Abstract
Despite its importance as a component of urban green spaces, as far as we are aware no study has focused on plant diversity in urban green corridors (GCs). Therefore, this study aimed at: (i) characterizing tree and shrub communities in Lisbon’s GCs and (ii) assessing whether GCs’ users value trees and shrubs. We counted Lisbon’s GCs users in the same places where we assessed the tree and shrub community. Along the nine GCs, we observed trees and shrubs belonging to 70 species, distributed across 35 families with most (≥50%) species and plants being trees, exotic, pollinated by insects, with fruit dispersion by animals, evergreen leaves, and producing dry fruits. Most GCs had a similar number of users (20–30 users h−1 survey−1) except for those of Central and Ribeirinho, which were more frequented (60 and 100 users h−1 survey−1, respectively). Most users (≥50%) were adults, walking accompanied, and performing leisure activities. Finally, the number of users was shown to be influenced by: (i) tree and shrub relative abundance, confirming that users preferred greener corridors; and (ii) function(s), showing that users preferred the most multifunctional GCs (i.e., GCs fulfilling ecological, cultural, and recreational functions). Our data suggest that Lisbon’s GCs favor more the inclusion of citizens than ecological functionality and resilience.
1. Introduction
Cities cover about 3% of the land on Earth, yet they produce about 72% of global greenhouse gas emissions. On top of that, 54% of the world’s population lives in urban areas (estimated to reach 66% by 2050) [1] and many cities and towns worldwide are presently dealing with the impacts of climate change (e.g., heatwaves, extreme droughts, torrential floods). Therefore, it is increasingly urgent to address climate change in the context of cities.
Concerning climate change adaptation and mitigation, plants (and larger species in particular) are recognized as valuable assets as they sequester carbon and reduce droughts, floods, and heat waves. As an example, it is estimated that 40% of Lisbon’s street trees provide irreplaceable services (energy saving, reduction of CO2, air pollution and floods, and property value increase) valued at €7.1 million annually [2]. Urban green spaces also contribute to social cohesion, environmental awareness, and human wellbeing (e.g., reducing of stress, noise and pollution, increased sun exposure, improved mental health and immune system, and reduced obesity and diabetes) [3]. Consequently, the implementation and revitalization of urban green spaces has been used as a strategy for urban populations to enjoy the environmental, social, and health benefits that nature provides.
Besides planting trees and other vegetation on streets and urban parks, green corridors (GCs) are another important component of urban green spaces. GCs emerged in the 19th and 20th centuries [4], and are made of natural and semi-natural linear infrastructures (e.g., trees and other vegetation) that connect other green spaces (including outside the city) to form an urban ecological network [5]. GCs constitute a continuous system, establishing links between areas of high concentrations of ecological, landscape, and cultural resources, while promoting their protection of human wellbeing and compatibility with human activity and wellbeing [6,7]. GCs provide (i) ecosystem services (e.g., habitat and resources for urban fauna, adaptation and mitigation of climate change impacts); (ii) genetic exchange between animals and plants; and (iii) improved mobility connecting points of the city with different uses (e.g., residential, commercial, education) and access to green spaces [8,9,10]. However, to provide these benefits, the implementation of GCs (and of other components of urban green spaces) must be properly planned to make cities ‘more natural’, functional, and resilient [11,12].
One strategy to properly plan urban green spaces is to study plant diversity, and in particular plant functional diversity, which is a measure of biodiversity that incorporates species attributes (e.g., mode of pollination and seed dispersal, leaf typology and type of fruit) [13,14]. So far, most studies on urban plant diversity have focused on taxonomic diversity rather than plant functional patterns [10]. The origin of plant species (native versus exotic) is one of the few traits that has been studied. Although the presence of many exotic species in GCs [15,16,17] may have undesirable effects on local plant communities [18], some studies show a positive effect on the environmental balance of urban areas [19], and therefore the use of exotic species in cities remains controversial. Thus, urban green spaces planning lacks studies on the functional patterns of urban vegetation and on functional traits. Finally, and contrary to other urban green spaces (e.g., urban parks) [20,21], as far as we are aware, there is no study on plant diversity in GCs.
As such, we characterized the plant diversity and its functional patterns in the nine GCs in the city of Lisbon (Portugal). We focused only on tree and shrub species because: (i) the cover of trees and shrubs in CGs has important environmental and social functions; (ii) being long-lived, trees and shrubs are the vegetation components most exposed and most resistant to the anthropogenic stresses that characterize the urban environment; and (iii) the maintenance of CGs implies the periodic removal of herbaceous plants. Although the ecological and environmental functions of GCs are unquestionable, it is important that these components of the urban green spaces are in tune with the needs and interests of the urban population, so that a GC can also have a cultural and/or recreational function. Depending on which function(s) a GC is planned to perform (ecological alone or combined with cultural and/or recreational), different biophysical structures will be implemented along the GC, with the potential to attract more or less people. Furthermore, if GCs provide the abovementioned social, health, and well-being benefits to the people who use them (i.e., users), our hypothesis is that we would find more users where vegetation was more diverse and/or more abundant. To test our hypothesis, we counted the number of GCs users (and their characteristics: apparent gender, mode of travel, age, etc.) in the same places where we assessed the tree and shrub community, as well as which vegetation variables influenced the number of users.
Our objectives were to: (i) characterize the plant diversity and its functional patterns in the nine GCs in the city of Lisbon (Portugal) and (ii) assess whether people value GCs’ plant diversity. Our study aims to provide scientific knowledge to local authorities (and to the Lisbon City Council in particular) so that they can maximize GCs’ ecological, environmental, social, health, and well-being benefits, and thus contribute to subsidizing urban planning actions that improve urban life quality.
2. Materials and Methods
2.1. Study Area
This study was carried out in the city of Lisbon (Portugal) which has a total area of 83.84 km2, of which 18% (~15.10 km2) correspond to urban green spaces (Figure 1). The climate is Mediterranean, characterized by hot and dry summers, contrasting with cold, humid, and rainy winters. Lisbon is located in a transition zone between the Atlantic Ocean and the Mediterranean Sea and between Africa and Eurasia, hence presenting unique characteristics, containing more biodiversity than the average European city [22]. As one of the oldest European capitals, Lisbon’s landscape and vegetation cover has become highly fragmentated. As a result, in the past 10 years the city has been going through a process of territory restructuring and re-arrangement accompanied by the construction urban green spaces (Municipal Master Plan) [23]. In fact, most of Lisbon’s GCs resulted from that very process.
