Assessment of the Heavy Metals Pollution and Ecological Risk in Sediments of Mediterranean Sea Drain Estuaries in Egypt and Phytoremediation Potential of Two Emergent Plants
Abstract
1. Introduction
2. Materials and Methods
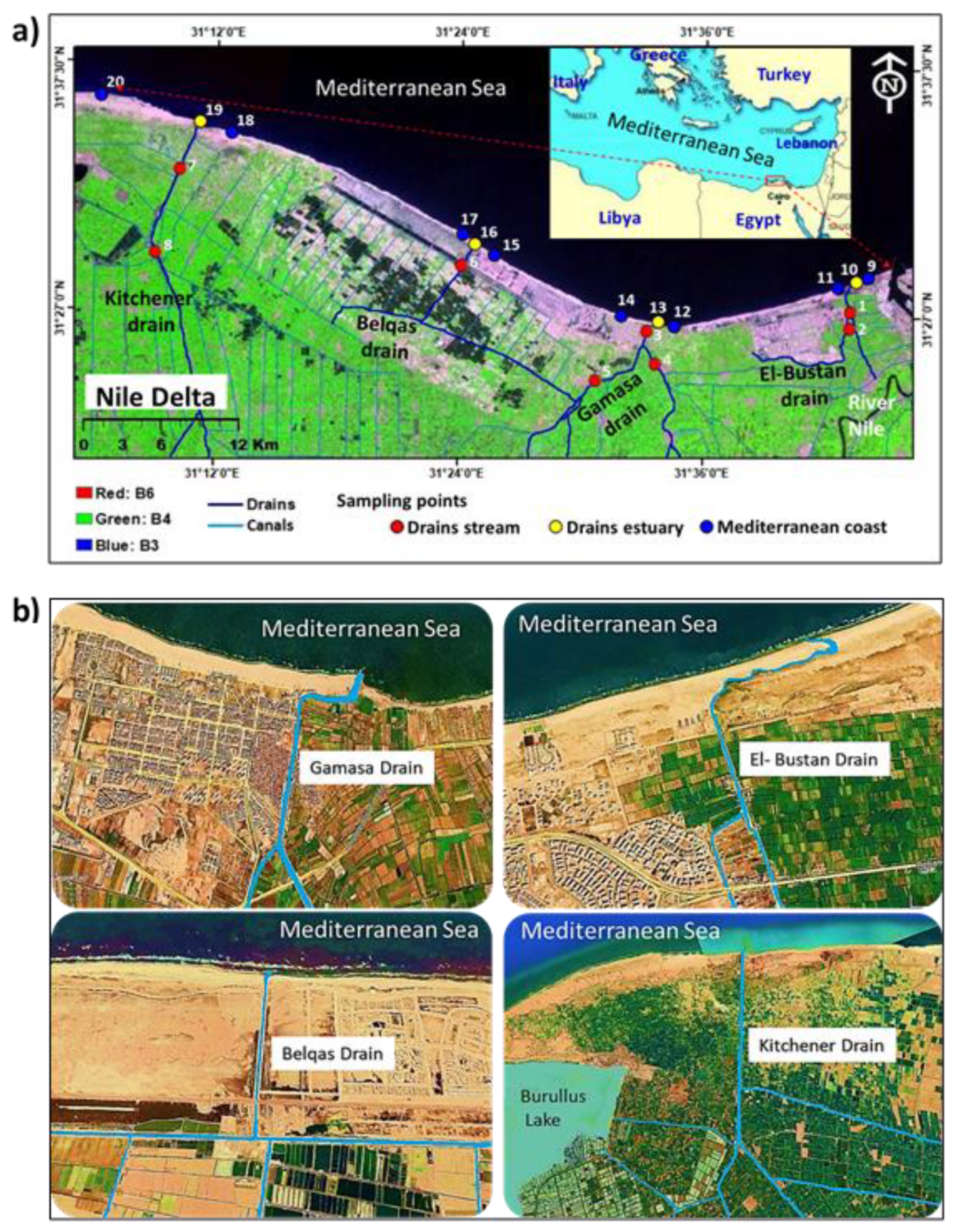
2.1. Study Area
2.2. Samples Collection and Preparation
2.3. Heavy Metals Analysis
2.4. Pollution Quantification
2.5. Phytoremediation Efficiency of Macrophyte Species
2.6. Statistical Analysis
3. Results and Discussion
3.1. Heavy Metals in Sediment
3.2. Statistical Analysis
3.2.1. Correlation Coefficient Analysis of Heavy Metals
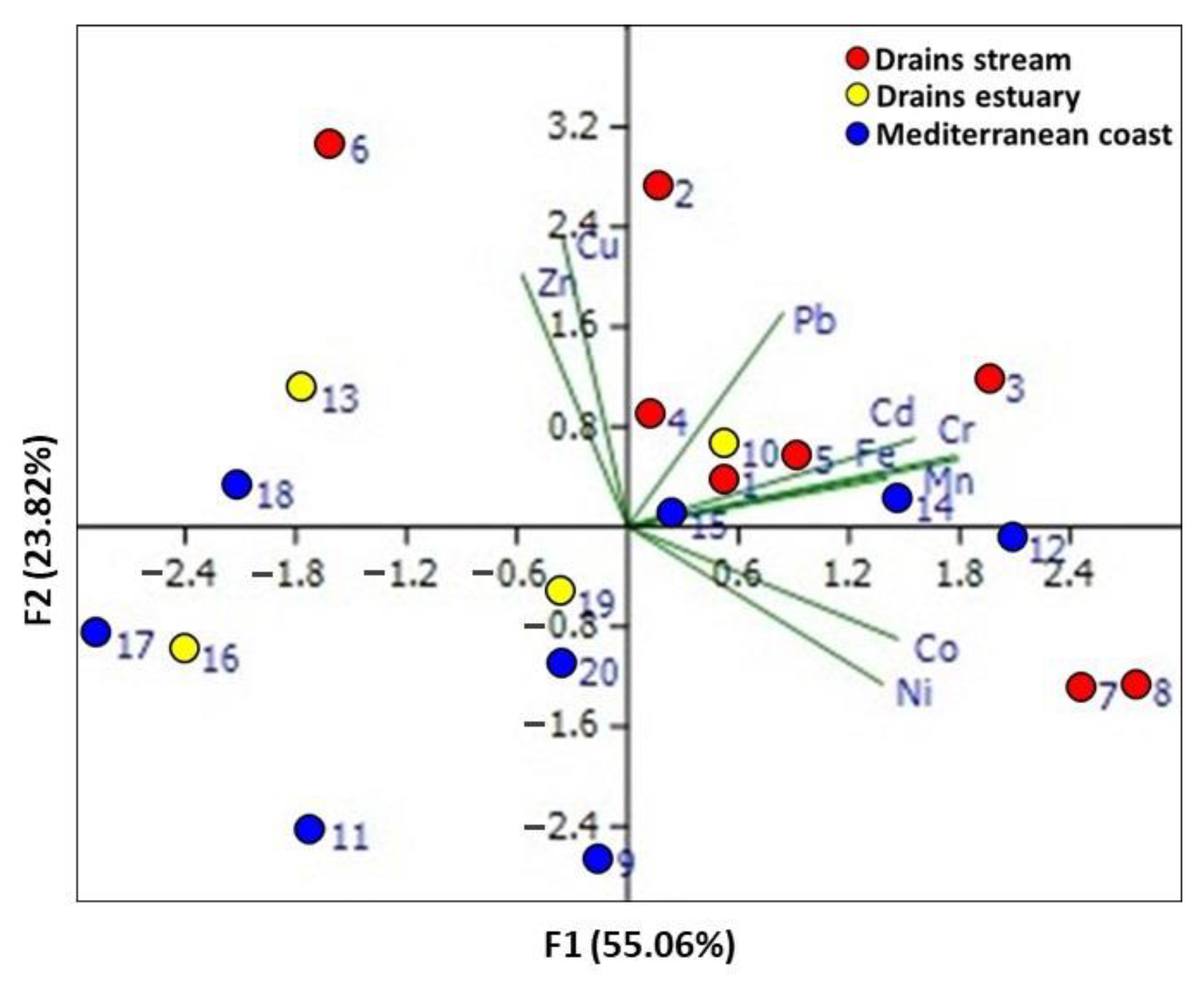
3.2.2. Principal Component Analysis (PCA)
3.3. Assessment of Sediment Contamination
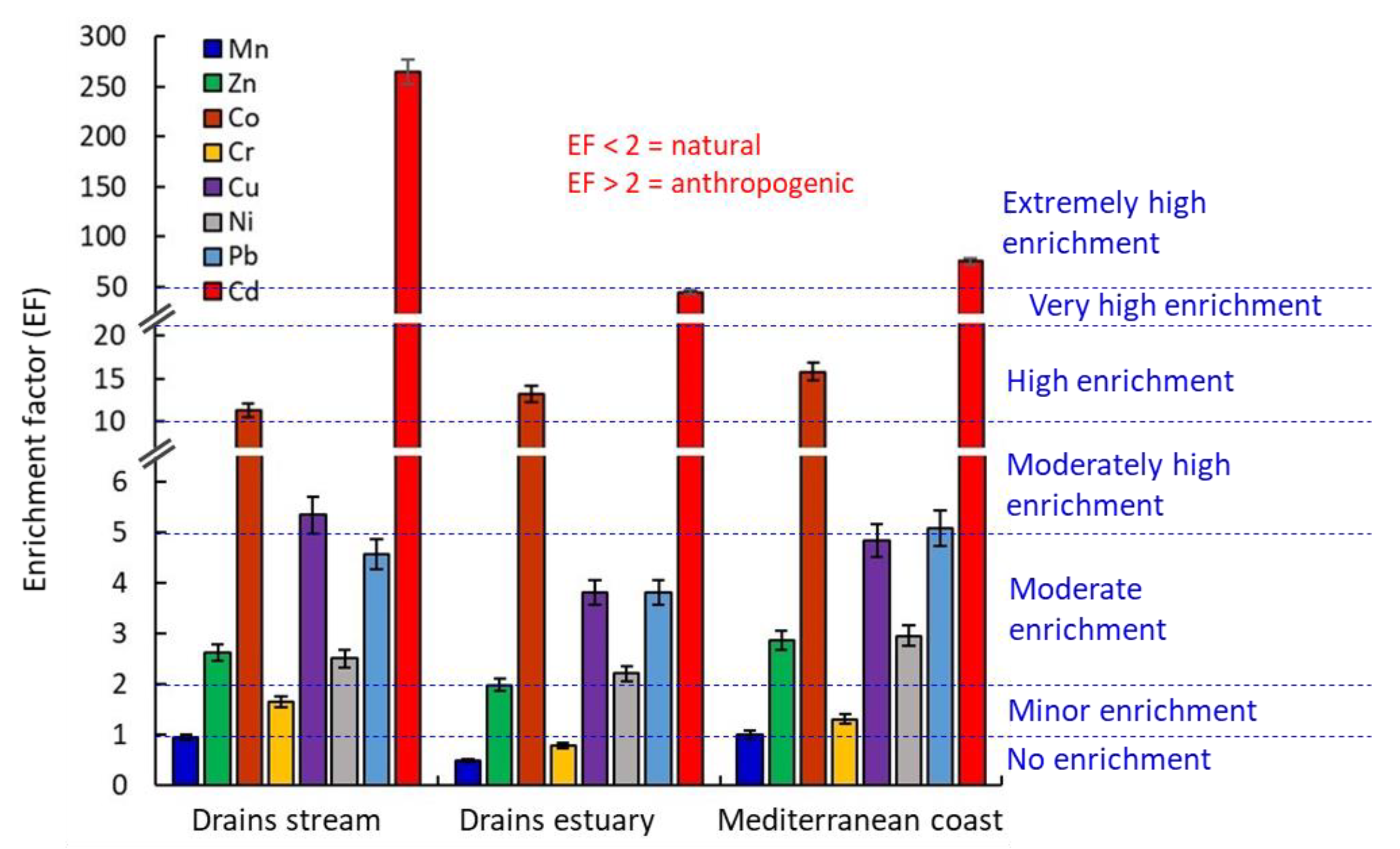
3.3.1. Enrichment Factor
3.3.2. Contamination Factor (Cf) and Contamination Degree (CD)
3.3.3. Geo-Accumulation Index (Igeo)
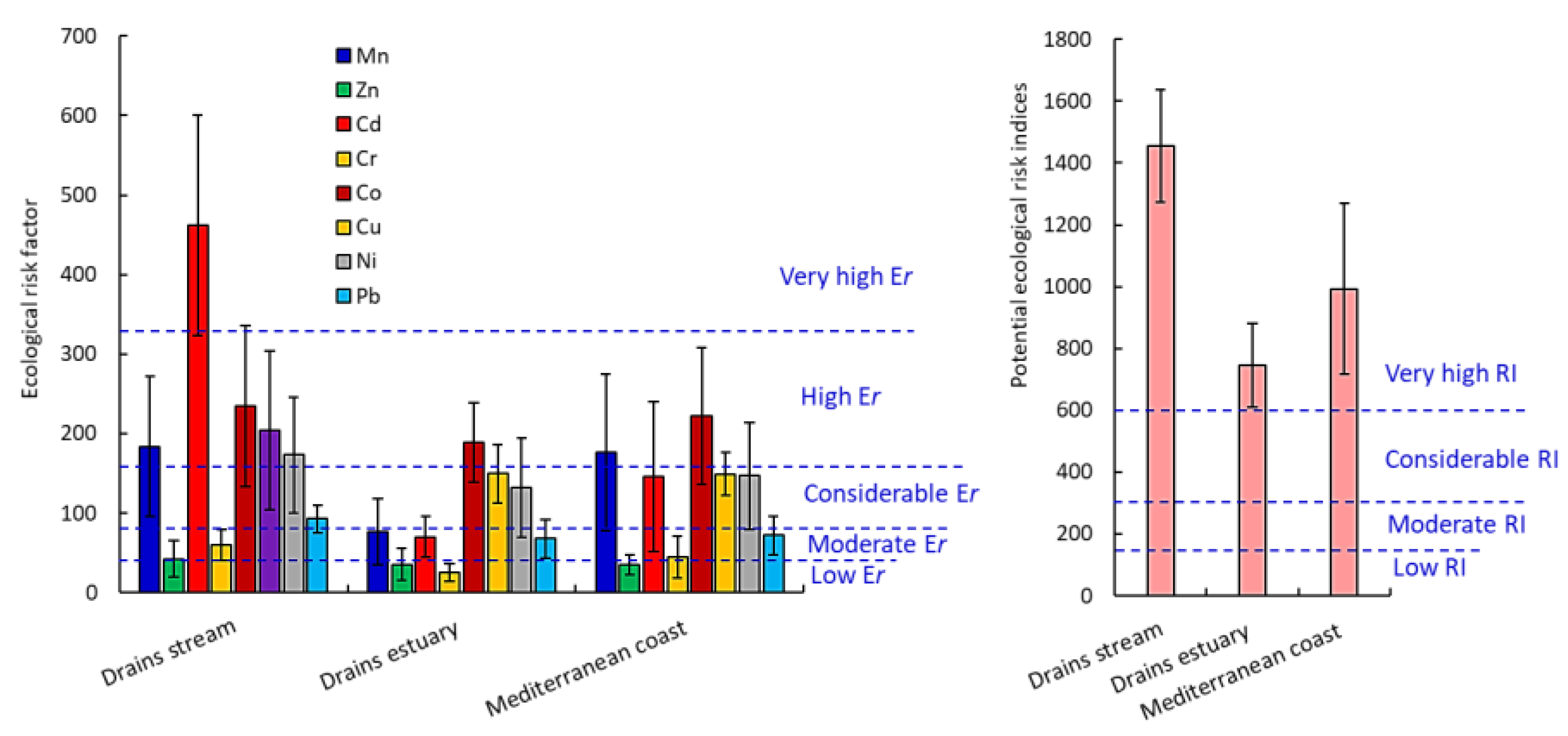
3.3.4. Ecological Risk Factor (Er) and Potential Ecological Risk Index (PERI)
3.4. Heavy Metal Phytoremediation
3.4.1. Heavy Metal Concentrations in Plants
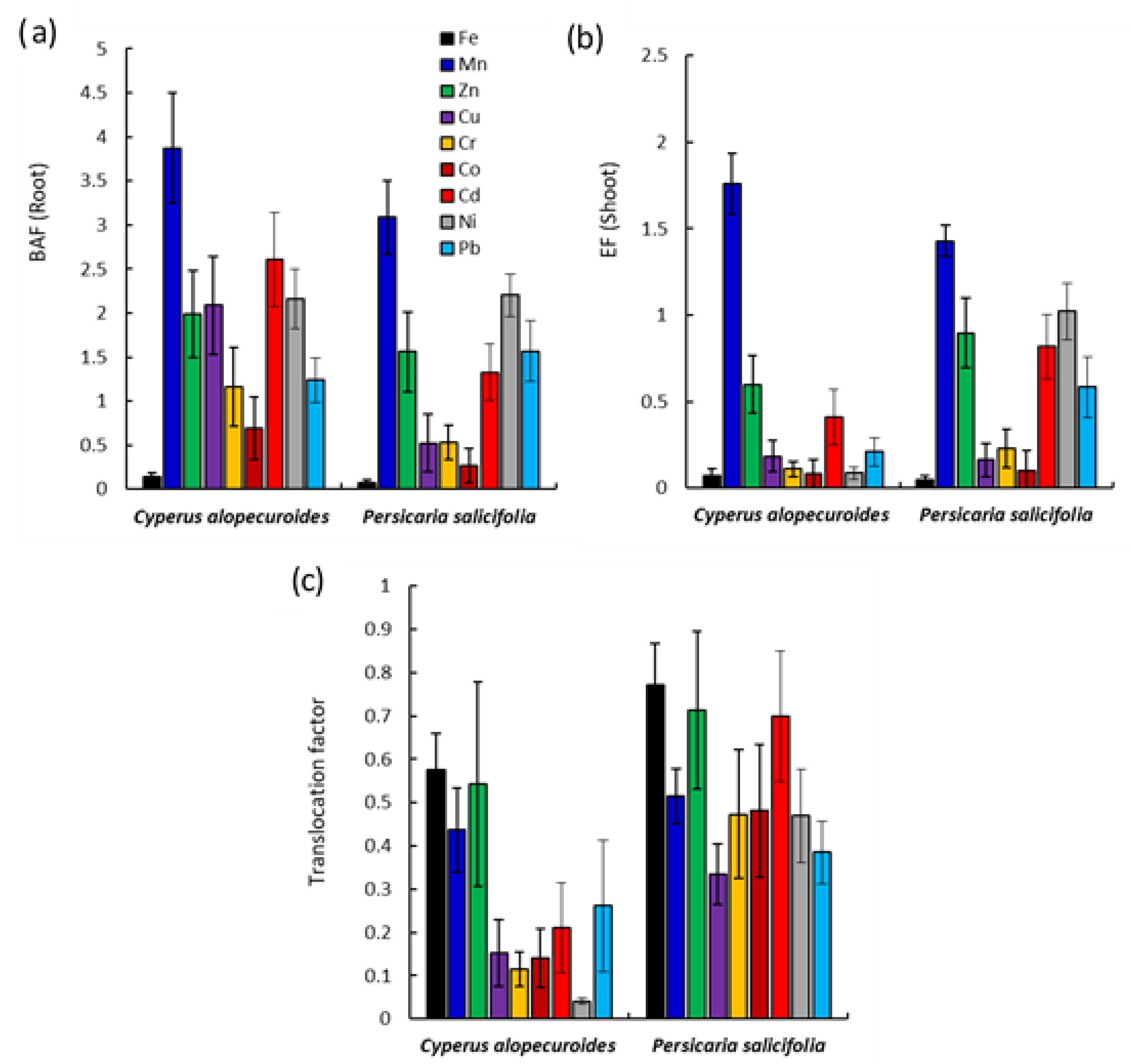
3.4.2. Assessment of Phytoremediation Efficiency of Plants Species
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Abukila, A.F. Assessing the drain estuaries’ water quality in response to pollution abatement. Water Sci. 2015, 29, 1–18. [Google Scholar] [CrossRef][Green Version]
- El-Alfy, M.A.; El-Amier, Y.A.; El-Eraky, T.E. Land use/cover and eco-toxicity indices for identifying metal contamination in sediments of drains, Manzala Lake, Egypt. Heliyon 2020, 6, e03177. [Google Scholar] [CrossRef]
- Elsokkary, I.; Abukila, A.F. Prospective speculation for safe reuse of agricultural drainage water in irrigation. Alex. Sci. Exch. J. 2012, 33, 134–152. [Google Scholar]
- Chang, W.K.; Ryu, J.; Yi, Y.; Lee, W.C.; Lee, C.W.; Kang, D.; Lee, C.H.; Hong, S.; Nam, J.; Khim, J.S. Improved water quality in response to pollution control measures at Masan Bay, Korea. Mar. Pollut. Bull. 2012, 64, 427–435. [Google Scholar] [CrossRef] [PubMed]
- Malone, T.C.; Newton, A. The globalization of cultural eutrophication in the coastal ocean: Causes and consequences. Front. Mar. Sci. 2020, 7, 670. [Google Scholar] [CrossRef]
