Increased Carbon Dioxide by Occupants Promotes Growth of Leafy Vegetables Grown in Indoor Cultivation System
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cultivation System and Fan Airspeed
2.2. Cultivation Space
2.3. Nutrient Solutions and Medium
2.4. Plant Materials
2.5. Chlorophyll Content and Root Activity
2.6. Soluble Sugar and Starch Contents
2.7. Soluble Proteins and Antioxidant Enzyme Activities
2.8. Statistical Analysis
3. Results
3.1. Environmental Measurement
3.1.1. Temperature and Relative Humidity
3.1.2. Carbon Dioxide Concentration
3.2. Plant Growth Parameters
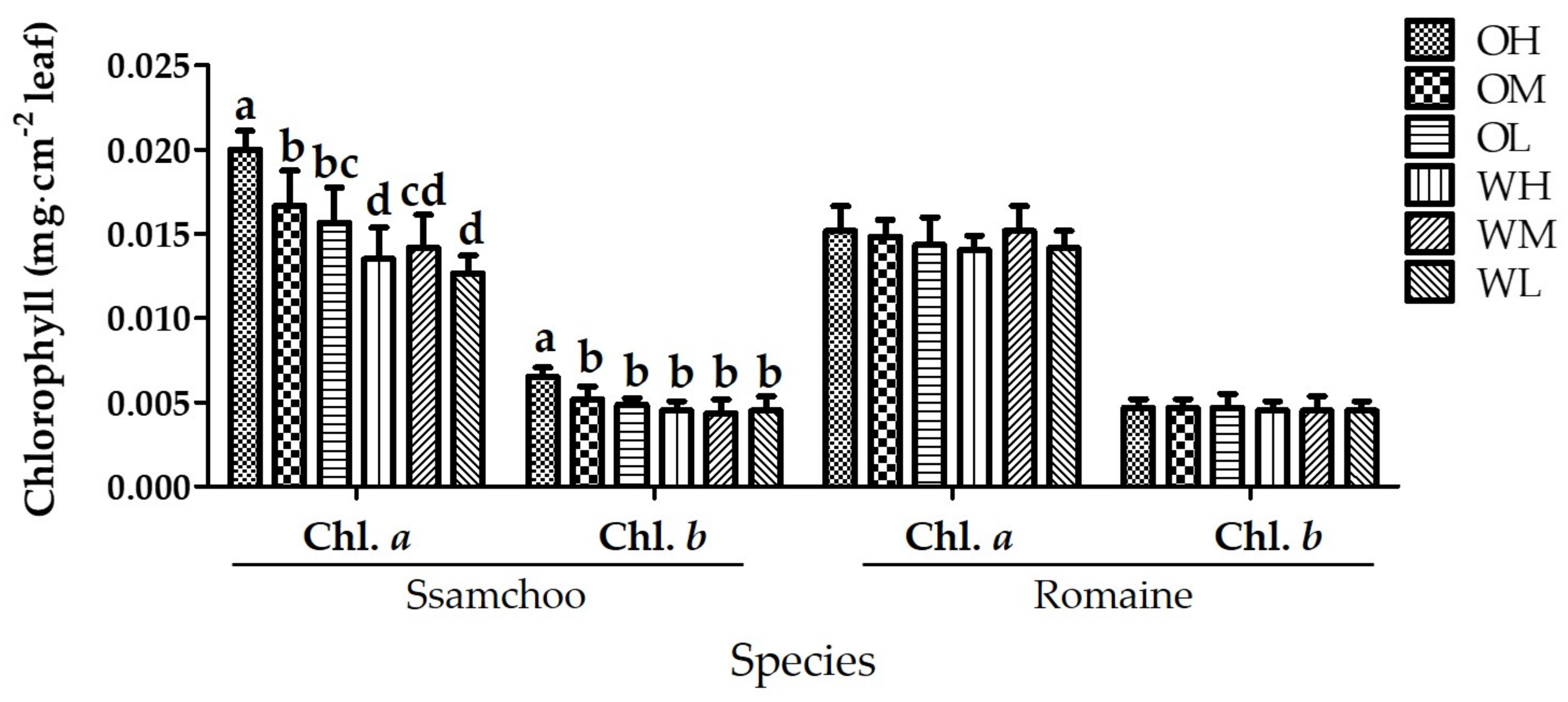
3.3. Chlorophyll Content and Root Activity
3.4. Soluble Protein, Soluble Sugar, and Starch Contents, and Antioxidant Enzyme Activities
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Robelia, B.; Murphy, T. What do people know about key environmental issues? A review of environmental knowledge surveys. Environ. Educ. Res. 2012, 18, 299–321. [Google Scholar] [CrossRef]
- Grasmück, D.; Scholz, R.W. Risk perception of heavy metal soil contamination by high-exposed and low-exposed inhabitants: The role of knowledge and emotional concerns. Risk Anal. Int. J. 2005, 25, 611–622. [Google Scholar] [CrossRef]
- Brönmark, C.; Hansson, L.-A. Environmental issues in lakes and ponds: Current state and perspectives. Environ. Conserv. 2002, 29, 290–307. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Henze, D.K.; Jack, D.; Kinney, P.L. The influence of air quality model resolution on health impact assessment for fine particulate matter and its components. Air Qual. Atmos. Health 2016, 9, 51–68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noh, K.; Jeong, B.R. Particulate matter in the cultivation area may contaminate leafy vegetables with heavy metals above safe levels in Korea. Environ. Sci. Pollut. Res. 2019, 26, 25762–25774. [Google Scholar] [CrossRef] [Green Version]
