Abstract
It cannot be denied the importance of groundwater (Gw) as a source for irrigation. It is considered the only source of water in some locations such as newly reclaimed lands. However, the groundwater quality could be affected by salinity or heavy metals because of human activities or natural reasons. Thence, groundwater desalination comes to above as a part of the solution. In this study, the modified active carbon by inorganic iron polymer (Fex(OH)y) (Fe-AC) and bentonite (Ben) were used in groundwater desalination. The treatment process of 2 liters of groundwater was carried out by using a fixed-bed column where the flow rate was 120 mL/hour for each 20 grams sorbent. The results showed that the EC value of groundwater (2.54 dS/m) was reduced to 1.12 dS/m for treated groundwater (TGw) by Fe-AC/Ben mixture. Furthermore, the effect of irrigation by Gw and TGw was tested on the Faba bean and soil properties. The vegetative characters were significantly affected by irrigation by saline Gw while plant characters were much better after irrigation with TGw as well as soil chemical properties. Accordingly, the desalination of groundwater by Fe-AC/Ben mixture considers an effective and economic method that can be applied to reduce groundwater salinity and its impact on soil and crop.
1. Introduction
Globally, there is no doubt that water considers a strategic resource due to the rapid increase in population, agriculture, and industrial sectors. Thence, using low-quality water such as saline groundwater, drainage water, and treated wastewater has become an important consideration and is a new policy that is applied. However, using saline groundwater can also cause real risks to soil, plant, and public health, once used in crop irrigation [1]. Therefore, reducing the risks of saline groundwater before reusing it in agriculture is a very important issue.
Hence, several techniques could be used to reduce groundwater salinity, while adsorption technology is considered as the best technique for desalination among these techniques due to its efficiency, simplicity, and economical to operate desalination [2].
Numerous natural and synthetic materials have been used as sorbents such as activated carbon (AC), which has several applications long ago such as catalysis, catalyst support [3], or adsorbents for contaminants and organisms [4]. In addition, the demand for AC increases throughout the world [5], referring to its low cost, high adsorption capacity, versatility, and its chemical/physical properties of the surface that consider the most important issues to define the characteristic of AC [6].
Currently, AC can be prepared from different agricultural wastes such as coconut shells [7], and sesame stalks [6] that also be helpful in waste minimization and materials reuse. As a common development to enhance the AC physical/chemical properties, countless practices have been used by utilizing some methods such as impregnation, ozone, surfactant, and microwave treatment [8,9]. As well, using some attractive features for the modification of AC with several materials such as metal oxides, organic or inorganic polycations could support, enhance and adjust the potential of AC surface properties for removing cations and anions [10,11]. Furthermore, the trivalent salts of iron such as poly ferric chloride that are commonly used in the water treatment process [12], could be used in modified AC. Whereas, iron salt polymers are Fe2(OH)24+ and Fe3(OH)45+. These molecules are synthesized by titrating a relatively concentrated iron solution with a base by different ratios [13]. Due to the high positive charge of iron hydroxides used in modified AC, the performance of the AC surface becomes more interactive toward the negatively charged contaminants besides heavy metals.
Furthermore, many studies using natural clay and its composites as an ecofriendly efficient adsorbent for removal of organic, inorganic, pathogenic contaminants, and decreasing the salinity of water because of their high specific surface area, chemical and mechanical stability, a variety of surface and structural properties, and low cost [14,15]. Subsequently, integrating modified AC with clay minerals, that have a great affinity and capacity to adsorb cations, can enhance the desalination process of saline water such as saline Gw.
Thus, the aims of this study were: (i) assess the effect of using modified AC/bentonite mixture on groundwater desalination, (ii) investigate the effect of using treated groundwater in irrigation on some soil and Faba bean plant properties.
2. Materials and Methods
2.1. Materials
2.1.1. Groundwater Samples
Groundwater samples were collected from the Sohag government, Egypt. The groundwater sample was stored in a clean dry plastic bottle in a refrigerator. Chemical analysis of Gw “EC, Heavy metals, TOC, pH, TDS, SAR and major cations and anions” before and after desalination process was carried out.
2.1.2. Agricultural Wastes
As an example of agricultural residuals plant from Sohag, Egypt, sesame stalks (St) were used. Thereafter, it was cleaned with pure water to remove dust, sun-dried, then dried at 110 °C for 24 h to eliminate moisture content before being used to make activated carbon.
2.1.3. Ferric Chloride (FeCl3)
Ferric Chloride that used in this study is extra Pure from (Alpha Chemika). Ferric Chloride solutions were prepared using distilled water.