- SWIM. Review and analysis of status of implementation of wastewater strategies and/or action plans national report- Egypt. Project funded by the European Union. Available online: http://www.swim-sm.eu/files/Natl._Rep._Ww_Strateg.-Eg_Final.Pdf (accessed on 6 July 2013).
- Negm, A.M.; Saavedra, O.; El-Adawy, A. Nile Delta biography: Challenges and opportunities; Springer: Cham, Switzerland, 2016. [Google Scholar]
- Balkhair, K.S.; Ashraf, M.A. Field accumulation risks of heavy metals in soil and vegetable crop irrigated with sewage water in western region of Saudi Arabia. Saudi J. Biol. Sci. 2016, 23, S32–S44. [Google Scholar] [CrossRef]
- Gall, J.E.; Boyd, R.S.; Rajakaruna, N. Transfer of heavy metals through terrestrial food webs: A review. Environ. Monit. Assess. 2015, 187, 201. [Google Scholar] [CrossRef]
- Fathy, S.A.; Abdel Hamid, F.F.; Shreadah, M.A.; Mohamed, L.A.; El-Gazar, M. Application of principal component analysis for developing water quality index for selected coastal areas of Alexandria Egypt. Recourses Environ. J. 2012, 2, 297–305. [Google Scholar]
- Shreadah, M.A.; Masoud, M.S.; Khattab, A.R.M.; El Zokm, G.M. Impacts of different drains on the seawater quality of El-Mex bay (Alexandria, Egypt). J. Ecol. Nat. Environ. 2014, 6, 287–303. [Google Scholar]
- Kostianoy, A.; Kostianaia, E.A.; Soloviev, D.M. Oil Pollution in the Mediterranean Waters of Egypt. In Environmental Remote Sensing in Egypt; Springer: Cham, Switzerland, 2020; pp. 305–328. [Google Scholar]
- Bianchini, A.; Cento, F.; Guzzini, A.; Pellegrini, M.; Saccani, C. Sediment management in coastal infrastructures: Techno-economic and environmental impact assessment of alternative technologies to dredging. J. Environ. Manag. 2019, 248, 109332. [Google Scholar] [CrossRef]
- Mills, G.; Fones, G. A review of in situ methods and sensors for monitoring the marine environment. Sens. Rev. 2012, 32, 17–28. [Google Scholar] [CrossRef]
- Shokr, M.S.; El Baroudy, A.A.; Fullen, M.A.; El-Beshbeshy, T.R.; Ramadan, A.R.; Abd El Halim, A.; Guerra, A.J.; Jorge, M.C. Spatial distribution of heavy metals in the middle nile delta of Egypt. Int. Soil Water Conserv. Res. 2016, 4, 293–303. [Google Scholar] [CrossRef]
