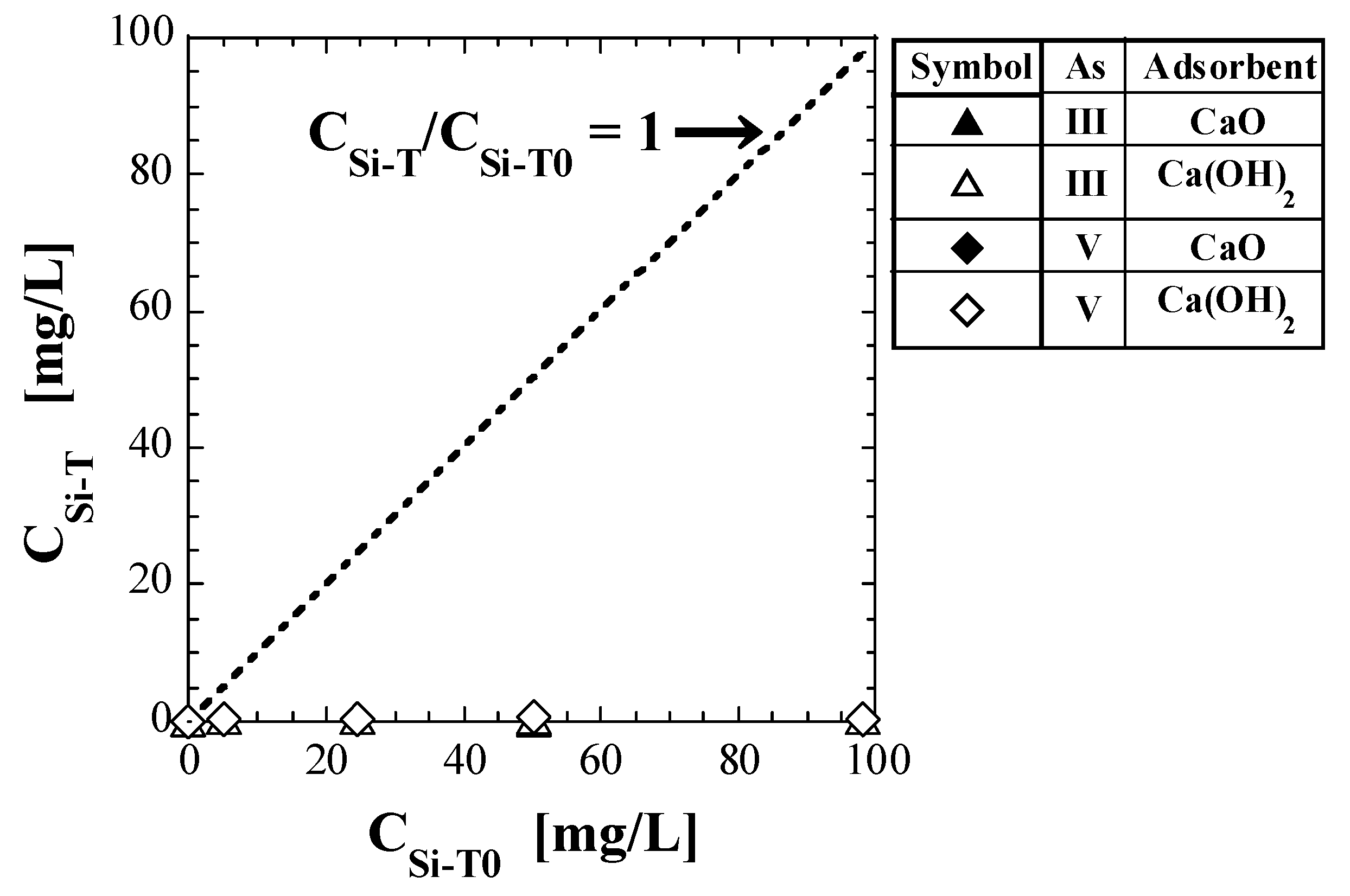Abstract
The spent adsorbents that remain after being used to purify As-contaminated water constitute waste containing a large amount of As. These spent adsorbents, after being disposed, are likely to come into contact with silicic acid leached from the soil or cementitious solidification materials. Thus, it is crucial the evaluate the effects of silicic acid on spent adsorbents. In this study, the effects of silicic acid on spent Ca-based (CaO and Ca(OH)2) adsorbents with arsenite were investigated. The As leaching ratio for the spent adsorbents decreased with an increase in the initial concentration of silicic acid in the liquid. Under the tested conditions, the As leaching ratio decreased from 8–9% to less than 0.7% in the presence of silicic acid at an initial Si-normalized concentration of 100 mg/L. The primary mechanism behind the inhibition of As leaching by silicic acid was determined to be re-immobilization via the incorporation of arsenite during the formation of calcium silicates. In the presence of silicic acid, spent Ca-based adsorbents with arsenite had a lower As leaching ratio than those with arsenate. Therefore, spent Ca-based adsorbents with arsenite were found to have a higher environmental stability than those with arsenate.
1. Introduction
There are numerous reports on groundwater contamination with arsenic (As), which is a global problem. In recent years, it has become a major challenge in regions across Asia (such as Bangladesh [1,2,3,4,5], Pakistan [6,7], Iran [8], India [9,10,11], Sri Lanka [12], Vietnam [13,14], Cambodia [15], and China [16,17,18]), Latin America (such as Argentina [19,20], Uruguay [21], and Mexico [22,23]) and West and South Africa [24,25]. Podgorski and Berg [26] reported the arsenic pollution in groundwater around the world; the proportion of affected area in Asia was 64%, in South America 14%, North America 10%, Africa 9%, Oceania 2% and Europe 1%. The proportion of total affected population in Asia was 94%, in Africa 3.7%, South America 1.6%, North America 0.6%, Europe 0.2% and Oceania 0.01%. In addition, the population per country exposed to As-contaminated groundwater was reported by Shaji et al. [27]. In some areas, in particular, such as South Asia and Southeast Asia, there is a high risk of health damage because groundwater contaminated with As is often used directly as drinking water.
Moreover, numerous studies have investigated methods for purifying As-contaminated water, such as the coprecipitation method using flocculants, ion exchange method using ion exchange resins, adsorption methods using various adsorbents such as activated carbon, silica gel, iron-based adsorbents, and aluminum-based adsorbents, microfiltration methods using microfiltration membranes, nanofiltration membranes, ultrafiltration membranes, or reverse osmosis filtration membranes, chemical or biological oxidation methods, and electrocoagulation methods. These have been outlined by Jadhav et al. (2015), Sarkar and Paul (2016), Ghosh et al. (2019), and Kumar et al. (2019) [28,29,30,31]. In developing countries, As treatment methods utilizing inexpensive adsorbents are considered more likely to be introduced with respect to economic and operational conditions. In addition, although the constituents of adsorbents often leach from the adsorbents, both Mg and Ca are essential and safe substances for human health. Furthermore, Mg-based [32,33,34] and Ca-based adsorbents [35,36,37,38] are expected to have high As removal performance.
In particular, certain calcium compounds are often used in combination with other adsorbents or as auxiliary agents to other treatment methods, in addition to being used alone as Ca-based adsorbents. Camacho et al. [35] looked to determine the effect of calcium addition as a stabilization agent on arsenic desorption from ferric water treatment residuals. They conducted laboratory and field tests using calcium agents such as lime (CaO or Ca(OH)2) and reported that: (1) desorption from laboratory ferric water treatment residuals did not generate any arsenic when calcium was present in solution, especially when excess calcium that did not join the surface of the treatment residual was present; similarly (2) arsenic leaching decreased when the field treatment residuals were treated with lime as a stabilizing agent; finally (3) the immobilization of arsenic in the treatment residuals may be achieved through treatment with lime and cement. Similarly, Montes-Hernande et al. [36] investigated the removal of oxyanions from an aqueous solution using the carbonation of Ca(OH)2 under moderate pressure (PCO2 = 20 bar) and temperature (30 °C). The study involved calcium hydroxide carbonation with compressed carbon dioxide in order to stabilize the solid matter. The authors reported that the Ca(OH)2 carbonation reaction allowed the successful removal of selenite (>90%), arsenate (>78%), and phosphate (almost 100%) from synthetic solutions. In another study, Olyaie et al. [37] evaluated the use of CaO2 nanoparticles in removing As (III) from contaminated water. CaO2 is an oxidant that decomposes in high humidity to produce Ca(OH)2 and H2O2. CaO2 nanoparticles effectively removed total As under natural pH conditions (6.5 and 8.5). The removal efficiency was enhanced by increasing the CaO2 nanoparticle dosage and reaction time, but decreased with increasing arsenic concentration and pH. Electrocoagulation has also been investigated as a method to remove arsenate; Hu et al. [38] studied the effect of calcium on arsenate removal by electrocoagulation with an aluminum electrode. The calcium salt used was CaCl2. The calcium ions could neutralize the negative surface charges of the precipitate and increase the binding energy of As–O; thus, the addition of calcium salt dramatically improved the removal efficiency of As(V). The addition of calcium also prevented the formation of a deposit layer on the anode surface, which caused an increase in the applied potential and decreased the concentration of dissolved aluminum.
On the other hand, spent adsorbents that have adsorbed As will become waste containing a large amount of As. Therefore, proper recovery treatments for spent adsorbents are essential. Without suitable treatment prior to disposal, As may leak from spent adsorbents, leading to secondary environmental pollution. Previous studies [39,40,41,42] have reported the leaching of As from spent Mg-based (MgO, Mg(OH)2, and MgCO3) and Ca-based (CaO and Ca(OH)2) adsorbents. One particular area that should be explored is the influence of silicic acid on spent adsorbents, as after disposal the spent adsorbents are likely to come into contact with silicic acid leached from soils or cementitious solidification materials. Therefore, Sugita et al. (2017) and Sugita et al. (2018) evaluated the effects of silicic acid on the leaching behavior of As from spent adsorbents; spent Mg-based and Ca-based adsorbents with arsenate were examined using silicic acid solution via shaking tests (leaching tests) [40,41]. Spent adsorbents based on Mg(OH)2 and MgCO3 were strongly affected by silicic acid, which significantly reduced their environmental stability and leaching of As. However, MgO-based adsorbents had extremely high environmental stability against silicic acid and hardly leached As [40]. In addition, the As leaching ratio for the spent adsorbents based on CaO and Ca(OH)2 decreased with increasing initial concentration of silicic acid in the test solution [41].
However, these studies focused on arsenate, As(V), and no similar tests have been conducted on arsenite, As(III). Arsenic is often reduced to arsenous acid underground. For example, a groundwater survey in Bangladesh reported more than 95% of soluble As is in the form of arsenous acid [1]. In general, arsenous acid is known to be more toxic than arsenic acid, and understanding the leaching behavior of arsenous acid is essential for understanding the leaching behavior of arsenic acid. In addition, As(III) differs greatly from As(V) in terms of its chemical characteristics, such as pH dependence. Clarifying the differences among the leaching characteristics of various valences of As is beneficial from a scientific point of view and may help evaluate the potential of spent adsorbents with hazardous substances to cause secondary environmental pollution. Therefore, an evaluation of the effects of silicic acid on spent adsorbents with arsenite would be valuable.