2.1.4. Bentonite
Bentonite (Ben), one of commonly present clay minerals in soils, was purchased from Alfa Aesar (Karlsruhe, Germany). Clay mineral was pretreated as described elsewhere [16].
Bentonite was dried and ground into fine powder. Then Ben was sieved to acquire geometrical sizes less than 1 μm, that reserved in an oven at 110 °C a period of 24 h, removed and cooled as described elsewhere [17]. After being dried, Ben was ground and sieved. The Ben was characterized and identified by qualitative X-ray powder diffractometry (XRD), FT-IR spectra, particle size distribution, and the BET surface area.
2.2. Methods
2.2.1. Preparation of Activated Carbon (AC)
The accurately weighted of St samples were carbonized at 300 °C in a muffle furnace (Fisher Scientific Isotempm Muffle Furnace) for 1.5 h. The St charcoal product was crushed and sieved in order to acquire ≤2 mm size. Then, St charcoal was mixed with NaOH within the ratio of 1:2 (weight/weight), The charcoal was held in the solution for 24 h at room temperature and then dried at 110 °C for 24 h. The dried material was activated at 500 °C for 2 h in the furnace at a low oxygen atmosphere. The sample was left to cool down before taking out of the furnace. After activation, the sample obtained was washed with 0.1 mol dm−3 HCl, and then with distilled water to adjust the pH closer to 6.5−7. Then, the AC sample was dried at 110 °C for 24 h and was stored in sealed flasks until used.
2.2.2. Preparation of Inorganic Polymer
Inorganic polycations polymer was synthesized by mixing freshly generated 0.1 mol dm−3 NaOH solutions with 0.1 mol dm−3 FeCl3 aqueous solutions. The reactant was then supplied drop by drop at a very slow (1 mL/min) pace until the reactant quantities achieved the molar ratios OH/Fe = 2 [10]. To avoid overconcentration of hydroxyl ions, rapid mixing (200 rpm) was required. Finally, the resulting solutions were aged for 10 days at room temperature before added to AC for modification [10].
2.2.3. Preparation of Modified Activated Carbon (Fe-AC)
To prepare Fe-AC, the modification of AC by Fex(OH)y was carried out under the following conditions: The pH of 2.5 g of AC sample is 2.3, and the concentration of Fex(OH)y is 622 mg L−1 ([Fe]). The solution stirred at room temperature for 5 h at 150 rpm. Finally, the material was filtered, and washed with distilled water until be chloride-free (detected by the AgNO3 test). The Fe-AC was dried at 30 °C.
2.2.4. Preparation of Bentonite
Clay nanocomposites were produced by mixing 2 g of bentonite to 50 mL distilled water and ultrasonically dispersing it for 30 minutes, as described before [18]. After that, a few drops of HCl were added to keep the pH at 3. After that, around 2 g of dry silk fibroin powder was added and stirred overnight at 50 °C. After stirring, the sample was dried in an oven. After that, the dried sample was ground into a fine powder.
2.2.5. Groundwater Desalination by Using Fixed-Bed Column Technique
Desalination of groundwater (GW) was achieved utilizing a fixed-bed column model with a glass column with a diameter of 3 cm and a length of 30 cm and an adsorption process at room temperature onto Fe-AC/Ben mixture. A layer of glass wool was packed in the bottom of the glass column, followed by a layer of sand, 20 g of Fe-AC/Ben, and a layer of glass wool above to support the adsorbent, while the top layer of glass wool was utilized to keep the adsorbent from floating. Gravitational flow was used to introduce Gw (influent) samples to the glass column at a rate of 120 mL/h. The TGw samples were collected and stored for further investigation. Complete analysis of TGw samples “EC, pH, TDS, major ions, TOC, and heavy metals” after desalination process occurred as mentioned earlier.
The data collected were subjected to analysis of variance (ANOVA) using Statistical Analysis System (SAS) and means were separated using LSD0.05.