- El-Alfy, M.A.; Darwish, D.H.; El-Amier, Y.A. Land use Land cover of the Burullus Lake shoreline (Egypt) and health risk assessment of metal-contaminated sediments. Hum Ecol Risk Assess 2021, 27, 898–920. [Google Scholar] [CrossRef]
- Ullah, H.; Noreen, S.; Rehman, A.; Waseem, A.; Zubair, S.; Adnan, M.; Ahmad, I. Comparative study of heavy metals content in cosmetic products of different countries marketed in Khyber Pakhtunkhwa, Pakistan. Arab. J. Chem. 2017, 10, 10–18. [Google Scholar] [CrossRef]
- El-Amier, Y.A.; Elsayed, A.; El-Esawi, M.A.; Noureldeen, A.; Darwish, H.; Fakhry, H. Optimizing the Biosorption Behavior of Ludwigia stolonifera in the Removal of Lead and Chromium Metal Ions from Synthetic Wastewater. Sustainability 2021, 13, 6390. [Google Scholar] [CrossRef]
- Wasi, S.; Tabrez, S.; Ahmad, M. Toxicological effects of major environmental pollutants: An overview. Environ. Monit. Assess. 2013, 185, 2585–2593. [Google Scholar] [CrossRef] [PubMed]
- El-Amier, Y.A.; Alghanem, S.M. Tree leaves as bioindicator of heavy metal pollution from soil and ambient air in urban environmental. Plant Arch. 2018, 18, 2559–2566. [Google Scholar]
- El Awady, F.R.; Abbas, M.A.; Abdelghany, A.M.; El-Amier, Y.A. Silver Modified Hydrophytes for Heavy Metal Removal from Different Water Resources. Biointerface Res. Appl. Chem. 2021, 11, 14555–14563. [Google Scholar]
- Cho-Ruk, K.; Kurukote, J.; Supprung, F.; Vetayasuporn, S. Perennial Plants in the phytoremediation of lead contaminated soils. Biotechnology 2006, 5, 1–4. [Google Scholar]
- Kumar, A.; Cabral Pinto, M.; Candeias, C.; Dinis, P.A. Baseline maps of potentially toxic elements on soils of Garhwal Himalaya, India: Assessment of their eco-environmental and human health risks. Land Degrad. Dev. 2021, 32, 3856–3869. [Google Scholar] [CrossRef]
- Rajput, V.; Minkina, T.; Semenkov, I.; Klink, G.; Tarigholizadeh, S.; Sushkova, S. Phylogenetic analysis of hyperaccumulator plant species for heavy metals and polycyclic aromatic hydrocarbons. Environ. Geochem. Health 2021, 43, 1629–1654. [Google Scholar] [CrossRef]
- El-Amier, Y.A.; Bonanomi, G.; Al-Rowaily, S.L.; Abd-ElGawad, A.M. Ecological risk assessment of heavy metals along three main drains in Nile Delta and potential phytoremediation by macrophyte plants. Plants 2020, 9, 910. [Google Scholar] [CrossRef]