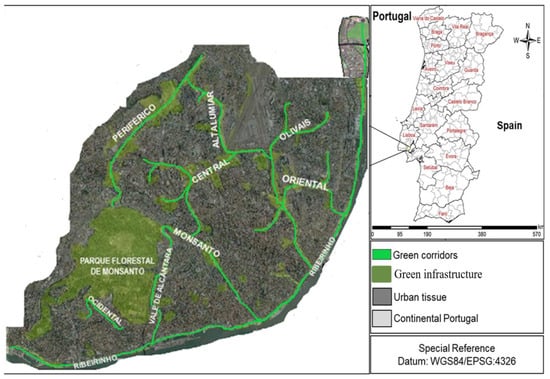
Figure 1.
Location and distribution of Lisbon’s GCs and green spaces in 2021 (source: Câmara Municipal de Lisboa).
Lisbon’s GCs were thus the target of our study: Ocidental, Alcântara, Olivais, Monsanto, Lumiar, Oriental, Ribeirinho, Central, and Periférico. The GCs differ in environmental context, extension, year of implementation, biophysical structures, and main function(s) (Table 1 and Figure 1). Sections of Alcântara, Ribeirinho, and Periférico GCs were still being implemented at the time of the tree and shrub surveys; hence, those unfinished sections were excluded from our study.

Table 1.
Main characteristics and designation of Lisbon’s GCs.
2.2. Characteristics of Tree and Shrub Diversity and Functional Patterns
The diversity of trees and shrubs in the nine GCs was assessed between September and November 2019, using the quadrant method (20 × 20 m) adapted from Durigan [24], sampling all trees and shrubs ≥5 cm high. The sampling effort differed between GCs and was not related with GC’s extension because: (i) as the locations for assessing plant diversity would be the same for counting the number of users, the sampling points had to guarantee good access, safety, sanitary environment, existence of recreational and sports facilities, and landscape diversity; (ii) some GCs (e.g., Ribeirinho, Central and Periférico) are discontinuous and have areas with limited green structures (e.g., avenues—Figure 1); (iii) the sections of some GCs (Alcântara, Ribeirinho, and Periférico) were still being implemented and were therefore excluded from the surveys; and iv) some GCs present very few different habitats along its extension, and therefore negligible differences in floristic composition overall. Taking into consideration the mentioned constraints, we made 58 surveys in total: 5 surveys in GC 1 (Ocidental), 4 surveys in GC 2 (Alcântara), 4 surveys in GC 3 (Olivais), 9 surveys in GC 4 (Monsanto), 5 surveys in GC 5 (Lumiar), 6 surveys in GC 6 (Oriental), 12 surveys in GC 7 (Ribeirinho), 4 surveys in GC 8 (Central), and 9 surveys in GC 9 (Periférico) (Table 2). The location of the quadrants was registered using a GPS device (Garmin eTrex® 30×, Olathe, KS, USA) and a digital camera (Canon SX540 HS—20.3 Megapixels, Tokyo, Japan).

Table 2.
Characterization of the tree and shrub community in Lisbon’s green corridors (GCs). List of the trees and shrubs observed between September and November 2019, their cumulative abundance (i.e., accounting for all the surveys performed in a given GC; the number of surveys per GC is shown in parenthesis below green corridor designation), and their functional traits (origin—O; size—S; pollination mode—P; seed dispersion mode—D; leaf lifespan—L; and fruit type—F).
To identify the tree and shrub species we combined analysis of plant physiological and morphological characteristics according to the APG IV classification system [25], specialized bibliography (botanical manual) [26,27] and the citizen science app PlantNet. From the tree and shrub community assessments, it was possible to calculate:
- (a)
- Relative abundance—number of tree and shrub individuals observed per survey;
- (b)
- Species richness (S)—number of tree and shrub species observed per survey;
- (c)
- Species evenness (J)—is a measure of diversity that quantifies how equal the community is numerically. Varies from 0 (zero) to 1 (one), with values closer to zero reflecting an uneven community is (i.e., one or more species are dominant) and vice versa.
Finally, plant species were grouped according to functional traits relevant for urban ecosystem functioning and environmental balance [14]. We studied the following traits adapted from Cornelissen et al. [13] and Duncan et al. [28]:
- (a)
- Species size: tree (≥6 m) versus shrub (≤5 m) [29]. Species size does not refer to the observed size of the individuals, but the maximum size described for a given species;
- (b)
- Species origin: native versus exotic. Although it is not consensual, we considered species origin as a functional trait in this study;
- (c)
- Pollination mode: anemophilous (pollination occurs through the wind) versus entomophilous (insects are the pollinating agents). Pollination mode was determined based on field observations and morphological analysis [30];
- (d)
- Seed dispersal mode: anemochory (dispersion is wind-facilitated) versus zoochory (dispersion is animal-facilitated);
- (e)
- Foliage lifespan: deciduous (plants shed their leaves during unfavorable seasons) versus evergreens (maintain their foliage permanently);
- (f)
- Fruit type: fleshy (the pericarp is succulent and attracts animals that will contribute to seed dispersion) versus dry (the pericarp is dry and normally the seed is wind dispersed).
2.3. Characteristics of GCs Users
To assess Lisbon’s GCs users, we counted the number of people using each of the nine GCs, in June, July, and October 2020 (no survey was made on very hot—>35 °C—or cloudy/rainy days). GCs users were counted during 1 h periods on weekdays in two time periods: in the morning between 9:00 and 12:00, and in the afternoon between 15:00 and 19:00. The locations for counting the number of users coincided with the 58 sampling points previously established for the tree and shrub surveys. As previously mentioned, these locations guaranteed: (1) good access, (2) safety, (3) clean environment, (4) recreational and sports facilities, and (5) landscape diversity. To ensure we did not overcount users (e.g., counted the same person twice), we made photographic records with a digital camera (Canon SX540 HS—20.3 Megapixels, Tokyo, Japan). GCs users were characterized in terms of:
- (a)
- Gender: male or female.
- (b)
- Age: children <11 years old; young between 12 and 24 years old; adults between 25 and 64 years old; and elderly >65 years old.
- (c)
- Company: alone or accompanied.
- (d)
- Mobility: walking, running, bicycle, others (skate, rollerblades, and scooter).
- (e)
- Purpose of using the GC: commuting to work/school, leisure, or sport.
2.4. Statistics
To confirm that our sampling effort to assess tree and shrub diversity was adequate, we built a species accumulation curve by the rarefaction method (data not shown) using Statistical Estimation of Species Richness and Shared Species from samples—Estimates (Version 9.1.0, 2019) [31].