2.2.6. Experimental Design
The experiment was performed in Lysimeters at the Experimental Farm of Shandaweel Agricultural Research Station, Agricultural Research Center (ARC), Egypt, during the two successive growing seasons of 2019/2020 and 2020/2021 to study the effect of Gw and TGw on the yield of faba bean and soil properties. Lysimeters were filled by sandy soil (87.6% sand, 8.2% silt, and 4.2% clay) as a model for reclaimed soil. The experiment was laid out in a randomized complete block design (RCBD) with three replications. The experiment was subject to three water treatments: freshwater (Fw), groundwater (Gw), and treated groundwater (TGw). Faba bean seeds were cultivated on 20 October in both seasons. Plants were irrigated using the same volume of water each time during the experiment. All the required agronomic practices were followed uniformly in all plots throughout the growing period. Throughout the vegetative growth stage (the growing stage was 80 days), three random plants were taken after the experiment end from each Lysimeter per treatment for determination of plant height (cm), a number of leaves per plant, fresh and dry weight per plant (g), leaf area per plant (cm2) according to El-sayed, 2019 [19]. At harvest, the weight of 100 seeds and seeds yield was calculated. The data collected were subjected to analysis of variance (ANOVA) using Statistical Analysis System (SAS) and means were separated using LSD0.05. In addition, soil chemical properties were estimated before and after the experiment [20].
3. Results and Discussion
3.1. Characterization of AC and Fe-AC Compounds
AC synthesized from sesame stalks and then modified with the use of Fe metallic polycations. The AC and Fe-AC complexes had surface areas of 190.039 and 378.404 m2/g, respectively. It is obvious that polycation species adsorption has a substantial impact on the surface of AC [21]. The average pore diameters of AC and Fe-AC complex were 1.3672 and 1.6687 nm, respectively, indicating that AC and AC-Fe complex are micropores materials. Due to t-test statistical results on surface area and average pore diameter, the modification procedure of AC with Fe-AC was highly significant. The values of probability (p) at t0.05 were 0.007 and 0.02 for surface area and the average pore diameter, respectively.
SEM images (Figure 1) revealed that the surface of AC was filled with cracks, channels, and some porous grains of varying sizes. The porous structure was generated because most of the organic volatiles were evolved [22], leaving behind the ruptured surface of AC with a small number of pores after activation at 500 °C [22]. Furthermore, due to the adsorption of Fe polycations species on AC, the SEM picture of Fe-AC has changed dramatically, becoming filled with ribbon-like structures that are almost compactly scattered.
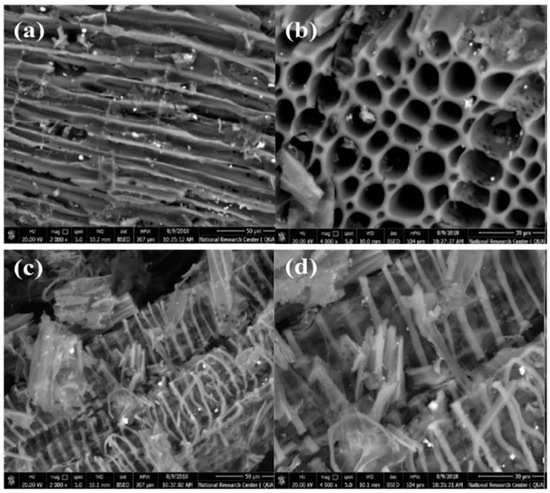
Figure 1.
Scanning electron microscope (SEM) of AC (a,b), and Fe-AC (c,d) in different an enlargement factor (a and c−2000×, and b and d−4000×).
The FT-IR spectra (Figure 2) were used to analyses the probable chemical bonds in the AC and Fe-AC complexes; the two spectra were almost identical. The stretching vibrations of –OH groups in the spectra of AC and AC–Fe showed a broad absorption peak in the range of 3200–3650 cm−1 (3216 cm−1 for AC, and 3355 cm−1 for Fe-AC), which could be attributed to the broad absorption peak in the range of 3200–3650 cm−1 (3216 cm−1 for AC, and 3355 cm−1 for Fe-AC) [23].
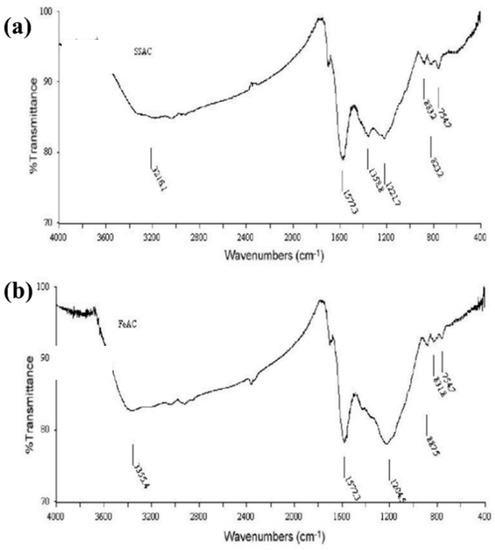
Figure 2.