- Rezania, S.; Taib, S.M.; Din, M.F.M.; Dahalan, F.A.; Kamyab, H. Comprehensive review on phytotechnology: Heavy metals removal by diverse aquatic plants species from wastewater. J. Hazard. Mater. 2016, 318, 587–599. [Google Scholar] [CrossRef]
- Bonanno, G. Comparative performance of trace element bioaccumulation and biomonitoring in the plant species Typha domingensis, Phragmites australis and Arundo donax. Ecotoxicol. Environ. Saf. 2013, 97, 124–130. [Google Scholar] [CrossRef]
- de Queiroz, R.D.C.S.; Maranduba, H.L.; Hafner, M.B.; Rodrigues, L.B.; de Almeida Neto, J.A. Life cycle thinking applied to phytoremediation of dairy wastewater using aquatic macrophytes for treatment and biomass production. J. Clean. Prod. 2020, 267, 122006. [Google Scholar] [CrossRef]
- Peng, K.; Luo, C.; Lou, L.; Li, X.; Shen, Z. Bioaccumulation of heavy metals by the aquatic plants Potamogeton pectinatus L. and Potamogeton malaianus Miq. and their potential use for contamination indicators and in wastewater treatment. Sci. Total Environ. 2008, 392, 22–29. [Google Scholar] [CrossRef]
- Arruti, A.; Fernández-Olmo, I.; Irabien, Á. Evaluation of the contribution of local sources to trace metals levels in urban PM2. 5 and PM10 in the Cantabria region (Northern Spain). J. Environ. Monit. 2010, 12, 1451–1458. [Google Scholar] [CrossRef] [PubMed]
- FAO. Land Resources Information Systems in the Near East. In Proceedings of the Regional Workshop, Cairo, Egypt, 3–7 September 2002. [Google Scholar]
- Abayazid, H.; Al-Shinnawy, I. Coastal lake sustainability: Threats and opportunities with climate change. IOSR J. Mech. Civ. Eng. 2012, 1, 33–41. [Google Scholar] [CrossRef]
- Boulos, L. Flora of Egypt, Volume 4: Monocotyledons (Alismataceae-Orchidaceae); All Hadara Publishing: Cairo, Egypt, 2005. [Google Scholar]
- ISO. Soil Quality—Microwave Assisted Extraction of the Aqua Regia Soluble Fraction for the Determination of Elements; ISO: Geneva, Switzerland, 2012. [Google Scholar]
- ISO. Water Quality—Determination of Selected Elements by Inductively Coupled Plasma Optical Emission Spectrometry (ICPOES); ISO 11885:2007; ISO: Geneva, Switzerland, 2009. [Google Scholar]
