Abstract
Interest in the presence of microplastics (MPs) in wastewater has grown significantly in recent years. In fact, wastewater treatment plants (WWTPs) represent the last barrier before the discharge of MPs into an aquatic ecosystem. The research has highlighted how MPs are in part effectively removed from the waters and accumulated inside the sewage sludge (SeS) produced by the WWTP, being a cause for concern, especially in the case of agricultural reuse. This work aims to analyze the existing literature on the (i) methodical procedure for MPs analysis (thermal, spectroscopic, optical analyses), (ii) qualitative and quantitative presence of MPs in SeS, (iii) effect on sludge properties, and (iv) the possible accumulation in amended soils. Based on the results already obtained in the literature, this work aims to provide critical insights to stimulate interest in the topic and direct future research on aspects that should be deepened. In particular, it emerges that there is a clear need for standardization of the collection methods and the analytical techniques for identifying and quantifying MPs, since their physico-chemical characterization and the study on aging and on the response towards acid or basic pre-treatments are fundamental for the understanding of microplastics ecotoxicological potential.
1. Introduction
The term microplastics (MPs) refers to the set of particles of plastic material that have a size of less than 5 mm, even if the literature does not agree on a unique definition [1,2,3,4]. Generally, MPs can be classified into two groups according to their initial configuration: (i) primary MPs, which were made in such dimensions, and (ii) secondary MPs, resulting from the fragmentation of larger particles [5]. For example, the first category includes the abrasive granules present in some cosmetic products and in shower gels (also named microbeads), and in shot-blasting abrasive products used in some industrial processes [3,6,7,8,9]. The second category includes the MPs released during the washing of synthetic clothes or those produced by the wear of tires on the road [3,6,7,8].
For some years now, interest in the research and study on MPs have been increasing mainly due to two diverse aspects. The first concerns the growing production and use of plastic [10,11] which will most likely lead to an increase in the concentration of MPs in the environment. Secondly, the chronic impact on the biosphere, although not completely clear, is emerging as significant considering also that MPs can serve as carrier for a widely range of pollutants due to their strong hydrophobicity [12,13,14,15]. Concerning the possible health effect of MPs on human health, Vethaak and Legler [16] recently highlighted that MPs can enter the human body by the inhalation of contaminated air and the ingestion of contaminated food and water. However, the health effects still remain unknown. Diverse studies hypothesized DNA damage, cytotoxicity effect, and other inflammation pathologies with immune response [17,18] but, according to Vethaak and Legler [16] not enough data have been collected on this topic due to the low data about human exposure.
Wastewater (WW) acts as a vector by transferring a large part of the primary and secondary MPs from the source (e.g., domestic environment, industrial environment, roads, etc.) to the destination (surface water bodies, soils) [14,19,20,21]. Therefore, the wastewater treatment plants (WWTPs) represent the last barrier before these substances are released into the surrounding environment [3].
In past years, most of the attention was placed on the final effluent and, therefore, the studies focused primarily on the effectiveness of the treatments conventionally present in the water line on the MPs [22,23,24]. The results showed that a conventional WWTP is able to remove up to 90% of MPs (depending on the characteristics of the influencing WW and the types of processes adopted) [3].
On the contrary, more than 90% of MPs found in WW are accumulated in sewage sludge (SeS), which in turn is used for land applications: the annual amount of MPs entering the soil in this way is greater than that enters the oceans [25,26]. As an example, Li et al. reported from around 10 to 25 × 103 MPs kg−1 dry sludge as average values in 11 different WWTPs in China provinces [27] and a good data summary of the MPs amounts in sludge in different places and countries can be found by Hatinoğlu and Sanin [11]. Liu et al. [28] also highlighted the high concentration of MPs in SeS (up to 2.40 × 105 particles kg−1) due to an accumulation phenomenon. For this reason, it is very important to isolate the MPs, to detect their chemical nature, their physico-chemical properties, the amounts of the MPs particles, and to investigate their effects in the SeS itself and soil amended with SeS.
Due to the growing interest on studying MPs, the full knowledge of their pathways in WWTPs represent a crucial factor to implement actions for mitigating the impact of MPs on the environment. This work aims to evaluate the findings of the research on four diverse aspects of MPs in SeS: (i) the methodical procedure of detection, (ii) the pathways of MPs transfer in WWTPs, (iii) the effect of MPs on SeS properties, and (iv) the possible accumulation in amended soils. The results of literature are reviewed and discussed with critical insights to point out research gaps and provide tips for future research.
2. Methodical Analysis of Literature
Peer-reviewed literature have been searched and selected according to preferred reporting items for systematic reviews and meta-analysis (PRISMA) guidelines [29].
To consider only peer-reviewed documents, the Scopus® database was used to search relevant literature on this topic. Moreover, other relevant reports (n = 2) and standardized methodical procedures (n = 2) obtained by search in Google Scholar® were selected. Data extraction from the database was made on 22nd September 2021 using the following query: TITLE-ABS-KEY (microplastic AND sewage AND sludge).
No publication period limits were imposed. Only documents in English were taken into consideration, and documents that did not present new data or other useful information were discarded. The documents were also screened individually to exclude papers that do not: (i) treat specifically subject in the scope of the research, (ii) report results on real WWTPs providing influent concentration of MPs (for data discussed in Section 3.2), or (iii provide data on SeS use for amending soils (for data discussed in Section 3.4). For two records, full text was not available for screening and the publications were excluded.
In Figure 1, the results of the literature search and the number of studies included according to PRISMA guidelines [29] are presented.
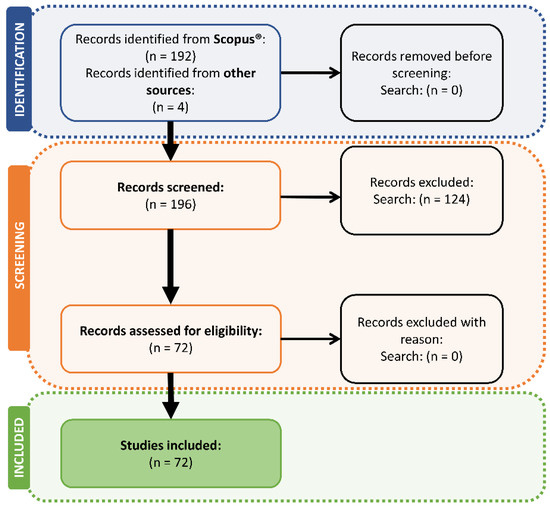
Figure 1.
Results of the literature search and studies included according to PRISMA guidelines.
3. Literature Findings and Discussion
3.1. Methodical Procedure of MPs Detection in Sewage Sludge
It is important to point out that SeS has not been comprised in the recent documents (August 2020) by the American Society for Testing and Materials (ASTM) regarding the collection and the treatments of waters and aqueous waste for the detection of MPs [30,31], so standard protocols do not exist yet and comparison among the different published data remains difficult [11]. Anyway, most of the experimental works use procedures and techniques similar to one another and to those followed for wastewater treatments, as well schematized and summarized in [11].
