Steel Slag and Autoclaved Aerated Concrete Grains as Low-Cost Adsorbents to Remove Cd2+ and Pb2+ in Wastewater: Effects of Mixing Proportions of Grains and Liquid-to-Solid Ratio
Abstract
1. Introduction
2. Materials and Methods
2.1. Adsorbents Preparation and Characterization
2.2. Procedures of Batch Experiments
2.2.1. Adsorption Isotherms for Cd2+ and Pb2+ in a Single-Metal Solution
2.2.2. Effect of Competitive Metal Ions on Cd2+ and Pb2+ Adsorption in Binary Metal Solution
2.2.3. Effect of Competitive Metal Ions on Cd2+ and Pb2+ Adsorption in Multi-Metal Solutions
3. Results and Discussion
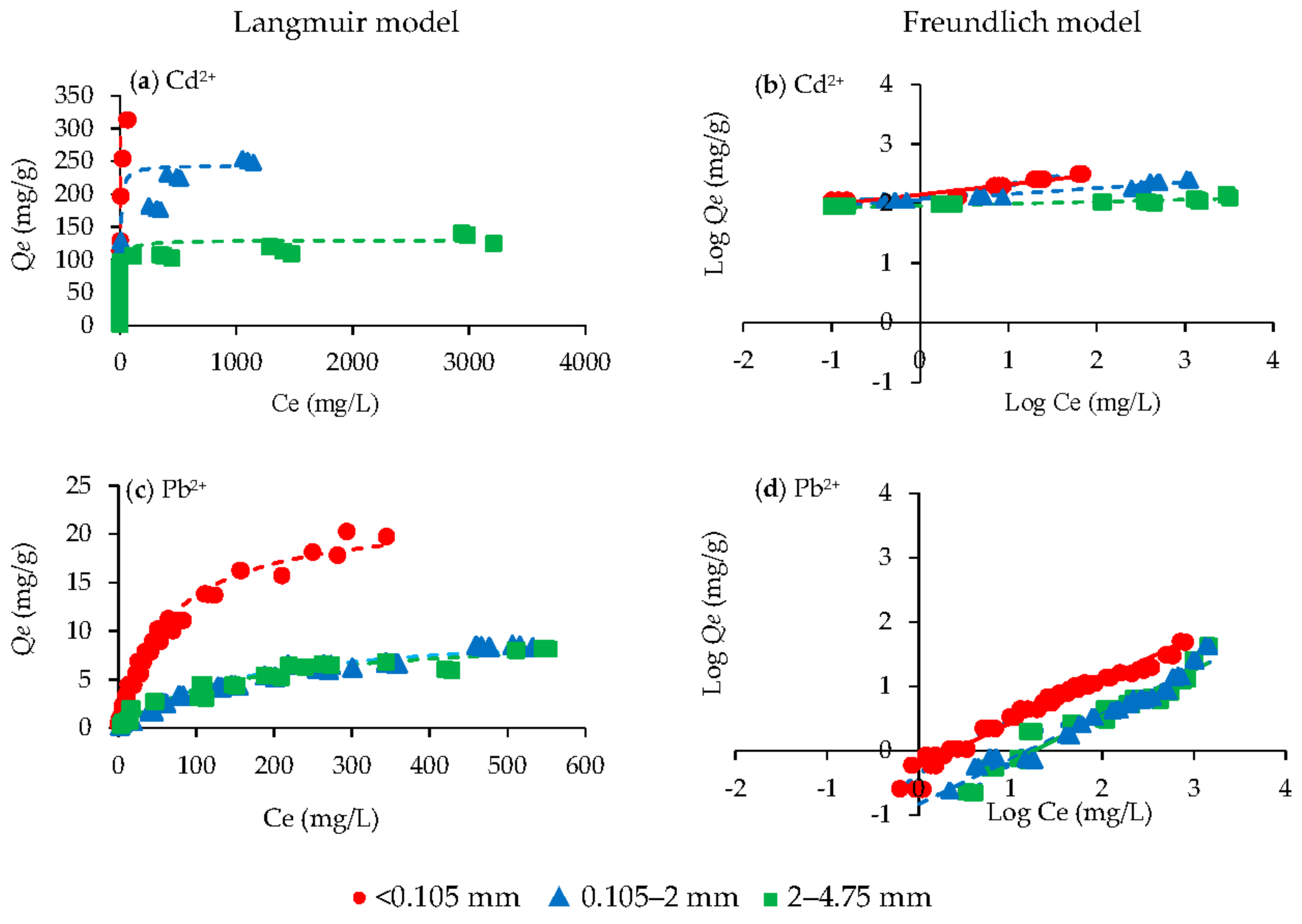
3.1. Adsorption Isotherms for Cd2+ and Pb2+ in Single Metal Solution
| Category | Adsorbent | Particle Size (mm) | BET Surface Area (m2/g) | Liquid-to-Solid Ratio (L/S) | pH Range | Cd2+ | Pb2+ | Ref. | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Ci Range (mg/L) | Qm (mg/g) | Ci Range (mg/L) | Qm (mg/g) | |||||||
| Industrial by-products (IBPs) (including construction and demolition waste) | Steel slag (SS) | 0.105–2 | 4.9 | 60 | 10–12 | 0–5000 | 130–313 | 0–1500 | 8.2–17.5 | This study |
| Fly ash | 0.01–0.02 | 2.8 | 50–10,000 | 2–10 | 0–5 | 3.8 | 0–5 | 5.1 | [35] | |
| Blast furnace slag | 0.15–0.3 | 1.1 | 50–10,000 | 2–10 | 0–5 | 5.1 | 0–5 | 4.9 | ||
| Arc furnace slag | 0.9–2 | 3.42 | 100 | 2–8 | 22.5–360 | 6.5 | 41–662 | 25 | [33] | |
| Autoclaved aerated concrete | 0.105–2 | 23.6 | 60 | 8–10 | 25–2000 | 16.5 | 25–2000 | 257 | [61] | |
| Crushed concrete fines | 0.105–2 | 11.2 | 60 | 8–11 | 25–2000 | 22.8 | 25–2000 | 129 | ||
| Crushed clay bricks | 0.105–2 | 15.9 | 10 | 6–7 | 0–2000 | 3.2 | 0–2000 | 5.5 | [79] | |
| Municipal solid waste slag | 0.105–2 | - | 10 | 8–9 | 0–2000 | 2.3 | 0–2000 | 3.3 | ||
| Cellular concrete | 1.5–2.5 | 177 | 1428 | 6.5–7.2 | 10–200 | 28.7 | 10–200 | 157 | [77] | |
| Geo-sorbents | Zeolite | - | 15.4 | 40–1000 | 6.5–6.7 | 0–50 | 6.72 | 0–50 | 9.97 | [43] |
| Ca-bentonite | <0.063 | 87 | 1000 | ≈5 | 1–100 | 31.3 | 5–150 | 85.5 | [80] | |
| Sepiolite | <0.1 | - | 100 | - | 50–600 | 19.2 | 50–600 | 50 | [81] | |
| Limestone | Powder | 119 | 6667 | 2–6 | 1–200 | 52.9 | 1–200 | 184 | [82] | |
| Montmorillonite | - | - | 100 | ≈2 | 10–500 | 1.2 | 10–500 | 3.25 | [83] | |
| Bio-sorbents | Coconut coir husk | <0.35 | 1.83 | 400 | ≈7 | 2.5–200 | 47.3 | 25–200 | 66.1 | [30] |
| Coconut husk/shell | 0.075 | 212 | 10 | ≈8.8 | 100–2000 | 3.5 | 100–2000 | 13.4 | [31] | |
| Biochar | 0.15 | 51.2 | 500 | 5–7 | 50–900 | 75 | 50–900 | 129 | [84] | |
| Green algae | 1–1.5 | - | 125 | 2–6 | 22–382 | 39.2 | 41–704 | 74.3 | [85] | |
| Lemon berry | 0.15 | - | 100 | 4.8–5 | 110–2800 | 129 | 200–5200 | 310 | [86] | |
3.2. Removal of Cd2+ and Pb2+ in Binary Metal Solution
3.3. Removal of Cd2+ and Pb2+ in Multi-Metal Solutions and Selectivity Sequence
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AAC | autoclaved aerated concrete |
| b | Langmuir constant (g/L) |
| BET | Brunauer–Emmett–Teller |
| CDW | construction and demolition waste |
| Ce | equilibrium concentration (mg/L) |
| Ci | initial concentration (mg/L) |
| DI | deionized water |
| EC | electrical conductivity (mS/cm) |
| EDX | energy-dispersive X-ray spectroscopy |
| Gs | specific gravity (-) |
| IBPs | industrial by-products |
| Kf | Freundlich adsorption capacity (L/g) |
| LOI | loss on ignition (%) |
| L/S | liquid-to-solid ratio |
| n | adsorption intensity (-) |
| pHe | equilibrium pH |
| Qe | equilibrium adsorption (mg/g) |
| Qm | maximum adsorption capacity (mg/g) |
| R | removal percentage (%) |
| SS | steel slag |
| SSA | surface area (m2/g) |
| wAD | gravimetric water content at air-dry [g/g, in %] |
| XRD | X-ray diffractometry |
References