- Jones, A.P. Indoor air quality and health. Atmos. Environ. 1999, 33, 4535–4564. [Google Scholar] [CrossRef]
- Persily, A.K. Indoor carbon dioxide concentrations in ventilation and indoor air quality standards. In Proceedings of the 36th AIVC Conference Effective Ventilation in High Performance Buildings, Madrid, Spain, 23–24 September 2015; pp. 810–819. [Google Scholar]
- Wyon, D.P. The effects of indoor air quality on performance and productivity. Indoor Air 2004, 14, 92–101. [Google Scholar] [CrossRef]
- Weschler, C.J.; Shields, H.C. The influence of ventilation on reactions among indoor pollutants: Modeling and experimental observations. Indoor Air 2000, 10, 92–100. [Google Scholar] [CrossRef]
- Persily, A.K.; Emmerich, S.J. Indoor air quality in sustainable, energy efficient buildings. HvacR Res. 2012, 18, 4–20. [Google Scholar]
- Jeong, B.K.; Fujiwara, K.; Kozai, T. Carbon dioxide enrichment in autotrophic micropropagation: Methods and advantages. HortTechnology 1993, 3, 332–334. [Google Scholar] [CrossRef]
- Noh, K.; Jeong, B.R. Optimizing temperature and photoperiod in a home cultivation system to program normal, delayed, and hastened growth and development modes for leafy Oak-leaf and Romaine lettuces. Sustainability 2021, 13, 10879. [Google Scholar] [CrossRef]
- Onanuga, A.O.; Adl, S. Effect of phytohormones, phosphorus and potassium on cotton varieties (Gossypium hirsutum) root growth and root activity grown in hydroponic nutrient solution. J. Agric. Sci. 2012, 4, 93. [Google Scholar] [CrossRef]