In this study, spent Ca-based adsorbents containing arsenous acid were studied, and shaking tests with silicic acid solution were conducted. In these tests, the leaching behavior of both As and Ca from the spent adsorbents with arsenite as well as the decreasing level of silicic acid in the liquid phase were investigated. Subsequently, the effects of silicic acid on the environmental stability of spent Ca-based adsorbents with arsenite were evaluated. To evaluate the environmental stability of spent adsorbents, using protocols detailed in previous studies [39,40,41], the following were assessed: (1) the difficulty of As leaching from the spent adsorbents; and (2) the difficulty in elution of the base material components of the adsorbents.
2. Materials and Methods
The reagents listed in this article were purchased from FUJIFILM Wako Pure Chemical Corporation (Osaka, Japan, formerly Wako Pure Chemical Industries, Ltd.), unless specified otherwise.
2.1. Spent Ca-Based Adsorbents
The spent Ca-based adsorbents with arsenite used in the experiments in this study were the same as those reported in our previous study [42]. An overview of the preparation of the spent adsorbents is provided below.
2.1.1. Ca-Based Adsorbents (Unspent)
Two types of Ca compound reagents, CaO and Ca(OH)2, were used in this study. These were commercially available and purchased from FUJI-FILM Wako Pure Chemical Co. The nominal values of the purity of CaO and Ca(OH)2 were 99.9% and 96%, respectively. In addition, the measured values of Ca content in CaO and the Ca(OH)2 were 71.2% and 53.5%, with a median particle size of 19.6 and 41.7 μm, and BET surface area of 2.7 and 14.3 m2/g, respectively [39].
2.1.2. Synthetic As-Contaminated Water
The powdered reagents of Sodium arsenite (NaAsO2, 90%) and sodium hydrogen arsenate heptahydrate (Na2HAsO4·7H2O, 99%) were dissolved in deionized water, and stock solutions of arsenite and arsenate with a concentration of 2000 mg-As/L were prepared. A portion of the stock solution was diluted with deionized water to prepare a 20 mg-As/L solution. This solution was used as synthetic As-contaminated water after adjusting the pH to near neutral by adding hydrochloric acid.
2.1.3. Preparation of Spent Ca-Based Adsorbents
The unspent Ca-based-adsorbent (1 g) was weighed in a TPX beaker. Synthetic As-contaminated water (200 mL) was added to the beaker and stirred with a magnetic stirrer (approximately 500 rpm). After stirring for about a day, suction filtration was performed using a Teflon filter (pore size 0.45 μm) for solid liquid separation. As and Ca in the filtrate were quantified via ICP-MS (Agilent 7700X or Shimadzu ICPM-8500) and ICP-AES (SII SPS3500DD), respectively. The analytical results of the filtrate are outlined below [41,42].
The data on the production of spent adsorbents with As(III) or As(V) are shown in Table 1 and Table 2, respectively. WAD is the amount of unspent adsorbent added to the synthetic As-contaminated water in mg, V is the liquid volume of the synthetic As-contaminated water in L, and WAD/V is the addition concentration of the unspent adsorbent in mg/L. pH0 is the pH of the solution immediately before adding the adsorbent, which is referred to as “the initial pH”. CAS0 is the initial As concentration in mg/L in As-contaminated water, CAS is the As concentration in the filtrate, and RAS is the As removal ratio, which is defined by the following equation:
RAS = (CAS0 − CAS)/CAS × 100.

Table 1.
Data on As for production of spent Ca-based adsorbents.

Table 2.
Data on Ca for production of spent Ca-based adsorbents.
WCa/V in Table 2 is the Ca addition concentration in mg/L, which is defined by the following equation:
where αCa is the Ca content of the Ca-based adsorbent.
WCa/V = WAD/V × αCa/100
Cca in Table 2 is the Ca concentration in the filtrate (= the concentration of Ca leached from the adsorbent). βCa is the Ca leaching ratio, which is defined by the following equation:
βCa = CCa/( WCa/V) × 100.
For both CaO and Ca(OH)2, the RAS for As(III) was slightly lower than that for As(V). On the other hand, for both As(III) and As(V), βCa for CaO was clearly lower than that for Ca(OH)2.
The adsorbents with As recovered by the solid liquid separation operation were dried at a low temperature (approximately 40 °C) for about half a day in a constant temperature dryer and then stored in a closed polypropylene bottle. The adsorbents with As were used as “spent adsorbents” in the shaking test, as described later. The outline of the calculation method for As content of the spent adsorbent is shown below [41,42].
The residual ratio of adsorbent γ as a percentage is defined by the following equation:
γ = 100 − βCa.
The amount of As adsorbed per unit mass of the adsorbent remaining as solid δ (mg/mg) is defined by the following equation:
δ = (CAS0 − CAS)/[( γ/100) × WAD/V].
The weighed value of the spent adsorbent is the total value of the adsorbent and As adsorbed on its surface. Therefore, the As content per unit mass of the recovered spent adsorbent QAS in mg-As/g can be easily obtained by the following equation:
QAS = δ/(1 + δ/1000).

Table 3.
As content per nit mass of spent adsorbent.
2.2. Silicic Acid Solution
The silicic acid solutions used in this study were the same as those reported in a previous study [40,41]. An overview of the preparation of the silicic acid solution is provided below.
The stock solution of silicic acid was prepared by dissolving approximately 1 g of the sodium silicate solution (21.2–23.8%, SiO2/Na2O = 3.0–3.6 mol ratio at nominal value) in 1 L of deionized water. The Si concentration in the stock solution was measured using an ICP-AES. A portion of the stock solution was diluted with deionized water to obtain the desired concentration. The silicic acid solution obtained after the pH was neutralized with hydrochloric acid and sodium hydroxide solution immediately before adding the spent adsorbent in the shaking test was used as the test solvent. No pH buffering agent was used in our studies because the use of pH buffering agents may affect the reaction between the adsorbent, silicic acid, and As. In this study, the pH of the solution immediately before adding the adsorbent (the pH of the test solvent) is referred to as “the initial pH” (denoted as pH0), and the pH of the eluate recovered by filtration after shaking is referred to as “the final pH” (denoted as pHf). The pH meter and electrode used in this study were a LAQUA F-72 and a Micro ToupH Electrode 9618S-10D, respectively, manufactured by HORIBA, Ltd. (Kyoto, Japan).
The Si concentration of the test solvent was divided into five levels: 0, 5, 25, 50, and 100 mg/L. The total Si concentration was measured using ICP-AES, and the concentration of monosilicic acid (silica monomer) was measured using the molybdenum yellow method. Silicic acid refers to H4SiO4 (or Si(OH)4), and silica refers to SiO2; however, in this study all concentrations of silicic acid species are described as values converted to Si concentration. Here, the Si concentration is referred as “Si-normalized concentration.” The Si-normalized concentrations quantified using ICP-AES and the molybdenum yellow method are referred to as “total Si-normalized concentration” and “Si-normalized monomer concentration,” respectively, and denoted as CSi-T and CSi-M. In addition, the difference between CSi-T and CSi-M was assumed to correspond to the concentration of polysilicic acid (silica polymer including colloidal silica), which is referred to as “Si-normalized polymer concentration” and is denoted as CSi-P. In addition, the Si polymer concentrations in the test solution before adding the spent adsorbent are denoted as CSi-T0, CSi-M0, and CSi-P0. Table 4 shows the data on the Si-normalized concentrations and pH0 for the test solvents used in the shaking tests. In the test solvent with a Si-normalized concentration of 100 mg/L, approximately 13 mg/L of polysilicic acid was present in the Si conversion. However, most of the silicic acid present in the solution was in the form of monosilicic acid, as shown in Table 4.

Table 4.
CSi-T0, CSi-M0, CSi-P0, and pH0 for test solvent used in this study.
2.3. Shaking Test with Spent Ca-Based Adsorbents and Silicic Acid Solution
The shaking tests were performed using a procedure described in previous studies [40,41], an overview of which is provided below.
About 0.1 g of the spent adsorbent and 50 mL of the test solvent (the aforementioned silicic acid solution or deionized water) were added to a reaction tube (50 mL polypropylene centrifuge tube). The tube was sealed with a cap and shaken in a thermostatic shaker (150–180 rpm at room temperature). After shaking for 24 h, the tube was placed in a centrifuge (4500 rpm for 20 min). After centrifugation, the supernatant was filtered through a syringe filter (0.45 μm pore size), and the filtrate (eluate) was collected in a polypropylene bottle. The concentration of As in the eluate was measured using ICP-MS, and those of Ca and Si were determined via ICP-AES. Si quantification using the molybdenum yellow method was also performed. In addition, in order to confirm the reproducibility of the shaking test, shaking tests using the spent adsorbents of CaO and Ca(OH)2 were conducted three times under the same conditions (CSi-T0 = 50 mg/L).