- Petrella, A.; Spasiano, D.; Acquafredda, P.; De Vietro, N.; Ranieri, E.; Cosma, P.; Rizzi, V.; Petruzzelli, V.; Petruzzelli, D. Heavy metals retention (Pb(II), Cd(II), Ni(II)) from single and multi-metal solutions by natural bio-sorbents from the olive oil milling operations. Process Saf. Environ. Prot. 2018, 114, 79–90. [Google Scholar] [CrossRef]
- Rad, L.R.; Momeni, A.; Ghazani, B.F.; Irani, M.; Mahmoudi, M.; Noghreh, B. Removal of Ni2+ and Cd2+ ions from aqueous solutions using electrospun PVA/zeolite nano-fibrous adsorbent. Chem. Eng. J. 2014, 256, 119–127. [Google Scholar] [CrossRef]
- Sellaoui, L.; Dotto, G.L.; Lamine, A.B.; Erto, A. Interpretation of single and competitive adsorption of cadmium and zinc on activated carbon using monolayer and exclusive extended monolayer models. Environ. Sci. Pollut. Res. 2017, 24, 19902–19908. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Demey, H.; Vincent, T.; Guibal, E. A novel algal-based sorbent for heavy metal removal. Chem. Eng. J. 2018, 332, 582–595. [Google Scholar] [CrossRef]
- Singh, O.V.; Labana, S.; Pandey, G.; Budhiraja, R.; Jain, R.K. Phytoremediation: An overview of metallic ion decontamination from soil. Appl. Microb. Biotech. 2013, 61, 405–412. [Google Scholar] [CrossRef]
- Duan, Q.; Lee, J.; Liu, Y.; Chen, H.; Hu, H. Distribution of heavy metal pollution in surface soil samples in China: A graphical review. Bull. Environ. Contam. Toxicol. 2016, 97, 303–309. [Google Scholar]
- Wu, G.; Kang, H.; Zhang, X.; Shao, H.; Chu, L.; Ruan, C. A critical review on the bio-removal of hazardous heavy metals from contaminated soils: Issues, progress, eco-environmental concerns and opportunities. J. Hazard. Mater. 2010, 174, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Elouear, Z.; Bouzid, J.; Boujelben, N. Removal of nickel and cadmium from aqueous solutions by sewage sludge ash: Study in single and binary systems. Environ. Technol. 2009, 30, 561–570. [Google Scholar] [CrossRef]
- Bertrand, P.S.J.; Silvestre, V.; Pinell, V. Lead-induced DNA damage in Vicia faba root cells: Potential involvement of oxidative stress. Mutat. Res./Genet. Toxicol. Environ. Mutagenesis 2011, 726, 123–128. [Google Scholar]
- Fu, F.; Wang, Q. Removal of heavy metal ions from wastewaters: A review. J. Environ. Manag. 2011, 92, 407–418. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Cao, Q.; Zheng, Y.M.; Huang, Y.Z.; Zhu, Y.G. Health risks of heavy metals in contaminated soils and food crops irrigated with wastewater in Beijing, China. Environ. Pollut. 2008, 152, 686–692. [Google Scholar] [CrossRef]
- Hui, S.; Chao, H.; Kot, C. Removal of mixed heavy metal ions in wastewater by zeolite 4A and residual products from recycled coal fly ash. J. Hazard. Mater. 2005, 127, 89–101. [Google Scholar] [CrossRef]
- Huisman, J.L.; Schouten, G.; Schultz, C. Biologically produced sulphide for purification of process streams, effluent treatment and recovery of metals in the metal and mining industry. Hydrome 2006, 83, 106–113. [Google Scholar] [CrossRef]
- Blöcher, C.; Dorda, J.; Mavrov, V.; Chmiel, H.; Lazaridis, N.K.; Matis, K.A. Hybrid flotation membrane filtration process for the removal of heavy metal ions from wastewater. Water Res. 2003, 37, 4018–4026. [Google Scholar] [CrossRef]
- Chan, B.K.C.; Dudeney, A.W.L. Reverse osmosis removal of arsenic residues from bioleaching of refractory gold concentrates. Miner. Eng. 2008, 21, 272–278. [Google Scholar] [CrossRef]
- Chehregani, A.; Noori, M.; Yazdi, H.L. Phytoremediation of heavy-metal-polluted soils: Screening for new accumulator plants in Angouran mine (Iran) and evaluation of removal ability. Ecotoxicol. Environ. Saf. 2009, 72, 1349–1353. [Google Scholar] [CrossRef]