- Vasseur, F.; Pantin, F.; Vile, D. Changes in light intensity reveal a major role for carbon balance in Arabidopsis responses to high temperature. Plant Cell Environ. 2011, 34, 1563–1576. [Google Scholar] [CrossRef]
- Ren, X.X.; Xue, J.Q.; Wang, S.L.; Xue, Y.Q.; Zhang, P.; Jiang, H.D.; Zhang, X.X. Proteomic analysis of tree peony (Paeonia ostii ‘Feng Dan’) seed germination affected by low temperature. J. Plant Physiol. 2018, 224, 56–67. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Li, Y.; Hu, J.; Lee, J.; Jeong, B.R. Pre-and/or postharvest silicon application prolongs the vase life and enhances the quality of cut peony (Paeonia lactiflora Pall.) Flowers. Plants 2021, 10, 1742. [Google Scholar] [CrossRef] [PubMed]
- Muneer, S.; Soundararajan, P.; Jeong, B.R. Proteomic and antioxidant analysis elucidates the underlying mechanism of tolerance to hyperhydricity stress in in vitro shoot cultures of Dianthus caryophyllus. J. Plant Growth Regul. 2016, 35, 667–679. [Google Scholar] [CrossRef]
- Ginnopolitis, C.; Rice, S. Superoxide dismutase purification and quantitative relationship with water soluble protein in seedling. Plant Physiol. 1977, 59, 315–318. [Google Scholar] [CrossRef] [Green Version]
- Cakmak, I.; Marschner, H. Magnesium deficiency and high light intensity enhance activities of superoxide dismutase, ascorbate peroxidase, and glutathione reductase in bean leaves. Plant Physiol. 1992, 98, 1222–1227. [Google Scholar] [CrossRef] [Green Version]
- Shah, K.; Kumar, R.G.; Verma, S.; Dubey, R. Effect of cadmium on lipid peroxidation, superoxide anion generation and activities of antioxidant enzymes in growing rice seedlings. Plant Sci. 2001, 161, 1135–1144. [Google Scholar] [CrossRef]
- Nakano, Y.; Asada, K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar]