3. Results
3.1. pH of Eluate
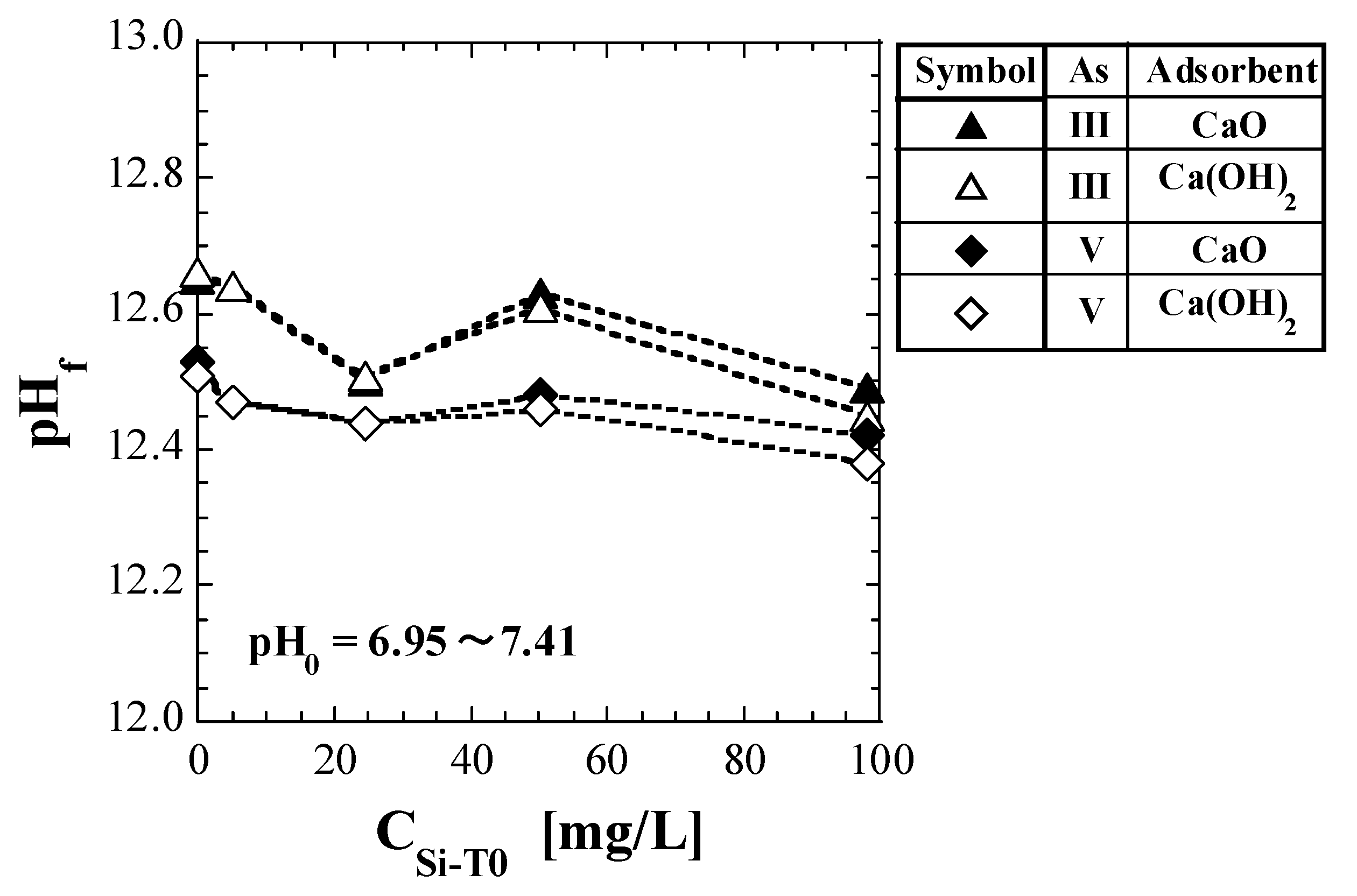
Table 5 lists the values of pHf measured during the shaking tests. The CSi-T0 values displayed in Table 5 are not the actual measured values and thus, for convenience, are shown by substituting the corresponding values of the Si-normalized concentration. Figure 1 shows a plot of pHf against CSi-T0.

Table 5.
Values of pH of eluate (pHf).
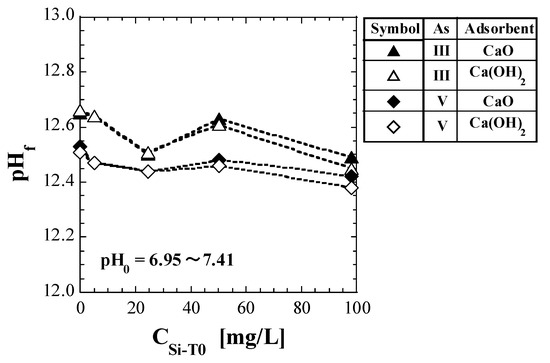
Figure 1.
Change in pH of eluate with initial total Si-normalized concentration.
For comparison with As(III), the results for the spent Ca-based adsorbents with As(V) (reported in a previous study [41]) are also shown in Table 5 and Figure 1. The pH0 values were near neutral (pH 6.95 to 7.41); however, the pH increased significantly after adding the spent adsorbents, consistent with the results of previous studies [41]. For both As(III) and As(V), no significant difference was observed between the pHf values of CaO and Ca(OH)2, as shown in Figure 1. In addition, for both CaO and Ca(OH)2, the values of pHf for As(III) were higher than those for As(V).
3.2. As Concentration in Eluate
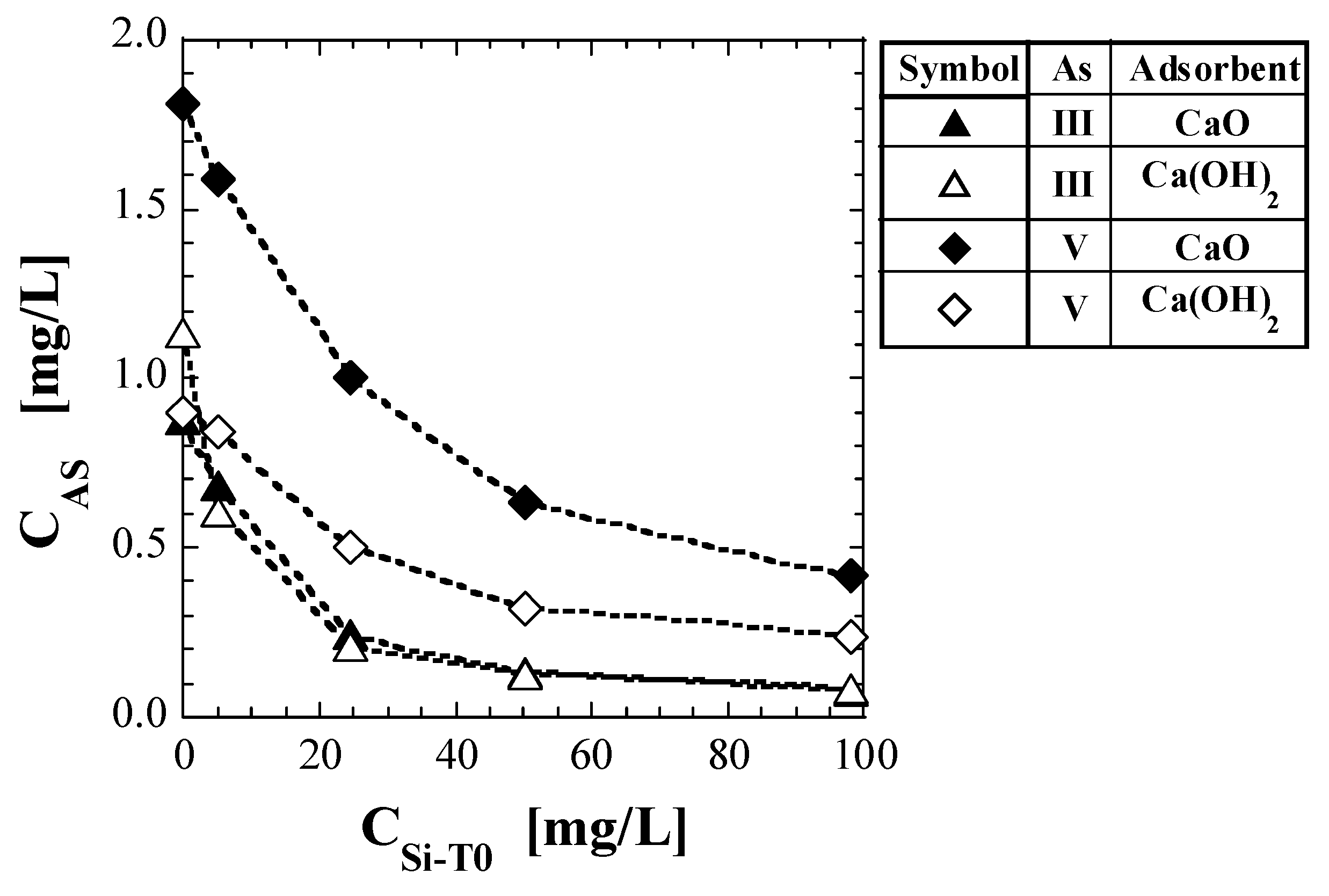
Table 6 shows the values of As concentration and CAS, as per the shaking tests. Figure 2 shows a plot of CAS against CSi-T0. The test results for As(V) reported in a previous study [41] are also shown in Table 6 and Figure 2. For both CaO and Ca(OH)2, the CAS tended to decrease with increasing CSi-T0. For As(III), no significant difference was noted between the CAS values of CaO and Ca(OH)2; however, the CAS value of Ca(OH)2 was clearly higher than that of CaO at CSi-T0 = 0 mg/L. CAS, which was around 1 when CSi-T0 = 0 mg/L, decreased to less than 0.1 mg/L when CSi-T0 = 100 mg/L. For As(V), regardless of CSi-T0, the CAS values for CaO were higher than those for Ca(OH)2. With respect to CaO, regardless of CSi-T0, the CAS values for As(V) were higher than those for As(III). With respect to Ca(OH)2, the CAS values for As(V) were higher than those for As(III), except that the CAS value for As(III) was clearly higher than that for As(V) at CSi-T0 = 0 mg/L.

Table 6.
Values of As concentration in eluate (CAS).
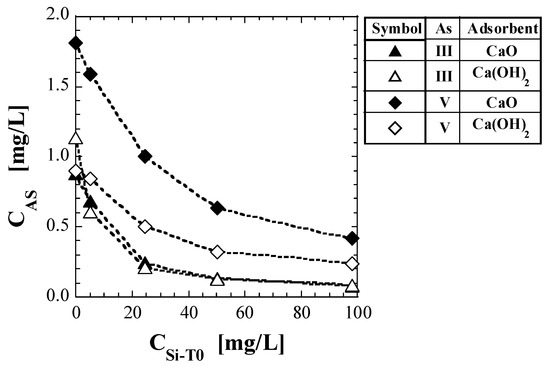
Figure 2.
Change in As concentration in eluate with initial total Si-normalized concentration.