FTIR spectra for (a) AC, and (b) FeAC.
The peak for AC at 1577 cm–1 could be caused by C = O stretching or the bending vibration of water absorbed, polymerized, and crystallized in the modified AC [23]. Peaks around 1350 and 1220 cm−1 were also realized, which could be due to oxygen functions such as strongly conjugated C–O stretching [24]. Furthermore, out-of-plane deformation vibrations of the C–H group in aromatic structures may be attributed to a weak band approximately 880 to 760 cm−1 [25]. In addition, the Fe-AC peaks about 1200 cm−1 could be explained by the asymmetric stretching vibration of Fe–O–Fe [26].
The asymmetric stretching and bending vibrations of Fe–OH–Fe was notified for the Fe-AC peak at 831 cm−1. There was also a peak for Fe-AC at 754 cm−1, which was attributed to Fe–OH bending vibrations, showing that the polymer is generated in the samples [23]. This suggests that, depending on the composition of the AC surface, the surface of the AC could be positively charged, favoring the adsorption of anions other than heavy metals.
3.2. Bentonite Characterization
Figure 3 shows the X-ray diffraction spectra of bentonite. The bentonite was found to be pure because of the spectra. The peaks of X-ray diffraction were compared to the well-known clay minerals literature [27]. The elemental analysis of bentonite was also determined using SEM/EDAX. The chemical composition of bentonite was determined using SEM/EDAX (SiO2 48.16 %, Al2O3 14.86 %, Fe2O3 4.80 %, CaO 1.16 %, Na2O 1.66 %, MgO 2.08 %, K2O 1.60 %, TiO2 0.94 %, P2O5 1.06 %). The Si:Al ratio in kaolinite is around 2:1, and the structure is made up of an octahedral aluminum layer a top of a tetrahedral silicon sheet, according to elemental analyses.
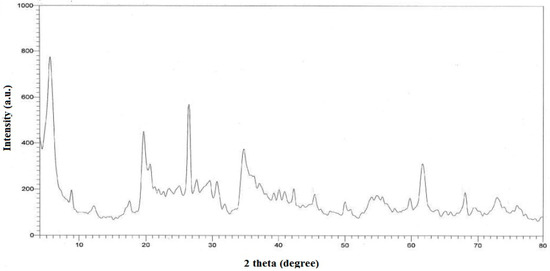
Figure 3.
XRD diffract graphs of bentonite.
Massive plates with some phase separations can be seen as a heterogeneous surface morphology in SEM pictures of bentonite samples (Figure 4). It revealed a soft surface with a layer disposition, where large lamellas tend to create massive agglomerates.
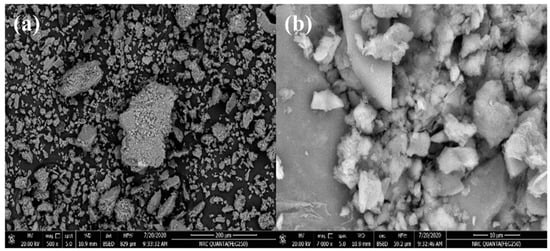
Figure 4.
SEM images of bentonite in different an enlargement factor (a 500×, and b 7000×).
The following are relevant properties of bentonite: bentonite particles were nanoscale (78.220 nm), the BET surface area was 51.3 m2/g, and the average pore diameter was 0.84 nm. Furthermore, bentonite has a zeta potential of −25.5, indicating that it interacts more with cations.
The bands at 3638 cm−1 and 3421 cm−1 in the FTIR spectra of bentonite (Figure 5) in this work are assigned to OH stretching vibrations in the Si–OH and Al–OH groups of the tetrahedral and octahedral sheets of bentonite. The bending vibration mode of adsorbed water created the band of low intensity centered at lower frequencies at 1645 cm−1 for both samples, which was in agreement with the values provided by [28]. The spectrum’s most strong bands were identified in the low-frequency area. The stretching modes of Si–O (out-of-plane) and Si–O stretching (in-plane) vibration for layered silicates produced the bands at 1035 cm−1 and 913 cm−1 for Ben, respectively. While the bending vibration of Al–Al–OH for Ben is connected with the band at 913 cm−1, which is in close accord with values published in various literature (Figure 5) [29].