- Lee, H.; Oh, Y.; Kim, J.; Choi, T. Report of Global Atmosphere Watch 2020; National Institute of Meteorological Sciences: Seogwipo-si, Korea, 2021; pp. 67–112.
- Gilmour, D.N.; Bazzani, C.; Nayga Jr, R.M.; Snell, H.A. Do consumers value hydroponics? Implications for organic certification. Agric. Econ. 2019, 50, 707–721. [Google Scholar] [CrossRef]
- Manos, D.P.; Xydis, G. Hydroponics: Are we moving towards that direction only because of the environment? A discussion on forecasting and a systems review. Environ. Sci. Pollut. Res. 2019, 26, 12662–12672. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Prakash, O.; Sahay, D.; Kumar, P.; Bala, M. Hydroponics (soil-less cultivation): A space effective and water efficient technology for sustainable agriculture. Int. J. Agric. Environ. Sustain. 2019, 1, 4–9. [Google Scholar]
- Mitchell, C.A.; Sheibani, F. LED advancements for plant-factory artificial lighting. In Plant Factory; Elsevier: Amsterdam, The Netherlands, 2020; pp. 167–184. [Google Scholar]
- Shimizu, H.; Saito, Y.; Nakashima, H.; Miyasaka, J.; Ohdoi, K. Light environment optimization for lettuce growth in plant factory. IFAC Proc. Vol. 2011, 44, 605–609. [Google Scholar] [CrossRef] [Green Version]
- Lee, R.J.; Bhandari, S.R.; Lee, G.; Lee, J.G. Optimization of temperature and light, and cultivar selection for the production of high-quality head lettuce in a closed-type plant factory. Hortic. Environ. Biotechnol. 2019, 60.2, 207–216. [Google Scholar] [CrossRef]
- Jung, D.H.; Kim, D.; Yoon, H.I.; Moon, T.W.; Park, K.S.; Son, J.E. Modeling the canopy photosynthetic rate of romaine lettuce (Lactuca sativa L.) grown in a plant factory at varying CO2 concentrations and growth stages. Hortic. Environ. Biotechnol. 2016, 57, 487–492. [Google Scholar] [CrossRef]
