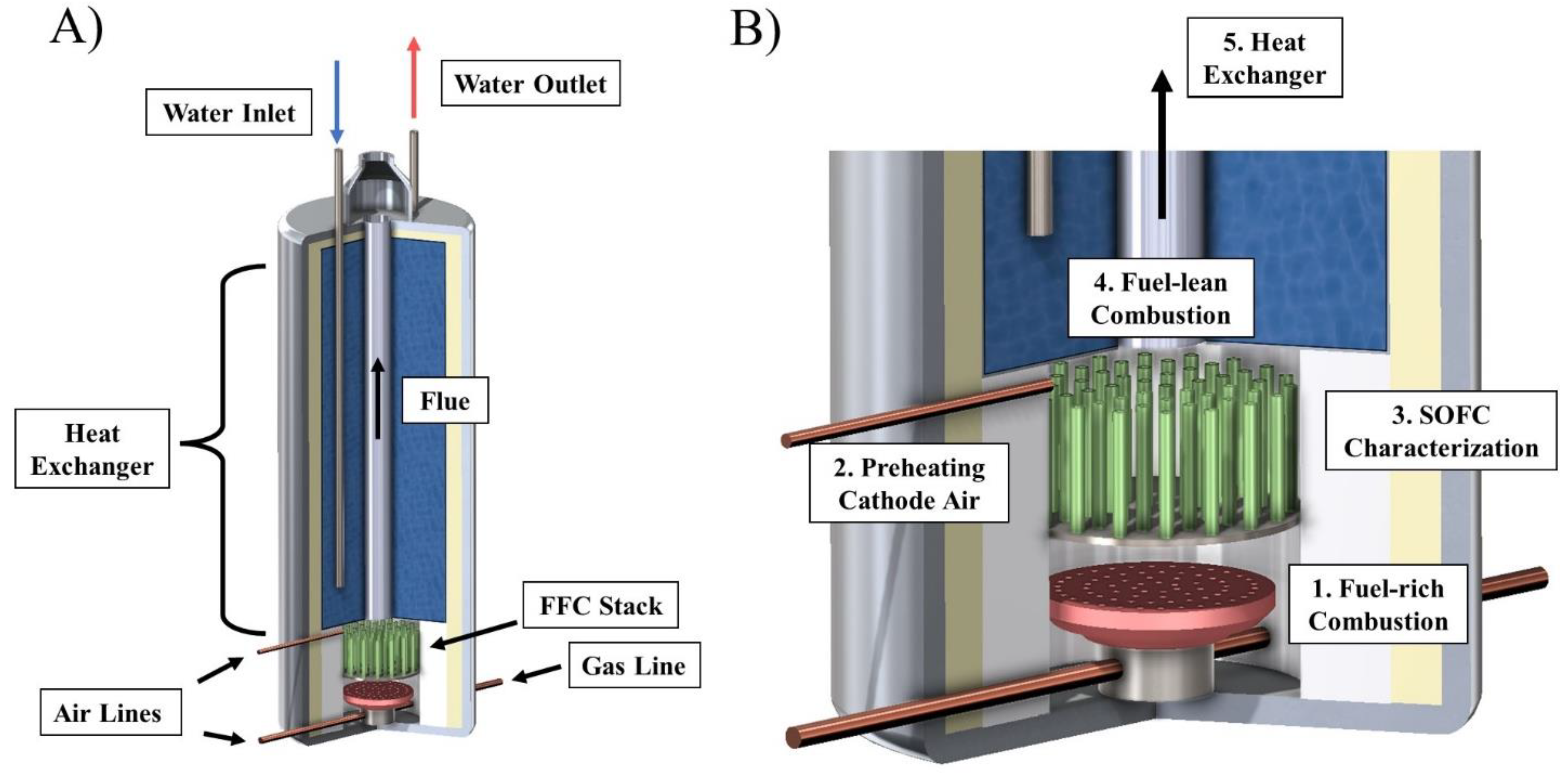
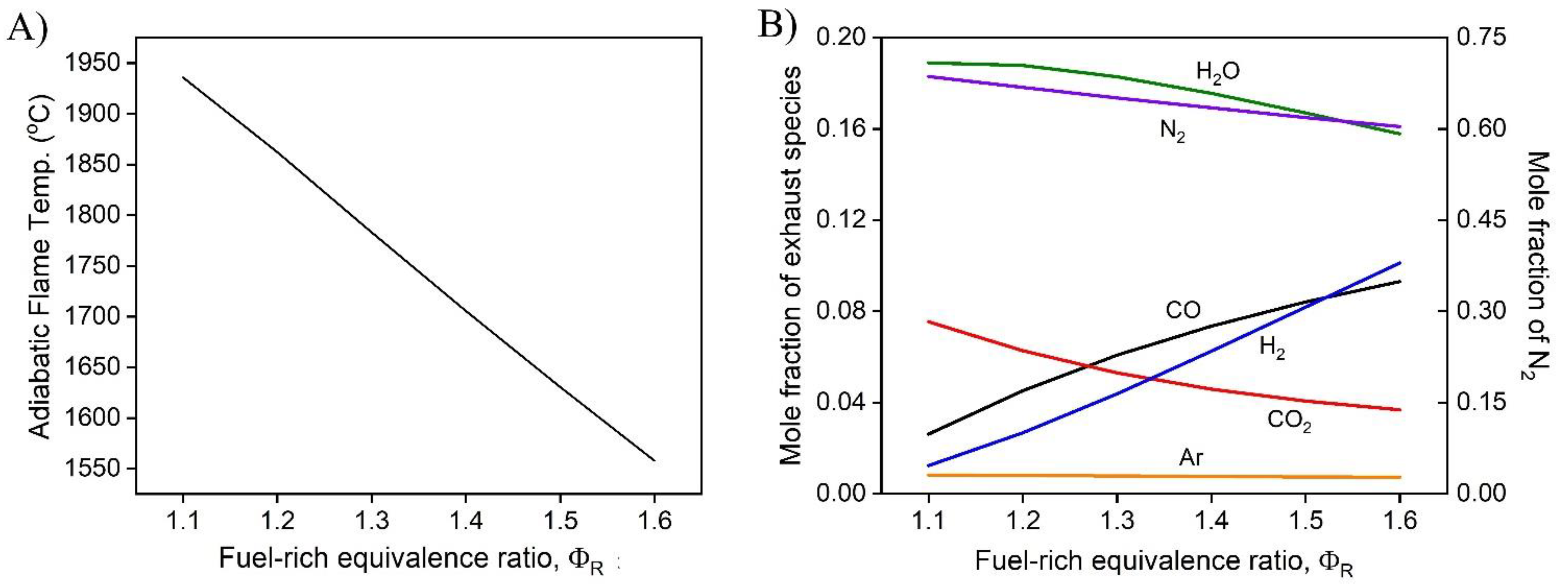
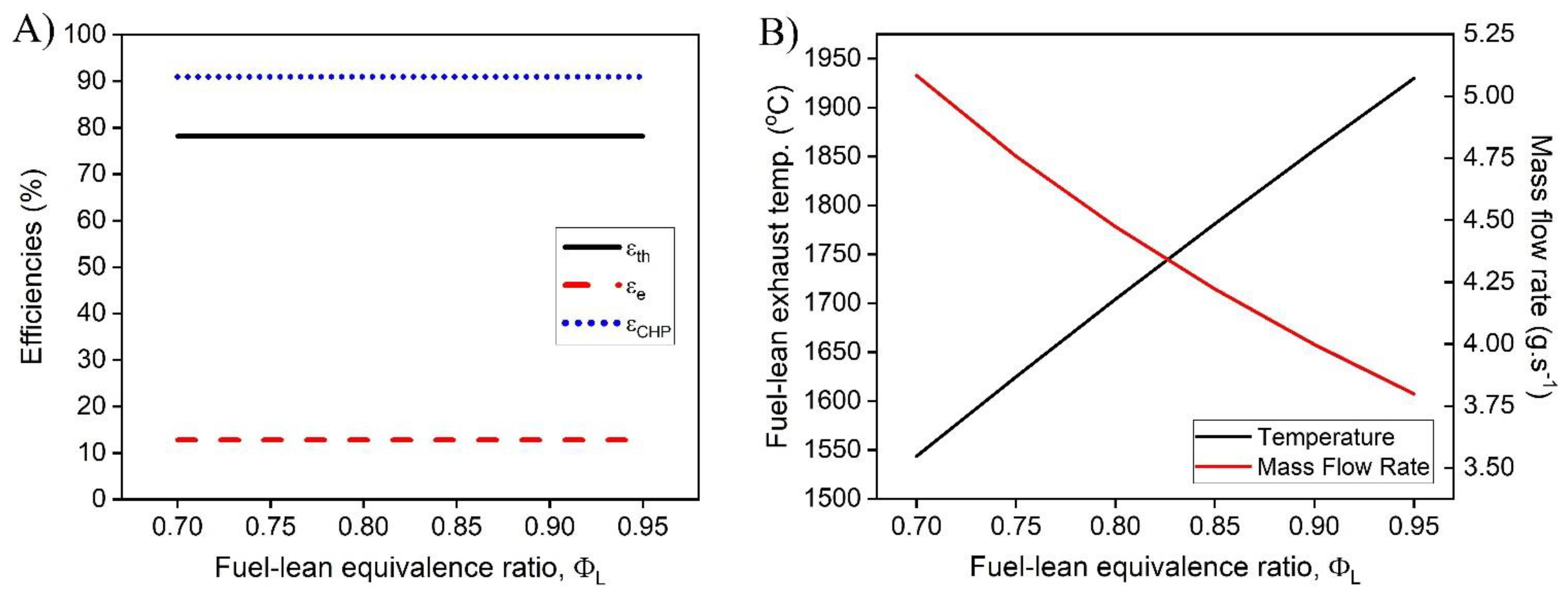
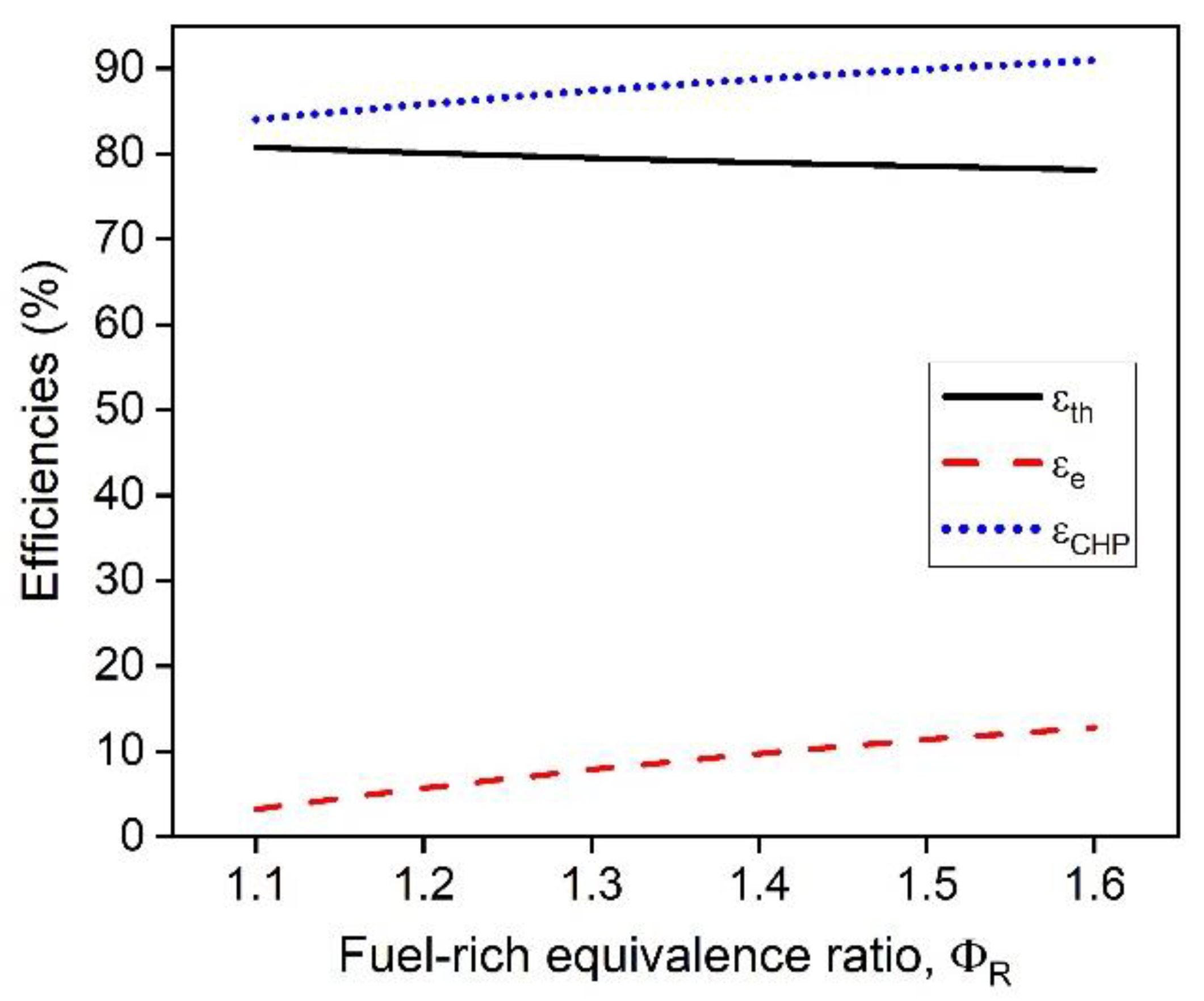
1. Introduction
The use of combined heat and power (CHP) systems is expected to increase in popularity worldwide with an estimated global capacity increase from 864.2 GW
e in 2018 to 1051 GW
e by 2025 [
1]. In the United States alone, there are over 4650 commercial and industrial installations with a total capacity of 80.8 GW
e [
2]. Additionally known as co-generation systems, CHP systems produce electricity while supplying thermal energy for heating purposes. The combined electrical and thermal energy generation in a CHP system results in an overall efficiency of 65–85% compared to 45–55% when the two devices are separated [