In general, SeS is collected by grab sampling methods, often in different points of the bulk materials or for different times to ensure the statistical significance [11,32,33]. In many studies, the SeS is dried before starting the analyses [34,35,36]; in general, wet or dry samples are sieved with different meshes sieves to separate the plastic size fractions [37,38,39].
Organic materials, microorganisms, and extracellular polymeric substances may interfere with the MPs extraction and identification and must be removed before the analysis: this step in general consists in a digestion, being water peroxide the most used and effective agent [40,41,42], or in the cost and time effective Fenton oxidation [32,42,43]. The extraction of the MPs is frequently realized by using sequential saturated salts solutions with increasing densities, so that the low-density MPs float to the surface [44,45,46]. The sequential extraction in NaI and ZnCl2 is recommended to separate and quantify both the low and high-density MPs, despite the high price and relatively toxic properties of these two salts [33,47].
After filtration, the MPs are analyzed by the conventional techniques used for solids samples, such as the non-destructive micro-Fourier transform infrared (FTIR) spectroscopy [46,48] and the attenuated total reflectance FTIR spectroscopy [49], Raman [50] and micro-Raman [51] measurements, thermoanalytical techniques such as the pyrolysis-gas chromatography/mass spectrometry (Pyr-GC/MS) [33,52], or the differential scanning calorimetry and the thermogravimetric analysis [53,54], in order to describe the chemical compositions of the plastics and their glass transition temperatures. The observation by conventional optical microscopy [46] or fluorescence microscopy [55] allows to describe their color, shape, and size, while by scanning electron microscopy the description of the morphology and of the microsize [21,53] is performed and porosimetry allows to determine the changes in the specific surface area [53,56]. For the physico-chemical characterization, a common operative procedure has not been established yet, and the very common optical microscopy observation is qualitative. The effect of both the surface characteristics and the MPs size on the chemical activity and the fate of the particles needs further research work.
3.2. From Wastewater to Sewage Sludge
In the literature there are 28 documents that simultaneously report the concentration of MPs in at least one sample of SeS extracted from a WWTP and the concentration of MPs in the influential WW [34,35,37,41,55,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79]. The number of publications has grown strongly in recent years with an almost exponential trend from the first publication to today: 2017 (n = 1), 2018 (n = 3), 2019 (n = 4); 2020 (n = 6), and 2021 (n = 14).
Table 1 shows the main results of the bibliographic analysis including for each publication: (i) the location of the WWTP studied, (ii) the potential and the flowrate, (iii) the type of WW treated, (iv) the concentration of MPs in the influential WW, (v) the type of water line treatments, (vi) the type of SeS studied, (vii) the treatments of SeS before the sampling point, (viii) the concentration of MPs in the SeS, and (ix) other results regarding the type of MPs.

Table 1.
Main results of the bibliographic analysis about the location of the WWTP studied, the potential and the flowrate, the type of WW treated, the concentration of MPs in the influential WW, the type of water line treatments, the type of SeS studied, the treatments of SeS before the sampling point, the concentration of MPs in the SeS, and results regarding the type of MPs. n.a.: not available; P: Primary sludge; S: Secondary/biological sludge; DM: Dry matter; UW: Urban wastewater; MW: Urban wastewater with a large contribution of industrial wastewater (mixed wastewater); IW: Industrial wastewater; UMW: Not specified if urban or mixed wastewater; A/A/O: Anaerobic/anoxic/aerobic; MBR: membrane bioreactor; BAF: Biologically active filter; SBR: Sequencing batch reactor; MBBR: Moving bed biofilm reactor; RFE: rapid filtration equipment; TF: trickling filters; MSBR: modified sequencing batch reactor; CAS: Conventional active sludge; UF: Ultrafiltration; NF: Nanofiltration; RO: Reverse osmosis.; AFB: aerobic fluidized bioreactor.
The results show several interesting aspects. At first, it can be noted that the concentration of MPs in the SeS is decidedly variable depending on the study under examination. For instance, Ziajahromi et al. [64] analyzed the MPs in treated thickened, digested, and dewatered SeS, finding a concentration of an order of magnitude higher than in Pittura et al. [63] (56.5 MPs g−1DW and 4.74 MPs g−1DW, respectively).
This strong difference can potentially be attributed to two main factors and the type of treatments present in the water line of the WWTP is one of these. Thanks to their hydrophobicity properties, MPs can be adsorbed on the surfaces of surface of biomass flocs in biological treatments, accumulating in SeS produced by the plant [11,21] (Table 1). The inclusion of pre-treatments in water line, such as those for removing oils, can help to increase the removal of MPs before biological processes preventing the accumulation phenomena [3].
On the contrary, according to Hatinoğlu and Sanin [11], the treatments generally present in the sludge line are not able to significant impact on MPs concentration. The presence of thickening or dewatering processes can only partially reduce the MPs content following the limited extraction of them with the supernatants. Despite the number of studies is limited, anaerobic digestion appears to be influenced by the presence of MPs (Section 3.3) but not be able to significantly influencing their content [11].
Although WWTPs represent the last “defense” before the discharge of the MPs into the environment, they have no direct function of elimination of the MPs. Treatments in WWTPs can only transfer MPs from the aqueous phase to the solid or sludge one, depending on the type of treatment.
The other main aspect influencing the concentration of MPs in SeS is the initial concentration in untreated WW. In accordance with previous literature works [14,80], the concentration of MPs in WW is highly variable, depending by many factors such as the habits of the population served and the type of WW considered. For instance, in the cases mentioned above [63,64], there were no substantial differences in the WWTP configuration both in the water line and in the sludge line, but the influential concentration of MPs in the study conducted by Ziajahromi et al. [64] was more than 25 times higher than that found in the study of Pittura et al. [63] (92 MPs L−1 and 3.64 MPs L−1, respectively) explaining the strong differences in results.
MPs can also be classified according to their shape. This aspect is of great importance because it is very useful for understanding the origin of particles [81]. From the analysis of the examined literature, among the MPs identified in the SeS, fibers and fragments substantially predominate [35,41,55,57,58,59,61,63,64,65,66,67,68,69,70,71,72,73,75,76] (Table 1). Both are already present in the influent WW, the former derive mainly from the washing of synthetic clothing at home or industrial level [82], while the latter are contained for instance in many cosmetics as scrubber, given their irregular shape [3,81,83] or derive from the fragmentation of larger particles [81].
From the results presented by the literature analysis, it does not seem evident a direct dependence between the processes inserted in the sludge line of the WWTP and the type of MPs that remain after the treatment of SeS (Table 1). The different prevalence of fibers or fragments is to be considered mainly related to the initial characteristics of the WW and therefore to the origin of the MPs themselves. However, on this aspect, the authors suggest the need to prepare ad hoc studies to determine the possible influence of treatment processes in WWTPs on the characterization of MPs in sludge keeping constant the other variables (e.g., the influent WW).