- Kozai, T. Resource use efficiency of closed plant production system with artificial light: Concept, estimation and application to plant factory. Proc. Jpn. Acad. Ser. B 2013, 89, 447–461. [Google Scholar] [CrossRef] [Green Version]
- Nakayama, S. Plant factory and its prospects. IFAC Proc. Vol. 1991, 24, 85–92. [Google Scholar] [CrossRef]
- Dong, J.; Gruda, N.; Lam, S.K.; Li, X.; Duan, Z. Effects of elevated CO2 on nutritional quality of vegetables: A review. Front. Plant Sci. 2018, 9, 924. [Google Scholar] [CrossRef]
- Lemon, E.R. CO2 and Plants: The Response of Plants to Rising Levels of Atmospheric Carbon Dioxide; CRC Press: Boca Raton, FL, USA, 2019; pp. 21–61. [Google Scholar]
- Singh, H.; Poudel, M.R.; Dunn, B.L.; Fontanier, C.; Kakani, G. Effect of greenhouse CO2 supplementation on yield and mineral element concentrations of leafy greens grown using nutrient film technique. Agronomy 2020, 10, 323. [Google Scholar] [CrossRef] [Green Version]
- Tian, Y. Study and design of the control system for household plant factory. In Advances in Computer Science Research, Proceedings of the 2018 3rd International Workshop on Materials Engineering and Computer Sciences (IWMECS 2018), Jinan, China, 27–28 January 2018; Atlantis Press: Amsterdam, The Netherlands, 2018; Volume 78, pp. 397–400. [Google Scholar]
- Chalabi, Z.; Biro, A.; Bailey, B.; Aikman, D.; Cockshull, K. SE—Structures and environment: Optimal control strategies for carbon dioxide enrichment in greenhouse tomato crops—part 1: Using pure carbon dioxide. Biosyst. Eng. 2002, 81, 421–431. [Google Scholar] [CrossRef]
- Makino, A.; Mae, T. Photosynthesis and plant growth at elevated levels of CO2. Plant Cell Physiol. 1999, 40, 999–1006. [Google Scholar] [CrossRef]