3]. CHP systems benefit from the ability to provide greater energy independence and lower energy costs, making them more economically competitive compared to stand-alone systems. The capacity of these installations varies depending on the industry sector implemented in, from kilowatts up to a gigawatt [
2]. Some of the most common CHP installations in the United States are in tandem with wastewater treatment plants, educational buildings (universities and schools), and chemical plants [
3,
4,
5]. In addition to the commercial and industrial sector, installation of CHP systems in residential buildings has become an important technology for energy savings and environmental conservation [
6,
7,
8,
9].
Additionally known as micro-combined heat and power (mCHP) systems, residential CHP systems normally have a capacity of no more than 15 kW
e [
10,
11]. Due to their smaller volumetric footprint, mCHP systems have a great market potential to be installed in apartments, houses, and duplexes across the world. The five major prime movers utilized in the majority of CHP systems include: reciprocating engines, gas turbines, steam turbines, fuel cells and microturbines [
3]. Unfortunately, heat engine technologies tend to have lower electrical efficiency at smaller scales [
5,
6]. Fuel cells, in contrast, can achieve high electrical efficiency at smaller scales, are quiet due to no moving parts, and have low to zero emissions [
6,
8,
12]. These features make fuel cells ideal prime movers for mCHP systems. Several manufactures have successfully commercialized residential fuel cell-based mCHP systems with thousands of mCHP installations performed in Japan, South Korea and Europe [
8,
9,
13,
14].
Fuel cells are electrochemical prime movers that convert chemical energy into electrical energy directly, bypassing the chemical-mechanical-electrical energy conversion processes needed by other prime movers (i.e., internal combustion engine and gas turbine systems) [
15]. The two main fuel cell technologies in mCHP systems include proton exchange membrane fuel cells (PEMFCs) and solid oxide fuel cells (SOFCs). In contrast with other fuel cell technologies, SOFCs are considered fuel flexible with the ability to utilize hydrogen, carbon monoxide, and methane as fuel with the byproducts of water and carbon dioxide. These devices consist of two cermet electrodes and a solid ceramic electrolyte. At high operating temperatures (500–1000 °C), the electrolyte permits oxygen ion diffusion to occur across the electrolyte via oxygen vacancies. Although they can use other fuels, hydrogen is the optimal fuel choice for SOFCs due to its faster electrochemical oxidation rate at the triple phase boundary while also eliminating the risk of carbon deposition from occurring [
16]. The reforming of fuel to hydrogen, however, is expensive and usually requires complex fuel reformers to be installed in mCHP systems.