- Alyüz, B.; Veli, S. Kinetics and equilibrium studies for the removal of nickel and zinc from aqueous solutions by ion exchange resins. J. Hazard. Mater. 2009, 167, 482–488. [Google Scholar] [CrossRef]
- Ouhadi, V.R.; Yong, R.N.; Shariatmadari, N.; Saeidijam, S.; Goodarzi, A.R.; Zanjani, M.S. Impact of carbonate on the efficiency of heavy metal removal from kaolinite soil by the electrokinetic soil remediation method. J. Hazard. Mater. 2010, 173, 87–94. [Google Scholar] [CrossRef]
- Li, Z.; Wang, L.; Meng, J.; Liu, X.; Xu, J.; Wang, F.; Brookes, P. Zeolite-supported nano-scale zero-valent iron: New findings on simultaneous adsorption of Cd(II), Pb(II), and As(III) in aqueous solution and soil. J. Hazard. Mater. 2018, 344, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Mishra, P.C.; Patel, R.K. Removal of lead and zinc ions from water by low cost adsorbents. J. Hazard. Mater. 2009, 168, 319–325. [Google Scholar] [CrossRef]
- Karnib, M.; Kabbanib, A.; Holail, H.; Olama, Z. Heavy metals removal using activated carbon, silica and silica activated carbon composite. Energy Procedia 2014, 50, 113–120. [Google Scholar] [CrossRef]
- Bailey, S.E.; Olin, T.J.; Bricka, R.M.; Adrian, D.D. A review of potentially low-cost sorbents for heavy metals. Water Res. 1999, 33, 2469–2479. [Google Scholar] [CrossRef]
- Erdem, E.; Karapinar, N.; Donat, R. The removal of heavy metal cations by natural zeolites. J. Colloid Interface Sci. 2004, 280, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Baker, H.M.; Massadeh, A.M. Natural Jordanian zeolite: Removal of heavy metal ions from water samples using column and batch methods. Environ. Monit. Assess. 2009, 157, 319–330. [Google Scholar] [CrossRef]
- Guo, Z.; Li, Y.; Zhang, S.; Niu, H.; Chen, Z.; Xu, J. Enhanced sorption of radiocobalt from water by Bi(III) modified montmorillonite: A novel adsorbent. J. Hazard. Mater. 2011, 192, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Galindo, L.S.G.; Neto, A.F.A. Removal of cadmium(II) and lead(II) ions from aqueous phase on sodic bentonite. Mater. Res. 2013, 16, 515–527. [Google Scholar] [CrossRef][Green Version]
- Ye, X.; Kang, S.; Wang, H.; Li, H.; Zhang, Y.; Wang, G.; Zhao, H. Modified natural diatomite and its enhanced immobilization of lead, copper and cadmium in simulated contaminated soils. J. Hazard. Mater. 2015, 289, 210–218. [Google Scholar] [CrossRef]
- Ghodbane, I.; Nouri, L.; Hamdaoui, O.; Chiha, M. Kinetic and equilibrium study for the sorption of cadmium(II) ions from aqueous phase by eucalyptus bark. J. Hazard. Mater. 2008, 152, 148–158. [Google Scholar] [CrossRef]
- Bozic, D.; Stankovic, V.; Gorgievski, M.; Bogdanovic, G.; Kovacevic, R. Adsorption of heavy metal ions by sawdust of deciduous trees. J. Hazard. Mater. 2009, 171, 684–692. [Google Scholar] [CrossRef]
- Sewwandi, B.G.N.; Vithanage, M.; Wijesekara, S.S.R.M.D.H.R.; Mowjood, M.I.M.; Hamamoto, S.; Kawamoto, K. Adsorption of Cd(II) and Pb(II) onto humic acid–treated coconut (Cocos nucifera) husk. J. Hazard. Toxic Radioact. Waste 2014, 18, 04014001. [Google Scholar] [CrossRef]
- Paranavithana, G.N.; Kawamoto, K.; Inoue, Y.; Saito, T.; Vithanage, M.; Kalpage, C.S.; Herath, G.B.B. Adsorption of Cd2+ and Pb2+ onto coconut shell biochar and biochar-mixed soil. Environ. Earth Sci. 2015, 75, 484–494. [Google Scholar] [CrossRef]
- Inyang, M.; Gao, B.; Yao, Y.; Xue, Y.; Zimmerman, A.; Mosa, A.; Pullammanappallil, P.; Ok, Y.S.; Cao, X. A review of biochar as a low-cost adsorbent for aqueous heavy metal removal. Crit. Rev. Environ. Sci. Technol. 2016, 46, 406–433. [Google Scholar] [CrossRef]
- Chen, X.; Hou, W.H.; Song, G.L.; Wang, Q.H. Adsorption of Cu, Cd, Zn and Pb ions from aqueous solutions by electric arc furnace slag and the effects of pH and grain size. Chem. Biochem. Eng. Q. 2011, 25, 105–114. [Google Scholar]