- Madhu, M.; Hatfield, J. Dynamics of plant root growth under increased atmospheric carbon dioxide. Agron. J. 2013, 105, 657–669. [Google Scholar] [CrossRef] [Green Version]
- Vasconez, P.A.V.; Vasconez, M.A.V.; Cardenas, C.; Nyheim, Ø.S.; Eraso, H.R. Can higher CO2 concentrations affect yield and quality parameters in lettuce and sugar beet crops? Rev. Verde Agroecol. Desenvolv. Sustentável 2021, 16, 27–32. [Google Scholar] [CrossRef]
- Kapalo, P.; Domniţa, F.; Bacoţiu, C.; Spodyniuk, N. The impact of carbon dioxide concentration on the human health-case study. J. Appl. Eng. Sci. 2018, 8, 61–66. [Google Scholar] [CrossRef] [Green Version]
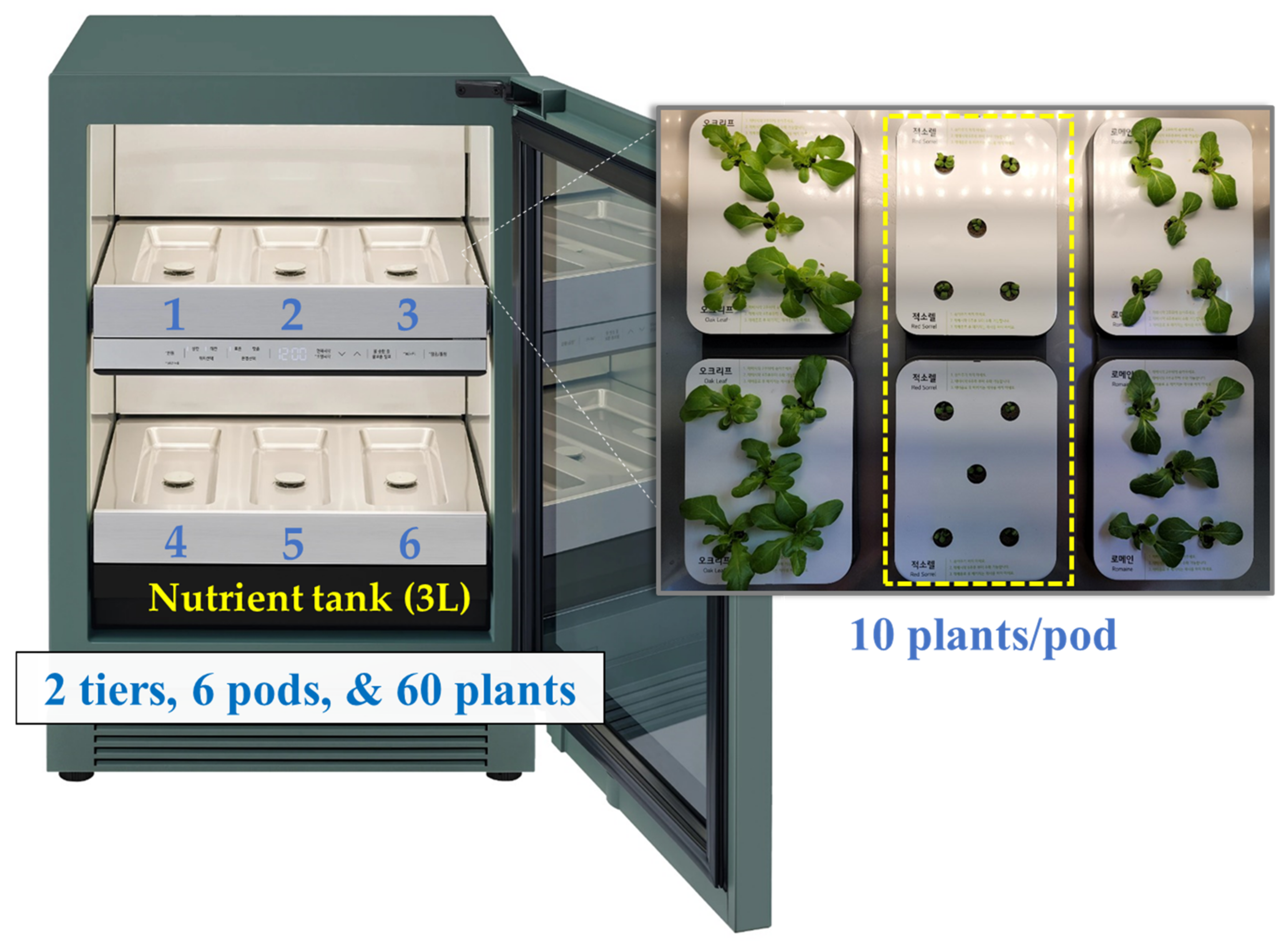
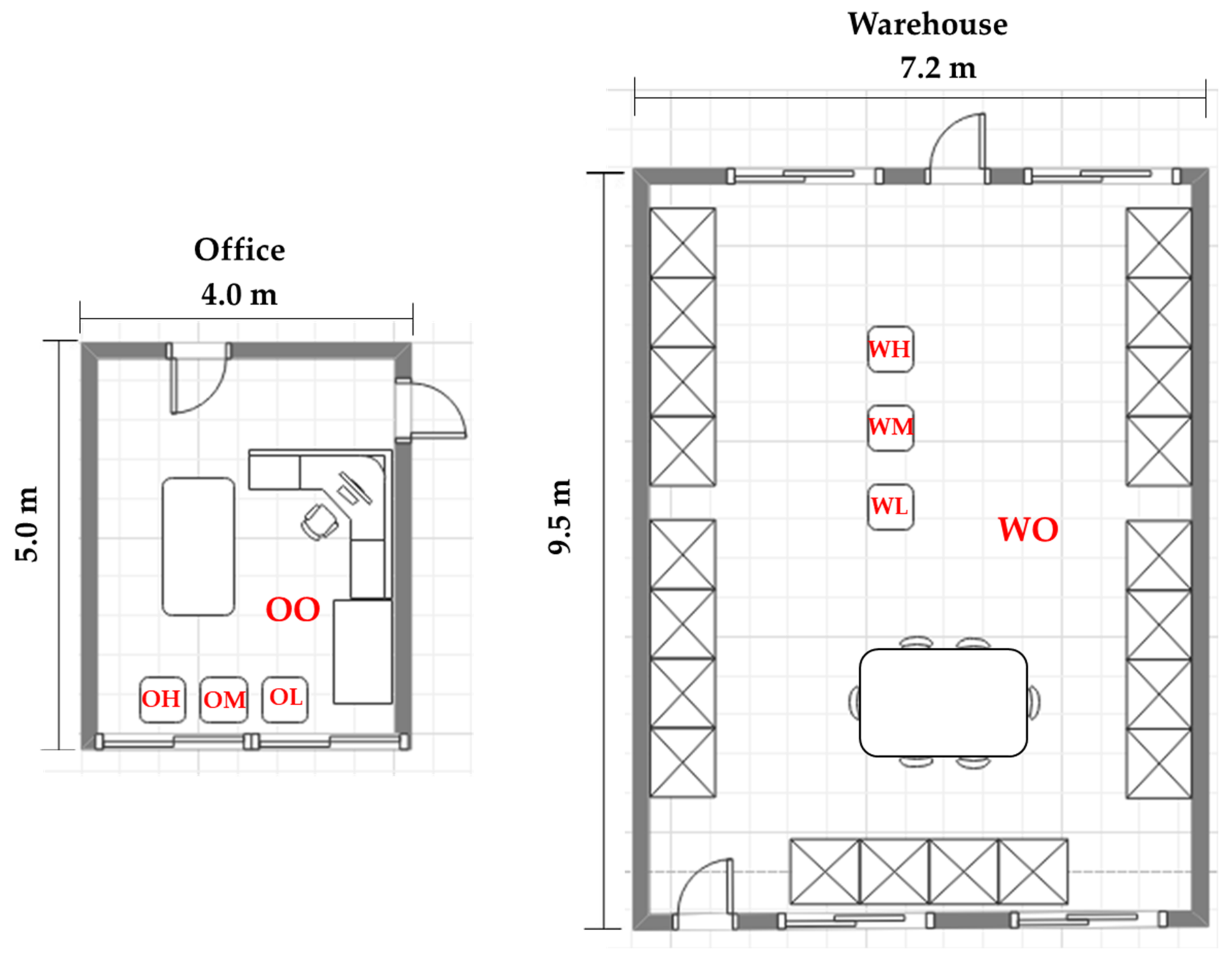
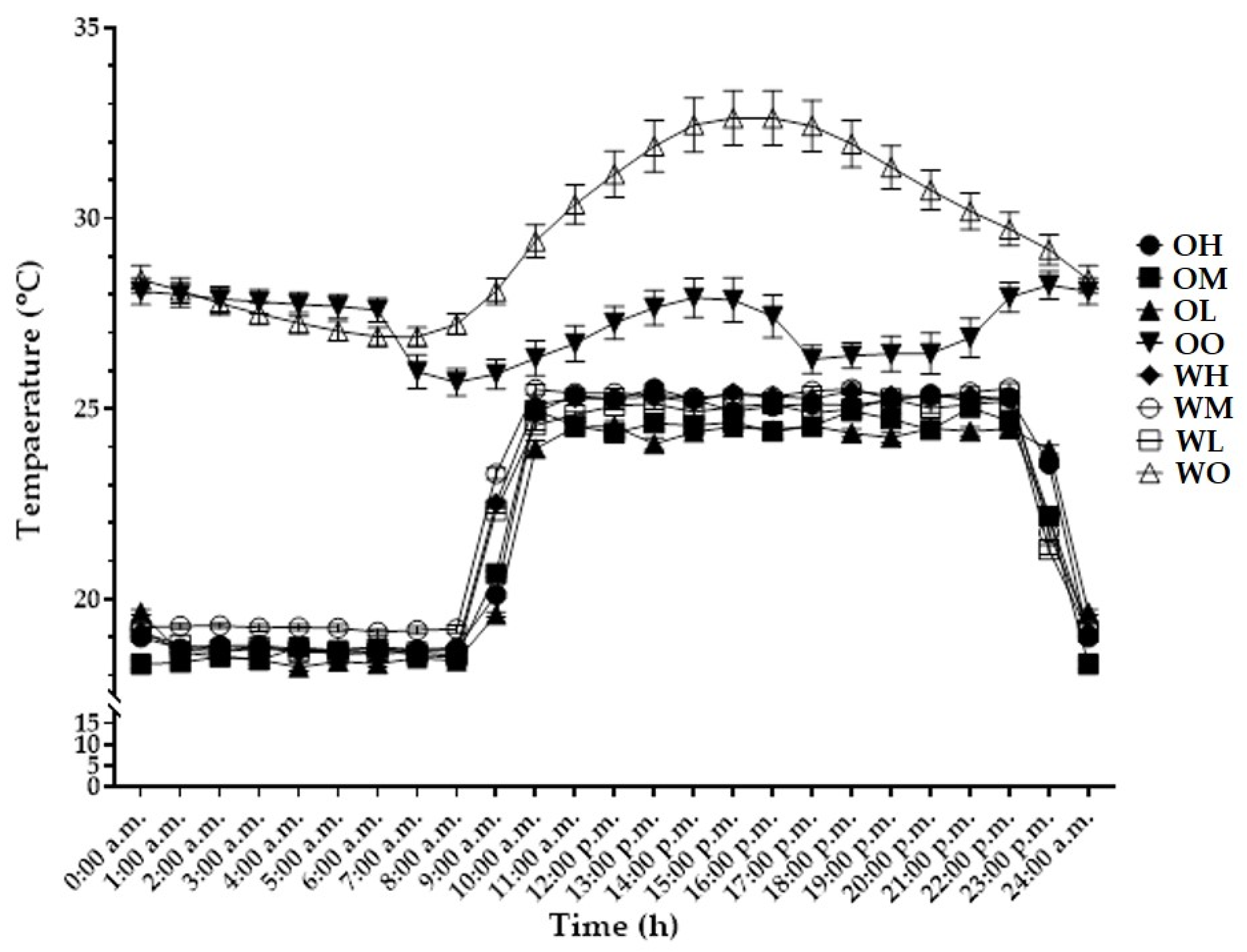
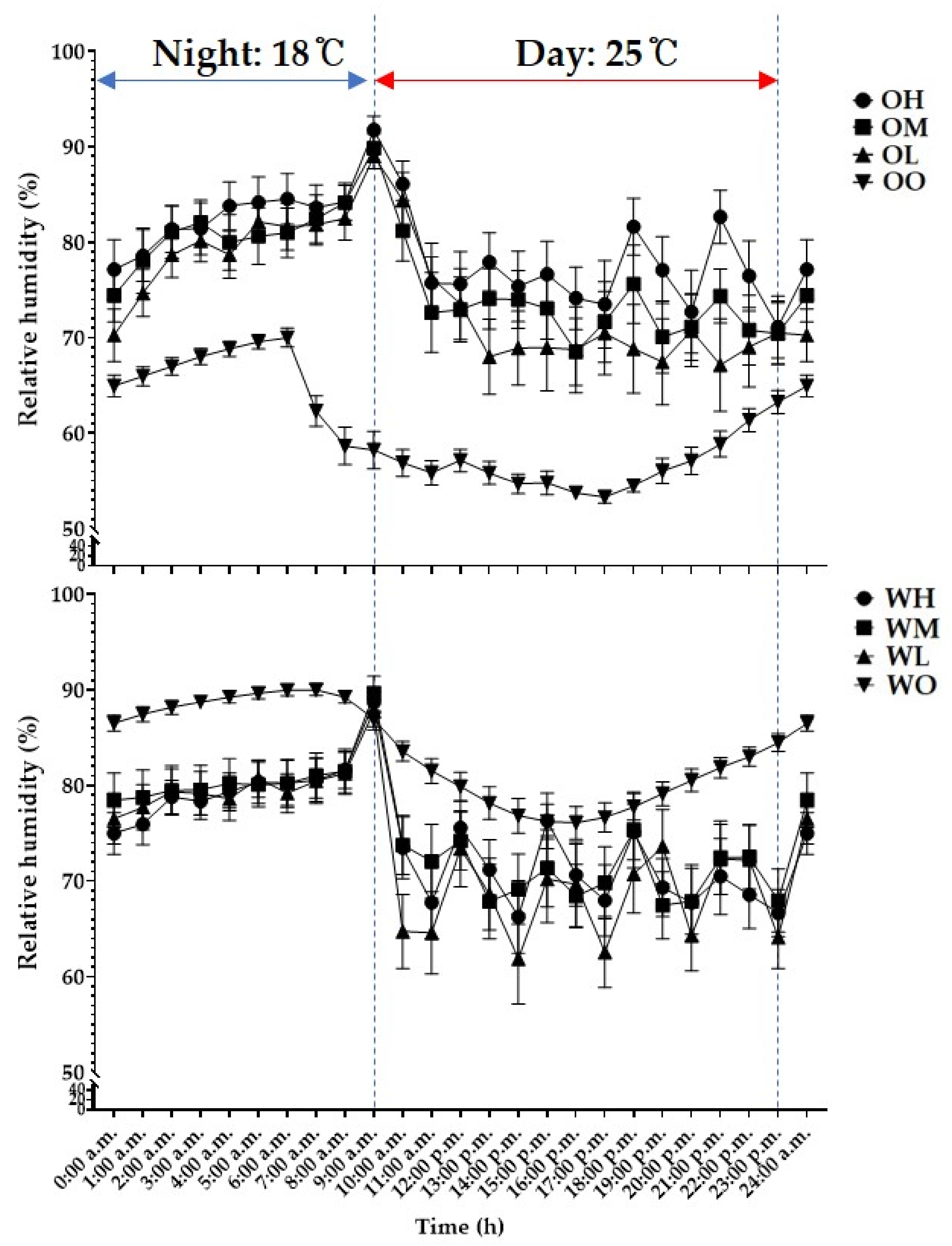
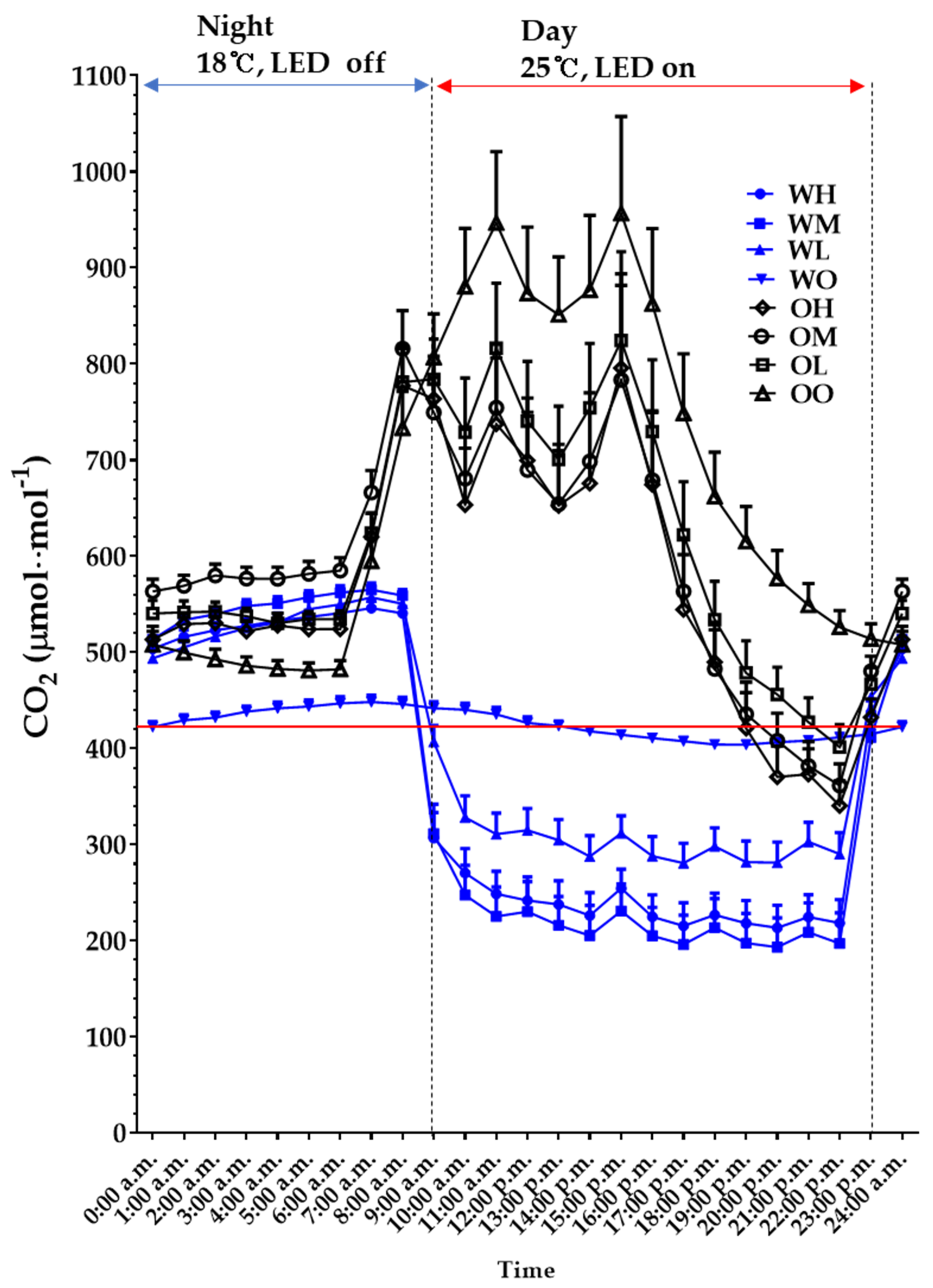
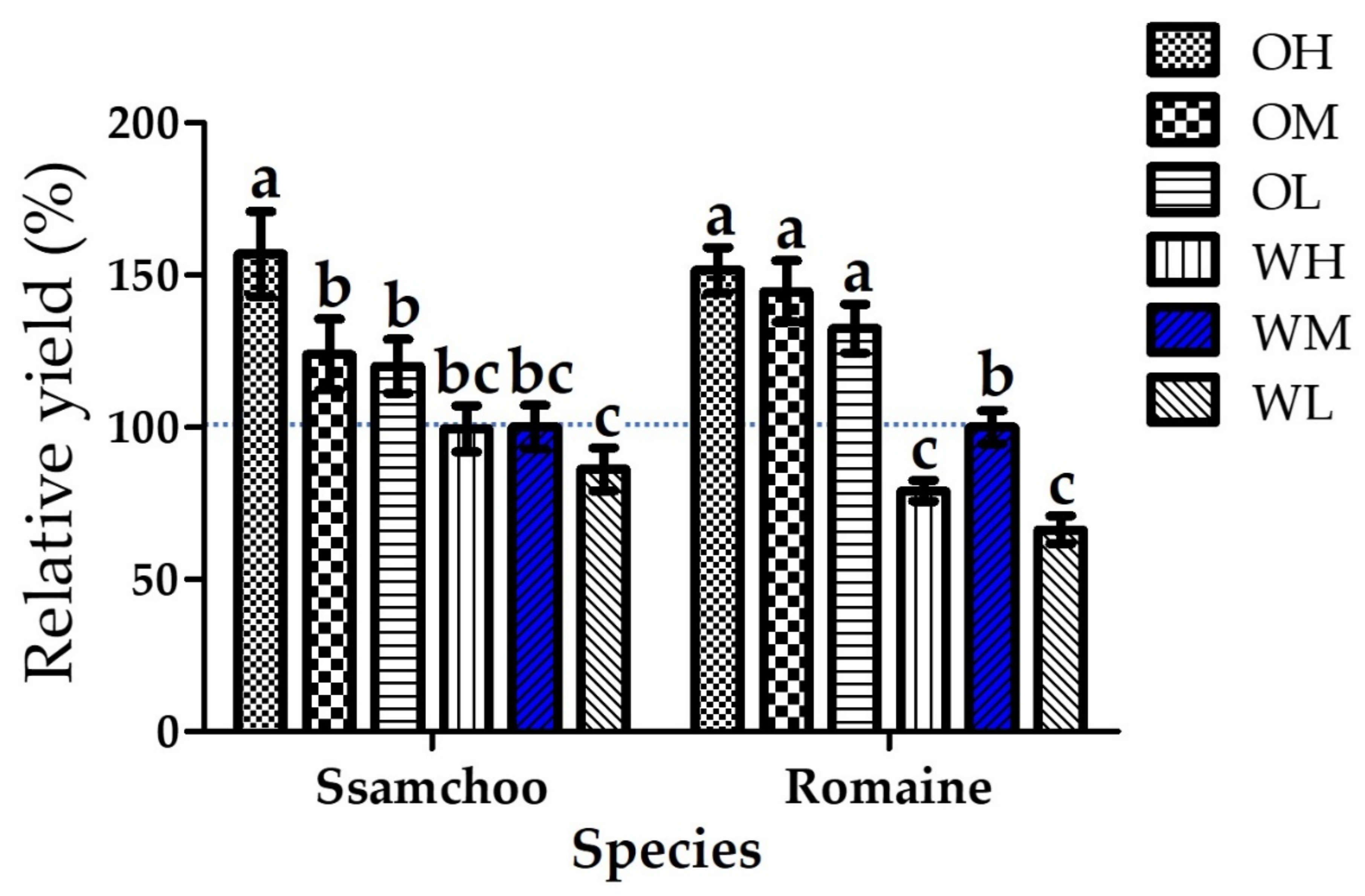
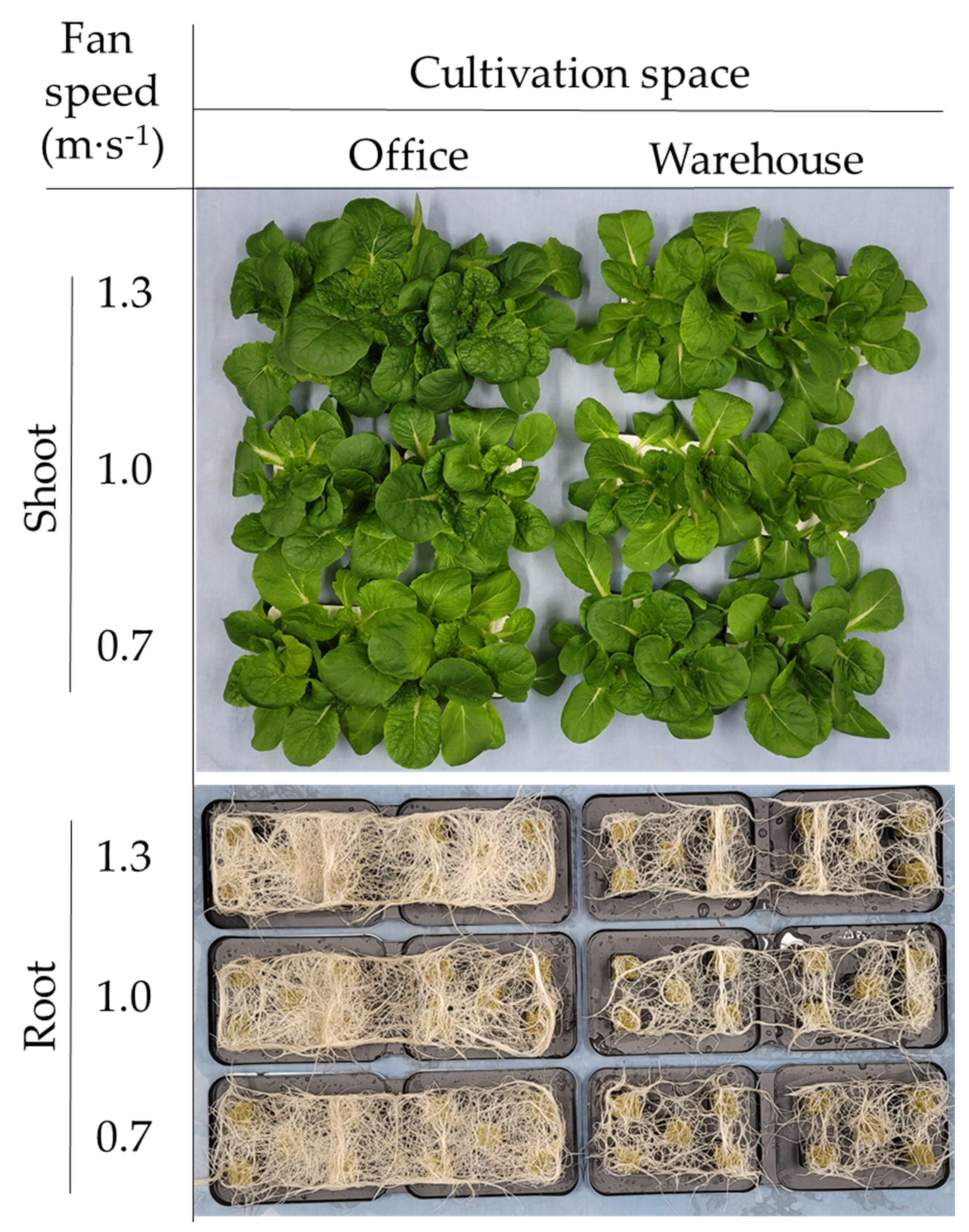

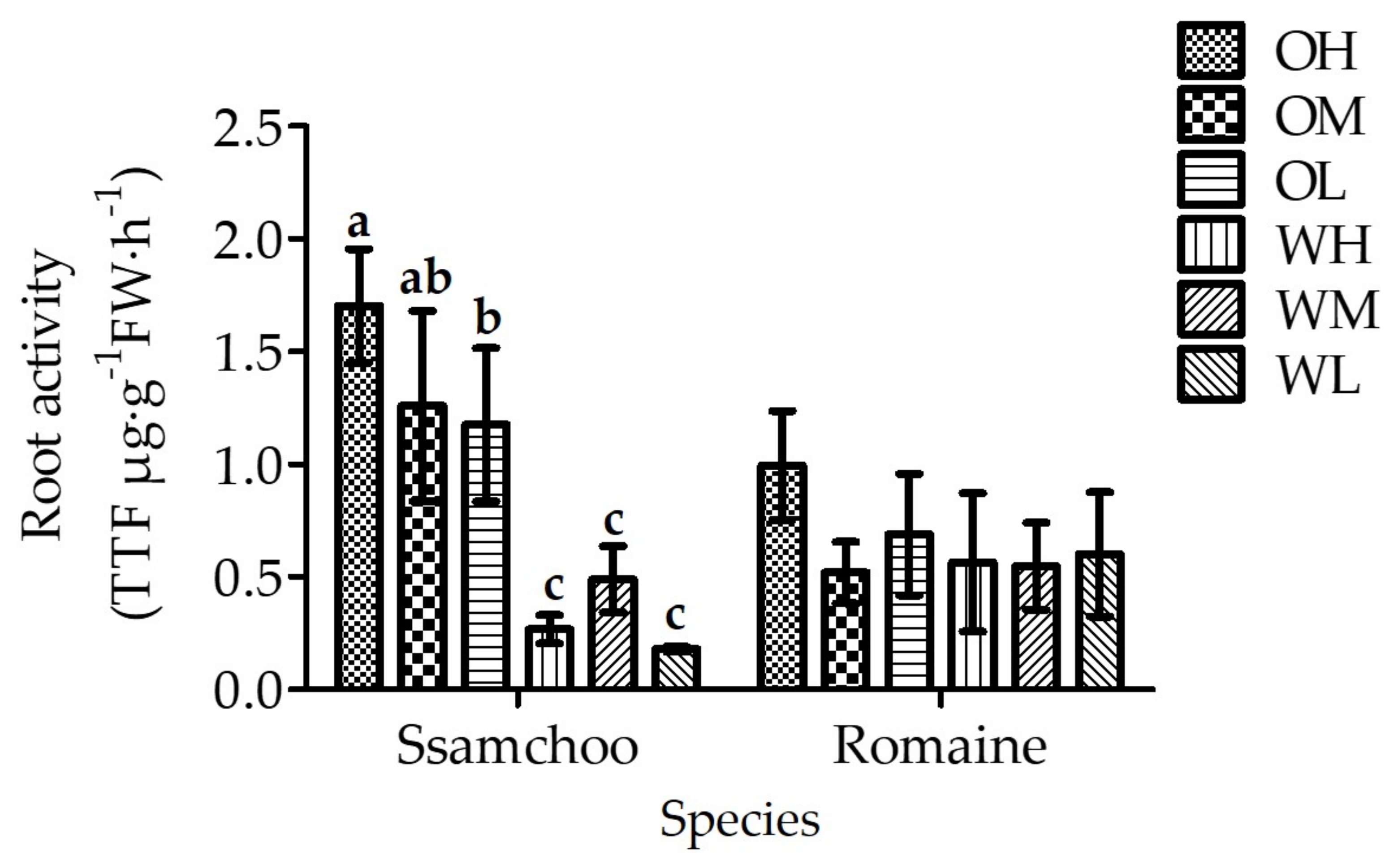
| Species (A) | Cultivation Space (B) | Fan Airspeed (m·s−1) (C) | Fresh Weight (g) | Shoot Length (cm) | Leaf | ||
|---|---|---|---|---|---|---|---|
| Length (cm) | Width (cm) | Number | |||||
| Ssamchoo | Office | 1.3 | 16.0 a y | 14.3 abcd | 13.9 abcd | 8.5 a | 8.1 bc |
| 1.0 | 12.6 b | 14.7 ab | 14.4 abc | 7.9 ab | 7.3 de | ||
| 0.7 | 12.2 bc | 15.1 a | 14.7 a | 7.9 ab | 7.1 ef | ||
| Warehouse | 1.3 | 10.2 bcd | 14.2 abcd | 13.8 abcd | 7.7 b | 6.6 ef | |
| 1.0 | 10.2 bcd | 13.7 cde | 13.4 cde | 7.4 bc | 6.9 ef | ||
| 0.7 | 8.8 de | 13.9 bcde | 13.5 bcd | 7.2 bc | 6.5 f | ||
| Romaine | Office | 1.3 | 11.6 bc | 14.9 a | 14.6 a | 6.8 cd | 8.3 abc |
| 1.0 | 11.0 bcd | 14.7 abc | 14.3 abc | 6.1 e | 8.9 a | ||