- Caeiro, S.; Costa, M.H.; Ramos, T.B.; Fernandes, F.; Silveira, N.; Coimbra, A.; Medeiros, G.; Painho, M. Assessing heavy metal contamination in Sado Estuary sediment: An index analysis approach. Ecol. Indic. 2005, 5, 151–169. [Google Scholar] [CrossRef]
- Franco-Uría, A.; López-Mateo, C.; Roca, E.; Fernández-Marcos, M.L. Source identification of heavy metals in pastureland by multivariate analysis in NW Spain. J. Hazard. Mater. 2009, 165, 1008–1015. [Google Scholar] [CrossRef]
- Hakanson, L. An ecological risk index for aquatic pollution control. A sedimentological approach. Water Res. 1980, 14, 975–1001. [Google Scholar] [CrossRef]
- Kowalska, J.; Mazurek, R.; Ga˛siorek, M.; Setlak, M.; Zaleski, T.; Waroszewski, J. Soil pollution indices conditioned by medieval metallurgical activity-A case study from Krakow (Poland). Environ. Pollut. 2016, 218, 1023–1036. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Bai, S. Contamination and potential mobility assessment of heavy metals in urban soils of Hangzhou, China: Relationship with different land uses. Environ. Earth Sci. 2010, 60, 1481–1490. [Google Scholar] [CrossRef]
- Müller, G. Die Schwermetallbelstung der sediment des Neckars und seiner Nebenflusse: Eine Bestandsaufnahme. Chem. Ztg. 1981, 105, 156–164. [Google Scholar]
- Baker, A.J.M. Accumulators and excluders-strategies in the response of plants to heavy metals. J. Plant Nutr. 1981, 3, 643–654. [Google Scholar] [CrossRef]
- Ghosh, M.; Singh, S.P. A review on phytoremediation of heavy metals and utilization of it’s by products. Asian J. Energy Env. 2005, 6, 18. [Google Scholar]
- Zou, J.; Dai, W.; Gong, S.; Ma, Z. Analysis of spatial variations and sources of heavy metals in farmland soils of Beijing suburbs. PLoS ONE 2015, 10, e0118082. [Google Scholar] [CrossRef]
- Beaver, M.B.; Beaver, J.R.; Mendenhall, W. Introduction to Probability and Statistics; Cengage Learning: New Delhi, India, 2012. [Google Scholar]
- USA-EPA. Screening Level Ecological Risk Assessment Protocol for HazardousWaste Combustion Facilities, Appendix E: Toxicity Reference Values; EPA530-D99–001C; USA EPA: Dallas, TX, USA, 2011; Volume 3.