Two technologies that have shown promise for mCHP applications are the direct flame SOFC (DFFCs [
17,
18,
19,
20]) and flame-assisted SOFC (FFCs [
21,
22]). These SOFCs utilize partial oxidation as a method to provide the thermal energy needed for operation while reforming the hydrocarbon fuel into hydrogen and carbon monoxide. By utilizing this method for fuel reformation, virtually any hydrocarbon could be used directly in a DFFC/FFC system without the need of any additional reformer or catalysts [
17,
19,
23]. This drastically simplifies SOFC integration into traditional balance of plant systems and removes the extended volume footprint required with current SOFC-based mCHP setups. Previous work with FFC systems utilizing common hydrocarbons found in natural gas (i.e., methane, ethane, propane, and
n-butane) have shown promising results with high power densities and fuel utilizations while maintaining stable performance over time [
24,
25,
26,
27].
Although there has been numerous studies and advancements in SOFC-based mCHP systems, research into the integration of FFCs into mCHP systems has been limited. Recent work investigating DFFC and FFC based mCHP systems have primarily utilized fuel cell experiments to investigate the potential of the technology in mCHP applications [
28,
29,
30,
31,
32,
33,
34] with only one study modeling the entire system and exploring the techno-economics [
29]. In 2020, Milcarek et al. integrated micro-tubular FFCs into a residential furnace and investigated the startup, performance and cycling of the mCHP system [
35]. The SOFCs demonstrated the ability to withstand 200 thermal cycles while providing quick response times, stable performance, and high CHP efficiency. However, it was concluded that hot water heaters and boilers would have greater benefit with this technology due to a larger market and longer annual operation, providing greater power generation without startup/cooldown losses. Additionally, with only one economic assessment performed focused in China [
29], questions regarding the feasibility of this technology in mCHP systems globally linger. Numerous economic assessments have been conducted with traditional SOFC mCHP systems but have yet to be analyzed for a FFC mCHP system.
In this work, a novel hybrid hot water system with an integrated FFC stack model was developed and a detailed techno-economic assessment was performed for various regions in the United States. Using experimental FFC performance data, the power generation of the model was analyzed at various operating conditions and the resulting effects on hot water generation were investigated. The CHP efficiency was calculated and reported. A thorough techno-economic analysis of the hybrid hot water heater was conducted based on four regions in the United States with varying pricing of natural gas and electricity. An estimated payback period using the net present value (NPV) of the hybrid hot water heater savings is reported for various operating conditions and hot water demands for each region considered. This work serves as the first look into incorporating FFCs into gas tank hot water heaters (creating a mCHP system without increasing the volume footprint of the system) and the first work to investigates the economic viability of a hybrid hot water heater in different geographical regions where natural gas prices, electricity prices, and hot water demands vary.