Despite trying different data transformations (e.g., log, ln), our plant diversity and users’ variables did not show a normal distribution (Shapiro-Wilk test, p < 0.05). Therefore, the effect of the GC on plant diversity and functional traits, and on users’ characteristics was tested separately using a non-parametric test (Kruskal-Wallis test followed by pairwise Mann-Whitney tests with Bonferroni correction, p < 0.05). To relate plant (diversity and functional traits) and users’ variables we used a principal component analysis (PCA), whereby we pooled all sampling points (n = 58). Preliminary analyses were performed to ensure there was no violation of the assumptions regarding the PCA application (e.g., Kaiser-Meyer-Olkin Measure of Sampling Adequacy and Bartlett’s test of sphericity). SPSS (version 27.0, IBM, Inc., Chicago, IL, USA) was used for all of these analyses.
The influence of plant diversity variables (tree and shrub richness and relative abundance) on the number of users was tested using Generalized Linear Models (GLMs) with Negative Binomial distribution (connection recording function) due to users’ characteristics (counting data and presenting super dispersion). We further included GC’s function(s) in the GLMs, which was shown to be significant. Finally, the effect of GC function(s) on the number of users was tested with the Tukey test using lsmeans (Least Square Media, p < 0.05). We used the statistical software R version 3.6.0 [32] for these analyses.
3. Results
3.1. Tree and Shrub Diversity and Functional Patterns
Along the nine GCs, we observed trees and shrubs belonging to 70 species, distributed across 35 families. Although the GCs differed in tree and shrub composition and abundance (Table 2 and Table 3), the most represented families were Rosaceae (10 species and 49 individuals) and Fabaceae (9 species and 24 individuals). The rare and less abundant species (1–5 individuals) were mostly exotic, while the most abundant species (>5 individuals) were native (e.g., Populus nigra, Buxus sempervirens, Pinus pinaster, and Quercus suber) or naturalized (Elaeagnus angustifolia—Table 2).

Table 3.
Characterization of the tree and shrub community in Lisbon’s GCs in terms of relative abundance and diversity (species richness and evenness). Different letters show significant differences between GCs (p < 0.05). Values are the mean ± SD (n = variable—see Table 2).
Most GCs were very heterogeneous along their extension, as shown by their high variation. We observed the highest tree and shrub relative abundance in GCs 4 (Monsanto) and 5 (Lumiar), while GCs 1 (Ocidental), 2 (Alcântara), and 3 (Olivais) had the lowest tree and shrub relative abundance (Table 3). We also observed the highest number of tree and shrub species (i.e., species richness) in GC 4 (Monsanto), together with GCs 1 (Ocidental), 5 (Lumiar), and 9 (Periférico), while GCs 2 (Alcântara), 3 (Olivais), and 8 (Central) were those with the lowest values of species richness. Although the variation was very small, GCs 1 (Ocidental), 2 (Alcântara), 3 (Olivais), and 8 (Central) showed more evenness between trees and shrubs, while GCs 4 (Monsanto), 5 (Lumiar), 6 (Oriental), 7 (Ribeirinho), and 9 (Periférico) had lower evenness values, reflecting a greater dominance of some species.
Considering the set of the nine Lisbon’s GCs, most of the observed species were trees (77%), had exotic origin (80%), were pollinated by insects (entomophilous—70%), fruits were dispersed by animals (zoochory—58%), with evergreen leaves (55%), and produced dry fruits (61%—Figure 2). However, some GCs showed a different pattern of functional traits. For example, in GCs 2 (Alcântara) and 3 (Olivais) we only observed trees that were mostly native (67% and 57%, respectively), displaying anemophilous pollination (67% and 57%, respectively), fruits with wind-dispersion (83% and 57%, respectively), deciduous leaves (67% and 86%, respectively), and dry fruits (83% and 71%, respectively).
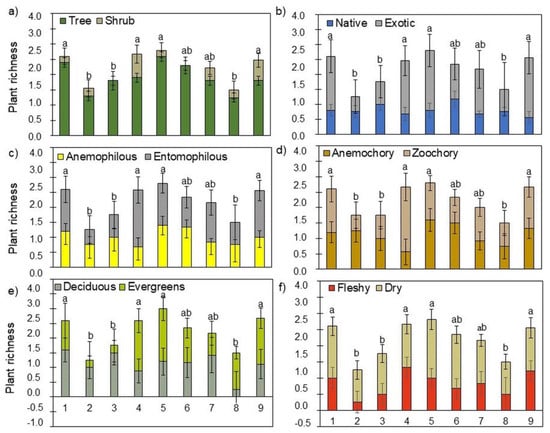
Figure 2.
Partitioning of the tree and shrub species observed in the GCs according to the following functional traits: origin (a), size (b), pollination mode (c), seed dispersion mode (d), leaf lifespan (e), and fruit type (f). Different letters show significant differences between GCs (p < 0.05). Bars and error bars are the mean and SD, respectively (n = variable—see Table 2).
Analysis of the functional traits considering the plants (trees and shrubs—Figure 3) showed a similar pattern to that of species (Figure 2) but highlighted differences in some functional traits. Thus, most of the plants we observed were trees (70%), exotic (64%), with pollination by insects (entomophilous—54%) and dispersal by animals (zoochory—53%), evergreen (55%), and that produce dry fruits (71%). Again, GCs 2 (Alcântara) and 3 (Olivais) showed a distinct pattern of functional traits.
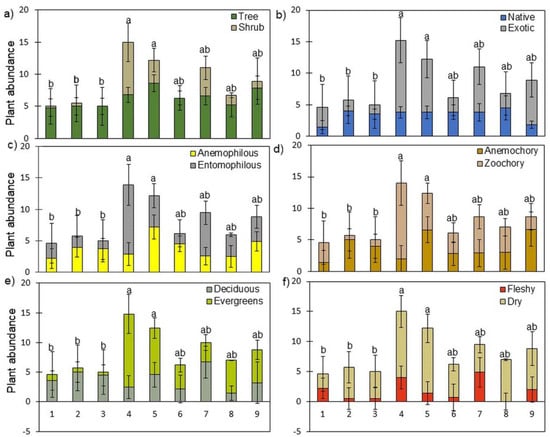
Figure 3.