3.3. Ca Concentration in Eluate
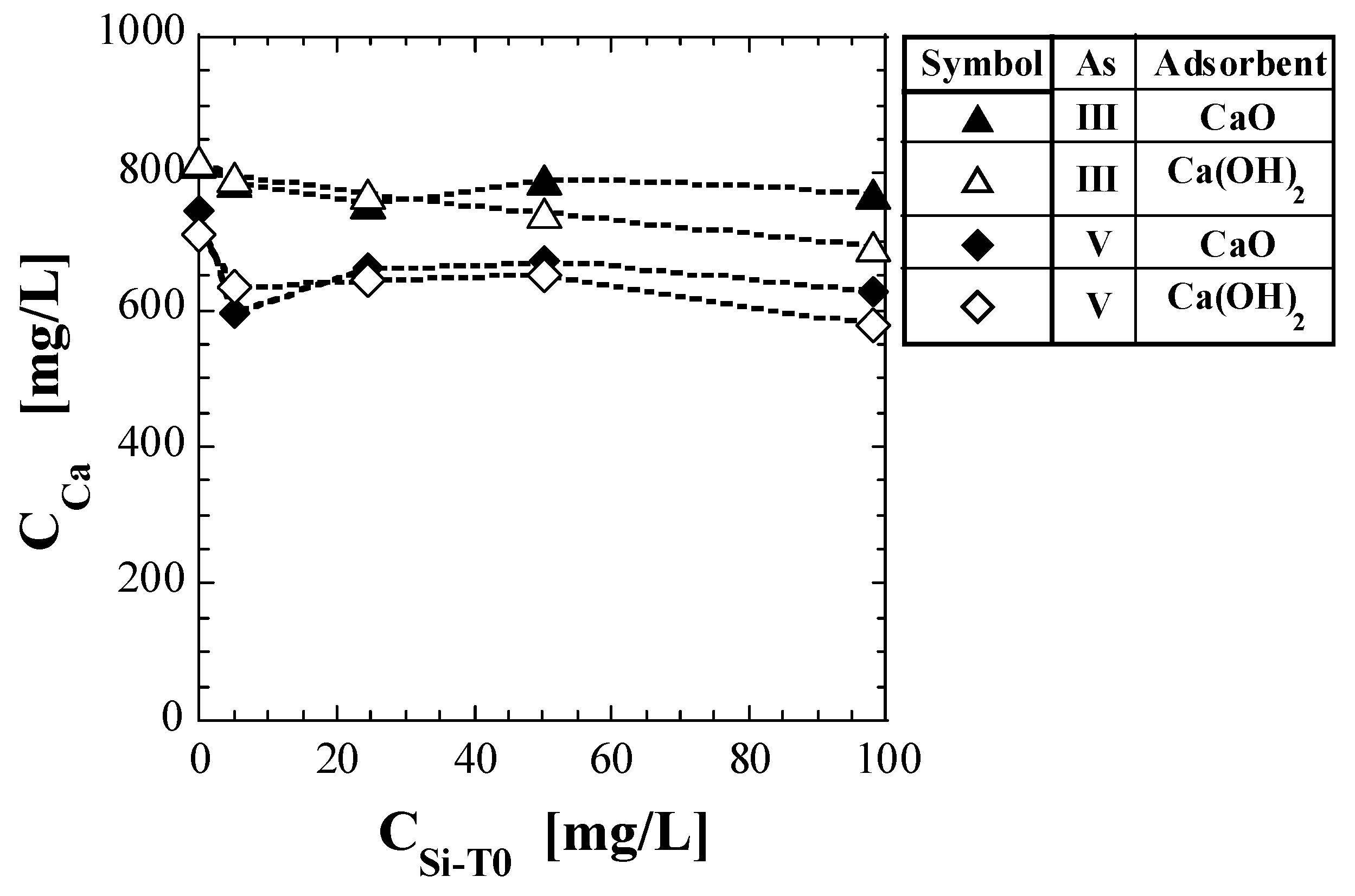
Table 7 shows the Ca concentration, CCa, as quantified via shaking tests. Figure 3 presents a plot of CCa against CSi-T0. The test results for As(V) reported in a previous study [41] are also shown in Table 7 and Figure 3. For the set of Ca(OH)2 and As(III), CCa gradually decreased with increasing CSi-T0. For the set of CaO and As(III), CCa did not appear to change significantly in the CSi-T0 range of 0–100 mg/L. For As(V), both the CCa for CaO and Ca(OH)2 decreased significantly in the CSi-T0 range of 0–5 mg/L and did not seem to change significantly in the CSi-T0 range of 5–100 mg/L. Thus, even if the base material of the spent adsorbent was the same, the value of CCa differed depending on whether As(III) or As(V) was adsorbed.

Table 7.
Values of Ca concentration in eluate (CCa).
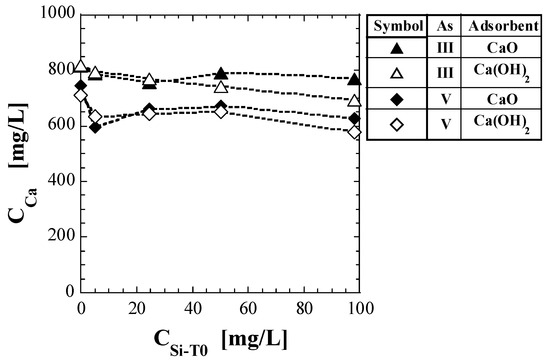
Figure 3.
Change in Ca concentration in eluate with initial total Si-normalized concentration.
3.4. Total Si-Normalized Concentration in Eluate
Table 8 shows the values of total Si-normalized concentration, CSi-T, quantified via shaking tests. Figure 4 shows a plot of CSi-T against CSi-T0. The test results for As(V) reported in a previous study [41] are also shown in Table 8 and Figure 4. The straight line of CSi-T/CSi-T0 = 1 is also shown in Figure 4. If CSi-T does not change from the initial value (CSi-T0), the plots should be on this straight line; however, all the plots are located well below the straight line.

Table 8.
Values of total Si-normalized concentration in eluate (CSi-T).
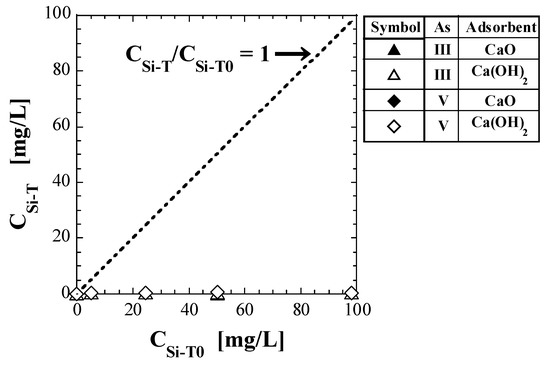
Figure 4.
Change in Si concentration in eluate with initial total Si-normalized concentration.
The CSi-M measurements were approximately equal to those of CSi-T. Therefore, in the following, only CSi-T was handled as the Si-normalized concentration.
3.5. Reproducibility of Experimental Data
The shaking tests with the silicic acid solution (CSi-T0 = 50 mg/L) and spent adsorbents (CaO and Ca(OH)2) with As(III) were conducted three times. The relative standard errors for the pHf, CAS, CCa, and CSi-T values estimated via these tests were 0.1%, 3%, 4%, and 1% for CaO and 0.2%, 2%, 5%, and 1% for Ca(OH)2, respectively. Based on these results, it was confirmed that the reproducibility of the experimental data was good.
4. Discussion
4.1. Relative Evaluation Using As Leaching Ratio
The As content of the spent adsorbent varied depending on the type of spent adsorbent (see Table 3). The As leaching ratio, EAS [%], defined by the following equation, was used for the evaluation:
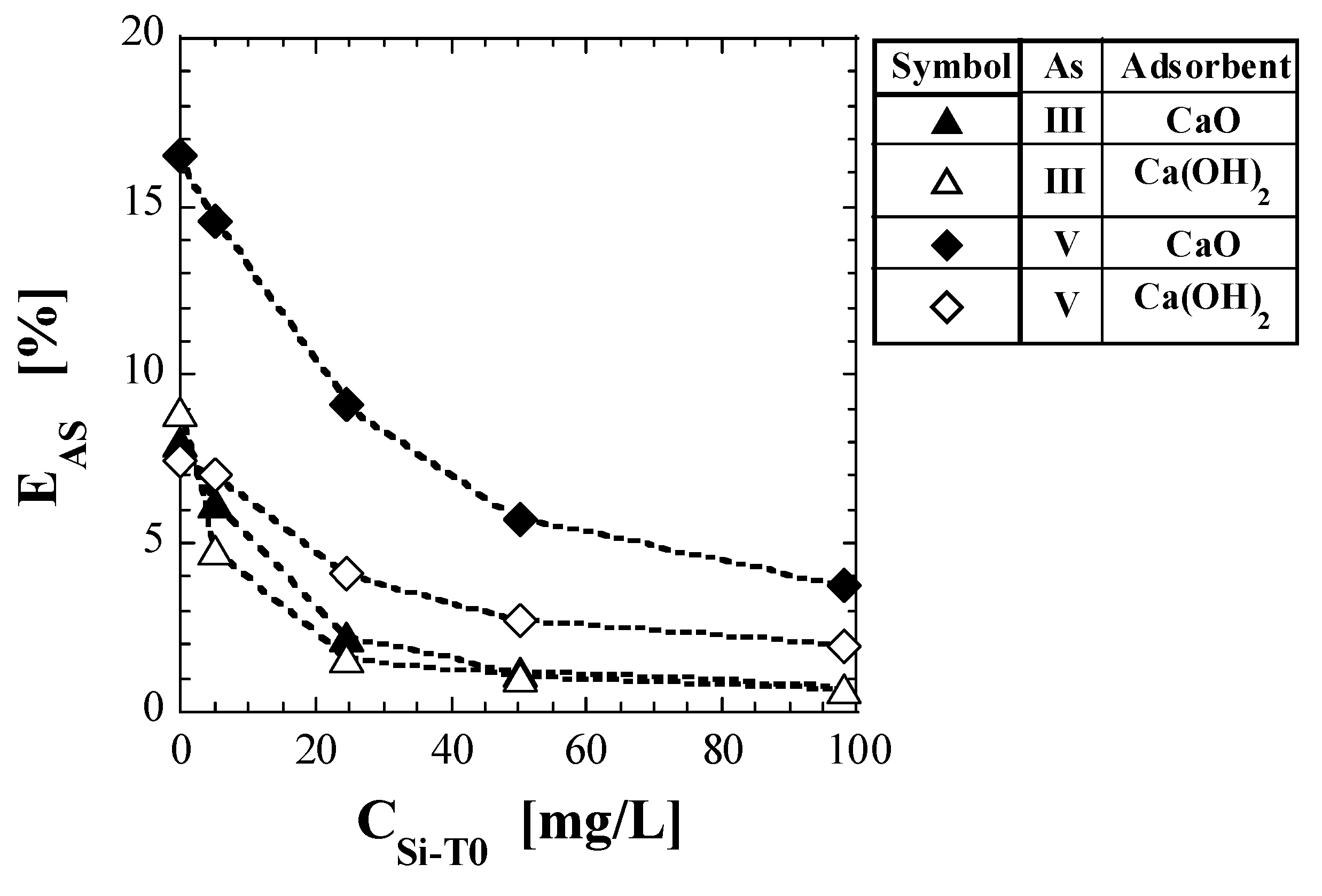
where WSP is the amount of spent adsorbent added to the test solvent [g], V is the liquid volume of the test solvent [L], and WSP/V is the addition concentration of the spent adsorbent [g/L]. Table 9 lists the values of the EAS obtained using Equation (7). Figure 5 shows a plot of EAS against CSi-T0. For comparison with As(III), the EAS for spent Ca-based adsorbents with As(V), reported in a previous study [41], is also shown in Table 9 and Figure 5. For all spent Ca-based adsorbents, the EAS values decreased significantly with increasing CSi-T0. For As(V), the EAS values of Ca(OH)2 were significantly lower than those of CaO, regardless of CSi-T0. For As(III), there was no significant difference between the EAS values of CaO and Ca(OH)2. EAS, which was 8–9% when CSi-T0 = 0 mg/L, decreased to less than 0.7% when CSi-T0 = 100 mg/L. On the other hand, the values of EAS for CaO and Ca(OH)2 when CSi-T0 = 100 mg/L were 3.78 and 1.94% for As(V), respectively, and both were less than 0.7% for As(III). Therefore, it appeared to be difficult for As leaching to occur in spent Ca-based adsorbents with As(III), compared to those with As(V) (Figure 5).
EAS = CAS/(QAS × WSP/V) × 100

Table 9.
Values of As leaching ratio (EAS).