| 0.7 | 10.1 bcd | 14.8 ab | 14.5 ab | 6.4 de | 8.9 a | ||
| Warehouse | 1.3 | 6.0 fg | 12.9 e | 12.5 e | 5.9 e | 8.1 bc | |
| 1.0 | 7.6 ef | 13.5 de | 13.1 de | 5.9 e | 8.6 ab | ||
| 0.7 | 5.1 g | 11.6 f | 11.2 f | 5.1 f | 7.8 cd | ||
| z F-test | A | *** | * | * | *** | *** | |
| B | *** | *** | *** | *** | *** | ||
| C | * | NS | NS | * | NS | ||
| A × B | NS | ** | ** | NS | NS | ||
| A × C | NS | * | * | NS | * | ||
| B × C | * | * | * | NS | NS | ||
| A × B × C | NS | NS | NS | NS | * | ||
| Species (A) | Cultivation Space (B) | Fan Speed (m·s−1) (C) | Content (mg·g−1 FW) | |||
|---|---|---|---|---|---|---|
| Soluble Protein | Soluble Sugar | Starch | Proline | |||
| Ssamchoo | Office | 1.3 | 34.1 | 2.8 a y | 1.5 b | 0.17 c |
| 1.0 | 32.7 | 1.9 de | 1.2 ef | 0.11 c | ||
| 0.7 | 33.7 | 2.3 bcd | 1.4 bc | 0.20 c | ||
| Warehouse | 1.3 | 36.2 | 2.4 abc | 1.2 def | 0.20 c | |
| 1.0 | 34.7 | 2.7 ab | 1.5 b | 0.15 c | ||
| 0.7 | 37.2 | 2.0 cde | 2.1 a | 0.20 c | ||
| Romaine | Office | 1.3 | 32.5 | 1.8 ef | 1.2 cdef | 0.49 b |
| 1.0 | 37.3 | 1.6 ef | 1.0 f | 0.83 a | ||
| 0.7 | 35.0 | 2.6 ab | 1.3 bcde | 0.20 c | ||
| Warehouse | 1.3 | 37.6 | 1.3 f | 1.4 bcd | 0.88 a | |
| 1.0 | 34.1 | 2.0 cde | 1.5 b | 0.20 c | ||
| 0.7 | 35.2 | 1.6 ef | 2.1 a | 0.51 b | ||
| z F-test | A | NS | *** | NS | *** | |
| B | NS | NS | *** | NS | ||
| C | NS | NS | *** | * | ||
| A × B | NS | * | * | NS | ||
| A × C | NS | * | NS | * | ||
| B × C | NS | *** | *** | ** | ||
| A × B × C | NS | NS | NS | *** | ||
| Species (A) | Cultivation Space (B) | Fan Speed (m·s−1) (C) | Activity (U·mg−1 Protein) | |||
|---|---|---|---|---|---|---|
| GPX | CAT | SOD | APX | |||
| Ssamchoo | Office | 1.3 | 17.7 b y | 44.2 a | 0.665 abc | 23.7 c |