Partitioning of the abundance of trees and shrubs observed in the GCs according to the following functional traits: origin (a), size (b), pollination mode (c), seed dispersion mode (d), leaf lifespan (e), and fruit type (f). Different letters show significant differences between GCs (p < 0.05). Bars and error bars are the mean and SD, respectively (n = variable—see Table 2).
3.2. GCs’ Users
Most GCs had a similar number of users. However, the most frequented GCs were 7 (Ribeirinho) and 8 (Central). The GCs with fewer users were 1 (Ocidental) and 2 (Alcântara) (Figure 4a). When characterizing Lisbon’s GCs users, we found no significant difference between users according to their gender (Figure 4b). However, most GCs users were young and adults (30% and 50%, respectively), walking (85%) accompanied (66%), performing leisure activities (61%) (Figure 4).
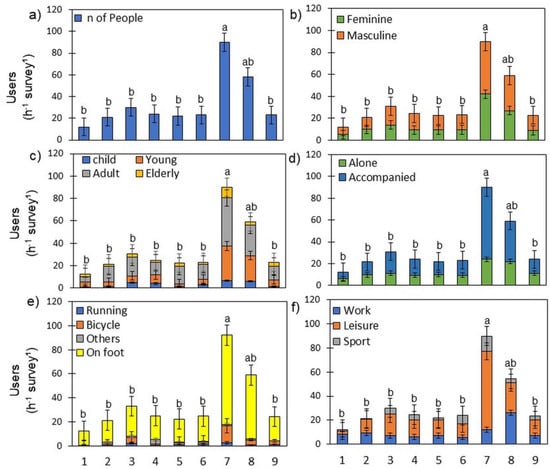
Figure 4.
Characterization of Lisbon’s GCs users (a) according to their: gender (b), age (c), with or without company (d), mobility type (e), and purpose of using the GC (f). Different letters show significant differences between GCs (p < 0.05). Bars and error bars are the mean and SD, respectively (n = variable—see Table 2).
3.3. Relationship between GCs’ Tree and Shrub Diversity and Users
Principal Component Analysis (PCA) of GCs’ tree and shrub diversity and users showed that the first two components explained 64% of the variance (Figure 5). PC1, which explained 42% of the variance, was associated with the number of users and users’ dominant characteristics (e.g., adults, walking accompanied). PC2, which explained 22% of the variance, was associated with the number of tree and shrub species (i.e., species richness). Therefore, there were two gradients along which the nine Lisbon’s GCs were distributed: very frequented GCs with high tree and shrub species richness (e.g., GC 7—Ribeirinho) and less frequented GCs and with low tree and shrub species richness (e.g., GC 2—Alcântara). Both tree and shrub relative abundance and plant functional traits showed low loadings (<0.8—Table S1) for the first two axes of the PCA. However, tree and shrub relative abundance was more associated with the number of users.
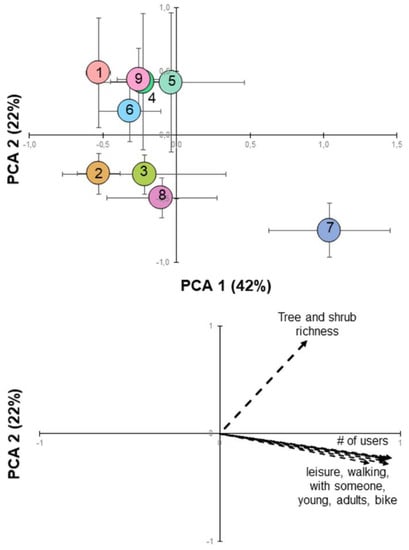
Figure 5.
Principal component analysis (PCA) of the GCs tree and shrub community and users (top). Symbols are the mean ± SD (n = variable—see Table 2); PC1 explains 42% of the variance while PC2 explains 22%. (bottom) Loading plot for the most important variables (loading >0.8; loadings for all variables are shown in Table S1) which are presented by vectors.
In fact, the tree and shrub relative abundance had a positive influence on the number of users (Table 4 and Figure 6a) but not tree and shrub richness (Table 4). The GLM estimate for the tree and shrub relative abundance was 0.04 (data not shown), meaning that when increasing the tree and shrub relative abundance by one unit, we would expect to have +1 user (3.74%). The function(s) for which a GC was designed also influenced the number of users, with people clearly preferring the most multifunctional GCs (i.e., GCs fulfilling ecological + cultural + recreational functions) (Figure 6b). We tested other models (without GC function(s), with other plant variables) but selected the model on Table 4 based on the normal distribution of the model residuals (Shapiro–Wilk test, p = 0.1321), Akaike information criterion (AIC = 514.6), and the Chi-square test (p < 0.05).

Table 4.
GLM results to assess if the number of GC users could be predicted by the tree and shrub diversity variables and/or GC function(s). Relative fitness of the model was evaluated via deviance, residuals distribution, and Akaike information criteria (AIC).
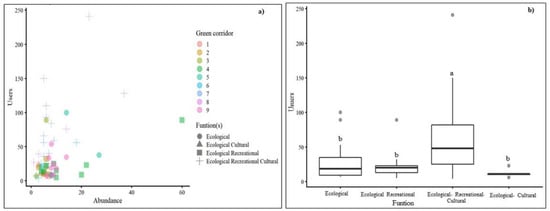
Figure 6.
Influence of the tree and shrub relative abundance (a) and GC function(s) (a + b) on the number of users. Different letters on the boxplots show significant differences (p < 0.05) between GC functions (b) (n = 58 sampling points).
4. Discussion
By characterizing, for the first time, urban GCs’ tree and shrub community and their users, we were able to confirm our hypothesis that people prefer “greener” (with more abundant trees and shrubs) corridors. Further, GCs function(s) also influenced the number of users, with people clearly preferring the most multifunctional GCs (i.e., GCs fulfilling ecological + cultural + recreational functions).
4.1. GCs Promote Urban Biodiversity and Human Wellbeing
Urban ecosystems do not have to be barriers to biodiversity, instead they can function as housing for plant diversity and consequently contribute to the maintenance of urban fauna, as suggested by the involvement of insects and other animals in the dominant pollination and seed dispersal modes found in GCs trees and shrubs (Table 2 and Figure 2 and Figure 3). Since there are no studies on plant diversity in GCs, we compared the tree and shrub richness we observed in the set of the nine GCs with that reported for street trees, as both components of urban green spaces have a similar linear structure. Using Brazilian cities as examples, de Almeida and Barbosa [33] observed 45 species in Cacoal-Rondônia and Kramer and Krupek [17] observed 98 species in Guarapuava-Paraná. Therefore, Lisbon’s GCs showed an intermediate tree and shrub species richness (Table 2).