- Agnieszka, G.K.; Baran, P.; Wdowin, M.; Franus, W. Waste dolomite powder as an adsorbent of Cd, Pb(II), and Zn from aqueous solutions. Environ. Earth Sci. 2017, 76, 521. [Google Scholar]
- Nguyen, T.C.; Loganathan, P.; Nguyen, T.V.; Kandasamy, J.; Naidu, R.; Vigneswaran, S. Adsorptive removal of five heavy metals from water using blast furnace slag and fly ash. Environ. Sci. Pollut. Res. 2018, 25, 20430–20438. [Google Scholar] [CrossRef]
- Gui, C.Y.; Neng, Z.K.; Sheng, Z.Y.; Yue, D.F. Removal of Pb2þ and Cd2þ by adsorption on clay-solidified grouting curtain for waste landfills. J. Cent. South Univ. Technol. 2006, 13, 166–170. [Google Scholar]
- Ok, Y.S.; Yang, J.E.; Zhang, Y.S.; Kim, S.J.; Chung, D.Y. Heavy metal adsorption by a formulated zeolite-portland cement mixture. J. Hazard. Mater. 2007, 147, 91–96. [Google Scholar] [CrossRef]
- Shaban, E.G.; Isam, M.G.; Abdallah, H.M.G. Cadmium(II) sorption from water samples by powdered marble wastes. Chem. Speciat. Bioavailab. 2015, 20, 249–260. [Google Scholar]
- Shaban, E.G.; Abdallah, H.M.G. Lead separation by sorption onto powdered marble waste. Arab. J. Chem. 2014, 7, 277–286. [Google Scholar]
- Visa, M.; Chelaru, A.M. Hydrothermally modified fly ash for heavy metals and dyes removal in advanced wastewater treatment. Appl. Surf. Sci. 2014, 303, 14–22. [Google Scholar] [CrossRef]
- Yoon, S.; Choi, M.; Hwang, Y.; Bae, S. Upcycling of steel slag for manufacture of Prussian-blue-encapsulated pectin beads and its use for efficient removal of aqueous cesium. J. Clean. Prod. 2021, 319, 128786. [Google Scholar] [CrossRef]
- Bibi, S.; Farooqi, A.; Hussain, K.; Haider, N. Evaluation of industrial based adsorbents for simultaneous removal of arsenic and fluoride from drinking water. J. Clean. Prod. 2015, 87, 882–896. [Google Scholar] [CrossRef]
- Nguyen, T.C.; Loganathan, P.; Nguyen, T.V.; Vigneswaran, S.; Kandasamy, J.; Naidu, R. Simultaneous adsorption of Cd, Cr, Cu, Pb, and Zn by an iron-coated Australian zeolite in batch and fixed-bed column studies. Chem. Eng. J. 2015, 270, 393–404. [Google Scholar] [CrossRef]
- Xin, M.; Yang, S.T.; Tang, H.; Liu, Y.; Wang, H. Competitive adsorption of heavy metal ions on carbon nanotubes and the desorption in simulated biofluids. J. Colloid Interface Sci. 2015, 448, 347–355. [Google Scholar]
- Abbas, A.; Al-Amer, A.M.; Laoui, T.; Al-Marri, M.J.; Nasser, M.S.; Khraisheh, M.; Atieh, M.A. Heavy metal removal from aqueous solution by advanced carbon nanotubes: Critical review of adsorption applications. Sep. Purif. Technol. 2016, 157, 141–161. [Google Scholar]
- Qiu, Q.; Jiang, X.; Lv, G.; Chen, Z.; Lu, S.; Ni, M.; Yan, J.; Deng, X. Adsorption of heavy metal ions using zeolite materials of municipal solid waste incineration fly ash modified by microwave-assisted hydrothermal treatment. Powder Technol. 2018, 335, 156–163. [Google Scholar] [CrossRef]
- Bilal, M.; Ihsanullah, I.; Younas, M.; Shah, M.U.H. Recent advances in applications of low-cost adsorbents for the removal of heavy metals from water: A critical review. Sep. Purif. Technol. 2021, 278, 119510. [Google Scholar] [CrossRef]
- Farinella, N.V.; Matos, G.D.; Arruda, M.A.Z. Grape bagasse as a potential biosorbent of metals in effluent treatments. Bioresour. Technol. 2007, 98, 1940–1946. [Google Scholar] [CrossRef]
- Pehlivan, E.; Yanık, B.H.; Ahmetli, G.; Pehlivan, M. Equilibrium isotherm studies for the uptake of cadmium and lead ions onto sugar beet pulp. Bioresour. Technol. 2008, 99, 3520–3527. [Google Scholar] [CrossRef]
- Kumara, G.M.P.; Saito, T.; Asamoto, S.; Kawamoto, K. Reviews on the applicability of construction and demolition waste as low-cost adsorbents to remove-heavy metals in wastewater. Int. J. Geomate 2018, 14, 44–51. [Google Scholar] [CrossRef]