Another aspect must be considered in the discussion of the results proposed by the literature, namely, the extraction and analysis method. Since, as already pointed out, there is not a standardized methodical procedure for extracting and analyzing microplastics from WW and SeS (Section 3.1), a direct comparison of the results is often difficult. However, the determination of the type of MPs in SeS represents an important step forward, which could be exploited to better evaluate the dispersion in the environment of MPs following the spread of SeS. A high concentration of fibers and fragments in the amended soil can act as an indicator of a clear WWTP-environment pathway (Section 3.4).
3.3. Effect on Sludge Properties
The effect of the MPs’ presence on the SeS characteristics and behavior are still under investigation and vary on the basis of the MPs composition and particle size.
Li et al. [84] studied the adsorption efficiency of different metals by MPs in SeS, reporting the following affinity scale: Pb > Cd > Zn > Cu > Co > Ni. In particular, considering Cd, one of the most toxic metals due to its solubility, mobility, and biological accumulation and its ability to disrupt proteins in the cells, they noted an important increase (one order of magnitude) of adsorption by MPs after the wastewater treatment process. Three different families of MPs were found in the investigated sludge, i.e., polyamide, rubbery MPs (polyethylene and polypropylene) and glassy MPs (polyvinyl chloride and polystyrene), having decreasing adsorption efficiency towards Cd. The highest efficiency was attributed to the higher specific surface area (average value of about 5 m2 g−1) and to the wrinkled and aggregated structures present on the MPs surfaces in the SeS with respect to the virgin particles. Moreover, FTIR measurements revealed higher intensity for the bands due to vibration of the O-containing groups (namely, C-O and O-H) on the sludge based MPs, probably due to the oxidative degradation of the MPs [85] and the subsequent attachment of organic matter on them [86].
This finding is in agreement with Turner et al. [87] and seems to point out that the attachment of organic matters on the MPs during the weathering strongly affects the metals adsorption. The 2D IR spectra correlation maps showed N-H bonds of the MPs as another preferential site for the metals adsorption, in agreement with the polymers efficiency order experimentally determined. The Cd adsorption was found influenced also by pH, with the highest efficiency depending from the chemical nature of the MPs but detectable in the range 6.0–7.7, hence close to the common pH values for SeS.
In another study, Li et al. [53] analyzed the changes in the physicochemical property of three MPs, namely, polyamide (PA), polyethylene (PE), and polystyrene (PS) by passing through the wastewater pipeline, grit, and biological aeration tanks, confirming that during all these treatments the surface area and the content of organic group containing O atoms increase, while the glass transition measured by calorimetry decreases; this confirms the oxidation and rupture of the polymeric chains during the treatments. Interestingly, the Cd adsorption capacity of the MPs increases with respect to the virgin materials after both the sulfidation in the pipeline and the biological treatment in aeration tank, but results to decrease after mechanical abrasion in the grit tank, in correspondence to the decrease of the carbonyl index.
Huang et al. [56] investigated the adsorption of Cu, Mn, Pb, and Zn by pristine and artificially aged low-density polyethylene (LDPE), showing that the amount of adsorbed metals increases with the aging time, together with the surface area of the plastic material, reaching values higher than 650 μg m−2 after 10 h aging.
Recently, several authors studied also the effects of MPs on methane and hydrogen production through anaerobic digestion of SeS, showing a strong dependence of the chemical nature of the plastic materials and their particle size and concentration. In general, low concentrations of MPs seem not to affect or slightly increase the methane production, while a highest number of MPs particles leads to the opposite effect [88,89]. For instance, Zhang et al. [90] highlighted that methane production in anaerobic system was not affected by polystyrene MPs in the case of low concentration (0.2 g L−1), while it can be reduced almost by 20% in presence of the same MPs in higher dosage (0.25 g L−1).
Hydrogen production was found to decrease during alkaline anaerobic fermentation of activated waste in presence of PET microplastic, which was found to inhibit hydrolysis, acidogenesis and acetogenesis [91]. Feng et al. [92] found that Pd-doped polystyrene nanoplastics (2.36 × 1010 particles mL−1) were able to reduce the methane production up to 14.29%. Further, aerobic digestion seems to be affected by MPs presence in SeS [93]. A very detailed and exhaustive summary of the recent literature and of the chemical and biological mechanisms evolving during the anaerobic and aerobic processes in presence of MPs can be found in [11,94]. Further, some results regarding the positive or negative effects induced by MPs and/or by the metals adsorbed on MPs as a function of their chemical nature on the evolution of the different reactions appear to be controversial, and more focused studies with common guidelines are needed to clarify these aspects, exploiting the potential ecotoxicity of the plastic wastes.
3.4. Microplastics in Soils Amended with Sewage Sludge
Although several authors try to roughly estimate the potential dispersion and accumulation of MPs in soils due to the spread of SeS [41,95,96], the number of research studies on this topic is low and severely limited to the last two years. In the literature, only four studies have been identified [97,98,99,100] (Table 2).

Table 2.
Main results of the bibliographic analysis about the type of sludge spread in soil, the rate and the frequency of application, the type of crop, and a comparison between the MPs concentration in amended soil and in not-amended soil. n.a.: Not available; MS: Municipal sludge; MIS: Mixed municipal and industrial sludge; DM: Dry matter.
Corradini et al. [97] examined 30 different plots of land in Chile, which had been amended with diverse frequency (0–5 times) in the previous 10 years with about 40 t ha−1 of SeS. Comparing the results with those observed in a “control” soil not amended, the impact of SeS spread was evident. The presence of MPs was considerably higher in amended soils than in the control case and 97% were fibers [97], typically present in SeS (Section 3.2) [68,73,75]. Corradini et al. [97] also evaluated that the spreading frequency did not play a key role in MPs accumulation when the spreads are very sparse. By testing one, two, and three applications in 10 years, the accumulation of MPs was similar and lower than that obtained by testing four and five applications [97].
Zhang et al. [98] studied the effects due to the application of different quantities of SeS (15 and 30 t ha−1) on diverse soils in China. Their results showed a concentration of MPs directly proportional to the sludge rate of application (up to 545.9 MPs kg−1) while the presence of MPs in the “control soil” (not amended) was significantly lower (5.0 MPs kg−1) [98].
van den Berg et al. [99] focused their attention on 16 different sites in south-eastern Spain (five used as “control” and eleven on which 20–22 t ha−1 of SeS was spread 1–8 times) finding a concentration of MPs up to three times higher in soils where SeS was spread than in “control” soils. Their study also confirmed the dependence between the number of SeS application and the accumulation of MPs, highlighting a significant positive linear correlation both for light density plastic (R = 0.593) and for heavy density plastic (R = 0.668) [99].
Finally, Yang et al. [100] confirmed the direct proportionality between the quantity of SeS used and the presence of MPs in the soils, examining four soils (three of which were amended). They also found a presence of MPs more than double in the case of the use of municipal SeS compared to mixed SeS (municipal and industrial), highlighting a possible strong dependence between the type of SeS and the quantity of MPs released after spreading in agriculture [100]. In accordance with the previous study by Corradini et al. [97], the qualitative analysis of MPs determined that fibers represent the MPs most present in the soil samples analyzed (from 66.7 to 82.5%) [100].