When analyzing the mean tree and shrub species richness per GC, we found low values (1.5–2.8 species per 400 m2—Table 3), which reflects the fact that GCs are composed of forest fragments that were implemented/revitalized in the last decade and share the space with other urban infrastructures (Table 1 and Figure 1). The low tree and shrub evenness in Lisbon GCs (Table 3) reflects the fact that only 3% of the species were dominant and almost 60% of species were rare and less abundant (Table 2), as observed in other studies [34]. Despite its artificial characteristics, Lisbon’s green infrastructure gained functionality with the implementation of the GCs network [35].
Although it is more likely to have suitable conditions for different plant communities along longer GCs (e.g., GC 9 is 5.81 km2) than in shorter ones (e.g., GC 1 is 0.4 km2), our sampling effort allowed us to detect most tree and shrub species (>80%, data not shown). Therefore, the differences in GCs’ tree and shrub composition and relative abundance (Table 2 and Table 3) may be related with the periods of GCs’ implementation, some of which were still being concluded at the time of our study (e.g., GC 2, 6, 7, and 9) (Table 1). Additionally, since 1977 (the year the implementation of the first GC in Lisbon started—GC 4) until 2020 (when the last sections of the most recent GCs were being completed—GC 2, 6, 7, and 9) there were 8 years of low precipitation, and between 2016 and 2019 there were 3 consecutive years of severe droughts. Since GCs were implemented in different years, the drought periods may have decreased tree and shrub survival, especially if droughts occurred soon after trees and shrubs were planted. Frequent droughts may also have contributed to changing plant community composition to promote more drought tolerant species. The drought periods and the low soil organic matter result in low water holding capacity (e.g., GC 6), which may have affected tree and shrub survival and functionality in the urban environment, and the reforestation plan of the Lisbon City Council [36]. The GCs with higher tree and shrub relative abundance were implemented when there were fewer droughts.
Tree and shrub functional traits are of great relevance in cities where the environment is constantly changing. Small plant species producing dry fruits tend to be favored by strongly disturbed environments [37,38] and urban management since small plants are more compatible with sidewalks and urban power lines [39], and fleshy fruits can cause accidents [33]. In fact, in Lisbon’s GCs, most species and plants produced dry fruits, but they were large trees (Table 2 and Figure 2 and Figure 3). The dominance of species and of trees and shrubs with entomophilous pollination (Figure 2c and Figure 3c) and zoochory (Figure 2d and Figure 3d) agrees with other studies in cities in Brazil [40,41], United States of America [15], Hong Kong [42], and Germany [16].
The large number of species and exotic trees and shrubs that we observed in the GCs (Table 2 and Figure 2b and Figure 3b) agrees with other studies on street trees [33,43]. However, the use of exotic species entails risks, namely: (i) considerable probability of competition with native species and subsequent native population decline [44]; (ii) homogenization of the urban flora composition [18]; (iii) damage to local fauna, with loss of habitats [45]; and iv) biological invasions [46]. The fact that most rare and low abundant species (1–5 individuals—Table 2) are exotic suggests that these plants are not yet reproducing and have not become invasive. However, in species invasions time-lags need to be considered [47], which calls for periodic monitoring of exotic plant species. By contrast, native species benefit from genetic variability, preserve local flora, etc. [48,49], which can turn cities into true ecological corridors connected with nearby forest fragments and increase the permeability of the urban environment [50]. Furthermore, the city and the flora will both benefit from such interactions because ecological processes essential to reproduction and species persistence will be maintained [42]. However, the use of native plant species in cities is not always possible due to the highly disturbed urban environment [51], reduced green spaces, and little or no connectivity between them [52]. Overall, and despite the constraints in using native plant species, these should be preferred in detriment of exotic plants.
Although deciduous species provide greater well-being [53] (i.e., in the summer they provide shade and reduce the heat waves typical of southern European cities, and in the winter they allow more light), in Lisbon’s GCs, most species and trees and shrubs were evergreens (Table 2 and Figure 2e and Figure 3e). This may reflect the fact that evergreen species do not require maintenance due to leaf fall (as deciduous species do), and evergreen species are more adapted to the water stress that characterizes the Mediterranean climate, and which is increasing due to climate change [36]. The advantages of using evergreen trees and shrubs could be maximized for building ecological functionality and resilience if the species used in Lisbon’s GCs were native, which is not the case.
The dominance of trees and shrubs whose seed dispersal is made by animals (i.e., zoochory) that we observed in Lisbon’s GCs can increase the amount and variety of resources available to the local fauna [39], such as bats [42], lizards [54], and birds [55]. Furthermore, the people who use the GCs can also collect the edible fruits, which can contribute to environmental education and improve people’s relationship with nature.
Human choices of which species will form urban vegetation act as strong selective filters for richness and functional types of plants found in cities [38,51]. Furthermore, the ecosystem services provided by urban green spaces are more important for the city’s environmental sustainability and human well-being than the number of species, so functional diversity should be considered as a conservation tool in the design of GCs [56].
4.2. Linking GCs’ Tree and Shrub Community and Users
The influence of GCs on urban biodiversity comes from the revitalization of existing green spaces and the implementation of new ones. Lisbon’s GCs also seem to influence human well-being as we observed a positive influence of the tree and shrub relative abundance on the number of users (Table 4 and Figure 6a). Considering that this census of GCs’ users was made during the pandemic, the total number of observed people is relevant (>4500 people) and, as observed in Spain, Italy, Israel, and Lithuania, probably reflects population’s rapprochement with nature and green elements triggered by the confinement during the previous months [57].
Most GCs’ users were adults (Figure 4), which is in line with a survey on the use of Lisbon’s green spaces [21] and reflects the dominance of this age group in Lisbon’s population (adults correspond to ~50%) [58,59]. By contrast, although 24% of Lisbon’s population are elderly, the low frequency of this age group in the GCs (10% elderly) (Figure 4c) may reflect reduced mobility and health concerns generated by the pandemic.
The fact that most GCs’ users were developing leisure or sports activities accompanied by other users may also be related to the pandemic, as socializing outdoors is known to be safer than indoors. Additionally, with the closing of gymnasiums and sports centers, and the fear of indoor activities, outdoor activities such as walking, hiking, and other outdoor sports became an option for many people. Thus, it is possible that the pandemic helped some people to start using GCs as a preferred place to socialize, relax, or practice sports.