- Yao, Z.T.; Ji, X.S.; Sarker, P.K.; Tang, J.H.; Ge, L.Q.; Xia, M.S.; Xi, Y.Q. Earth-Science Reviews A comprehensive review on the applications of coal fly ash. Earth-Sci. Rev. 2015, 141, 105–121. [Google Scholar] [CrossRef]
- Gao, D.; Wang, F.P.; Wang, Y.T.; Zeng, Y.N. Sustainable utilization of steel slag from traditional industry and agriculture to catalysis. Sustainability 2020, 12, 9295. [Google Scholar] [CrossRef]
- Reddy, K.R.; Gopakumar, A.; Chetri, J.K. Critical review of applications of iron and steel slags for carbon sequestration and environmental remediation. Rev. Environ. Sci. Biotechnol. 2019, 18, 127–152. [Google Scholar] [CrossRef]
- Yusuf, M.; Chuah, L.; Khan, M.A.; Choong, T.S. Adsorption of nickel on electric arc furnace slag: Batch and column studies. Sep. Sci. Technol. 2014, 49, 388–397. [Google Scholar] [CrossRef]
- Iucolano, F.; Campanile, A.; Caputo, D.; Liguori, B. Sustainable management of autoclaved aerated concrete wastes in gypsum composites. Sustainability 2021, 13, 3961. [Google Scholar] [CrossRef]
- Narayanan, N.; Ramamurthy, K. Structure and properties of aerated concrete: A review. Cem. Concr. Compos. 2000, 22, 321–329. [Google Scholar] [CrossRef]
- Hoda, A.; Salarirad, M.M.; Behnamfard, A. Characterization and utilization of clay-based construction and demolition wastes as adsorbents for zinc (II) removal from aqueous solutions: An equilibrium and kinetic study. Environ. Prog. Sust. Energy 2014, 33, 777–789. [Google Scholar]
- Taha, A.A.; Wu, Y.; Wang, H.; Li, F. Preparation and application of functionalized cellulose acetate/silica composite nano-fibrous membrane via electrospinning for Cr(VI) ion removal from aqueous solution. J. Environ. Manag. 2012, 112, 10–16. [Google Scholar] [CrossRef]
- Chuang, C.L.; Fan, M.; Xu, M.; Brown, R.C.; Sung, S.; Saha, B.; Huang, C.P. Adsorption of arsenic(V) by activated carbon prepared from oat hulls. Chemosp 2005, 61, 478–483. [Google Scholar] [CrossRef] [PubMed]
- Monarrez-Cordero, B.E.; Trevizo, A.S.; Carrillo, L.M.B.; Vidaurri, L.G.S.; Yoshida, M.M.; Madrid, P.A. Simultaneous and fast removal of As3+, As5+, Cd2+, Cu2+, Pb2+ and F− from water with composite Fe-Ti oxides nanoparticles. J. Alloys Comp. 2018, 757, 150–160. [Google Scholar] [CrossRef]
- Kumara, G.M.P.; Kawamoto, K.; Saito, T.; Hamamoto, S.; Asamoto, S. Evaluation of autoclaved aerated concrete (AAC) fines for removal of Cd(II) and Pb(II) from wastewater. J. Environ. Eng. 2019, 145, 04019078. [Google Scholar] [CrossRef]
- Gustavo, F.C.; Affonso, C.C.J.; Juan, C.N.M.; David, F.C. Competitive and non-competitive cadmium, copper and lead sorption/desorption on wheat straw affecting sustainability in vineyards. J. Clean. Prod. 2016, 139, 1496–1503. [Google Scholar]
- Park, J.H.; Ju, S.C.; Yong, S.O.; Seong, H.K.; Jong, S.H.; Ronald, D.D.; Dong, C.S. Comparison of single and competitive metal adsorption by pepper stem biochar. Arch. Agron. Soil Sci. 2016, 62, 617–632. [Google Scholar] [CrossRef]
- Xue, Y.; Hou, H.; Zhu, S. Competitive adsorption of copper(II), cadmium(II), lead(II) and zinc(II) onto basic oxygen furnace slag. J. Hazard. Mater. 2009, 162, 391–401. [Google Scholar] [CrossRef] [PubMed]
- Kumara, G.M.P.; Matsuno, A.; Nga, T.T.V.; Giang, N.H.; Kawamoto, K. Simultaneous removal of Pb(II) and Cd(II) from binary and multi-metals solutions using autoclaved aerated concrete and steel slag grains as low-cost adsorbents. In Proceedings of the 17th Sardinia Conference, Forte Village, Santa Margherita Di Pula, Italy, 30 September–4 October 2019. [Google Scholar]
- Nippon Slag Association. About Iron and Steel Slag. 2003. Available online: https://www.slg.jp/e/slag/product/doboku.html (accessed on 10 September 2021).
