that the maximum thermal efficiency decreases for the Lw gas mixture with hydrogen, both for hotplate burners and gas cookers, in relation to the efficiency for the Lw gas itself, which is 4.6%.
An analysis of
Figure 12,
Figure 13 and
Figure 14, relating to the tested gas-fired instantaneous water heaters and the gas-fired heating boiler, clearly indicates that, along with the Wobbe index and net calorific value decreases resulting from adding hydrogen to the Lw-group nitrogen-rich natural gas, the heat inputs are also decreasing, as compared to the values obtained for the Lw gas itself. The nominal heat input for the gas-fired heating boiler decreased by 4.8%, while the efficiency for operation in the 80/60 °C temperature regime decreased by 1.8%, but only by 0.5% for operation in the 50/30 °C temperature regime. The measured decrease in the burner heat input in the case of the gas-fired instantaneous water heater with an open combustion chamber amounted to 2.7%, while its efficiency increased by 1.0%. Even better results were obtained by the gas-fired instantaneous water heater with a closed combustion chamber. The burner heat input decreased by 2.9% following the hydrogen addition to the Lw natural gas, and an efficiency increase of 1.1% was observed.
If we look at the percentage efficiency differences of the appliances obtained for the Lw nitrogen-rich natural gas and the mixture of Lw natural gas with hydrogen, it can clearly be seen that the efficiency decrease is slight (gas-fired heating boiler), or that the efficiency increases (gas-fired instantaneous water heater), despite lowering the heat input of the burners for the tested appliances, that are, in fact, consuming a lower energy amount delivered by gas. Without a doubt, this is related to the fact that adding hydrogen to nitrogen-rich natural gas results in a higher amount of steam in the flue gas, as compared to the flue gas from the Lw natural gas itself. It has been described above how this influences the efficiency of such types of appliances.
In the case of the gas-fired instantaneous water heater with an open combustion chamber, an efficiency increase is observed that is even higher than for the gas-fired heating boiler, despite the fact that it is not a condensing device. Here, however, apart from the influence of the higher amount of steam in the flue gas upon the thermal efficiency increase, another cause of this phenomenon can be also indicated: the burner heat input decrease, an effect of lowering the supply gas’s calorific value resulting from the hydrogen addition, which resulted in improving the heat exchange from the flue gas to the water flowing through the heat exchanger. This says that the nominal heat input of the burner operating in the gas-fired instantaneous water heater with an open combustion chamber is too high in relation to the heat exchanger size. This example shows the importance of the proper selection of the burner heat input in relation to the heat reception ability in the heat exchanger.
An even better result, with regard to the efficiency increase (an increase of 1.1%), was obtained by the gas-fired instantaneous water heater with a closed combustion chamber. In this case, the abovementioned observations for the gas-fired instantaneous water heater with an open combustion chamber, with regard to the efficiency increase, are also valid.
5. Conclusions
It can be stated, when summarizing the results of testing the influence of a hydrogen addition to Lw nitrogen-rich natural gas on the operation of domestic gas-fired appliances used for the preparation of meals (gas hob with burners equipped with regulated combustion air throttle, gas cooker with bucket-type burners) and hot water for heating and hygienic purposes (gas-fired heating boiler, gas-fired instantaneous water heaters), that adding approximately 13.2% hydrogen to the Lw nitrogen-rich natural gas does not result in a worsening of the safety of their operation, namely, an increase in the content of carbon monoxide, nitrogen oxides, and hydrocarbons in the flue gas, or the instability of the operation of the burners.
Attention should be placed on the decrease in the heat input of the burners, and on the resulting thermal efficiency, especially in cases of appliances used for the preparation of meals (gas hob and cooker), and in gas-fired heating boilers operating in a 80/60 °C temperature regime. The causes of such a state of things are discussed in the “Analysis of Results” paragraph. Nevertheless, such a decrease should not be noticeable from the user’s point of view because:
In the case of domestic appliances used for the preparation of meals, only in the first phase of meal preparation is the full heat input of the burner used. Following the attainment of the temperature required by the meal (which usually takes several minutes), the heat input of the burner is lowered;
Gas-fired heating boilers usually do not operate at maximum heat input and, in order to force their operation in a condensing regime, the 50/30 °C temperature operating regime is set. As shown by boiler tests (see
Figure 13), the decrease in efficiency is minimal at such settings (0.5%);
Tests of gas-fired instantaneous water heaters with open and closed combustion chambers have shown that, despite the heat input decrease, the thermal efficiency of such types of appliances has increased.
A decrease in the Lw gas energy parameters should not itself reflect on gas bills because, in many countries, including Poland, the system of settlements for gas fuel, which is based on the energy amount delivered, is in operation. Taking the above into consideration, the end user will pay a lower gas bill following the addition of hydrogen, and the calorific value of the gas will decrease.
To recapitulate, on the grounds of the research conducted within the framework of this study, the safe volume of hydrogen that can be pumped into nitrogen-rich natural gas in order for the resulting mixture to be safely and effectively combusted in the domestic gas-fired appliances tested within the framework of this study, and without the necessity for making any changes to their design, equals approximately 13.2%.