Multifactorial analysis of the GCs’ tree and shrub community (and their characteristics) and users (and their characteristics) showed that the number of tree and shrub species (i.e., richness) was not the main factor explaining the number of users. Although the tree and shrub relative abundance had a smaller influence than expected (loading < 0.8, Table S1), this biodiversity variable influenced positively the number of users (Table 4 and Figure 6a). Further, we could observe that the most multifunctional GCs (GCs fulfilling ecological, cultural, and recreational functions) were the most frequented by the local community and tourists (Table 4 and Figure 6b), especially in the GC sections with more recreational and sports infrastructures. GC 7 (Ribeirinho), which differed from the other GCs in the PCA (Figure 5), is a good example of other factors that attract users: besides having recreational and sports infrastructures (Table 1), as it extends along the Tagus River (Figure 1), it provides a particularly visually attractive landscape, which explains why it has more users than any other GC in Lisbon (Figure 4, Figure 5 and Figure 6). Urban public instruments (e.g., garden benches, sports, and recreational equipment), GCs’ maintenance and the presence of drinking fountains along the GCs appear to be factors that attract users.
5. Conclusions
The tree and shrub communities in GCs, and their functional patterns, may connect urban green spaces and function as ecological refuges for urban fauna, thus promoting urban biodiversity. However, the number of exotic tree and shrub species that are currently used in Lisbon GCs is excessive, which contrasts with that of native trees and shrubs. The design and implementation of Lisbon’s GCs favor more the inclusion of citizens than ecological functionality and resilience. Therefore, and although Lisbon’s GCs improve urban mobility, environmental comfort, and human well-being, urban green spaces projects should promote ecological complexity, increasing the size and connectivity between green spaces. While there is a positive influence of tree and shrub relative abundance on users, users prefer more multifunctional GCs (and GC sections). Thus, as urban populations grow, implementing and revitalizing urban GCs promoting the adequate set of functional traits (e.g., native species pollinated by insects and with fruit dispersion by animals) can be an important strategy to promote ecologically resilient urban ecosystems that provide benefits to people and nature.
Supplementary Materials
The following is available online at https://www.mdpi.com/article/10.3390/su132313228/s1, Table S1: PCA component matrix for the first 2 components.
Author Contributions
All authors contributed meaningfully to this study. Conceptualization and methodology were developed by J.R.d.A., R.d.O.N. and T.D. Formal analysis was done by J.R.d.A. and T.D. Investigation and data curation were done by J.R.d.A. Funding acquisition was done by T.D. All authors have read and agreed to the published version of the manuscript.
Funding
This study was financially supported by Portuguese funds through Fundação para a Ciência e a Tecnologia (project UIDB/00329/2020 and researcher contract to Teresa Dias).
Acknowledgments
We are grateful to: (i) Diego Luis Florencio and Gabriela Marcely for their help in the fieldwork; (ii) Ane Patrícia Cacique for her help with the statistical analyses; (iii) Cristina Cruz for her comments and suggestions which greatly improved the first draft of this paper; (iv) Joana Roma for the proof-reading; and (v) the Editor and the Reviewers for their comments and suggestions, which greatly improved the present paper.
Conflicts of Interest
The authors declare no conflict of interest.
References
- United Nations. World Urbanization Prospects; Department of Economic and Social Affairs, United Nation: New York, NY, USA, 2014. [Google Scholar]
- Soares, A.L.; Rego, F.C.; McPherson, E.G.; Simpson, J.R.; Peper, P.J.; Xiao, Q. Benefits and costs of street trees in Lisbon, Portugal. Urban For. Urban Green. 2011, 10, 69–78. [Google Scholar] [CrossRef]
- Lachowycz, K.; Jones, A.P. Towards a better understanding of the relationship between greenspace and health: Development of a theoretical framework. Landsc. Urban Plan. 2013, 118, 62–69. [Google Scholar] [CrossRef]
- Ribeiro, L.; Barão, T. Greenways for recreation and maintenance of landscape quality: Five case studies in Portugal. Landsc. Urban Plan. 2006, 76, 79–97. [Google Scholar] [CrossRef]
- Ferreira, J.C.; Machado, J.R. Infra-estruturas verdes para um futuro urbano sustentável. O contributo da estrutura ecológica e dos corredores verdes. Rev. LabVerde 2010. [Google Scholar] [CrossRef]
- Ferreira, J.C.; Silva, C.; Tenedorio, J.A.; Pontes, S.; Encarnação, S.; Marques, L. Coastal greenways: Interdisplinarity and integration challenges for the management of developed coastal areas. J. Coastal Res. 2006, 39, 1833–1837. Available online: https://www.jstor.org/stable/25743078 (accessed on 7 June 2021).