- Trong, L.N.; Asamoto, S.; Matsui, K. Sorption isotherm and length change behavior of autoclaved aerated concrete. Cem. Concr. Compos. 2018, 94, 136–144. [Google Scholar] [CrossRef]
- Shabalala, A.N.; Stephen, O.E.; Souleymane, D.; Fitsum, S. Pervious concrete reactive barrier for removal of heavy metals from acid mine drainage-column study. J. Hazard. Mater. 2017, 323, 641–653. [Google Scholar] [CrossRef]
- Yuanming, S.; Li, B.; Yang, E.H.; Liu, Y.; Ding, T. Feasibility study on utilization of municipal solid waste incineration bottom ash as aerating agent for the production of autoclaved aerated concrete. Cem. Concr. Compos. 2015, 56, 51–58. [Google Scholar]
- Marzouk, I.; Hannachi, C.; Dammak, L.; Hamrouni, B. Removal of chromium by adsorption on activated alumina. Desalination Water Treat. 2011, 51, 279–286. [Google Scholar] [CrossRef]
- Naiya, T.K.; Bhattacharya, A.K.; Das, S.K. Adsorption of Cd (II) and Pb (II) from aqueous solutions on activated alumina. J. Colloid Interface Sci. 2009, 333, 14–26. [Google Scholar] [CrossRef]
- Lopez, F.A.; Martin, M.I.; Perez, C.; Lopez-Delgado, A.; Alguacil, F.J. Removal of copper ions from aqueous solutions by a steel-making by-product. Water Res. 2003, 37, 3883–3890. [Google Scholar] [CrossRef]
- Organization for Economic Co-operation and Development Publications (OECD). Guidelines for the Testing of Chemicals. 2000. Available online: https://www.oecd.org/chemicalsafety/testing/oecdguidelinesforthetestingofchemicals.htm (accessed on 15 September 2021).
- Metcalf & Eddy, Inc.; Tchobanoglous, G.; Burton, F.L.; Stensel, H.D. Chap. 2 Constituents in Wastewater. In Wastewater Engineering: Treatment and Reuse, 4th ed.; McGraw-Hill Series in Civil and Environmental Engineering; McGraw-Hill Science Engineering: Boston, MA, USA, 2002. [Google Scholar]
- Shafiq, M.; Alazba, A.A.; Amin, M.T. Kinetic and isotherm studies of Ni2+ and Pb2+ adsorption from synthetic wastewater using eucalyptus camdulensis—derived biochar. Sustainability 2021, 13, 3785. [Google Scholar] [CrossRef]
- Saadi, R.; Saadi, Z.; Fazaeli, R. Monolayer and multilayer adsorption isotherm models for sorption from aqueous media. Korean J. Chem. Eng. 2015, 32, 787–799. [Google Scholar] [CrossRef]
- Martemianov, D.; Xie, B.B.; Yurmazova, T.; Khaskelberg, M.; Wang, F.; Wei, C.H.; Preis, S. Cellular concrete-supported cost-effective adsorbents for aqueous arsenic and heavy metals abatement. J. Environ. Chem. Eng. 2017, 5, 3930–3941. [Google Scholar] [CrossRef]
- Damrongsiri, S. Feasibility of using demolition waste as an alternative heavy metal immobilising agent. J. Environ. Manag. 2017, 192, 197–202. [Google Scholar] [CrossRef]
- Kumara, G.M.P.; Kawamoto, K. Applicability of crushed clay brick and municipal solid waste slag as low-cost adsorbents to refine high concentrate Cd (II) and Pb (II) contaminated wastewater. Int. J. Geomate 2019, 17, 133–142. [Google Scholar] [CrossRef]
- Bourliva, A.; Michailidis, K.; Sikalidis, C.; Filippidis, A.; Betsiou, M. Adsorption of Cd (II), Cu (II), Ni (II) and Pb (II) onto natural bentonite: Study in mono- and multimetal systems. Environ Earth Sci. 2015, 73, 5435–5445. [Google Scholar] [CrossRef]
- Seyedeh, S.H.G.; Landi, A.; Khademi, H.; Hojati, S. Removal of Cd2+ and Pb2+ ions from aqueous solutions using Iranian natural zeolite and sepiolite. J. Environ. Stud. 2014, 40, 43–45. [Google Scholar]
- He, S.; Li, Y.; Weng, L.; Wang, J.; He, J.; Liu, Y.; Zhang, K.; Wu, Q.; Zhang, Y.; Zhang, Z. Competitive adsorption of Cd2+, Pb2+ and Ni2+ onto Fe3+-modified argillaceous limestone: Influence of pH, ionic strength and natural organic matters. Sci. Total Environ. 2018, 637–638, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Youness, A.; Olguín, M.T.; Abatal, M.; Ali, B.; Mendez, S.E.D.; Santiago, A.A. Comparison of the divalent heavy metals (Pb, Cu and Cd) adsorption behavior by montmorillonite-KSF and their calcium- and sodium-forms. Superlattices Microstruct. 2019, 127, 165–175. [Google Scholar]
- Yang, D.; Liu, Y.; Liu, S.; Li, Z.; Tan, X.; Huang, X.; Zeng, G.; Zhou, Y.; Zheng, B.; Cai, X. Competitive removal of Cd(II) and Pb(II) by biochars produced from water hyacinths: Performance and mechanism. RSC Adv. 2016, 6, 5223–5232. [Google Scholar]
- Bulgariu, D.; Bulgariu, L. Equilibrium and kinetics studies of heavy metal ions biosorption on green algae waste biomass. Bioresour. Technol. 2012, 103, 489–493. [Google Scholar] [CrossRef] [PubMed]
- Arslanoglu, H.; Altundogan, H.S.; Tumen, F. Heavy metals binding properties of esterified lemon. J. Hazard. Mater. 2009, 164, 1406–1413. [Google Scholar] [CrossRef] [PubMed]
- Duan, J.; Su, B. Removal characteristics of Cd(II) from acidic aqueous solution by modified steel-making slag. Chem. Eng. J. 2014, 246, 160–167. [Google Scholar] [CrossRef]
- Dimitrova, S.V. Metal sorption on blast-furnace slag. Water Res. 1996, 30, 228–232. [Google Scholar] [CrossRef]
- Dimitrova, S.V.; Mehandgiev, D.R. Lead removal from aqueous solutions by granulated blast-furnace slag. Water Res. 1998, 32, 3289–3292. [Google Scholar] [CrossRef]
- Dimitrova, S.V.; Mehandgiev, D.R. Interaction of Blast-furnace slag with heavy metal ions in water solutions. Water Res. 2000, 34, 1957–1961. [Google Scholar] [CrossRef]
- Takeno, N. Atlas of Eh-pH Diagrams; Intercomparison of Thermo Dynamic Database. 2005. Available online: https://www.gsj.jp/researches/openfile/openfile2005/openfile0419.html (accessed on 3 September 2021).