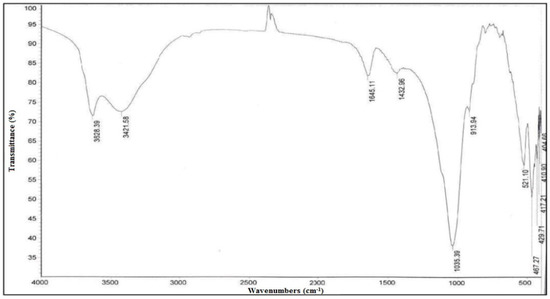
Figure 5.
FT-IR spectra of bentonite.
3.3. Groundwater Desalination
The usage of saline groundwater as a source of irrigation water is being evaluated as a viable option for food security. Salinity, on the other hand, results in a large decrease in agricultural crop output and a quick deterioration of soil quality. The Gw desalination and treatment procedure was carried out utilizing a fixed bed column model with a rated flow of 120 mL/h and adsorption over Fe-AC / Ben [19]. The chemical analysis of Gw improved after treatment with Ben/Fe-AC, as shown in Table 1. (EC, pH, TDC, major cations, and major anions). The desalination procedure had a significant impact on the EC value, which dropped from 2.54 to 1.12 dS/m. All cations and anions are also significantly affected and reduced.

Table 1.
Chemical analysis of Gw and TGw.
In addition, the desalination process had a considerable impact on all heavy metal concentrations and total organic carbon in Gw, as indicated in Table 2 and as a result of the LSD results. All heavy metals had been entirely eliminated, with the exception of iron and zinc, which were removed at ratios of 96.8 and 98.5, respectively.

Table 2.
Heavy metals and total organic carbon concentrations in Gw and TGw.
The highly active site contents, function groups, and BET surface area of the Ben and Fe-AC complexes account for these results. Furthermore, Ben’s activity toward cations and its ability to remove cations, heavy metals, and organic carbon, but modified active carbon’s efficacy in removing anions was high.
3.4. Evaluation of Gw and TGw Quality for Irrigation
Table 3 shows the parameters used to determine the acceptability and feasibility of TGw for irrigation: sodium adsorption ratio (SAR), salinity (EC), soluble sodium percentage (SSP), and residual sodium carbonate (RSC).

Table 3.
The SAR, EC, SSP and RSC values of non-TGW and TGW.
The salinity of Gw poses a serious difficulty when utilized in irrigation because of its impact on soil permeability and water infiltration at high sodium concentrations. For determining the sodium effect, the SAR is a crucial parameter. Gw has been categorized as a class (S2), which implies it offers a considerable danger but can be used responsibly. TGw, on the other hand, was assigned an outstanding water class (S1), indicating that it poses little or no risk in terms of SAR water classification and can be utilized in irrigation for practically all soils (McGeorge, 1954).
Gw salinity is a hazardous environmental element that has a dual impact on plant and soil properties, leading in reduced crop productivity and increased soil salinity. The EC results for Gw and TGw are shown in Table 3. The Gw comes into the C4 class in the table of irrigation water classification by salinity, signifying very high salinity water that can be used for soil with restricted drainage and plants with good salt tolerance. While the EC of TGw is in the C3 salinity class, it indicates that medium salinity water can be used after adding the right amount of leaching and that some plants can be grown without additional salinity management techniques.
Moreover, the SSP, such as the SAR, is used to measure the sodium hazard of irrigation water, but in contrast with the SAR, which only correlates sodium with Ca2+ and Mg2+, it expresses the proportion of sodium out of total cations. Gw’s estimated SSP value (Table 3) stated that this water is acceptable for irrigation in the SSP table, whereas TGw’s calculated SSP value indicated that this water is suitable for irrigation in the same classification [30].
The residual sodium carbonate (RSC) is also used to determine the quality of water, with a high RSC suggesting a high pH. All samples with RSC less than zero are more suited for irrigation, according to the USSL, than those with RSC are more than zero [31]. As a result, Gw is suitable for irrigation, while TGw produced by the Fe-AC/Ben complex is an excellent choice.
3.5. The Effect of Using Gw, and TGw in Irrigation
In order to study the effect of Gw and TGw on some plant and soil properties, Faba bean seeds were sown in lysimeters and were irrigated by different irrigation sources (Freshwater, Groundwater, and treated groundwater).
3.5.1. Effect of Irrigation with Gw and TGw on Some Properties of Soil Samples
The soil under examination had a sandy texture and a CaCO3 concentration of 3.7%. Table 4 shows the chemical characteristics of the soil before and after Gw and TGw irrigation. The results showed that OC concentration and pH of the soil changed somewhat under different irrigation sources, with little increase after using Gw rather than TGw. These findings are based on the contents of Gw and TGw’s TOCs, as described in Table 2.