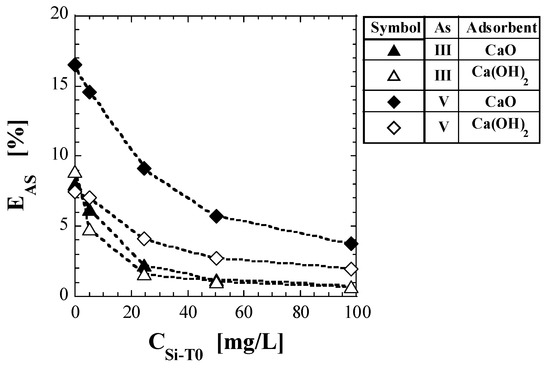
Figure 5.
Change in As leaching ratio with initial total Si-normalized concentration.
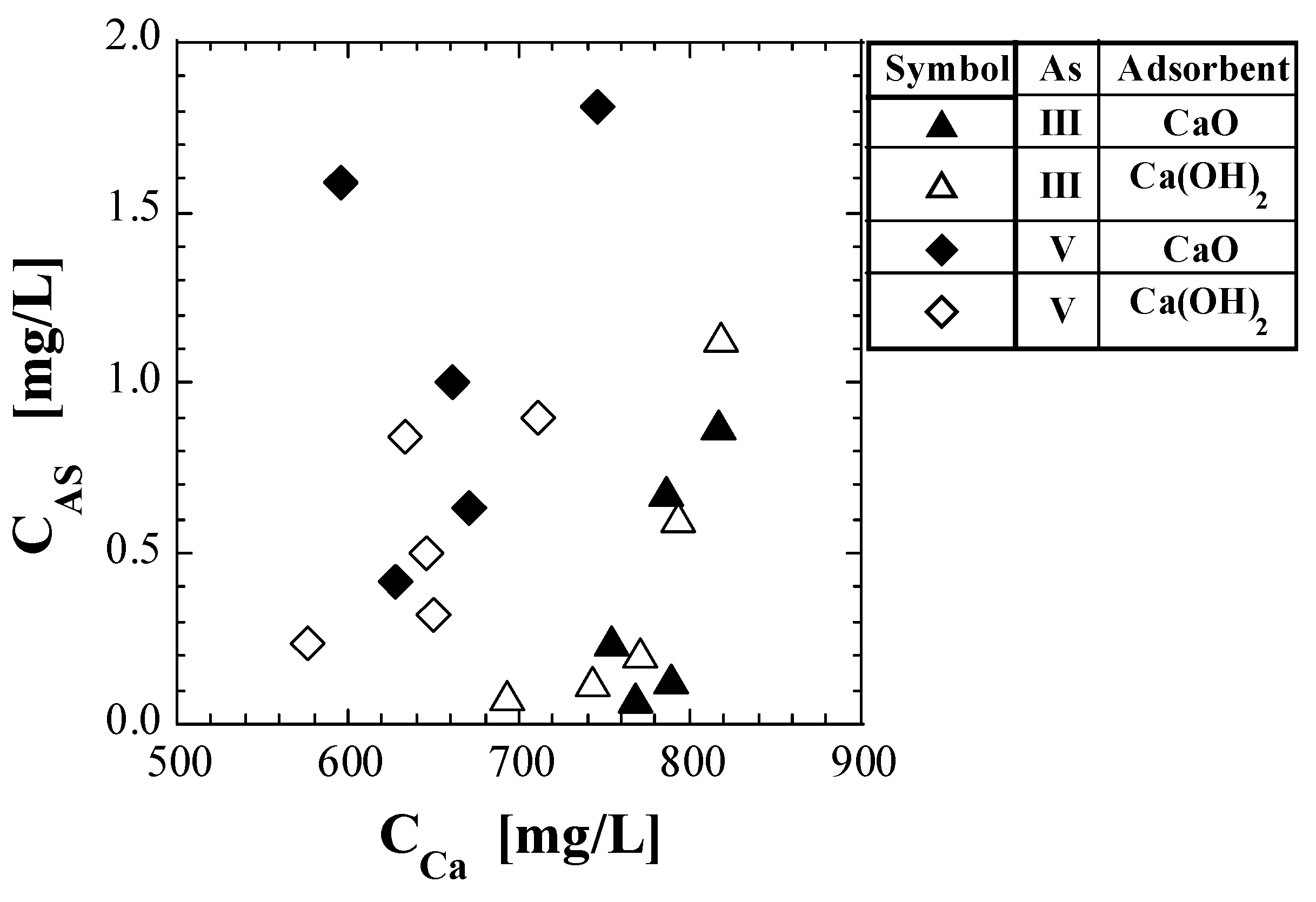
If the leaching of As from the spent adsorbents is simply due to the dissolution of the spent adsorbent, there should be a quantitative correlation between the leaching amount of As and that of Ca. To test this hypothesis, CAS was plotted against CCa (Figure 6). In the set of As(III) and Ca(OH)2, CAS increased with increasing CCa. However, in the other sets, there was no clear correlation between the CAS and CCa. A previous study [41] concluded that the mechanism of As leaching from spent Ca-based adsorbents with As(V) is the desorption reaction of arsenate on its surface. Considering the results in Figure 6, the mechanism of As leaching from the spent Ca-based adsorbents with As(III) is presumed to be the desorption reaction of arsenite.
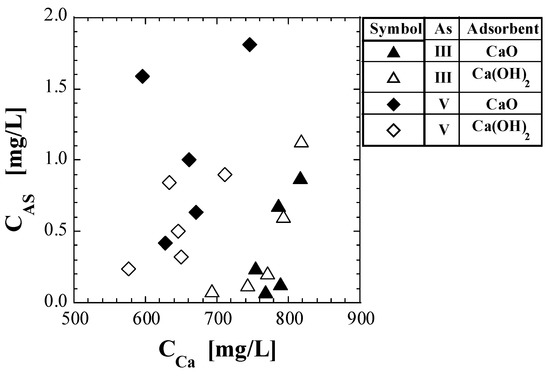
Figure 6.
Plot of As concentration against Ca concentration in eluate.
4.2. Dissolved Forms of Siliacic Acid in Liquid
Similar to previous studies [40,41], the dissolved form of silicic acid in liquid was considered based on the dissociation formulas of silicic acid.
H4SiO4 ←→ H3SiO4− + H+
H3SiO4− ←→ H2SiO42− + H+
When the equilibrium constants of the dissociation Equations (8) and (9) are denoted as Ka1 and Ka2, respectively, the following equations are applicable:
where the units of the applicable molecular formula are mol/L, pKa1 = 9.86, and pKa2 = 13.1 (25 °C) [43].
[H3SiO4−]/[H4SiO4] = 10 exp (pH − pKa1)
[H2SiO42−]/[H3SiO4-] = 10 exp (pH − pKa2)
The values of pHf quantified via the shaking tests on As(III) are in the range 12.45–12.66 (see Table 5). Substituting these values into Equation (10) yields values in the range of 389–631. When converted to the ionization ratio, these values corresponded to a range of 99.7–99.8%. This means that most of the silicic acid in the solution was ionized. Substituting the values of pHf into Equation (11) yields values of 0.22–0.36. When converted to a percentage, this corresponds to 73–82%. This means that the main dissolved form is H3SiO4−, which is a monovalent ion, with the remaining 18–27% being H2SiO42−, a divalent ion. A previous study on As(V) reported that 78–84% of the dissolved form was H3SiO4− and 16–22% was H2SiO42− [40,41]. The difference in the analytical values for As(III) and As(V) is due to the difference in pHf. In addition, in the test solvent before adding the spent adsorbent, the ionization ratio of silicic acid was estimated to be 0.1–0.4%. Therefore, most of the silicic acid in the test solvent is considered to exist as an uncharged monosilicic acid [40,41].
4.3. Dissolved Forms of Arsenous Acid in Liquid
As(III) in the spent Ca-based adsorbents is presumed to be adsorbed (immobilized) on the solid surface as follows: Solid-O-Ca-O-As(OH)2 followed by a reaction wherein As(III) on the surface of the adsorbent is desorbed in a neutral or alkaline solution, as represented by Equations (12) and (13), respectively.
Solid-O-Ca-O-As(OH)2 + H2O → Solid-O-Ca-OH + As(OH)3
Solid-O-Ca-O-As(OH)2 + OH− → Solid-O-Ca-O-As(OH)2 + As(OH)2O−
In addition, the dissolved form of As(III) leached from the spent adsorbent is represented by the following dissociation reaction formulas for arsenous acid:
where pKa1 = 9.1, pKa2 = 12.1, and pKa3 = 13.4 (25 °C) [43]. In this study, as pKa1 << pHf, the abundance of H3AsO3 was considered to be extremely small and negligible. The values of pKa2 and pKa3 were applied to Equations (15) and (16), respectively. Then, substituting the pHf values (12.45–12.66), the values of (HAsO32−)/(H2AsO3−) and (AsO33−)/(HAsO32−) were calculated to be 2.24–3.63 and 0.11–0.18, respectively. Based on the results, the main dissolved form of As leached from the spent Ca-based adsorbents was presumed to be HAsO32−, a hydrogen arsenite ion.
H3AsO3 ←→ H2AsO3− + H+
H2AsO3− ←→ HAsO32− + H+
HAsO32− ←→ AsO33− + H+
It is possible that the valence of As may change during the shaking test, however, in this study neither oxidizing nor reducing agents were used, and the shaking time was only one day. Therefore, we assumed that the valence of As did not change between them.
4.4. Stoichiometric Considerations on Spent Ca-Based Adsorbents
Similar to previous studies [40,41], stoichiometric considerations were made using the values obtained by converting the mass-based concentration CX (mg/L) to the molar-based concentration MX [mmol/L].
4.4.1. Formation Reactions of Calcium Silicate Species
The dissolution reactions of the base materials of the spent Ca-based adsorbents in water are expressed by Equations (17) and (18), respectively:
CaO + H2O → Ca(OH)2
Ca(OH)2 → Ca2+ + 2OH−
In this study, CaO was assumed to dissolve after being converted to Ca(OH)2 by the hydration reaction represented by Equation (17). The Ca2+ leached from the Ca-based adsorbents may then react with the silicic acid species in the solution to form calcium silicate species. The silicic acid species present in the pH range of the test solution in this study were mainly H4SiO4 and H3SiO4− (see Section 4.2); therefore, the silicic acid species that react with Ca2+ to produce calcium silicate species were assumed to be the two aforementioned silicic acid species. Assuming H4SiO4 as the reactive silicic acid species, the reaction formulas for the formation of the calcium silicate species are as follows:
Ca2+ + 2H4SiO4 + 2OH− → CaSi2O2(OH)6 + 2H2O → CaSi2O5 + 5H2O
Ca2+ + H4SiO4 + 2OH− → CaSiO(OH)4 + H2O → CaSiO3 + 3H2O
2Ca2+ + H4SiO4 + 4OH− → Ca2SiO2(OH)4 + 2H2O → Ca2SiO4 + 4H2O
3Ca2+ + H4SiO4 + 6OH− → Ca3SiO3(OH)4 + 3H2O → Ca3SiO5 + 5H2O
4Ca2+ + H4SiO4 + 8OH− → Ca4SiO4(OH)4 + 4H2O → Ca4SiO6 + 6H2O
Assuming that one Ca atom is chemically bonded to two O atoms and that one Si atom is chemically bonded to four O atoms, the formation of calcium silicate species with a Ca/Si molar ratio of 0.5–4 is stoichiometrically conceivable, as shown by Equations (19)–(23). In addition, when H3SiO4− is assumed to be the reactive silicic acid species, “H4SiO4” on the left side of the above formulas must be replaced with “H3SiO4− + H+”. In addition to the above reactions, the silicic acid species in the solution may be adsorbed upon reacting with the OH groups on the adsorbent surface, as shown in Equation (24); this reaction is essentially a dehydration–condensation reaction:
Solid-O-Ca-OH + H4SiO4 → Solid-O-Ca-O-Si(OH)3 + H2O.