4. Conclusions
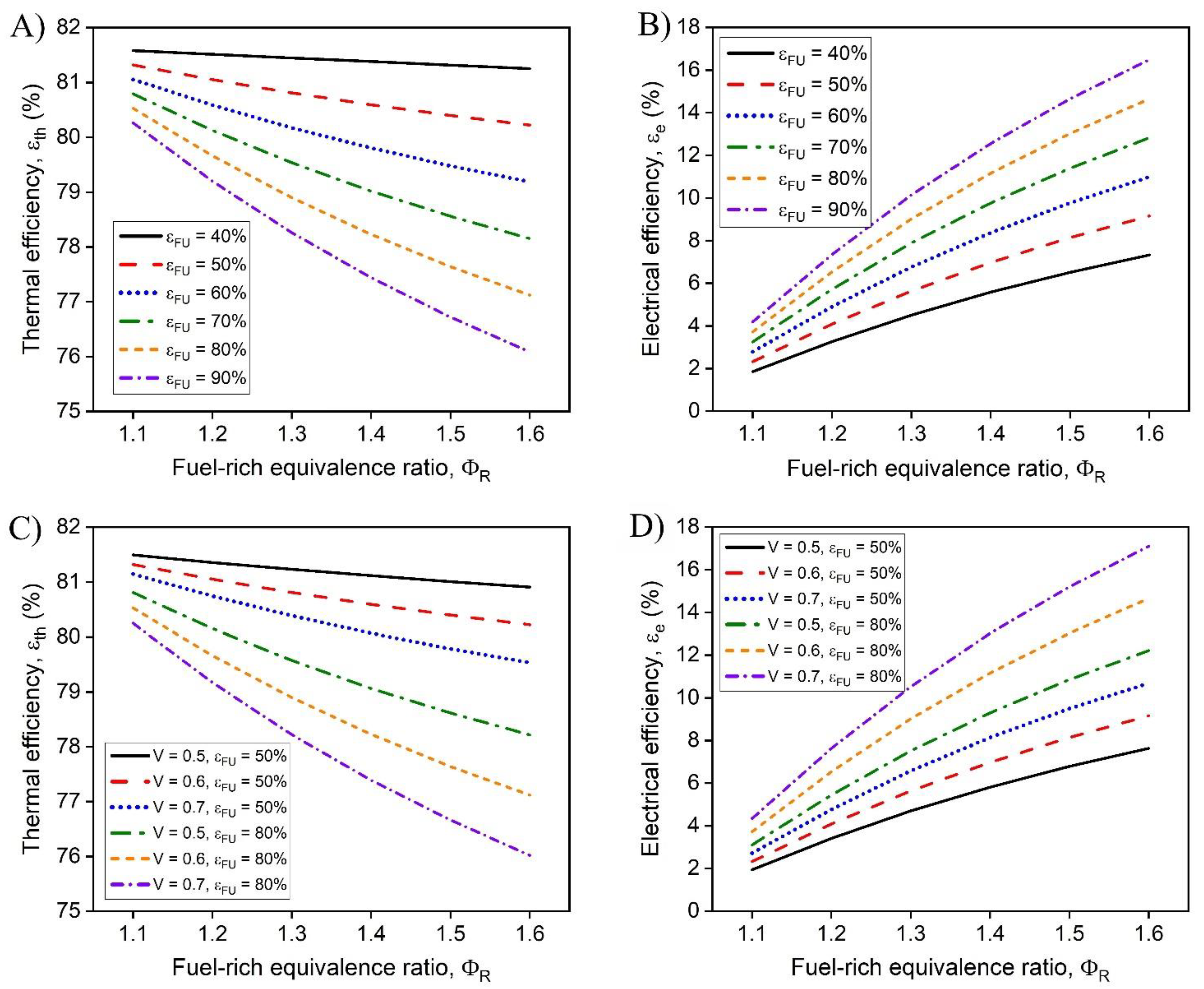
In this work, a hybrid hot water heater with an integrated FFC model was developed and a techno-economic analysis was performed. The performance of the hot water heater was dependent on the operating conditions including the fuel-rich combustion equivalence ratio, the operating voltage of the FFC, and the fuel utilization of the FFC. The dependence of the fuel-lean combustion equivalence ratio after the FFC was investigated and was determined to provide minimal effect on the overall performance of the system. The temperature of the exhaust leaving the hybrid combustion system was determined to be dependent on the mass flow rate of the system while operating at a set fuel-rich equivalence ratio, operating voltage, and fuel utilization. The hybrid hot water heater model demonstrated that CHP efficiencies greater than >90% with electrical efficiencies as high as 16% are possible. As the fuel-rich equivalence increases, the electrical and thermal efficiencies vary more at different fuel utilizations, providing greater performance increase for each increase in fuel-rich equivalence ratio. To obtain optimal performance, high fuel utilization coupled with higher operating voltages are desirable. With practical FFC operating conditions, ( = 70% and V = 0.6 V) the CHP efficiency was 91.0% with an electrical efficiency of 12.8%.