Considering these studies, despite the limited number of data, the accumulation of MPs in the soils due to the spreading of SeS appears to be significant and directly proportional to the amount of soil improver spread highlighting a significant pathway for the diffusion of MPs in the environment. This correlation is more clear, also referring to the high prevalence of fibers in amended soils, typically present in SeS [68,73,75]. This could represent a problem considering also the possible existence of pathways of the smaller fraction of MPs from contaminated soils to human and biotic communities in general [13,101], not yet fully confirmed (Figure 2).
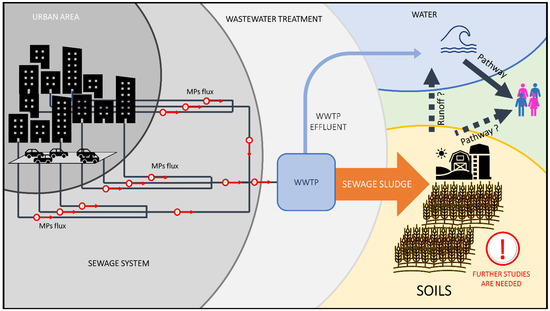
Figure 2.
Pathways of MPs in and out of WWTPs and accumulation in soils amended with SeS.
However, these studies have not shed light on many aspects that should still be adequately addressed before being able to fully define the impact of SeS spread in the release of MPs in the environment. The impact of the diverse types of SeS, the influence of the initial concentration of MPs, environmental factors (e.g., precipitation, etc.), and the influence of diverse crops and types of soil are just some of the aspects that should be clarified in future research.
Despite the difficulties in conducting studies on this issue such as long monitoring periods (years), large amounts of data required (type, frequency, quantity and quality of SeS spread, etc.), and the lack of a standardized method for the extraction and quantification of MPs in soils, the authors believe that studies on this topic are increasingly necessary and need to be stimulated.
Although, to date, there are no limits on the presence of MPs in the SeS for agricultural reuse or in the soils in which they are spread [102], attention on this issue remains high. In 2020, the EU launched an evaluation of the Sewage Sludge Directive 86/278/EEC [103] in perspective of future revision of the legislation to consider also the presence of emerging contaminants in SeS [104].
4. Conclusions and Future Perspectives
The analysis of the literature showed that, to date, there is not a standard method for the extraction and analysis of MPs from SeS, causing difficulties in the direct comparison of the results obtained in the different studies regarding the quantity and quality of MPs in the SeS. Moreover, the effect of both the surface characteristics and the MPs size on the chemical activity and the fate of the particles needs further research work. The quantity of MPs in sludge seems to be mainly a function of two aspects: (i) the configuration of water line treatments in WWTPs and (ii) MPs presence in the influential WW. To date, no clear direct influence of the type of treatments present in the sludge line and MPs found in sludge line can be highlighted. From the analyzed studies, the presence of MPs can affect the properties of the SeS by altering the adsorption capacity of metals and modifying the digestibility of the sludge itself. Further, some results regarding the positive or negative effects induced by MPs and/or by the metals adsorbed on MPs appear to be controversial, and more focused studied with common guidelines are needed to clarify these aspects. Regarding the presence of MPs in soil improvers, although more data are required, based on preliminary studies, the sludge spreading seems to be a significant pathway for the diffusion of MPs in the environment. However, several aspects, such as the impact of the diverse types of SeS, the influence of the initial concentration of MPs, environmental factors, the influence of diverse crops and types of soil, remain unclear and should be better clarified in future research. The determination of the type of MPs represents an aspect which could be exploited to better evaluate the dispersion in the environment of MPs following the spread of SeS using fibers and fragments (very common in SeS) as an indicator of a WWTP-environment pathway.
Author Contributions
Conceptualization, M.C.C. and M.C.M.; methodology, M.C.M.; validation, M.C.C. and C.M.; formal analysis, M.C.M. and F.M.C.; data curation, M.C.M.; writing—original draft preparation, M.C.C., M.C.M., F.M.C. and C.M.; writing—review and editing, M.C.M. and C.M.; visualization, F.M.C.; supervision, M.C.C. and C.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| A/A/O | anaerobic/anoxic/aerobic; |
| AFB | aerobic fluidized bioreactor; |
| ASTM | American society for testing and materials; |
| BAF | biologically active filter; |
| CAS | conventional active sludge; |
| DM | dry matter; |
| FTIR | micro-Fourier transform infrared; |
| IW | industrial wastewater; |
| LDPE | low-density polyethylene; |
| MBBR | moving bed biofilm reactor; |
| MBR | membrane bioreactor; |
| MIS | mixed municipal and industrial sludge; |
| MPs | microplastics; |
| MS | municipal sludge; |
| MSBR | modified sequencing batch reactor; |
| MW | urban wastewater with a large contribution of industrial wastewater (mixed wastewater); |
| n.a. | not available; |
| NF | nanofiltration; |
| P | primary sludge; |
| PA | polyamide; |
| PE | polyethylene; |
| PET | polyethylene terephthalate; |
| PRISMA | preferred reporting items for systematic reviews and meta-analysis; |
| PS | polystyrene; |
| Pyr-GC/MS | pyrolysis-gas chromatography/mass spectrometry; |
| RFE | rapid filtration equipment; |
| RO | reverse osmosis; |
| S | secondary/biological sludge; |
| SBR | sequencing batch reactor; |
| SeS | sewage sludge; |
| TF | trickling filters; |
| UF | ultrafiltration; |
| UMW | not specified if urban or mixed wastewater; |
| UW | urban wastewater; |
| WW | wastewater; |
| WWTPs | wastewater treatment plants. |
References
- Graca, B.; Szewc, K.; Zakrzewska, D.; Dołęga, A.; Szczerbowska-Boruchowska, M. Sources and fate of microplastics in marine and beach sediments of the Southern Baltic Sea—A preliminary study. Environ. Sci. Pollut. Res. 2017, 24, 7650–7661. [Google Scholar] [CrossRef] [Green Version]
- Rocha-Santos, T.; Duarte, A.C. A critical overview of the analytical approaches to the occurrence, the fate and the behavior of microplastics in the environment. TrAC Trends Anal. Chem. 2015, 65, 47–53. [Google Scholar] [CrossRef]