4.4.2. Estimation of Calcium Silicate Species Formed
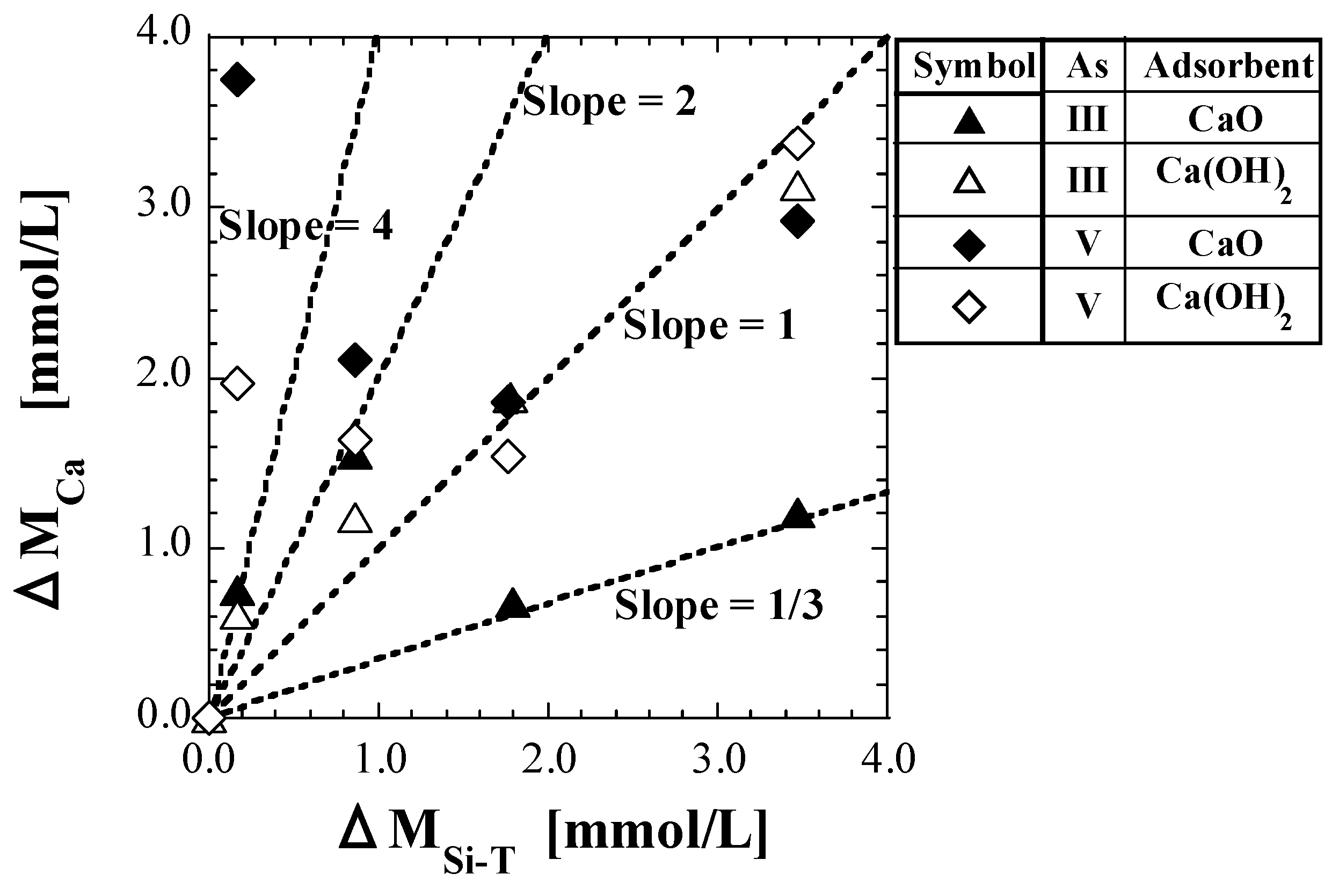
As described at the beginning of Section 4.4, CCa in mg/L converted into molar units is denoted as MCa, mmol/L. The value of MCa at CSi-T0 = 0 mg/L is denoted as MCa0, and the difference between MCa0 and MCa, MCa0 − MCa, is denoted as ΔMCa. Similarly, for CSi-T, the value of MSi-T at CSi-T0 = 0 mg/L is denoted as MSi-T0, and the difference between MSi-T0 and MSi-T, MSi-T0 − MSi-T, is denoted as ΔMSi-T. Figure 7 shows the plots of ΔMCa against ΔMSi-T. In addition, Table 10 shows the values of ΔMCa/ΔMSi-T corresponding to the plot lines.
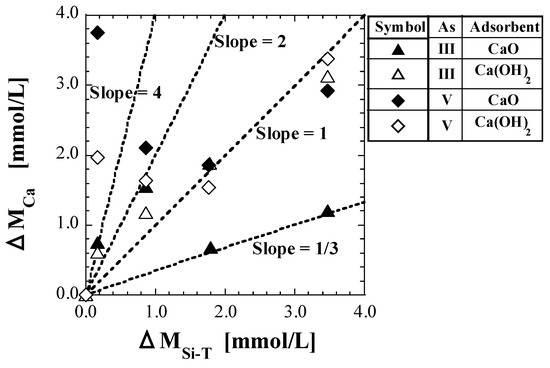
Figure 7.
Plot of ΔMCa against ΔMSi-T.

Table 10.
Values of ΔMCa/ΔMSi-T.
If the only cause of the decrease in both MCa and MSi-T was the production of the calcium silicate species caused by a reaction between Ca2+ leached from the spent adsorbent and the silicic acid species in the solution, the value of ΔMCa/ΔMSi-T should be between 0.5 and 4, based on Equations (19)−(23). Most of ΔMCa/ΔMSi-T were found to be in the range 0.5–4, as shown in Table 10. For As(III), because the values of ΔMCa/ΔMSi-T at CSi-T0 = 5 mg/L are approximately 4 for both CaO and Ca(OH)2, the average composition of the calcium silicate species produced can be estimated to be Ca4SiO6. In addition, because the values at CSi-T0 = 25 mg/L were approximately 1.5, the coexistence of Ca2SiO4 and CaSiO3 could be estimated. Furthermore, because the values at CSi-T0 = 50 and 100 mg/L for Ca(OH)2 are approximately 1, the average composition of the calcium silicate species produced can be estimated to be CaSiO3. On the other hand, the values at CSi-T0 = 50 and 100 mg/L for CaO are 0.3–0.4, which is close to 1/3. This implies that CaSi3O7 has a chemical composition, and the hydration form can be represented as “Si(OH)3O-Ca-O-Si(OH)2O-Si(OH)3.” To produce this calcium silicate species, the polymerization of silicic acid must have occurred. However, as there was no significant difference in pH and CSi-T0 compared to the other test conditions, it may not be theoretically conceivable that the polymerization of silicic acid occurred only in the set of As(III) and CaO. Therefore, CaO, reactions in which only silicic acid species decrease in the solution without decreasing Ca2+ concentration should be considered.
Replacing the hydration reaction of CaO with that of the surface layer of the adsorbent, Equation (17) can be rewritten as Equation (25):
Solid-O-Ca-O-Ca-O-Solid + H2O → Solid-O-Ca-OH + HO-Ca-O-Solid.
Assuming that the adsorption of silicic acid species on the spent adsorbent of CaO occurs after the reaction of Equation (25), there is essentially no mechanistic difference between the reaction of CaO with silicic acid species and reaction of Ca(OH)2 with silicic acid species. However, as mentioned above, for CaO there must be some reaction in which only silicic acid species decrease without decreasing the concentration of Ca2+ in the solution. Therefore, assuming the mechanisms by which silicic acid species react directly with unhydrated CaO seems to be more appropriate. Equations (26) and (27) are proposed as the reaction of silicic acid species with a solid surface layer of CaO:
Solid-O-Ca-O-Ca-O-Solid + H4SiO4 → Solid-O-Ca-O-Si(OH)3 + HO-Ca-O-Solid
Solid-O-Ca-O-Ca-O-Solid + H4SiO4 → Solid-O-Ca-O-Si(OH)2O-Solid + Ca2+ + 2OH−.
According to the reaction shown in Equation (26), the silicic acid species in the solution are directly adsorbed on the surface layer of the CaO adsorbent without reacting with Ca2+ in the solution. According to the reaction shown in Equation (27), the silicic acid species are adsorbed with the release of Ca2+ from the surface of the adsorbent.
Similar discussions on As(V) have been reported in detail in a previous study [41]. The values of ΔMCa/ΔMSi-T at CSi-T0 = 5 mg/L for As(V) were significantly different from those for As(III) and greatly exceeded 4, at more than 20 for CaO and more than 10 for Ca(OH)2, as shown in Table 10. In the test solvent containing silicic acid, the leaching of Ca and As might be inhibited to some extent by adsorbing the silicic acid species directly on the surface of the spent adsorbents and covering them. In low Si-normalized concentrations (approximately 5 mg/L), the proportion of Ca being inhibited by the direct adsorption of silicic acid was inferred to be larger than the proportion of Ca2+ that was consumed with silicic acid in the production of calcium silicate species [41].
Because the values of ΔMCa/ΔMSi-T are different between As(III) and As(V), as shown in Figure 7 and Table 10, the differences in the coordination number and dissociation constants of As may be considered to have some effect on the mechanisms of silicic acid adsorption on the adsorbent surface as well as on calcium silicate formation; however, such effects are currently unknown.