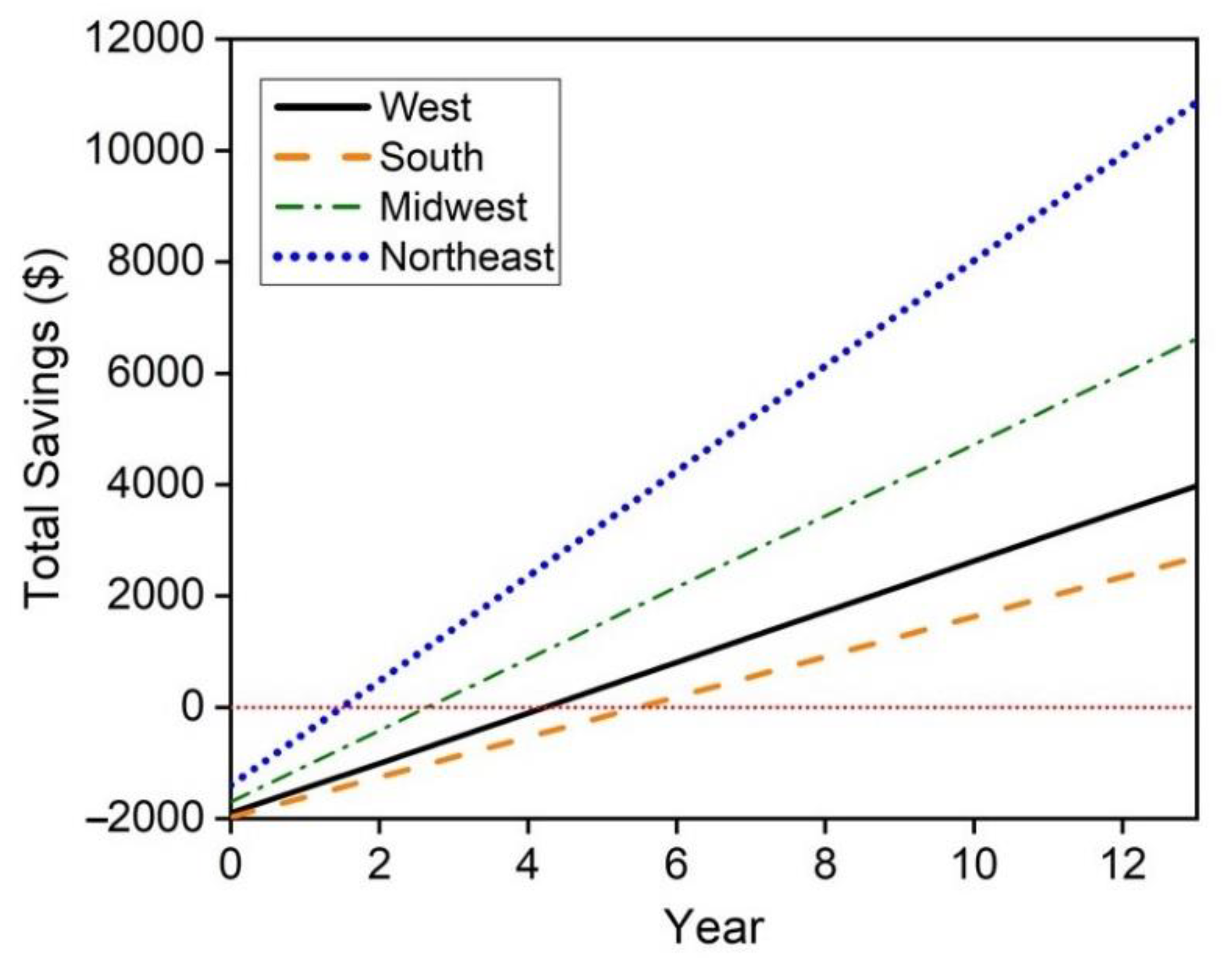
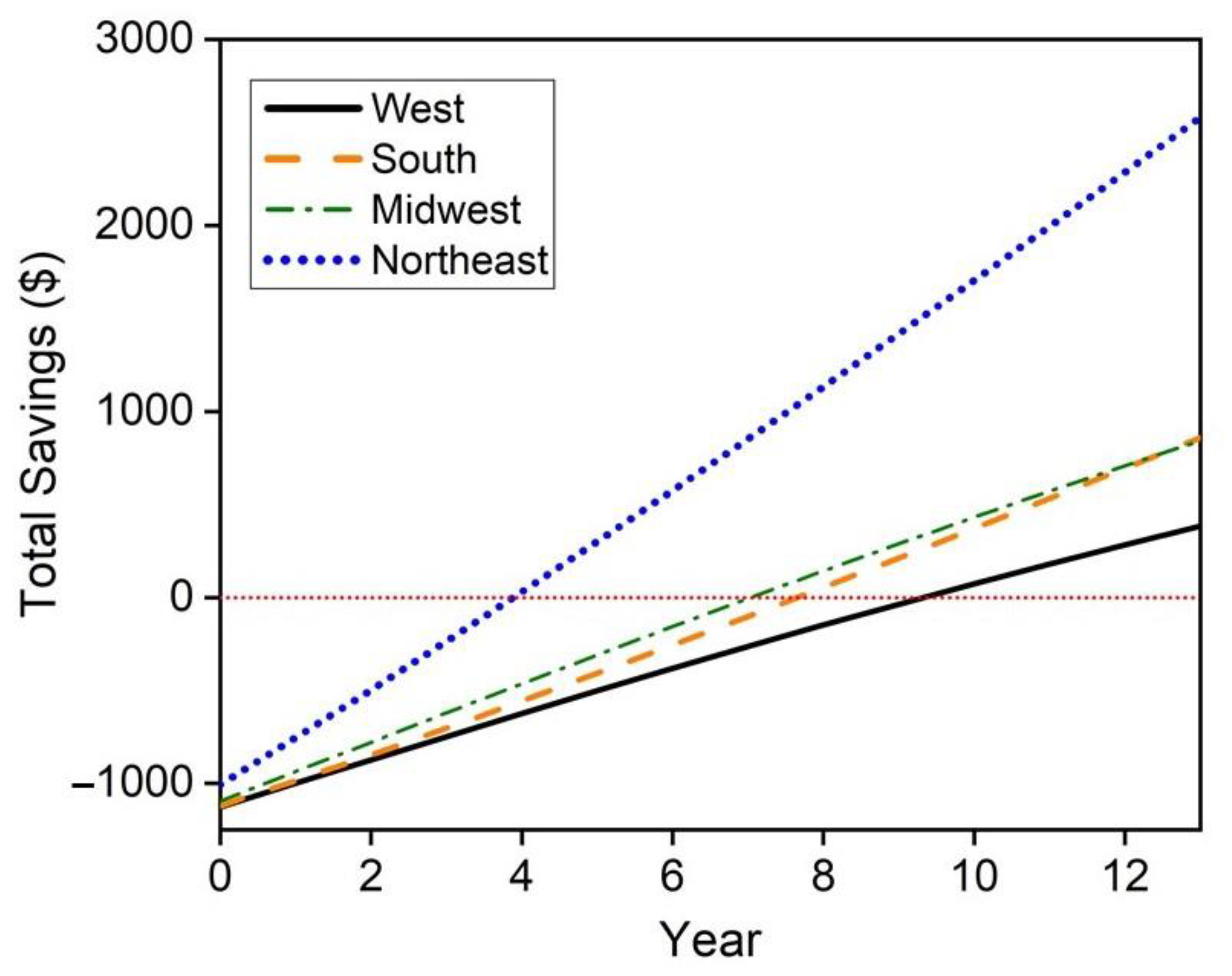
The hybrid hot water heater replacement of electric hot water heaters proves to be economically feasible in all regions of the United States with the longest payback period being less than 6 years in the South region. However, this may not be feasible depending on natural gas availability. A maximum cost savings of $10,868 in the Northeast region of the United States is achieved and a minimum cost savings of $2696 is achieved in the South. Replacement of natural gas hot water heaters with the hybrid system is less economically viable with payback periods spanning from approximately 4 years in the Northeast to between 9–10 years in the west. These savings only consider a 13 year lifetime, however the mCHP system may also achieve replacement cost savings if the FFC does not see noticeable degradation within this 13 year time period. Future work in this techno-economic analysis will consider these replacement savings and the degradation of the FFC throughout its lifetime.