- CSQGD. Canadian Soil Quality Guidelines for the Protection of Environment and Human Health; Canadian Council of Ministers of the Environment: Winnipeg, MB, Canada, 2007. [Google Scholar]
- Li, F.Y.; Fan, Z.P.; Xiao, P.F.; Oh, K.; Ma, X.P.; Hou, W. Contamination, chemical speciation and vertical distribution of heavy metals in soils of an old and large industrial zone in Northeast China. Environ. Geol. 2009, 54, 1815–1823. [Google Scholar] [CrossRef]
- Bessa, A.Z.E.; Ngueutchoua, G.; Janpou, A.K.; El-Amier, Y.A.; Nguetnga, O.A.N.N.M.; Kayou, U.R.K.; Bisse, S.B.; Mapuna, E.C.N.; Armstrong-Altrin, J.S. Heavy metal contamination and its ecological risks in the beach sediments along the Atlantic Ocean (Limbe coastal fringes, Cameroon). Earth Syst. Environ. 2021, 5, 433–444. [Google Scholar] [CrossRef]
- Ramasamy, V.; Senthil, S.; Paramasivam, K.; Suresh, G. Potential toxicity of heavy metals in beach and intertidal sediments: A comparative study. Acta Ecol. Sin. 2021. [Google Scholar] [CrossRef]
- Guo, B.; Hong, C.; Tong, W.; Xu, M.; Huang, C.; Yin, H.; Lin, Y.; Fu, Q. Health risk assessment of heavy metal pollution in a soil-rice system: A case study in the Jin-Qu Basin of China. Sci. Rep. 2020, 10, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, J.A.; Lopez-Arias, M.; Grau Corbi, J.M. Heavy metals contents in agricultural topsoils in the Ebro basin (Spain). Application of the multivariate geostatistical methods to study spatial variations. Environ. Pollut. 2006, 144, 1001–1012. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Yu, Z.G.; Zeng, G.M.; Jiang, M.; Yang, Z.Z.; Cui, F.; Zhu, M.Y.; Shen, L.Q.; Hu, L. Effects of sediment geochemical properties on heavy metal bioavailability. Environ. Int. 2014, 73, 270–281. [Google Scholar] [CrossRef] [PubMed]
- Facchinelli, A.; Sacchi, E.; Mallen, L. Multivariate statistical and GIS-based approach to identify heavy metal sources in soils. Environ. Pollut. 2001, 114, 313–324. [Google Scholar] [CrossRef]
- Sun, Y.; Zhou, Q.; Xie, X.; Liu, R. Spatial, sources and risk assessment of heavy metal contamination of urban soils in typical regions of Shenyang, China. J. Hazard. Mater. 2010, 174, 455–462. [Google Scholar] [CrossRef]
- Liu, J.; Yin, P.; Chen, B.; Gao, F.; Song, H.; Li, M. Distribution and contamination assessment of heavy metals in surface sediments of the Luanhe River Estuary, northwest of the Bohai Sea. Mar. Pollut. Bull. 2016, 109, 633–639. [Google Scholar] [CrossRef]
- Sezgin, N.; Ozcan, H.K.; Demir, G.; Nemlioglu, S.; Bayat, C. Determination of heavy metal concentrations in street dusts in Istanbul E-5 highway. Environ. Int. 2004, 29, 979–985. [Google Scholar] [CrossRef]
- Sood, A.; Uniyal, P.; Prasanna, R.; Ahluwalia, A. Phytoremediation potential of aquatic macrophyte, Azolla. Ambio 2012, 41, 122–137. [Google Scholar] [CrossRef]
- Dhir, B.; Sharmila, P.; Saradhi, P.P. Potential of aquatic macrophytes for removing contaminants from the environment. Crit. Rev. Environ. Sci. Technol. 2009, 39, 754–781. [Google Scholar] [CrossRef]
- Sawidis, T.; Chettri, M.K.; Zachariadis, G.A.; Stratis, J.A. Heavy metals in aquatic plants and sediments from water systems in Macedonia, Greece. Ecotoxicol. Environ. Saf. 1995, 32, 73–80. [Google Scholar] [CrossRef]
- Bonanno, G.; Giudice, R.L. Heavy metal bioaccumulation by the organs of Phragmites australis (common reed) and their potential use as contamination indicators. Ecol. Indic. 2010, 10, 639–645. [Google Scholar] [CrossRef]