4.4.3. Effects of Silicic Acid on Leaching Behavior of As(III)
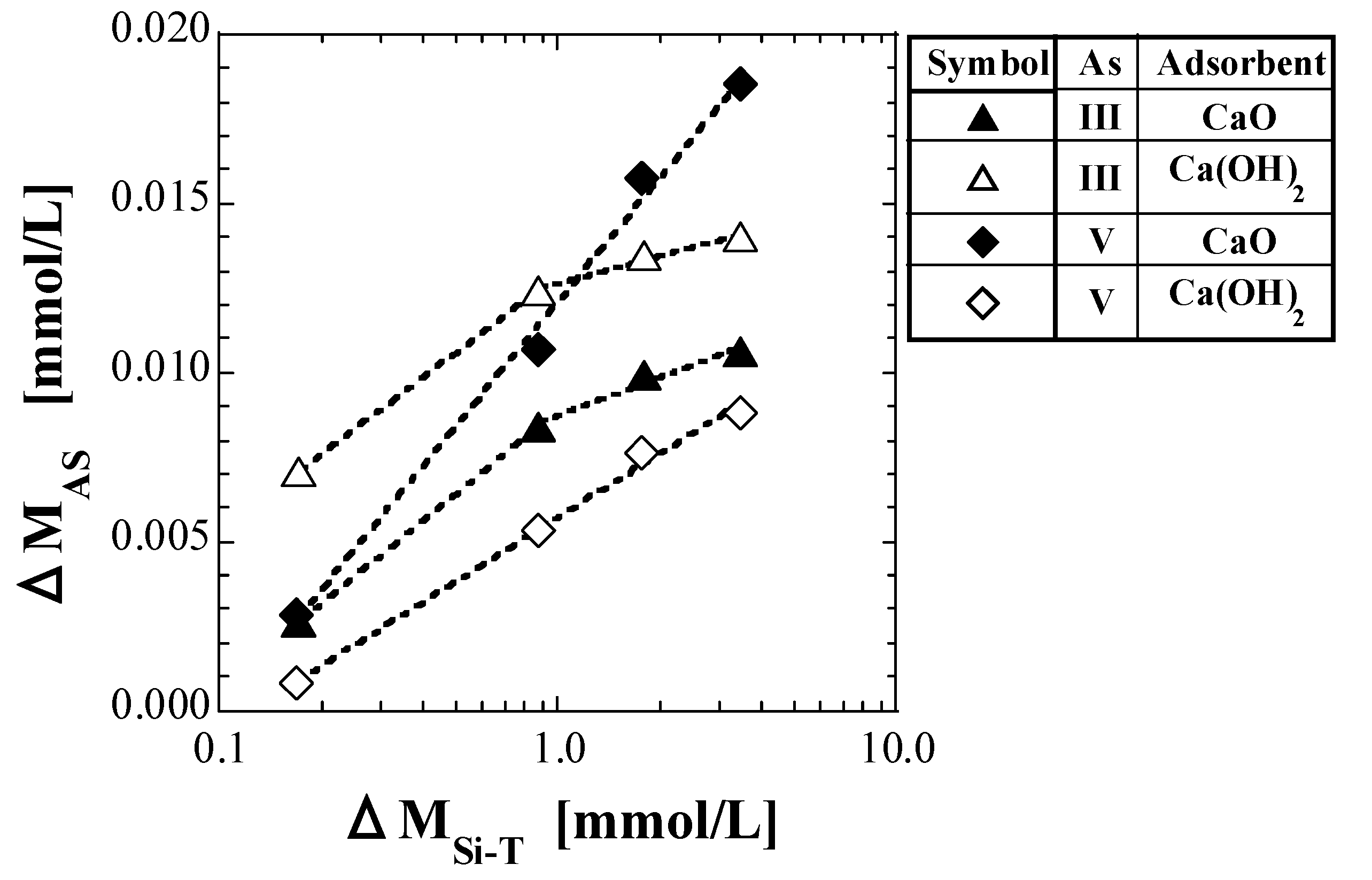
As in Section 4.4.1, CAS in mg/L converted into molar units is denoted as MAS, mmol/L, the value of MAS at CSi-T0 = 0 mg/L is denoted as MAS0, and the difference between MAS0 and MAS (MAS0 − MAS) is denoted as ΔMAS. Figure 8 shows a semi-logarithmic plot of ΔMAS against ΔMSi-T.
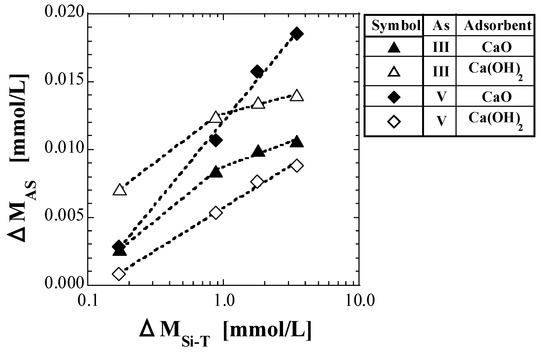
Figure 8.
Plot of ΔMAS against ΔMSi-T.
For As(V), as shown in Figure 8, both CaO and Ca(OH)2 appear to present a proportional relationship between ΔMAS and the logarithmic value of ΔMSi-T. The proportional relationships in Figure 8 are represented by the following equation:
where A and B are the slopes and intercepts of the lines, respectively. Equation (28) indicates that ΔMAS increases with increasing ΔMSi-T.
ΔMAS = A × log ΔMSi-T + B
In a previous study on As(V) [41], the As which leached from the spent adsorbent with As(V) into the solution was speculated to be incorporated in place of some silicic acid species during the formation of calcium silicate species. A similar reaction is considered to have occurred in the current study with As(III); however, both the approximate curves of CaO and Ca(OH)2 for As(III) appear to have a change in slope at around ΔMSi-T = 1 mmol/L. The value of CSi-T0 corresponding to ΔMSi-T = 1 mmol/L in this study was approximately 25 mg/L. Therefore, it is considered that the proportion of As incorporated instead of silicate species during the production of calcium silicate species changed depending on whether CSi-T0 was higher or lower than 25 mg/L. The values of A and B obtained using Equation (28) are listed in Table 11 and Table 12, respectively.

Table 11.
Values of A obtained using Equation (28).

Table 12.
Values of B obtained using Equation (28).
Because the pH of leachate from cementitious solidification materials is generally highly alkaline, there is a concern that the reaction represented by Equation (13) may promote the leaching of As from spent adsorbents. In addition, because a large amount of Ca is leached from spent Ca-based adsorbents, the spent Ca-based adsorbents with As(III) are considered to have an environmental stability as low as those with As(V). However, assuming that the leachate from soil and cementitious solidification materials contains a large amount of silicate and Ca components in advance, the leaching of Ca components from spent Ca-based adsorbents is likely to be inhibited, and thus the environmental stability of the spent adsorbents may be enhanced, as reported in a previous study [41].
5. Conclusions
After being used to purify As-contaminated water, the spent adsorbents contained a large amount of As. When spent adsorbents are discarded in the environment without proper treatment, secondary environmental pollution may be caused by the leaching of As. In a previous study [41], the effects of silicic acid on the environmental stability of spent Ca-based adsorbents with arsenate were reported. In this study, the effects of silicic acid on the environmental stability of spent Ca-based adsorbents with arsenite were examined.
Similar to the case of spent Ca-based adsorbents with arsenate, those with arsenite were strongly affected by silicic acid in the solution, and the amount of As leaching apparently decreased with increasing initial silicic acid concentration. The As concentration in the eluate, which was around 1 in the absence of silicic acid, decreased to less than 0.1 mg/L when the initial Si-normalized concentration was 100 mg/L. The corresponding inhibitory mechanism of silicic acid on As leaching was determined to primarily involve re-immobilization via incorporation of the As leached from the spent adsorbents during the production of the calcium silicate species. In addition, in the solution containing silicic acid the As leaching ratio was clearly lower for the spent Ca-based adsorbents with arsenite than for those with arsenate. The As leaching ratios of CaO and Ca(OH)2 under the initial Si-normalized concentration of 100 mg/L were 3.78 and 1.94% for arsenate, respectively, while they were each less than 0.7% for arsenite. Thus, the spent adsorbents with arsenite demonstrate higher environmental stability than those with arsenate.
This study revealed that the environmental stability of spent Ca-based adsorbents differs depending on the valence of the adsorbed As. Future analyses should utilize chemical equilibrium models to further develop and validate the results presented here.