- WHO. Microplastics in Drinking-Water; WHO: Geneva, Switzerland, 2019. [Google Scholar]
- Frias, J.P.G.L.; Nash, R. Microplastics: Finding a consensus on the definition. Mar. Pollut. Bull. 2019, 138, 145–147. [Google Scholar] [CrossRef]
- Iyare, P.U.; Ouki, S.K.; Bond, T. Microplastics removal in wastewater treatment plants: A critical review. Environ. Sci. Water Res. Technol. 2020, 6, 2664–2675. [Google Scholar] [CrossRef]
- Kole, P.J.; Löhr, A.J.; Van Belleghem, F.; Ragas, A. Wear and Tear of Tyres: A Stealthy Source of Microplastics in the Environment. Int. J. Environ. Res. Public Health 2017, 14, 1265. [Google Scholar] [CrossRef]
- De Falco, F.; Di Pace, E.; Cocca, M.; Avella, M. The contribution of washing processes of synthetic clothes to microplastic pollution. Sci. Rep. 2019, 9, 6633. [Google Scholar] [CrossRef]
- Guerranti, C.; Martellini, T.; Perra, G.; Scopetani, C.; Cincinelli, A. Microplastics in cosmetics: Environmental issues and needs for global bans. Environ. Toxicol. Pharmacol. 2019, 68, 75–79. [Google Scholar] [CrossRef]
- Thompson, R.C. Microplastics in the Marine Environment: Sources, Consequences and Solutions. In Marine Anthropogenic Litter; Springer International Publishing: Berlin/Heidelberg, Germany, 2015; pp. 185–200. [Google Scholar]
- Borrelle, S.B.; Ringma, J.; Law, K.L.; Monnahan, C.C.; Lebreton, L.; McGivern, A.; Murphy, E.; Jambeck, J.; Leonard, G.H.; Hilleary, M.A.; et al. Predicted growth in plastic waste exceeds efforts to mitigate plastic pollution. Science 2020, 369, 1515–1518. [Google Scholar] [CrossRef]
- Hatinoğlu, M.D.; Sanin, F.D. Sewage sludge as a source of microplastics in the environment: A review of occurrence and fate during sludge treatment. J. Environ. Manag. 2021, 295, 113028. [Google Scholar] [CrossRef]
- Wang, C.; Zhao, J.; Xing, B. Environmental source, fate, and toxicity of microplastics. J. Hazard. Mater. 2021, 407, 124357. [Google Scholar] [CrossRef]
- Selonen, S.; Dolar, A.; Jemec Kokalj, A.; Skalar, T.; Parramon Dolcet, L.; Hurley, R.; van Gestel, C.A.M. Exploring the impacts of plastics in soil—The effects of polyester textile fibers on soil invertebrates. Sci. Total Environ. 2020, 700, 134451. [Google Scholar] [CrossRef]
- Sun, J.; Dai, X.; Wang, Q.; van Loosdrecht, M.C.M.; Ni, B.-J. Microplastics in wastewater treatment plants: Detection, occurrence and removal. Water Res. 2019, 152, 21–37. [Google Scholar] [CrossRef] [PubMed]
- He, Z.-W.; Yang, W.-J.; Ren, Y.-X.; Jin, H.-Y.; Tang, C.-C.; Liu, W.-Z.; Yang, C.-X.; Zhou, A.-J.; Wang, A.-J. Occurrence, effect, and fate of residual microplastics in anaerobic digestion of waste activated sludge: A state-of-the-art review. Bioresour. Technol. 2021, 331, 125035. [Google Scholar] [CrossRef] [PubMed]
- Vethaak, A.D.; Legler, J. Microplastics and human health. Science 2021, 371, 672–674. [Google Scholar] [CrossRef] [PubMed]
- Yong, C.; Valiyaveettil, S.; Tang, B. Toxicity of Microplastics and Nanoplastics in Mammalian Systems. Int. J. Environ. Res. Public Health 2020, 17, 1509. [Google Scholar] [CrossRef] [Green Version]
- Blackburn, K.; Green, D. The potential effects of microplastics on human health: What is known and what is unknown. Ambio 2021. [Google Scholar] [CrossRef]
- Carr, S.A.; Liu, J.; Tesoro, A.G. Transport and fate of microplastic particles in wastewater treatment plants. Water Res. 2016, 91, 174–182. [Google Scholar] [CrossRef]
- Ziajahromi, S.; Neale, P.A.; Rintoul, L.; Leusch, F.D.L. Wastewater treatment plants as a pathway for microplastics: Development of a new approach to sample wastewater-based microplastics. Water Res. 2017, 112, 93–99. [Google Scholar] [CrossRef]
- Mahon, A.M.; O’Connell, B.; Healy, M.G.; O’Connor, I.; Officer, R.; Nash, R.; Morrison, L. Microplastics in Sewage Sludge: Effects of Treatment. Environ. Sci. Technol. 2017, 51, 810–818. [Google Scholar] [CrossRef]
- Mason, S.A.; Garneau, D.; Sutton, R.; Chu, Y.; Ehmann, K.; Barnes, J.; Fink, P.; Papazissimos, D.; Rogers, D.L. Microplastic pollution is widely detected in US municipal wastewater treatment plant effluent. Environ. Pollut. 2016, 218, 1045–1054. [Google Scholar] [CrossRef] [PubMed]
- Talvitie, J.; Mikola, A.; Koistinen, A.; Setälä, O. Solutions to microplastic pollution—Removal of microplastics from wastewater effluent with advanced wastewater treatment technologies. Water Res. 2017, 123, 401–407. [Google Scholar] [CrossRef] [Green Version]
- Estahbanati, S.; Fahrenfeld, N.L. Influence of wastewater treatment plant discharges on microplastic concentrations in surface water. Chemosphere 2016, 162, 277–284. [Google Scholar] [CrossRef]
- Zhang, B.; Yang, X.; Chen, L.; Chao, J.; Teng, J.; Wang, Q. Microplastics in soils: A review of possible sources, analytical methods and ecological impacts. J. Chem. Technol. Biotechnol. 2020, 95, 2052–2068. [Google Scholar] [CrossRef]
- Hurley, R.R.; Nizzetto, L. Fate and occurrence of micro(nano)plastics in soils: Knowledge gaps and possible risks. Curr. Opin. Environ. Sci. Health 2018, 1, 6–11. [Google Scholar] [CrossRef]
- Li, X.; Chen, L.; Mei, Q.; Dong, B.; Dai, X.; Ding, G.; Zeng, E.Y. Microplastics in sewage sludge from the wastewater treatment plants in China. Water Res. 2018, 142, 75–85. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, J.; Liu, H.; Guo, X.; Zhang, X.; Yao, X.; Cao, Z.; Zhang, T. A review of the removal of microplastics in global wastewater treatment plants: Characteristics and mechanisms. Environ. Int. 2021, 146, 106277. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- ASTM D8332-20 Standard. Practice for Collection of Water Samples with High, Medium, or Low Suspended Solids for Identification and Quantification of Microplastic Particles and Fibers; American Society for Testing and Materials International: West Conshohocken, PA, USA, 2020. [Google Scholar]
- ASTM D8333-20 Standard. Practice for Preparation of Water Samples with High, Medium, or Low Suspended Solids for Identification and Quantification of Microplastic Particles and Fibers Using Raman Spectroscopy, IR Spectroscopy, or Pyrolysis-GC/MS; American Society for Testing and Materials International: West Conshohocken, PA, USA, 2020. [Google Scholar]