- Shi, X.; Qin, M.; Li, B.; Zhang, D. A Framework for Optimizing Green Infrastructure Networks Based on Landscape Connectivity and Ecosystem Services. Sustainability 2021, 13, 10053. [Google Scholar] [CrossRef]
- Suarez-Rubio, M.; Thomlinson, J.R. Landscape and patch-level factors influence bird communities in an urbanized tropical island. Biol. Conserv. 2009, 142, 1311–1321. [Google Scholar] [CrossRef]
- Roy, S.; Byrne, J.; Pickering, C. A systematic quantitative review of urban tree benefits, costs, and assessment methods across cities in different climatic zones. Urban For. Urban Green. 2012, 11, 351–363. [Google Scholar] [CrossRef]
- de Freitas, W.K.; Pinheiro, M.A.S.; Abrahão, L.L.F. Análise da Arborização de Quatro Praças no Bairro da Tijuca, RJ, Brasil. Floresta E Ambiente 2015, 22, 23–31. [Google Scholar] [CrossRef]
- Briz, J.; De Felipe, I. Incorporacion de la naturaleza em cada Rincón de la ciudad: Naturalización urbana. Arquitectura y Paisaje 2004, 120, 12–19. [Google Scholar]
- Boada, M.; Sanchez, S. Naturaleza y cultura, biodiversidad urbana. In Ecoinovação Para Melhoria Ambiental de Produtos e Serviços-Experiências Espanholas e Brasileiras Nos Setores Industrial, Urbano e Agrícola; Ometto, A.R., Peres, R.B., Saavedra, Y.M.B., Eds.; Diagrama Editorial: São Carlos, Brazil, 2012; pp. 131–142. [Google Scholar]
- Cornelissen, J.H.C.; Lavorel, S.; Garnier, E.; Díaz, S.; Buchmann, N.; Gurvich, D.E.; Reich, P.; ter Steege, H.; Morgan, H.D.; Van Der Heijden, M.G.A.; et al. A handbook of protocols for standardised and easy measurement of plant functional traits worldwide. Aust. J. Bot. 2003, 51, 335–380. [Google Scholar] [CrossRef]
- Monalisa-Francisco, N.; Ramos, F.N. Composition and Functional Diversity of the Urban Flora of Alfenas-MG, Brazil. Floresta E Ambient. 2019, 26, 11. [Google Scholar] [CrossRef]
- Aronson, M.F.; Handel, S.N.; Clemants, S.E. Fruit type, life form and origin determine the success of woody plant invaders in an urban landscape. Biol. Invasions 2007, 9, 465–475. [Google Scholar] [CrossRef]
- Knapp, S.; Kühn, I.; Schweiger, O.; Klotz, S. Challenging urban species diversity: Contrasting phylogenetic patterns across plant functional groups in Germany. Ecol. Lett. 2008, 11, 1054–1064. [Google Scholar] [CrossRef] [PubMed]
- Kramer, J.A.; Krupek, R.A. Caracterização florística e ecológica da arborização de praças públicas do município de Guarapuava, PR. Rev. Árvore 2012, 36, 647–658. [Google Scholar] [CrossRef]
- McKinney, M.L. Urbanization as a major cause of biotic homogenization. Biol. Conserv. 2006, 127, 247–260. [Google Scholar] [CrossRef]
- Schlaepfer, M.A.; Sax, D.F.; Olden, J.D. The Potential Conservation Value of Non-Native Species. Conserv. Biol. 2011, 25, 428–437. [Google Scholar] [CrossRef]
- Jim, C. Characteristics of Urban Park Trees in Hong Kong in Relation to Greenspace Planning and Development. Acta Hortic. 2004, 643, 123–128. [Google Scholar] [CrossRef]
- Luz, A.C.; Buijs, M.; Aleixo, C.; Metelo, I.; Grilo, F.; Branquinho, C.; Santos-Reis, M.; Pinho, P. Should I stay or should I go? Modelling the fluxes of urban residents to visit green spaces. Urban For. Urban Green. 2019, 40, 195–203. [Google Scholar] [CrossRef]
- CML; Câmara Municipal de Lisboa. Biodiversidade na Cidade de Lisboa. Uma Estratégia Para 2020. 2015. Available online: https://issuu.com/camara_municipal_lisboa/docs/biodiversidade_estrat_2020 (accessed on 7 June 2021).
- CML. Plano Diretor Municipal de Lisboa. 2012. Available online: https://dre.pt/dre/analise-juridica/aviso/11622-2012-1787349 (accessed on 7 June 2021).
- Durigan, G.; de Siqueira, M.F.; Franco, G.A.D.C.; Bridgewater, S.; Ratter, J.A. The vegetation of priority areas for Cerrado conservation in São Paulo Sate, Brazil. Edinburgh J. Bot. 2003, 60, 217–241. [Google Scholar] [CrossRef]
- Chase, M.W.; Christenhusz, M.J.M.; Fay, M.F.; Byng, J.W.; Judd, W.S.; Soltis, D.E.; Mabberley, D.J.; Sennikov, A.N.; Soltis, P.S.; The Angiosperm Phylogeny Group; et al. An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG IV. Bot. J. Linn. Soc. 2016, 181, 1–20. [Google Scholar] [CrossRef]
- ICNF-Instituto da Conservação da Natureza e das Florestas. Espécies Arbóreas Indígenas em Portugal Continental. Guia de Utilização 2016, 1, 1–44. Available online: https://www.icnf.pt/api/file/doc/2ed27 (accessed on 7 June 2021).
- Sociedade Portuguesa de Botânica. Flora-On: Flora de Portugal Interactiva. 2014. Available online: www.flora-on.pt (accessed on 7 June 2021).
- Duncan, R.P.; Clemants, S.E.; Corlett, R.T.; Hahs, A.K.; McCarthy, M.A.; McDonnell, M.J.; Schwartz, M.W.; Thompson, K.; Vesk, P.A.; Williams, N.S.G. Plant traits and extinction in urban areas: A meta-analysis of 11 cities. Glob. Ecol. Biogeogr. 2011, 20, 509–519. [Google Scholar] [CrossRef]
- Salviatí, E.J. Tipos vegetais aplicados ao paisagismo. Paisag. Ambiente 1993, 9–45. Available online: https://www.revistas.usp.br/paam/article/download/133781/129652/257110. (accessed on 7 June 2021).
- Van Der Pijl, L. Principles of Dispersal in Higher Plants; Springer-Verlarg: Berlin/Heidelberg, Germany, 1982; 161p. [Google Scholar]
- Colwell, R.K.; Chao, A.; Gotelli, N.J.; Lin, S.-Y.; Mao, C.X.; Chazdon, R.L.; Longino, J.T. Models and estimators linking individual-based and sample-based rarefaction, extrapolation and comparison of assemblages. J. Plant. Ecol. 2012, 5, 3–21. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019. [Google Scholar]
- de Almeida, J.R.; Barbosa, C.G. Diagnóstico da Arborização Urbana da Cidade de Cacoal-Ro. Rev. Soc. Bras. Arborização Urb. 2010, 5, 61–81. [Google Scholar] [CrossRef]
- Pauleit, S.; Jones, N.; Garcia-Martin, G.; Garcia-Valdecantos, J.L.; Rivière, L.M.; Vidal-Beaudet, L.; Bodson, M.; Randrup, T.B. Tree establishment practice in towns and cities—Results from a European survey. Urban For. Urban Green. 2002, 1, 83–96. [Google Scholar] [CrossRef]
- Mata, D. (Câmara Municipal de Lisboa, Lisboa, Portugal). Personal communication, 2020.
- CML; Câmara Municipal de Lisboa. Plantação de Árvores e Arbustos: Aumento da Densidade de Cobertura Arbórea ao Longo da Infraestrutura Verde Urbana de Lisboa. 2021. Available online: https://life-lungs.lisboa.pt/acoes/plantacao-de-arvores-e-arbustos/rega (accessed on 7 June 2021).