Table 4.
Chemical Analysis of soil samples under study (in the peast extractable).
Additionally, several chemical characteristics of the soil, such as EC (ds/m), cations (meq/L): Ca2+, Mg2+, Na+, K+, and anions (meq/L): CO32−, HCO3−, Cl−, SO42− before and after irrigation with Gw and TGw were measured. The findings revealed that the irrigation sources had an impact on the chemical attributes of the soil samples under consideration.
The soil pH and TOC increased insignificantly when Gw was used to irrigate the soil under investigation compared to TGw. This could be because to Gw’s alkaline personality [32]. The values of EC, major cations, and major anions increased after irrigation with Gw, but EC and major anions and cations values decreased and improved dramatically following irrigation with TGw [33]. The soil’s pH and EC are important factors that affect the availability of nutrients for plant roots. Similarly, the major cations and anions in the soil were reduced by TGw irrigation, according to the findings. As a result, after irrigation with TGw rather than Gw, some soil qualities improved dramatically.
3.5.2. Effect of Irrigation with Gw and TGw on Faba Bean Properties (Vegetative Growth Characters)
Table 5 shows some of the characteristics of Faba bean plants after irrigation with Gw and TGw. Plant height, fresh and dry weight, number of leaves per plant, and photosynthetic pigments (Chlorophyll a, Chlorophyll b, and carotenoids) were all significantly affected after irrigation with Gw and TGw due to LSD values. While the number of branches, leaf width, root zone, and leaf area were all insignificantly affected.

Table 5.
Means of vegetative growth characters of Faba bean plant after 60 days (Means of two seasons).
Utilizing TGw by Fe-AC/Ben complex in irrigation led to better values for all studied characters and was more similar to using freshwater than using Gw. The EC value of Gw and the high concentration of main ions such as Na+, Ca2+, Mg2+, Cl−, SO42−, and HCO3− cause a considerable reduction in several plant vegetative growth features when it is irrigated. Furthermore, soil and irrigation water pH alter plant nutrient availability [34]. Gw had a pH of 7.72, while TGw had a pH of 7.35, according to Table 1. As a result, Gw irrigation had a greater impact on nutrient availability and plant development than TGw irrigation. Furthermore, heavy metal toxicity had a substantial impact on plant characteristics, with heavy metal concentrations significantly reduced after the treatment process. Furthermore, Gw pollutants are associated with a decrease in photosynthetic pigments. Table 6 shows that irrigation source affected seed production and weight of 100 seeds due to LSD value and substantial changes in several plant parameters such as plant height, fresh and dry weight, and photosynthetic pigments.

Table 6.
Faba bean yield under different water irrigate sources.
Consequently, the desalination of Gw through adsorption onto the Fe-AC/Ben complex in a fixed bed column model is a very cost-effective, easy, and safe technique of treating Gw for irrigation purposes.
4. Conclusions
Due to its salinity, this study found that using groundwater for irrigation without further treatment caused problems for soil and plant on long term. As a result, the desalination of Gw using Fe-AC/Ben mixture had a considerable impact on lowering Gw salinity as well as major cations and anions. In addition, the use of TGw has a considerable impact on soil and plant attributes as well. Hence, the desalination process of Gw by Fe-AC/Ben is a better, safe, and cost-effective approach for reusing conventional water resources such as groundwater.
Author Contributions
Conceptualization, M.E.A.E.-S. and J.W.; methodology, M.E.A.E.-S. and I.A.A.; validation M.E.A.E.-S., I.A.A. and J.W.; formal analysis, M.E.A.E.-S.; investigation, M.E.A.E.-S., I.A.A. and J.W.; resources, M.E.A.E.-S., I.A.A. and J.W.; data curation, M.E.A.E.-S.; writing—original draft preparation, M.E.A.E.-S.; writing—review and editing, M.E.A.E.-S., J.W. and I.A.A.; visualization, J.W. and M.E.A.E.-S.; supervision, M.E.A.E.-S. and J.W.; funding acquisition, J.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors reported no potential conflict of interest.