| Adsorbent | Particle Size (mm) | pH | EC (mS/cm) | LOI (%) | wAD (%) | Gs | SSA (m2/g) | Reference | |
|---|---|---|---|---|---|---|---|---|---|
| DI | 1M KCl | ||||||||
| <0.105 | 12.9 | 13.1 | 6.1 | 14.5 | 2.5 | 3.05 | 8.0 | ||
| SS | 0.105–2 | 12.4 | 12.6 | 6.9 | 4.8 | 1.9 | 4.9 | This study | |
| 2–4.75 | 13.1 | 13.1 | 5.0 | 4.8 | 1.9 | 2.6 | |||
| <0.105 | 10.0 | 8.7 | 1.7 | 10.3 | 4.4 | 23.6 | |||
| AAC | 0.105–2 | 10.0 | 8.3 | 1.8 | 10.8 | 3.9 | 2.49 | 23.6 | [61] |
| 2–4.75 | 9.9 | 8.9 | 1.4 | 9.8 | 4.0 | 21.9 | |||
| Adsorbent | Composition (wt %) | Reference | ||||||
|---|---|---|---|---|---|---|---|---|
| SiO2 | CaO | Al2O3 | Fe2O3 | K2O | MgO | Others | ||
| SS | 16.8 | 41.7 | 3.2 | 22.5 | 0.2 | 3.5 | 12.1 | This study |
| AAC | 48.6 | 33.8 | 2.8 | 1.9 | 0.5 | 0.4 | 12.0 | [61] |
| Adsorbent | Types of Experiment | Liquid-to-Solid Ratio (L/S) | Initial Metal Concentration, (Ci = mg/L) |
|---|---|---|---|
| SS | 1. Adsorption isotherm in single-metal solution (Cd2+, Pb2+) | 60 (Cd2+), 10 (Pb2+) | 0–5000 (Cd2+), 0–1500 (Pb2+) |
| SS SS+AAC [4:1] SS+AAC [1:1] SS+AAC [1:4] AAC | 2. Removal % in binary-metal solution (Cd2+ + Pb2+) | 60 | 1000 |
| 3. Removal % in multi-metal solution (Cd2+ + Pb2+ + Cu2+ + Ni2+ + Zn2+) | 60 | 1000 | |
| 4. Effect of L/S ratio on removal % in multi-metal solution | 5, 10, 60, 100, 250 | 1000 |
| Adsorbent | Metal | Particle Size (mm) | Langmuir | Freundlich | Reference | ||||
|---|---|---|---|---|---|---|---|---|---|
| Qe (mg/g) | b (L/mg) | r2 | Kf (mg/g) | 1/n | r2 | ||||
| SS | Cd2+ | <0.105 | 3131 | – | – | 105 | 0.27 | 0.98 | This study |
| 0.105–2 | 244 | 0.18 | 0.99 | 114 | 0.17 | 0.89 | |||
| 2–4.75 | 130 | 0.09 | 0.99 | 92.0 | 0.04 | 0.77 | |||
| Pb2+ | <0.105 | 17.5 | 0.02 | 0.97 | 0.57 | 0.67 | 0.97 | ||
| 0.105–2 | 8.6 | 0.01 | 0.91 | 0.15 | 0.70 | 0.96 | |||
| 2–4.75 | 8.2 | 0.01 | 0.93 | 0.13 | 0.72 | 0.94 | |||
| AAC | <0.105 | 16.5 | 1.72 | 0.99 | 9.89 | 0.11 | 0.91 | [61] | |
| Cd2+ | 0.105–2 | 16.5 | 0.91 | 0.99 | 9.92 | 0.10 | 0.93 | ||
| 2–4.75 | 15.2 | 0.69 | 0.99 | 8.82 | 0.11 | 0.89 | |||
| <0.105 | 2581 | – | – | 137 | 0.20 | 0.97 | |||
| Pb2+ | 0.105–2 | 2571 | – | – | 121 | 0.24 | 0.98 | ||
| 2–4.75 | 250 | 0.05 | 0.99 | 74.3 | 0.22 | 0.98 | |||
| Adsorbent | L/S | Selectivity Sequence | R (%) | ||||
|---|---|---|---|---|---|---|---|
| Cd2+ | Pb2+ | Cu2+ | Ni2+ | Zn2+ | |||
| SS | 5 | Cd2+ ≈ Cu2+ ≈ Ni2+ > Zn2+ > Pb2+ | 100 | 86.1 | 100 | 100 | 99.8 |
| 10 | Cd2+ ≈ Cu2+ ≈ Ni2+ > Zn2+ > Pb2+ | 100 | 86.9 | 100 | 100 | 99.7 | |
| 60 | Pb2+ > Cu2+ > Cd2+ > Ni2+ ≈ Zn2+ | 55.2 | 99.5 | 63.4 | 20.9 | 20.6 | |
| 100 | Pb2+ > Cu2+ > Cd2+ > Zn2+ > Ni2+ | 51.7 | 76.2 | 63.8 | 0.9 | 8.0 | |
| 250 | Pb2+ > Cd2+ > Cu2+ > Zn2+ > Ni2+ | 45.9 | 62.0 | 31.7 | 0.0 | 6.1 | |