- Cicero-Fernández, D.; Peña-Fernández, M.; Expósito-Camargo, J.A.; Antizar-Ladislao, B. Role of Phragmites australis (common reed) for heavy metals phytoremediation of estuarine sediments. Int. J. Phytoremediation 2016, 18, 575–582. [Google Scholar] [CrossRef]
- FAO; WHO. Joint FAO/WHO Food Standards Programme Codex Committee on Contaminants in Foods, 5th ed.; WHO: Geneva, Switzerland, 2011. [Google Scholar]
- Wang, X.; Sato, T.; Xing, B.; Tao, S. Health risks of heavy metals to the general public in Tianjin, China via consumption of vegetables and fish. Sci. Total Environ. 2005, 350, 28–37. [Google Scholar] [CrossRef] [PubMed]
- El-Amier, Y.A.; El-Alfy, M.A.; Nofal, M.M. Macrophytes potential for removal of heavy metals from aquatic ecosystem, Egypt: Using metal accumulation index (MAI). Plant Arch. 2018, 18, 2131–2144. [Google Scholar]
- Khandare, R.V.; Watharkar, A.D.; Pawar, P.K.; Jagtap, A.A.; Desai, N.S. Hydrophytic plants Canna indica, Epipremnum aureum, Cyperus alternifolius and Cyperus rotundus for phytoremediation of fluoride from water. Environ. Technol. Innov. 2021, 21, 101234. [Google Scholar] [CrossRef]
- Pilon-Smits, E. Phytoremediation. Annu. Rev. Plant Biol. 2005, 56, 15–39. [Google Scholar] [CrossRef]
- Ghavri, S.V.; Bauddh, K.; Kumar, S.; Singh, R.P. Bioaccumulation and translocation potential of Na+ and K+ in native weeds grown on industrially contaminated soil. Int. J. Chem. Tech. Res. 2013, 5, 1869–1875. [Google Scholar]
- Jiang, B.; Xing, Y.; Zhang, B.; Cai, R.; Zhang, D.; Sun, G. Effective phytoremediation of low-level heavy metals by native macrophytes in a vanadium mining area, China. Environ. Sci. Pollut. Res. 2018, 25, 31272–31282. [Google Scholar] [CrossRef]
- Klink, A.A. Comparison of tracemetal bioaccumulation and distribution in Typha latifolia and Phragmites australis: Implication for phytoremediation. Environ. Sci. Pollut. Res. 2017, 24, 3843–3852. [Google Scholar] [CrossRef]

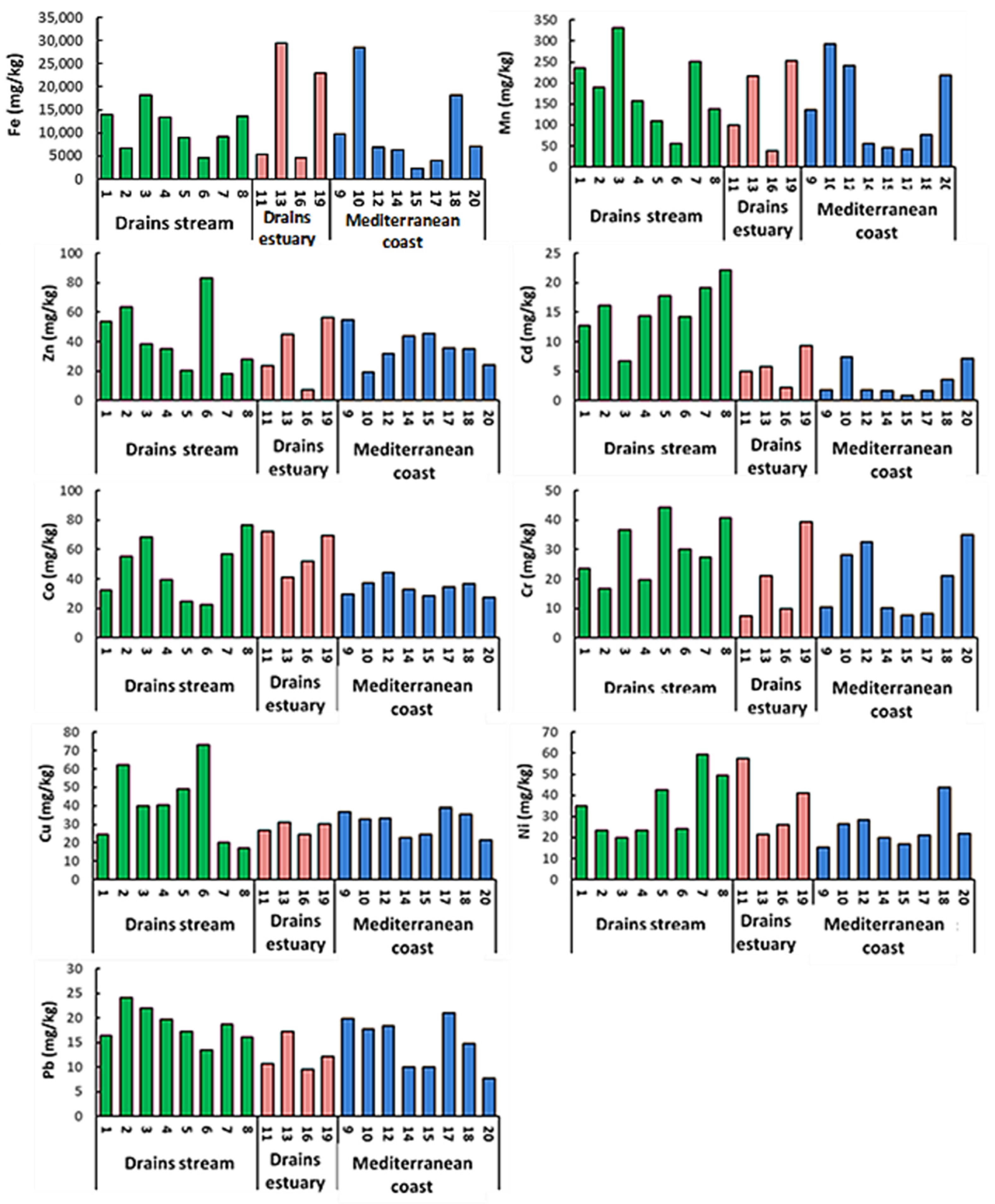


| Statistics Data | Fe | Mn | Zn | Cd | Co | Cr | Cu | Ni | Pb |
|---|---|---|---|---|---|---|---|---|---|
| Drains stream | |||||||||
| N | 8 | 8 | 8 | 8 | 8 | 8 | 8 | 8 | 8 |
| Min. | 4629.23 | 56.06 | 18.25 | 6.73 | 22.41 | 16.82 | 17.31 | 19.79 | 13.56 |
| Max. | 18,179.17 | 331.46 | 83.20 | 22.16 | 76.43 | 44.44 | 73.21 | 59.44 | 24.19 |
| Mean | 11,095.61 | 183.87 | 42.54 | 15.41 | 47.03 | 29.97 | 40.86 | 34.68 | 18.51 |
| Median | 4435.25 | 87.57 | 22.64 | 4.63 | 20.21 | 9.96 | 20.05 | 14.53 | 3.43 |
| ±SD | 11,316.37 | 174.00 | 36.78 | 15.29 | 47.49 | 28.78 | 40.09 | 29.65 | 17.97 |
| Skewness | 0.09 | 0.30 | 0.80 | −0.59 | 0.17 | 0.19 | 0.42 | 0.73 | 0.39 |
| Kurtosis | −0.67 | −0.21 | −0.22 | 1.09 | −1.56 | −1.36 | −0.95 | −0.89 | −0.32 |
| CV% | 39.97 | 47.63 | 53.21 | 30.04 | 42.98 | 33.23 | 49.07 | 41.89 | 18.54 |
| Drains estuary | |||||||||
| N | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 |
| Min. | 4688.33 | 38.85 | 7.34 | 2.27 | 41.30 | 7.53 | 24.48 | 21.56 | 9.57 |
| Max. | 29,432.92 | 253.13 | 56.21 | 9.33 | 72.03 | 39.44 | 31.19 | 57.62 | 17.28 |
| Mean | 15,597.30 | 151.85 | 33.04 | 5.58 | 58.70 | 19.50 | 28.07 | 36.56 | 12.43 |
| Median | 12,512.50 | 99.70 | 21.81 | 2.91 | 14.61 | 14.58 | 3.10 | 16.30 | 3.40 |
| ±SD | 14,133.97 | 157.71 | 34.31 | 5.37 | 60.73 | 15.51 | 28.31 | 33.53 | 11.44 |
| Skewness | 0.23 | −0.19 | −0.24 | 0.43 | −0.42 | 1.14 | −0.26 | 0.75 | 1.45 |
| Kurtosis | −4.69 | −3.64 | −2.42 | 1.25 | −3.37 | 0.34 | −3.34 | −1.24 | 2.18 |
| CV% | 80.22 | 65.66 | 66.01 | 52.15 | 24.90 | 74.78 | 11.04 | 44.59 | 27.35 |
| Mediterranean coast | |||||||||
| N | 8 | 8 | 8 | 8 | 8 | 8 | 8 | 8 | 8 |
| Min. | 2258.53 | 42.63 | 19.45 | 0.78 | 27.55 | 7.65 | 21.73 | 15.54 | 7.82 |