References
- Meneses, M.; Pasqualino, J.; Castells, F. Environmental assessment of urban wastewater reuse: Treatment alternatives and applications. Chemosphere 2010, 81, 266–272. [Google Scholar] [CrossRef] [PubMed]
- El-Sayed, M.E.A.; Ahmed, A.; Farghaly, O.A.; Abd-Elmottaleb, M.; Elnasr, T.A.S.; Hassan, M.A.M. Preparation and Using Modified Nanohydroxyapatite Molecules for Wastewater Treatment. Water Conserv. Sci. Eng. 2018, 3, 331–337. [Google Scholar] [CrossRef]
- Dias, J.M.; Alvim-Ferraz, M.C.; Almeida, M.F.; Rivera-Utrilla, J.; Sanchez-Polo, M. Waste materials for activated carbon preparation and its use in aqueous-phase treatment: A review. J. Environ. Manag. 2007, 85, 833–846. [Google Scholar] [CrossRef]
- Gupta, V.K.; Suhas. Application of low-cost adsorbents for dye removal—A review. J. Environ. Manag. 2009, 90, 2313–2342. [Google Scholar] [CrossRef]
- Tran, H.N.; You, S.-J.; Hosseini-Bandegharaei, A.; Chao, H.-P. Mistakes and inconsistencies regarding adsorption of contaminants from aqueous solutions: A critical review. Water Res. 2017, 120, 88–116. [Google Scholar] [CrossRef]
- Jiang, L.; Liang, G.; Gao, Y. Optimization of Activated Carbons Production from Sesame Stalks Using Response Surface Methodology. Proc. Int. Conf. Chem. Mater. Food Eng. 2015, 22, 118–122. [Google Scholar] [CrossRef][Green Version]
- Cobb, A.; Warms, M.; Maurer, E.P.; Chiesa, S. Low-Tech Coconut Shell Activated Charcoal Production. Int. J. Serv. Learn. Eng. Humanit. Eng. Soc. Entrep. 2012, 7, 93–104. [Google Scholar] [CrossRef]
- Kristiana, I.; Joll, C.; Heitz, A. Powdered activated carbon coupled with enhanced coagulation for natural organic matter removal and disinfection by-product control: Application in a Western Australian water treatment plant. Chemosphere 2011, 83, 661–667. [Google Scholar] [CrossRef]
- Bhatnagar, A.; Hogland, W.; Marques, M.; Sillanpää, M. An overview of the modification methods of activated carbon for its water treatment applications. Chem. Eng. J. 2013, 219, 499–511. [Google Scholar] [CrossRef]
- Nguemtchouin, M.G.M.; Ngassoum, M.B.; Kamga, R.; Deabate, S.; Lagerge, S.; Gastaldi, E.; Chalier, P.; Cretin, M. Characterization of inorganic and organic clay modified materials: An approach for adsorption of an insecticidal terpenic compound. Appl. Clay Sci. 2015, 104, 110–118. [Google Scholar] [CrossRef]
- Cho, D.-W.; Chon, C.-M.; Kim, Y.; Jeon, B.-H.; Schwartz, F.W.; Lee, E.-S.; Song, H. Adsorption of nitrate and Cr(VI) by cationic polymer-modified granular activated carbon. Chem. Eng. J. 2011, 175, 298–305. [Google Scholar] [CrossRef]
- Spellman, F.R. Handbook of Environmental Engineering. Handb. Environ. Eng. 2015, 49, 1–707. [Google Scholar] [CrossRef]
- Abeysinghe, S. Keggin-Type Aluminum Nanoclusters: Synthesis, Structural Characterization and Environmental Implications; The University of Iowa: Iowa City, IA, USA, 2012. [Google Scholar]
- Wibowo, E.; Rokhmat, M.; Sutisna; Murniati, R.; Khairurrijal; Abdullah, M. Thermally Activated Clay to Compete Zeolite for Seawater Desalination. Adv. Mater. Res. 2015, 1112, 154–157. [Google Scholar] [CrossRef]
- Sekihata, F. Preparation of Desalination Agent from CA-Type Clay Minerals. Int. J. GEOMATE 2020, 19, 123–129. [Google Scholar] [CrossRef]
- El-Sayed, M.E.; Khalaf, M.M.; Gibson, D.; Rice, J.A. Assessment of clay mineral selectivity for adsorption of aliphatic/aromatic humic acid fraction. Chem. Geol. 2019, 511, 21–27. [Google Scholar] [CrossRef]
- Abdel-Rahman, L.H.; Al-Farhan, B.S.; Abu-Dief, A.M.; Yousef, D.; El-Sayed, M.E.A. Kinetic study of humic acid adsorption onto smectite: The role of individual and blend background electrolyte. AIMS Mater. Sci. 2019, 6, 1176–1190. [Google Scholar] [CrossRef]