- Knapp, S.; Dinsmore, L.; Fissore, C.; Hobbie, S.E.; Jakobsdottir, I.; Kattge, J.; King, J.Y.; Klotz, S.; McFadden, J.P.; Cavender-Bares, J. Phylogenetic and functional characteristics of household yard floras and their changes along an urbanization gradient. Ecology 2012, 93, S83–S98. [Google Scholar] [CrossRef]
- Williams, N.S.G.; Schwartz, M.W.; Vesk, P.A.; McCarthy, M.A.; Hahs, A.K.; Clemants, S.E.; Corlett, R.T.; Duncan, R.P.; Norton, B.A.; Thompson, K.; et al. A conceptual framework for predicting the effects of urban environments on floras. J. Ecol. 2009, 97, 4–9. [Google Scholar] [CrossRef]
- Guimarães, M. Há mais aves nos grandes centros urbanos hoje? Ciência Cult. 2006, 58, 14–15. Available online: http://cienciaecultura.bvs.br/scielo.php?script=sci_arttext&pid=S0009-672520060002 (accessed on 7 June 2021).
- Kinoshita, L.S.; Torres, R.B.; Forni-Martins, E.R.; Spinelli, T.; Ahn, Y.J.; Constâncio, S.S. Composição florística e síndromes de polinização e de dispersão da mata do Sítio São Francisco, Campinas, SP, Brasil. Acta Bot. Bras. 2006, 20, 313–327. [Google Scholar] [CrossRef]
- Vale, A.; Navarro, L.; Rojas, D.; Alvarez, J.C. Breeding system and pollination by mimicry of the orchid Tolumnia guibertiana in Western Cuba. Plant. Species Biol. 2011, 26, 163–173. [Google Scholar] [CrossRef]
- Corlett, R.T. Interactions between birds, fruit bats and exotic plants in urban Hong Kong, South China. Urban. Ecosyst. 2005, 8, 275–283. [Google Scholar] [CrossRef]
- Wang, H.-F.; MacGregor-Fors, I.; López-Pujol, J. Warm-temperate, immense, and sprawling: Plant diversity drivers in urban Beijing, China. Plant. Ecol. 2012, 213, 967–992. [Google Scholar] [CrossRef]
- Vidra, R.L.; Shear, T.H.; Stucky, J.M. Effects of vegetation removal on native understory recovery in an exotic-rich urban forest1. J. Torrey Bot. Soc. 2007, 134, 410–419. [Google Scholar] [CrossRef]
- Corbet, S.A.; Bee, J.; DasMahapatra, K.; Gale, S.; Gorringe, E.; La Ferla, B.; Moorhouse, T.; Trevail, A.; Van Bergen, Y.; Vorontsova, M. Native or Exotic? Double or Single? Evaluating Plants for Pollinator-friendly Gardens. Ann. Bot. 2001, 87, 219–232. [Google Scholar] [CrossRef]
- Shackleton, C.M.; Shackleton, R.T. Knowledge, perceptions and willingness to control designated invasive tree species in urban household gardens in South Africa. Biol. Invasions 2016, 18, 1599–1609. [Google Scholar] [CrossRef]
- Seebens, H.; Essl, F.; Dawson, W.; Fuentes, N.; Moser, D.; Pergl, J.; Pyšek, P.; van Kleunen, M.; Weber, E.; Winter, M.; et al. Global trade will accelerate plant invasions in emerging economies under climate change. Glob. Chang. Biol. 2015, 21, 4128–4140. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, B.C. Xeriscaping: Sources of new native ornamental plants. In Progress in New Crops; Janick, J., Ed.; ASHS: Arlington, VA, USA, 1996; pp. 536–539. Available online: https://hort.purdue.edu/newcrop/proceedings1996/V3-536.html (accessed on 7 June 2021).
- Heiden, G.; Barbieri, R.L.; Stumpf, E.R.T. Considerações sobre o uso de plantas ornamentais nativas. Rev. Bras. De Hortic. Ornam. 2006, 12, 2–7. [Google Scholar] [CrossRef][Green Version]
- Munshi-South, J. Urban landscape genetics: Canopy cover predicts gene flow between white-footed mouse (Peromyscus leucopus) populations in New York City. Mol. Ecol. 2012, 21, 1360–1378. [Google Scholar] [CrossRef]
- Knapp, S.; Kühn, I.; Stolle, J.; Klotz, S. Changes in the functional composition of a Central European urban flora over three centuries. Perspect. Plant. Ecol. Evol. Syst. 2010, 12, 235–244. [Google Scholar] [CrossRef]
- Ordóñez, C.; Duinker, P.N. Ecological integrity in urban forests. Urban. Ecosyst. 2012, 15, 863–877. [Google Scholar] [CrossRef]
- Abreu, L.V.; Labaki, L.C. Conforto térmico propiciado por algumas espécies arbóreas: Avaliação do raio de influência através de diferentes índices de conforto. Ambiente Construído 2010, 10, 103–117. [Google Scholar] [CrossRef]
- González-García, A.; Belliure, J.; Gómez-Sal, A.; Dávila, P. The role of urban greenspaces in fauna conservation: The case of the iguana Ctenosaura similis in the “patios” of León city, Nicaragua. Biodivers. Conserv. 2009, 18, 1909–1920. [Google Scholar] [CrossRef]
- Pauw, A.; Louw, K. Urbanization Drives a Reduction in Functional Diversity in a Guild of Nectar-feeding Birds. Ecol. Soc. 2012, 17. [Google Scholar] [CrossRef]
- Flynn, D.F.B.; Gogol-Prokurat, M.; Nogeire, T.; Molinari, N.; Richers, B.T.; Lin, B.B.; Simpson, N.; Mayfield, M.M.; DeClerck, F. Loss of functional diversity under land use intensification across multiple taxa. Ecol. Lett. 2009, 12, 22–33. [Google Scholar] [CrossRef] [PubMed]
- Ugolini, F.; Massetti, L.; Calaza-Martínez, P.; Cariñanos, P.; Dobbs, C.; Ostoić, S.K.; Marin, A.M.; Pearlmutter, D.; Saaroni, H.; Šaulienė, I.; et al. Effects of the COVID-19 pandemic on the use and perceptions of urban green space: An international exploratory study. Urban For. Urban Green. 2020, 56, 126888. [Google Scholar] [CrossRef] [PubMed]
- INE—Instituto Nacional de Estatística. Censos 2011 da População Residente que Trabalha ou Estuda por Município. 2011. Available online: http://www.ine.pt (accessed on 7 June 2021).
- CML; Câmara Municipal de Lisboa. II Diagnóstico Social de Lisboa 2015–2016. 2016. Available online: www.am-lisboa.pt/documentos/1518709936A8sST5fr2Qg86FJ5 (accessed on 7 June 2021).
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).