- Lusher, A.L.; Hurley, R.R.; Vogelsang, C.; Nizzetto, L.; Olsen, M. Mapping Microplastics in Sludge; Norwegian Institute for Water Research: Oslo, Norway, 2017. [Google Scholar]
- Okoffo, E.D.; O’Brien, S.; O’Brien, J.W.; Tscharke, B.J.; Thomas, K.V. Wastewater treatment plants as a source of plastics in the environment: A review of occurrence, methods for identification, quantification and fate. Environ. Sci. Water Res. Technol. 2019, 5, 1908–1931. [Google Scholar] [CrossRef]
- Lares, M.; Ncibi, M.C.; Sillanpää, M.; Sillanpää, M. Occurrence, identification and removal of microplastic particles and fibers in conventional activated sludge process and advanced MBR technology. Water Res. 2018, 133, 236–246. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Kim, Y. Treatment characteristics of microplastics at biological sewage treatment facilities in Korea. Mar. Pollut. Bull. 2018, 137, 1–8. [Google Scholar] [CrossRef]
- Murphy, F.; Ewins, C.; Carbonnier, F.; Quinn, B. Wastewater Treatment Works (WwTW) as a Source of Microplastics in the Aquatic Environment. Environ. Sci. Technol. 2016, 50, 5800–5808. [Google Scholar] [CrossRef] [Green Version]
- Hongprasith, N.; Kittimethawong, C.; Lertluksanaporn, R.; Eamchotchawalit, T.; Kittipongvises, S.; Lohwacharin, J. IR microspectroscopic identification of microplastics in municipal wastewater treatment plants. Environ. Sci. Pollut. Res. 2020, 27, 18557–18564. [Google Scholar] [CrossRef] [PubMed]
- Magnusson, K.; Norén, F. Screening of Microplastic Particles in and Down-Stream a Wastewater Treatment Plant; IVL Swedish Environmental Research Institute: Stockholm, Sweden, 2014. [Google Scholar]
- Ren, P.; Dou, M.; Wang, C.; Li, G.; Jia, R. Abundance and removal characteristics of microplastics at a wastewater treatment plant in Zhengzhou. Environ. Sci. Pollut. Res. 2020, 27, 36295–36305. [Google Scholar] [CrossRef]
- Bretas Alvim, C.; Mendoza-Roca, J.A.; Bes-Piá, A. Wastewater treatment plant as microplastics release source—Quantification and identification techniques. J. Environ. Manag. 2020, 255, 109739. [Google Scholar] [CrossRef]
- Edo, C.; González-Pleiter, M.; Leganés, F.; Fernández-Piñas, F.; Rosal, R. Fate of microplastics in wastewater treatment plants and their environmental dispersion with effluent and sludge. Environ. Pollut. 2020, 259, 113837. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Chen, L.; Ji, Y.; Li, M.; Dong, B.; Qian, G.; Zhou, J.; Dai, X. Effects of chemical pretreatments on microplastic extraction in sewage sludge and their physicochemical characteristics. Water Res. 2020, 171, 115379. [Google Scholar] [CrossRef]
- Hurley, R.R.; Lusher, A.L.; Olsen, M.; Nizzetto, L. Validation of a Method for Extracting Microplastics from Complex, Organic-Rich, Environmental Matrices. Environ. Sci. Technol. 2018, 52, 7409–7417. [Google Scholar] [CrossRef] [Green Version]
- Li, Q.; Wu, J.; Zhao, X.; Gu, X.; Ji, R. Separation and identification of microplastics from soil and sewage sludge. Environ. Pollut. 2019, 254, 113076. [Google Scholar] [CrossRef]
- Lv, L.; Qu, J.; Yu, Z.; Chen, D.; Zhou, C.; Hong, P.; Sun, S.; Li, C. A simple method for detecting and quantifying microplastics utilizing fluorescent dyes—Safranine T, fluorescein isophosphate, Nile red based on thermal expansion and contraction property. Environ. Pollut. 2019, 255, 113283. [Google Scholar] [CrossRef]
- Bayo, J.; Olmos, S.; López-Castellanos, J. Microplastics in an urban wastewater treatment plant: The influence of physicochemical parameters and environmental factors. Chemosphere 2020, 238, 124593. [Google Scholar] [CrossRef]
- Silva, A.B.; Bastos, A.S.; Justino, C.I.L.; da Costa, J.P.; Duarte, A.C.; Rocha-Santos, T.A.P. Microplastics in the environment: Challenges in analytical chemistry—A review. Anal. Chim. Acta 2018, 1017, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Wesch, C.; Bredimus, K.; Paulus, M.; Klein, R. Towards the suitable monitoring of ingestion of microplastics by marine biota: A review. Environ. Pollut. 2016, 218, 1200–1208. [Google Scholar] [CrossRef]
- Elert, A.M.; Becker, R.; Duemichen, E.; Eisentraut, P.; Falkenhagen, J.; Sturm, H.; Braun, U. Comparison of different methods for MP detection: What can we learn from them, and why asking the right question before measurements matters? Environ. Pollut. 2017, 231, 1256–1264. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, B.; Claveau-Mallet, D.; Hernandez, L.M.; Xu, E.G.; Farner, J.M.; Tufenkji, N. Separation and Analysis of Microplastics and Nanoplastics in Complex Environmental Samples. Acc. Chem. Res. 2019, 52, 858–866. [Google Scholar] [CrossRef] [Green Version]
- Araujo, C.F.; Nolasco, M.M.; Ribeiro, A.M.P.; Ribeiro-Claro, P.J.A. Identification of microplastics using Raman spectroscopy: Latest developments and future prospects. Water Res. 2018, 142, 426–440. [Google Scholar] [CrossRef]
- Mallow, O.; Spacek, S.; Schwarzböck, T.; Fellner, J.; Rechberger, H. A new thermoanalytical method for the quantification of microplastics in industrial wastewater. Environ. Pollut. 2020, 259, 113862. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, M.; Mei, Q.; Niu, S.; Wang, X.; Xu, H.; Dong, B.; Dai, X.; Zhou, J.L. Aging microplastics in wastewater pipeline networks and treatment processes: Physicochemical characteristics and Cd adsorption. Sci. Total Environ. 2021, 797, 148940. [Google Scholar] [CrossRef]
- Rozman, U.; Turk, T.; Skalar, T.; Zupančič, M.; Čelan Korošin, N.; Marinšek, M.; Olivero-Verbel, J.; Kalčíková, G. An extensive characterization of various environmentally relevant microplastics—Material properties, leaching and ecotoxicity testing. Sci. Total Environ. 2021, 773, 145576. [Google Scholar] [CrossRef]
- Liu, X.; Yuan, W.; Di, M.; Li, Z.; Wang, J. Transfer and fate of microplastics during the conventional activated sludge process in one wastewater treatment plant of China. Chem. Eng. J. 2019, 362, 176–182. [Google Scholar] [CrossRef]
- Huang, X.; Zemlyanov, D.Y.; Diaz-Amaya, S.; Salehi, M.; Stanciu, L.; Whelton, A.J. Competitive heavy metal adsorption onto new and aged polyethylene under various drinking water conditions. J. Hazard. Mater. 2020, 385, 121585. [Google Scholar] [CrossRef]