| SS+AAC [4:1] | 5 | Cd2+ ≈ Pb2+ ≈ Cu2+ ≈ Ni2+ ≈ Zn2+ | 100 | 100 | 100 | 100 | 100 |
| 10 | Cd2+ ≈ Cu2+ ≈ Ni2+ > Pb2+ > Zn2+ | 100 | 96.4 | 100 | 100 | 44.5 | |
| 60 | Cu2+ > Pb2+ > Cd2+ > Zn2+ > Ni2+ | 46.1 | 87.8 | 99.3 | 17.9 | 23.2 | |
| 100 | Pb2+ > Cu2+ > Cd2+ > Zn2+ > Ni2+ | 38.3 | 60.1 | 42.9 | 0.0 | 6.3 | |
| 250 | Cd2+ > Pb2+ > Cu2+ > Zn2+ > Ni2+ | 39.1 | 37.1 | 28.4 | 0.2 | 5.3 | |
| SS+AAC [1:1] | 5 | Cd2+ ≈ Pb2+ ≈ Cu2+ ≈ Ni2+ ≈ Zn2+ | 100 | 100 | 100 | 100 | 100 |
| 10 | Cd2+> ≈ Pb2+ ≈ Cu2+ ≈ Zn2+ > Ni2+ | 100 | 100 | 100 | 95.2 | 99.9 | |
| 60 | Pb2+ ≈ Cu2+ > Cd2+ > Zn2+ > Ni2+ | 28.6 | 99.8 | 99.8 | 11.5 | 20.3 | |
| 100 | Pb2+ > Cu2+ > Cd2+ > Zn2+ > Ni2+ | 26.8 | 59.9 | 39.3 | 0.2 | 5.4 | |
| 250 | Pb2+ > Cd2+ > Cu2+ > Zn2+ > Ni2+ | 26.4 | 45.5 | 22.3 | 0.0 | 4.3 | |
| SS+AAC [1:4] | 5 | Ni2+ > Cu2+ > Zn2+ ≈ Pb2+ > Cd2+ | 99.4 | 99.5 | 99.9 | 100 | 99.8 |
| 10 | Cu2+ > Pb2+ > Zn2+ > Cd2+ > Ni2+ | 47.2 | 99.2 | 100 | 23.9 | 71.4 | |
| 60 | Cu2+ > Pb2+ > Cd2+ > Zn2+ > Ni2+ | 21.9 | 97.6 | 98.5 | 0.0 | 6.5 | |
| 100 | Pb2+ > Cu2+ > Cd2+ > Zn2+ > Ni2+ | 18.4 | 69.0 | 43.4 | 0.0 | 1.3 | |
| 250 | Pb2+ > Cu2+ ≈ Cd2+ > Zn2+ > Ni2+ | 17.8 | 39.6 | 18.0 | 0.0 | 0.1 | |
| AAC | 5 | Pb2+ ≈ Cu2+ > Zn2+ > Cd2+ > Ni2+ | 41.3 | 100 | 100 | 44.7 | 71.1 |
| 10 | Cu2+ > Pb2+ > Ni2+ > Cd2+ > Zn2+ | 32.1 | 99.4 | 100 | 32.9 | 20.6 | |
| 60 | Pb2+ > Cu2+ > Cd2+ ≈ Ni2+ > Zn2+ | 19.4 | 100 | 60.1 | 17.9 | 5.3 | |
| 100 | Pb2+ > Cu2+ > Cd2+ > Zn2+ > Ni2+ | 15.0 | 60.4 | 41.0 | 2.9 | 4.2 | |
| 250 | Pb2+ > Cu2+ > Cd2+ > Zn2+ > Ni2+ | 14.0 | 30.7 | 25.2 | 0.0 | 2.9 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumara, G.M.P.; Kawamoto, K. Steel Slag and Autoclaved Aerated Concrete Grains as Low-Cost Adsorbents to Remove Cd2+ and Pb2+ in Wastewater: Effects of Mixing Proportions of Grains and Liquid-to-Solid Ratio. Sustainability 2021, 13, 10321. https://doi.org/10.3390/su131810321
Kumara GMP, Kawamoto K. Steel Slag and Autoclaved Aerated Concrete Grains as Low-Cost Adsorbents to Remove Cd2+ and Pb2+ in Wastewater: Effects of Mixing Proportions of Grains and Liquid-to-Solid Ratio. Sustainability. 2021; 13(18):10321. https://doi.org/10.3390/su131810321
Chicago/Turabian StyleKumara, Gajanayake Mudalige Pradeep, and Ken Kawamoto. 2021. "Steel Slag and Autoclaved Aerated Concrete Grains as Low-Cost Adsorbents to Remove Cd2+ and Pb2+ in Wastewater: Effects of Mixing Proportions of Grains and Liquid-to-Solid Ratio" Sustainability 13, no. 18: 10321. https://doi.org/10.3390/su131810321
APA StyleKumara, G. M. P., & Kawamoto, K. (2021). Steel Slag and Autoclaved Aerated Concrete Grains as Low-Cost Adsorbents to Remove Cd2+ and Pb2+ in Wastewater: Effects of Mixing Proportions of Grains and Liquid-to-Solid Ratio. Sustainability, 13(18), 10321. https://doi.org/10.3390/su131810321