Author Contributions
Conceptualization, H.S.; methodology, H.S. and J.H.; formal analysis, H.S. and J.H.; investigation, T.O. and H.S.; resources, H.S., M.Z. and J.H.; data curation, T.O. and H.S.; writing—original draft preparation, H.S. and T.O.; writing—review and editing, H.S., T.O., M.Z., J.H. and Y.K.; supervision, H.S. and M.Z.; project administration, M.Z. and Y.K.; funding acquisition, M.Z., Y.K. and H.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data that support the findings of this study are available from the corresponding authors upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- van Geen, A.; Zheng, Y.; Cheng, Z.; Aziz, Z.; Horneman, A.; Dhar, R.K.; Mailloux, B.; Stute, M.; Weinman, B.; Good-bred, S.; et al. A transect of groundwater and sediment properties in Araihazar, Bangladesh: Further evidence of decoupling be-tween As and Fe mobilization. Chem. Geol. 2006, 228, 85–96. [Google Scholar] [CrossRef]
- Harvey, C.F.; Ashfaque, K.N.; Yu, W.; Badruzzaman, A.B.M.; Ali, M.A.; Oates, P.M.; Michael, H.A.; Neumann, R.B.; Beckie, R.; Islam, S.; et al. Groundwater dynamics and arsenic contamination in Bangladesh. Chem. Geol. 2006, 228, 112–136. [Google Scholar] [CrossRef]
- Senanayake, N.; Mukherji, A. Irrigating with arsenic contaminated groundwater in West Bengal and Bangladesh: A review of interventions for mitigating adverse health and crop outcomes. Agric. Water Manag. 2014, 135, 90–99. [Google Scholar] [CrossRef]
- Khan, K.M.; Parvez, F.; Zoeller, R.T.; Hocevar, B.A.; Kamendulis, L.M.; Rohlman, D.; Eunus, M.; Graziano, J. Thyroid hormones and neurobehavioral functions among adolescents chronically exposed to groundwater with geogenic arsenic in Bangladesh. Sci. Total Environ. 2019, 678, 278–287. [Google Scholar] [CrossRef]
- Huq, M.E.; Fahad, S.; Shao, Z.; Sarven, M.S.; Khan, I.A.; Alam, M.; Saeed, M.; Ullah, H.; Adnan, M.; Saud, S.; et al. Arsenic in a groundwater environment in Bangladesh: Occurrence and mobilization. J. Environ. Manag. 2020, 262, 110318. [Google Scholar] [CrossRef]
- Shahid, M.; Niazi, N.K.; Dumat, C.; Naidu, R.; Khalid, S.; Rahman, M.M.; Bibi, I. A meta-analysis of the distribution, sources and health risks of arsenic-contaminated groundwater in Pakistan. Environ. Poll. 2018, 242, 307–319. [Google Scholar] [CrossRef]
- Malik, A.; Parvaiz, A.; Mushtaq, N.; Hussain, I.; Javed, T.; Rehman, H.U.; Farooqi, A. Characterization and role of derived dissolved organic matter on arsenic mobilization in alluvial aquifers of Punjab, Pakistan. Chemosphere 2020, 251, 126374. [Google Scholar] [CrossRef]
- Hamidian, A.H.; Razeghi, N.; Zhang, Y.; Yang, M. Spatial distribution of arsenic in groundwater of Iran, a review. J. Geochem. Explor. 2019, 201, 88–98. [Google Scholar] [CrossRef]
- Chakraborti, D.; Das, B.; Rahman, M.M.; Nayak, B.; Pal, A.; Sengupta, M.K.; Ahamed, S.; Hossain, M.A.; Chowdhury, U.K.; Biswas, B.K.; et al. Arsenic in groundwater of the Kolkata Municipal Corporation (KMC), India: Critical review and modes of mitigation. Chemosphere 2017, 180, 437–447. [Google Scholar] [CrossRef]
- Bhowmick, S.; Pramanik, S.; Singh, P.; Mondal, P.; Chatterjee, D.; Nriagu, J. Arsenic in groundwater of West Bengal, India: A review of human health risks and assessment of possible intervention options. Sci. Total Environ. 2018, 612, 148–169. [Google Scholar] [CrossRef] [PubMed]
- Bindal, S.; Singh, C.K. Predicting groundwater arsenic contamination: Regions at risk in highest populated state of India. Water Res. 2019, 159, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Chandrajith, R.; Diyabalanage, S.; Dissanayake, C.B. Geogenic fluoride and arsenic in groundwater of Sri Lanka and its implications to community health. Groundw. Sustain. Dev. 2020, 10, 100359. [Google Scholar] [CrossRef]
- Hoang, T.H.; Bang, S.; Kim, K.-W.; Nguyen, M.H.; Dang, D.M. Arsenic in groundwater and sediment in the Mekong River delta, Vietnam. Environ. Poll. 2010, 158, 2648–2658. [Google Scholar] [CrossRef]
- Stopelli, E.; Duyen, V.T.; Mai, T.T.; Tran, P.T.K.; Viet, P.H.; Lightfoot, A.; Kipfer, R.; Schneider, M.; Eiche, E.; Kontny, A.; et al. Spatial and temporal evolution of groundwater arsenic contamination in the Red River delta, Vietnam: Interplay of mobilisation and retardation processes. Sci. Total Environ. 2020, 717, 137143. [Google Scholar] [CrossRef] [PubMed]
- Richards, L.A.; Magnone, D.; Sültenfuß, J.; Chambers, L.; Bryant, C.; Boyce, A.J.; van Dongen, B.E.; Ballentine, C.J.; Sovann, C.; Uhlemann, S.; et al. Dual in-aquifer and near surface processes drive arsenic mobilization in Cambodian groundwaters. Sci. Total Environ. 2019, 659, 699–714. [Google Scholar] [CrossRef] [PubMed]
- Wen, D.; Zhang, F.; Zhang, E.; Wang, C.; Han, S.; Zheng, Y. Arsenic, fluoride and iodine in groundwater of China. J. Geochem. Explor. 2013, 135, 1–21. [Google Scholar] [CrossRef] [Green Version]
- Guo, H.; Wen, D.; Liu, Z.; Jia, Y.; Guo, Q. A review of high arsenic groundwater in Mainland and Taiwan, China: Distribution, characteristics and geochemical processes. Appl. Geochem. 2014, 41, 196–217. [Google Scholar] [CrossRef]
- Zhang, L.; Qin, X.; Tang, J.; Liu, W.; Yang, H. Review of arsenic geochemical characteristics and its significance on arsenic pollution studies in karst groundwater, South-west China. Appl. Geochem. 2017, 77, 80–88. [Google Scholar] [CrossRef]
- Mariño, E.E.; Ávila, G.T.; Bhattacharya, P.; Schulz, C.J. The occurrence of arsenic and other trace elements in groundwaters of the southwestern Chaco-Pampean plain, Argentina. J. S. Am. Earth Sci. 2020, 100, 102547. [Google Scholar] [CrossRef]
- Alcaine, A.A.; Schulz, C.; Bundschuh, J.; Jacks, G.; Thunvik, R.; Gustafsson, J.-P.; Mörth, C.-M.; Sracek, O.; Ahmada, A.; Bhattacharya, P. Hydrogeochemical controls on the mobility of arsenic, fluoride and other geogenic co-contaminants in the shallow aquifers of northeastern La Pampa Province in Argentina. Sci. Total Environ. 2020, 715, 136671. [Google Scholar] [CrossRef] [PubMed]
- Machado, I.; Falchi, L.; Bühl, V.; Mañay, N. Arsenic levels in groundwater and its correlation with relevant inorganic parameters in Uruguay: A medical geology perspective. Sci. Total Environ. 2020, 721, 137787. [Google Scholar] [CrossRef] [PubMed]
- Navarro, O.; González, J.; Júnez-Ferreira, H.E.; Bautista, C.-F.; Cardona, A. Correlation of Arsenic and Fluoride in the Groundwater for Human Consumption in a Semiarid Region of Mexico. Procedia Eng. 2017, 186, 333–340. [Google Scholar] [CrossRef]
- Gómez-Hernández, A.; Rodríguez, R.; Río, A.L.; Ruiz-Huerta, E.A.; Armienta, M.A.; Dávila-Harris, P.; Sen-Gupta, B.; Delgado-Rodríguez, O.; Ríos, A.D.A.; Martínez-Villegas, N. Alluvial and gypsum karst geological transition favors spreading arsenic contamination in Matehuala, Mexico. Sci. Total Environ 2020, 707, 135340. [Google Scholar] [CrossRef]
- Bretzler, A.; Lalanne, F.; Nikiema, J.; Podgorski, J.; Pfenninger, N.; Berg, M.; Schirmer, M. Groundwater arsenic contamination in Burkina Faso, West Africa: Predicting and verifying regions at risk. Sci. Total Environ. 2017, 584–585, 958–970. [Google Scholar] [CrossRef] [PubMed]
- Abiye, T.A.; Bhattacharya, P. Arsenic concentration in groundwater: Archetypal study from South Africa. Groundw. Sustain. Dev. 2019, 9, 100246. [Google Scholar] [CrossRef]
- Podgorski, J.; Berg, M. Global threat of arsenic in groundwater. Science 2020, 368, 845–850. [Google Scholar] [CrossRef] [PubMed]
- Shaji, E.; Santosh, M.; Sarath, K.V.; Prakash, P.; Deepch, V.; Divy, B.V. Arsenic contamination of groundwater: A global synopsis with focus on the Indian Peninsula. Geosci. Front. 2021, 13, 101079. [Google Scholar] [CrossRef]
- Jadhav, S.V.; Bringas, E.; Yadav, G.D.; Rathod, V.K.; Ortiz, I.; Marathe, K.V. Arsenic and fluoride contaminated groundwaters: A review of current technologies for contaminants removal. J. Environ. Manag. 2015, 162, 306–325. [Google Scholar] [CrossRef]
- Sarkar, A.; Paul, B. The global menace of arsenic and its conventional remediation-A critical review. Chemosphere 2016, 158, 37–49. [Google Scholar] [CrossRef]
- Ghosh, S.; Debsarkar, A.; Dutta, A. Technology alter-natives for decontamination of arsenic-rich groundwater—A critical review, Environ. Technol. Innov. 2019, 13, 277–303. [Google Scholar] [CrossRef]
- Kumar, R.; Patel, M.; Singh, P.; Bundschuh, J.; Pittman, C.U., Jr.; Trakal, L.; Mohan, D. Emerging technologies for arsenic removal from drinking water in rural and peri-urban areas: Methods, experience from, and options for Latin America. Sci. Total Environ. 2019, 694, 133427. [Google Scholar] [CrossRef] [PubMed]
- Opiso, E.M.; Sato, T.; Morimoto, K.; Asai, A.; Anraku, S.; Numako, C.; Yoneda, T. Incorporation of arsenic during the formation of Mg-bearing minerals at alkaline condition. Miner. Eng. 2010, 23, 230–237. [Google Scholar] [CrossRef]
- Park, Y.Y.; Tran, T.; Lee, Y.H.; Nam, Y., II; Senanayake, G.; Kim, M.J. Selective removal of arsenic(V) from a molybdate plant liquor by precipitation of magnesium arsenate. Hydrometallurgy 2010, 104, 290–297. [Google Scholar] [CrossRef] [Green Version]
- Tresintsi, S.; Simeonidis, K.; Katsikini, M.; Paloura, E.C.; Bantsis, G.; Mitrakas, M. A novel approach for arsenic adsorbents regeneration using MgO. J. Hazard. Mater. 2014, 265, 217–225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Camacho, J.; Wee, H.-Y.; Kramer, T.A.; Autenrieth, R. Arsenic stabilization on water treatment residuals by calcium addition. J. Hazard. Mater. 2009, 165, 599–603. [Google Scholar] [CrossRef] [PubMed]
- Montes-Hernandeza, G.; Concha-Lozan, N.; Renard, F.; Quirico, E. Removal of oxyanions from synthetic wastewater via carbonation process of calcium hydroxide: Applied and fundamental aspects. J. Hazard. Mater. 2009, 166, 788–795. [Google Scholar] [CrossRef]
- Olyaie, E.; Banejad, H.; Afkhami, A.; Rahmani, A.; Khodaveisi, J. Development of a cost-effective technique to remove the arsenic contamination from aqueous solutions by calcium peroxide nanoparticles. Sep. Purif. Technol. 2012, 95, 10–15. [Google Scholar] [CrossRef]
- Hua, C.-Y.; Lo, S.-L.; Kuan, W.-H. High concentration of arsenate removal by electrocoagulation with calcium. Sep. Purif. Technol. 2014, 126, 7–14. [Google Scholar] [CrossRef]
- Sugita, H.; Oguma, T.; Zhang, M.; Hara, J.; Takahashi, S. Environmental stability of spent magnesium-based and calcium-based arsenic adsorbents-Effects of soils. J. Jpn. Soc. Civ. Eng. Ser. G Environ. Res. 2016, 72, 437–448. [Google Scholar] [CrossRef] [Green Version]
- Sugita, H.; Oguma, T.; Zhang, M.; Hara, J.; Kawabe, Y. Effects of silicic acid on environmental stability of spent magnesium-based arsenic adsorbents. J. Jpn. Soc. Civ. Eng. Ser. G Environ. Res. 2017, 73, 407–418. [Google Scholar] [CrossRef]
- Sugita, H.; Oguma, T.; Zhang, M.; Hara, J.; Kawabe, Y. Effects of silicic acid on environmental stability of spent calcium-based arsenic adsorbents. J. Jpn. Soc. Civ. Eng. Ser. G Environ. Res. 2018, 74, 493–502. [Google Scholar] [CrossRef]
- Sugita, H.; Oguma, T.; Hara, J.; Zhang, M.; Kawabe, Y. Effects of solution pH on spent Mg-based and Ca-based adsorbents -leaching behavior of arsenite and arsenate acids. Jpn. Geotech. J. 2020, 15, 441–453. [Google Scholar] [CrossRef]
- The Chemical Society of Japan (CSJ). Kagaku Binran (Handbook of Chemistry), Pure Chemistry II, 4th ed.; Maruzen: Tokyo, Japan, 1993; p. 317. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).