- Dang, Q.; Lu, S.; Yu, S.; Sun, P.; Yuan, Z. Silk Fibroin/Montmorillonite Nanocomposites: Effect of pH on the Conformational Transition and Clay Dispersion. Biomacromolecules 2010, 11, 1796–1801. [Google Scholar] [CrossRef]
- El-sayed, M.E.A. Assess the influence of using treated wastewater by nano hydroxyapatite and its modification on some soil and faba bean plant properties. N. Y. Sci. J. 2019, 12, 1–7. [Google Scholar] [CrossRef]
- Carter, M.R.; Gregorich, E.G. Soil Sampling and Methods of Analysis, 2nd ed.; CRC Press: Ottawa, ON, Canada, 2007; ISBN 9780849335860. [Google Scholar]
- Al-Saidi, H.M.; Farghaly, O.A.; El-sayed, M.E.A.; Elmottaleb, M.A.-; Taha, A.; Ahmed, M.G.Z. Effect of Al & Fe inorganic pol-ymers on the enhancement of the surface properties of Activated carbon prepared from sesame stalks. Life Sci. J. 2020, 17. [Google Scholar] [CrossRef]
- Menéndez-Díaz, J.A.; Martín-Gullón, I. Types of carbon adsorbents and their production. Interface Sci. Technol. 2006, 7, 1–47. [Google Scholar]
- Sun, T.; Sun, C.-H.; Zhu, G.-L.; Miao, X.-J.; Wu, C.-C.; Lv, S.-B.; Li, W.-J. Preparation and coagulation performance of poly-ferric-aluminum-silicate-sulfate from fly ash. Desalination 2011, 268, 270–275. [Google Scholar] [CrossRef]
- Deng, H.; Yang, L.; Tao, G.; Dai, J. Preparation and characterization of activated carbon from cotton stalk by microwave assisted chemical activation—Application in methylene blue adsorption from aqueous solution. J. Hazard. Mater. 2009, 166, 1514–1521. [Google Scholar] [CrossRef]
- Rao, M.N.; Chakrapani, C.; Reddy, B.V.R.; Babu, C.S.; Rao, Y.H.; Rao, S.; Rajesh, K.; Kalasala, C.M. Preparation of activated Kaza’s carbons from Bio-Materials and their characterization. IJABPT 2011, 2, 610–618. [Google Scholar]
- Sun, T.; Liu, L.-L.; Wan, L.-L.; Zhang, Y.-P. Effect of silicon dose on preparation and coagulation performance of poly-ferric-aluminum-silicate-sulfate from oil shale ash. Chem. Eng. J. 2010, 163, 48–54. [Google Scholar] [CrossRef]
- Septian, A.; Oh, S.; Shin, W.S. Sorption of antibiotics onto montmorillonite and kaolinite: Competition modelling. Environ. Technol. 2019, 40, 2940–2953. [Google Scholar] [CrossRef] [PubMed]
- Dutta, A.; Singh, N. Surfactant-modified bentonite clays: Preparation, characterization, and atrazine removal. Environ. Sci. Pollut. Res. 2015, 22, 3876–3885. [Google Scholar] [CrossRef]
- Abdullahi, S.L.; Audu, A.A. Comparative analysis on chemical composition of bentonite clays obtained from Ashaka and tango deposits in Gombe State, Nigeria. ChemSearch J. 2017, 8, 35–40. [Google Scholar]
- Todd, D.; Mays, L. Groundwater Hydrology; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2005. [Google Scholar]
- McGeorge, W.T. Diagnosis and Improvement of Saline and Alkaline Soils. Soil Sci. Soc. Am. J. 1954, 18, 348. [Google Scholar] [CrossRef]
- Alghobar, M.A.; Ramachandra, L.; Suresha, S. Effect of Sewage Water Irrigation on Soil Properties and Evaluation of the Accumulation of Elements in Grass Crop in Mysore City, Karnataka, India. Am. J. Environ. Prot. 2014, 3, 283. [Google Scholar] [CrossRef]
- Ahemad, M. Remediation of metalliferous soils through the heavy metal resistant plant growth promoting bacteria: Paradigms and prospects. Arab. J. Chem. 2019, 12, 1365–1377. [Google Scholar] [CrossRef]
- Al-Tahir, O.A.; Al-Abdulsalam, M.A. Growth of faba bean (Vicia faba L.) as influenced by irrigation water salinity and time of salinization. Agric. Water Manag. 1997, 34, 161–167. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).