- Yang, Z.; Li, S.; Ma, S.; Liu, P.; Peng, D.; Ouyang, Z.; Guo, X. Characteristics and removal efficiency of microplastics in sewage treatment plant of Xi’an City, northwest China. Sci. Total Environ. 2021, 771, 145377. [Google Scholar] [CrossRef] [PubMed]
- Tadsuwan, K.; Babel, S. Microplastic contamination in a conventional wastewater treatment plant in Thailand. Waste Manag. Res. J. Sustain. Circ. Econ. 2021, 39, 754–761. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, J.; Xie, Y.; Zhong, S.; Gao, P. Occurrence and removal of microplastics from wastewater treatment plants in a typical tourist city in China. J. Clean. Prod. 2021, 291, 125968. [Google Scholar] [CrossRef]
- Sun, J.; Zhu, Z.-R.; Li, W.-H.; Yan, X.; Wang, L.-K.; Zhang, L.; Jin, J.; Dai, X.; Ni, B.-J. Revisiting Microplastics in Landfill Leachate: Unnoticed Tiny Microplastics and Their Fate in Treatment Works. Water Res. 2021, 190, 116784. [Google Scholar] [CrossRef]
- Zhang, Z.; Su, Y.; Zhu, J.; Shi, J.; Huang, H.; Xie, B. Distribution and removal characteristics of microplastics in different processes of the leachate treatment system. Waste Manag. 2021, 120, 240–247. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, H.; Xu, J.; Su, X.; Lu, M.; Wang, Z.; Zhang, Y. Occurrence and Characteristics of Microplastics in a Wastewater Treatment Plant. Bull. Environ. Contam. Toxicol. 2021, 107, 677–683. [Google Scholar] [CrossRef] [PubMed]
- Pittura, L.; Foglia, A.; Akyol, Ç.; Cipolletta, G.; Benedetti, M.; Regoli, F.; Eusebi, A.L.; Sabbatini, S.; Tseng, L.Y.; Katsou, E.; et al. Microplastics in real wastewater treatment schemes: Comparative assessment and relevant inhibition effects on anaerobic processes. Chemosphere 2021, 262, 128415. [Google Scholar] [CrossRef]
- Ziajahromi, S.; Neale, P.A.; Telles Silveira, I.; Chua, A.; Leusch, F.D.L. An audit of microplastic abundance throughout three Australian wastewater treatment plants. Chemosphere 2021, 263, 128294. [Google Scholar] [CrossRef] [PubMed]
- Naji, A.; Azadkhah, S.; Farahani, H.; Uddin, S.; Khan, F.R. Microplastics in wastewater outlets of Bandar Abbas city (Iran): A potential point source of microplastics into the Persian Gulf. Chemosphere 2021, 262, 128039. [Google Scholar] [CrossRef] [PubMed]
- Bretas Alvim, C.; Bes-Piá, M.A.; Mendoza-Roca, J.A. Separation and identification of microplastics from primary and secondary effluents and activated sludge from wastewater treatment plants. Chem. Eng. J. 2020, 402, 126293. [Google Scholar] [CrossRef]
- Jiang, J.; Wang, X.; Ren, H.; Cao, G.; Xie, G.; Xing, D.; Liu, B. Investigation and fate of microplastics in wastewater and sludge filter cake from a wastewater treatment plant in China. Sci. Total Environ. 2020, 746, 141378. [Google Scholar] [CrossRef]
- Tang, N.; Liu, X.; Xing, W. Microplastics in wastewater treatment plants of Wuhan, Central China: Abundance, removal, and potential source in household wastewater. Sci. Total Environ. 2020, 745, 141026. [Google Scholar] [CrossRef] [PubMed]
- Raju, S.; Carbery, M.; Kuttykattil, A.; Senthirajah, K.; Lundmark, A.; Rogers, Z.; SCB, S.; Evans, G.; Palanisami, T. Improved methodology to determine the fate and transport of microplastics in a secondary wastewater treatment plant. Water Res. 2020, 173, 115549. [Google Scholar] [CrossRef] [PubMed]
- Kazour, M.; Terki, S.; Rabhi, K.; Jemaa, S.; Khalaf, G.; Amara, R. Sources of microplastics pollution in the marine environment: Importance of wastewater treatment plant and coastal landfill. Mar. Pollut. Bull. 2019, 146, 608–618. [Google Scholar] [CrossRef] [PubMed]
- Lv, X.; Dong, Q.; Zuo, Z.; Liu, Y.; Huang, X.; Wu, W.-M. Microplastics in a municipal wastewater treatment plant: Fate, dynamic distribution, removal efficiencies, and control strategies. J. Clean. Prod. 2019, 225, 579–586. [Google Scholar] [CrossRef]
- Magni, S.; Binelli, A.; Pittura, L.; Avio, C.G.; Della Torre, C.; Parenti, C.C.; Gorbi, S.; Regoli, F. The fate of microplastics in an Italian Wastewater Treatment Plant. Sci. Total Environ. 2019, 652, 602–610. [Google Scholar] [CrossRef] [PubMed]
- Gies, E.A.; LeNoble, J.L.; Noël, M.; Etemadifar, A.; Bishay, F.; Hall, E.R.; Ross, P.S. Retention of microplastics in a major secondary wastewater treatment plant in Vancouver, Canada. Mar. Pollut. Bull. 2018, 133, 553–561. [Google Scholar] [CrossRef]
- Talvitie, J.; Mikola, A.; Setälä, O.; Heinonen, M.; Koistinen, A. How well is microlitter purified from wastewater?—A detailed study on the stepwise removal of microlitter in a tertiary level wastewater treatment plant. Water Res. 2017, 109, 164–172. [Google Scholar] [CrossRef] [Green Version]
- Vardar, S.; Onay, T.T.; Demirel, B.; Kideys, A.E. Evaluation of microplastics removal efficiency at a wastewater treatment plant discharging to the Sea of Marmara. Environ. Pollut. 2021, 289, 117862. [Google Scholar] [CrossRef]
- Nakao, S.; Akita, K.; Ozaki, A.; Masumoto, K.; Okuda, T. Circulation of fibrous microplastic (microfiber) in sewage and sewage sludge treatment processes. Sci. Total Environ. 2021, 795, 148873. [Google Scholar] [CrossRef]
- Rasmussen, L.A.; Iordachescu, L.; Tumlin, S.; Vollertsen, J. A complete mass balance for plastics in a wastewater treatment plant—Macroplastics contributes more than microplastics. Water Res. 2021, 201, 117307. [Google Scholar] [CrossRef]
- Salmi, P.; Ryymin, K.; Karjalainen, A.K.; Mikola, A.; Uurasjärvi, E.; Talvitie, J. Particle balance and return loops for microplastics in a tertiary-level wastewater treatment plant. Water Sci. Technol. 2021, 84, 89–100. [Google Scholar] [CrossRef]
- Cunsolo, S.; Williams, J.; Hale, M.; Read, D.S.; Couceiro, F. Optimising sample preparation for FTIR-based microplastic analysis in wastewater and sludge samples: Multiple digestions. Anal. Bioanal. Chem. 2021, 413, 3789–3799. [Google Scholar] [CrossRef]
- Blair, R.M.; Waldron, S.; Gauchotte-Lindsay, C. Average daily flow of microplastics through a tertiary wastewater treatment plant over a ten-month period. Water Res. 2019, 163, 114909. [Google Scholar] [CrossRef]
- Martellone, L.; Lucentini, L.; Mattei, D.; De Vincenzo, M.; Favero, G.; Bogialli, S.; Litti, L.; Meneghetti, M.; Corami, F.; Rosso, B. Sampling Strategies and Pretreatment Methods for Microplastics in Aquatic Environments (In Italian); Rapporti ISTISAN 21/2; Italian National Institute of Health: Rome, Italy, 2021.