| 1.0 | 9.2 d | 18.4 de | 0.801 a | 25.9 c | ||
| 0.7 | 25.2 a | 26.8 b | 0.727 ab | 26.7 c | ||
| Warehouse | 1.3 | 12.4 c | 41.5 a | 0.541 bc | 33.2 b | |
| 1.0 | 8.7 d | 26.0 b | 0.819 a | 40.6 a | ||
| 0.7 | 24.3 a | 16.2 de | 0.644 abc | 22.8 cd | ||
| Romaine | Office | 1.3 | 9.4 d | 23.4 bc | 0.462 c | 18.8 de |
| 1.0 | 8.1 d | 12.1 f | 0.504 bc | 24.2 c | ||
| 0.7 | 8.6 d | 15.7 e | 0.510 bc | 17.2 e | ||
| Warehouse | 1.3 | 8.0 d | 19.9 cd | 0.693 abc | 31.9 b | |
| 1.0 | 8.9 d | 17.9 de | 0.615 abc | 8.9 f | ||
| 0.7 | 8.6 d | 8.6 f | 0.200 d | 17.2 e | ||
| z F-test | A | *** | *** | *** | *** | |
| B | * | NS | NS | ** | ||
| C | *** | *** | * | *** | ||
| A × B | * | NS | NS | ** | ||
| A × C | *** | *** | * | *** | ||
| B × C | * | *** | * | *** | ||
| A × B × C | NS | NS | NS | *** | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Noh, K.; Jeong, B.R. Increased Carbon Dioxide by Occupants Promotes Growth of Leafy Vegetables Grown in Indoor Cultivation System. Sustainability 2021, 13, 13288. https://doi.org/10.3390/su132313288
Noh K, Jeong BR. Increased Carbon Dioxide by Occupants Promotes Growth of Leafy Vegetables Grown in Indoor Cultivation System. Sustainability. 2021; 13(23):13288. https://doi.org/10.3390/su132313288
Chicago/Turabian StyleNoh, Kyungdeok, and Byoung Ryong Jeong. 2021. "Increased Carbon Dioxide by Occupants Promotes Growth of Leafy Vegetables Grown in Indoor Cultivation System" Sustainability 13, no. 23: 13288. https://doi.org/10.3390/su132313288
APA StyleNoh, K., & Jeong, B. R. (2021). Increased Carbon Dioxide by Occupants Promotes Growth of Leafy Vegetables Grown in Indoor Cultivation System. Sustainability, 13(23), 13288. https://doi.org/10.3390/su132313288