| Max. | 28,513.38 | 292.69 | 54.83 | 7.46 | 44.32 | 35.00 | 38.84 | 43.82 | 21.01 |
| Mean | 10,380.92 | 138.77 | 36.27 | 3.25 | 33.96 | 19.21 | 30.81 | 24.23 | 14.94 |
| Median | 8749.67 | 99.43 | 11.60 | 2.63 | 5.56 | 11.49 | 6.76 | 9.04 | 5.08 |
| ±SD | 7046.06 | 105.92 | 35.40 | 1.81 | 33.65 | 15.82 | 33.12 | 21.39 | 16.23 |
| Skewness | 1.56 | 0.54 | 0.11 | 1.12 | 0.76 | 0.36 | −0.40 | 1.65 | −0.29 |
| Kurtosis | 2.03 | −1.62 | −0.50 | −0.53 | 0.32 | −2.04 | −1.88 | 3.16 | −1.86 |
| CV% | 84.29 | 71.65 | 31.98 | 80.81 | 16.37 | 59.79 | 21.95 | 37.30 | 34.03 |
| p-value | 0.93 ns | 0.28 ns | 0.42 ns | 0.002 ** | 0.16 ns | 0.019 * | 0.14 ns | 0.024 * | 0.04 * |
| References | |||||||||
| Geochemical background | 47,200 | 850 | 95 | 0.30 | 19 | 90 | 45 | 68 | 20 |
| Guidelines US-EPA [46] | - | 550 | 60 | 0.01–41 | 9.1 | 54 | 25 | 19 | 19 |
| CSQGD [47] | - | - | - | 1.4 | 40 | 64 | - | 50 | 70 |
| Toxic response factor | - | 1 | 1 | 30 | 5 | 2 | 5 | 5 | 5 |
| Metals | Fe | Mn | Zn | Cd | Co | Cr | Cu | Ni | Pb |
|---|---|---|---|---|---|---|---|---|---|
| Fe | 1 | ||||||||
| Mn | 0.615 ** | 1 | |||||||
| Zn | −0.02 | −0.057 | 1 | ||||||
| Cd | 0.093 | 0.249 | 0.056 | 1 | |||||
| Co | 0.202 | 0.437 * | −0.224 | 0.251 | 1 | ||||
| Cr | 0.380 | 0.458 * | −0.056 | 0.784 ** | 0.181 | 1 | |||
| Cu | −0.114 | −0.122 | 0.595 ** | 0.222 | −0.428 * | 0.100 | 1 | ||
| Ni | 0.057 | 0.074 | −0.436 * | 0.447 * | 0.520 * | 0.297 | −0.479 * | 1 | |
| Pb | 0.228 | 0.400 | 0.173 | 0.309 | 0.120 | 0.122 | 0.439 * | −0.099 | 1 |
| Component | Rotation Sums of Squared Loadings | ||
|---|---|---|---|
| Eigenvalue | Variance (%) | Cumulative (%) | |
| 1 | 2.81 | 31.23 | 31.23 |
| 2 | 2.14 | 23.82 | 55.05 |
| 3 | 1.36 | 15.10 | 70.15 |
| Element | Rotated component matrix | ||
| PC1 | PC2 | PC3 | |
| Fe | 0.351 | 0.096 | −0.518 |
| Mn | 0.449 | 0.134 | −0.419 |
| Zn | −0.143 | 0.504 | 0.072 |
| Cd | 0.389 | 0.175 | 0.508 |
| Co | 0.366 | −0.225 | 0.078 |
| Cr | 0.445 | 0.141 | 0.081 |
| Cu | −0.088 | 0.579 | 0.271 |
| Ni | 0.346 | −0.315 | 0.450 |
| Pb | 0.210 | 0.427 | −0.067 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Amier, Y.A.; Bessa, A.Z.E.; Elsayed, A.; El-Esawi, M.A.; AL-Harbi, M.S.; Samra, B.N.; Kotb, W.K. Assessment of the Heavy Metals Pollution and Ecological Risk in Sediments of Mediterranean Sea Drain Estuaries in Egypt and Phytoremediation Potential of Two Emergent Plants. Sustainability 2021, 13, 12244. https://doi.org/10.3390/su132112244
El-Amier YA, Bessa AZE, Elsayed A, El-Esawi MA, AL-Harbi MS, Samra BN, Kotb WK. Assessment of the Heavy Metals Pollution and Ecological Risk in Sediments of Mediterranean Sea Drain Estuaries in Egypt and Phytoremediation Potential of Two Emergent Plants. Sustainability. 2021; 13(21):12244. https://doi.org/10.3390/su132112244
Chicago/Turabian StyleEl-Amier, Yasser A., Armel Zacharie Ekoa Bessa, Ashraf Elsayed, Mohamed A. El-Esawi, Mohammad S. AL-Harbi, Bassem N. Samra, and Wafaa K. Kotb. 2021. "Assessment of the Heavy Metals Pollution and Ecological Risk in Sediments of Mediterranean Sea Drain Estuaries in Egypt and Phytoremediation Potential of Two Emergent Plants" Sustainability 13, no. 21: 12244. https://doi.org/10.3390/su132112244
APA StyleEl-Amier, Y. A., Bessa, A. Z. E., Elsayed, A., El-Esawi, M. A., AL-Harbi, M. S., Samra, B. N., & Kotb, W. K. (2021). Assessment of the Heavy Metals Pollution and Ecological Risk in Sediments of Mediterranean Sea Drain Estuaries in Egypt and Phytoremediation Potential of Two Emergent Plants. Sustainability, 13(21), 12244. https://doi.org/10.3390/su132112244