- Rochman, C.M. Microplastics research—From sink to source. Science 2018, 360, 28–29. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Chatterjee, S. Microplastic pollution, a threat to marine ecosystem and human health: A short review. Environ. Sci. Pollut. Res. 2017, 24, 21530–21547. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Mei, Q.; Chen, L.; Zhang, H.; Dong, B.; Dai, X.; He, C.; Zhou, J. Enhancement in adsorption potential of microplastics in sewage sludge for metal pollutants after the wastewater treatment process. Water Res. 2019, 157, 228–237. [Google Scholar] [CrossRef]
- Ceccarini, A.; Corti, A.; Erba, F.; Modugno, F.; La Nasa, J.; Bianchi, S.; Castelvetro, V. The Hidden Microplastics: New Insights and Figures from the Thorough Separation and Characterization of Microplastics and of Their Degradation Byproducts in Coastal Sediments. Environ. Sci. Technol. 2018, 52, 5634–5643. [Google Scholar] [CrossRef] [PubMed]
- Wijesekara, H.; Bolan, N.S.; Bradney, L.; Obadamudalige, N.; Seshadri, B.; Kunhikrishnan, A.; Dharmarajan, R.; Ok, Y.S.; Rinklebe, J.; Kirkham, M.B.; et al. Trace element dynamics of biosolids-derived microbeads. Chemosphere 2018, 199, 331–339. [Google Scholar] [CrossRef] [PubMed]
- Turner, A.; Holmes, L.A. Adsorption of trace metals by microplastic pellets in fresh water. Environ. Chem. 2015, 12, 600. [Google Scholar] [CrossRef]
- Wei, W.; Huang, Q.-S.; Sun, J.; Wang, J.-Y.; Wu, S.-L.; Ni, B.-J. Polyvinyl Chloride Microplastics Affect Methane Production from the Anaerobic Digestion of Waste Activated Sludge through Leaching Toxic Bisphenol-A. Environ. Sci. Technol. 2019, 53, 2509–2517. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Hao, Q.; Chen, Z.; Bao, T.; Ni, B.-J. Polystyrene nanoplastics reshape the anaerobic granular sludge for recovering methane from wastewater. Water Res. 2020, 182, 116041. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhao, M.; Li, C.; Miao, H.; Huang, Z.; Dai, X.; Ruan, W. Evaluation the impact of polystyrene micro and nanoplastics on the methane generation by anaerobic digestion. Ecotoxicol. Environ. Saf. 2020, 205, 111095. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Zhang, Y.-T.; Huang, Q.-S.; Ni, B.-J. Polyethylene terephthalate microplastics affect hydrogen production from alkaline anaerobic fermentation of waste activated sludge through altering viability and activity of anaerobic microorganisms. Water Res. 2019, 163, 114881. [Google Scholar] [CrossRef]
- Feng, Y.; Duan, J.-L.; Sun, X.-D.; Ma, J.-Y.; Wang, Q.; Li, X.-Y.; Tian, W.-X.; Wang, S.-G.; Yuan, X.-Z. Insights on the inhibition of anaerobic digestion performances under short-term exposure of metal-doped nanoplastics via Methanosarcina acetivorans. Environ. Pollut. 2021, 275, 115755. [Google Scholar] [CrossRef]
- Wei, W.; Chen, X.; Peng, L.; Liu, Y.; Bao, T.; Ni, B.-J. The entering of polyethylene terephthalate microplastics into biological wastewater treatment system affects aerobic sludge digestion differently from their direct entering into sludge treatment system. Water Res. 2021, 190, 116731. [Google Scholar] [CrossRef]
- Mohammad Mirsoleimani Azizi, S.; Hai, F.I.; Lu, W.; Al-Mamun, A.; Ranjan Dhar, B. A review of mechanisms underlying the impacts of (nano)microplastics on anaerobic digestion. Bioresour. Technol. 2021, 329, 124894. [Google Scholar] [CrossRef] [PubMed]
- Nizzetto, L.; Bussi, G.; Futter, M.N.; Butterfield, D.; Whitehead, P.G. A theoretical assessment of microplastic transport in river catchments and their retention by soils and river sediments. Environ. Sci. Process. Impacts 2016, 18, 1050–1059. [Google Scholar] [CrossRef]
- Baensch-Baltruschat, B.; Kocher, B.; Kochleus, C.; Stock, F.; Reifferscheid, G. Tyre and road wear particles—A calculation of generation, transport and release to water and soil with special regard to German roads. Sci. Total Environ. 2021, 752, 141939. [Google Scholar] [CrossRef]
- Corradini, F.; Meza, P.; Eguiluz, R.; Casado, F.; Huerta-Lwanga, E.; Geissen, V. Evidence of microplastic accumulation in agricultural soils from sewage sludge disposal. Sci. Total Environ. 2019, 671, 411–420. [Google Scholar] [CrossRef]
- Zhang, L.; Xie, Y.; Liu, J.; Zhong, S.; Qian, Y.; Gao, P. An Overlooked Entry Pathway of Microplastics into Agricultural Soils from Application of Sludge-Based Fertilizers. Environ. Sci. Technol. 2020, 54, 4248–4255. [Google Scholar] [CrossRef]
- Van den Berg, P.; Huerta-Lwanga, E.; Corradini, F.; Geissen, V. Sewage sludge application as a vehicle for microplastics in eastern Spanish agricultural soils. Environ. Pollut. 2020, 261, 114198. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Li, L.; Li, R.; Xu, L.; Shen, Y.; Li, S.; Tu, C.; Wu, L.; Christie, P.; Luo, Y. Microplastics in an agricultural soil following repeated application of three types of sewage sludge: A field study. Environ. Pollut. 2021, 289, 117943. [Google Scholar] [CrossRef]
- Lu, L.; Luo, T.; Zhao, Y.; Cai, C.; Fu, Z.; Jin, Y. Interaction between microplastics and microorganism as well as gut microbiota: A consideration on environmental animal and human health. Sci. Total Environ. 2019, 667, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Collivignarelli, M.C.; Abbà, A.; Frattarola, A.; Carnevale Miino, M.; Padovani, S.; Katsoyiannis, J.; Torretta, V. Legislation for the Reuse of Biosolids on Agricultural Land in Europe: Overview. Sustainability 2019, 11, 6015. [Google Scholar] [CrossRef] [Green Version]
- Council of the European Union. Council Directive 86/278/EEC of 12 June 1986 on the Protection of the Environment, And in Particular of the Soil, When Sewage Sludge Is Used in Agriculture; Council of the European Union: Luxembourg, 1986; pp. 6–12.
- EC Sewage Sludge. Available online: https://ec.europa.eu/environment/topics/waste-and-recycling/sewage-sludge_en (accessed on 1 October 2021).
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).