Utilization of Regional Natural Brines for the Indoor Cultivation of Salicornia europaea
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Natural Brines
2.3. Plant Material and Cultivation Conditions
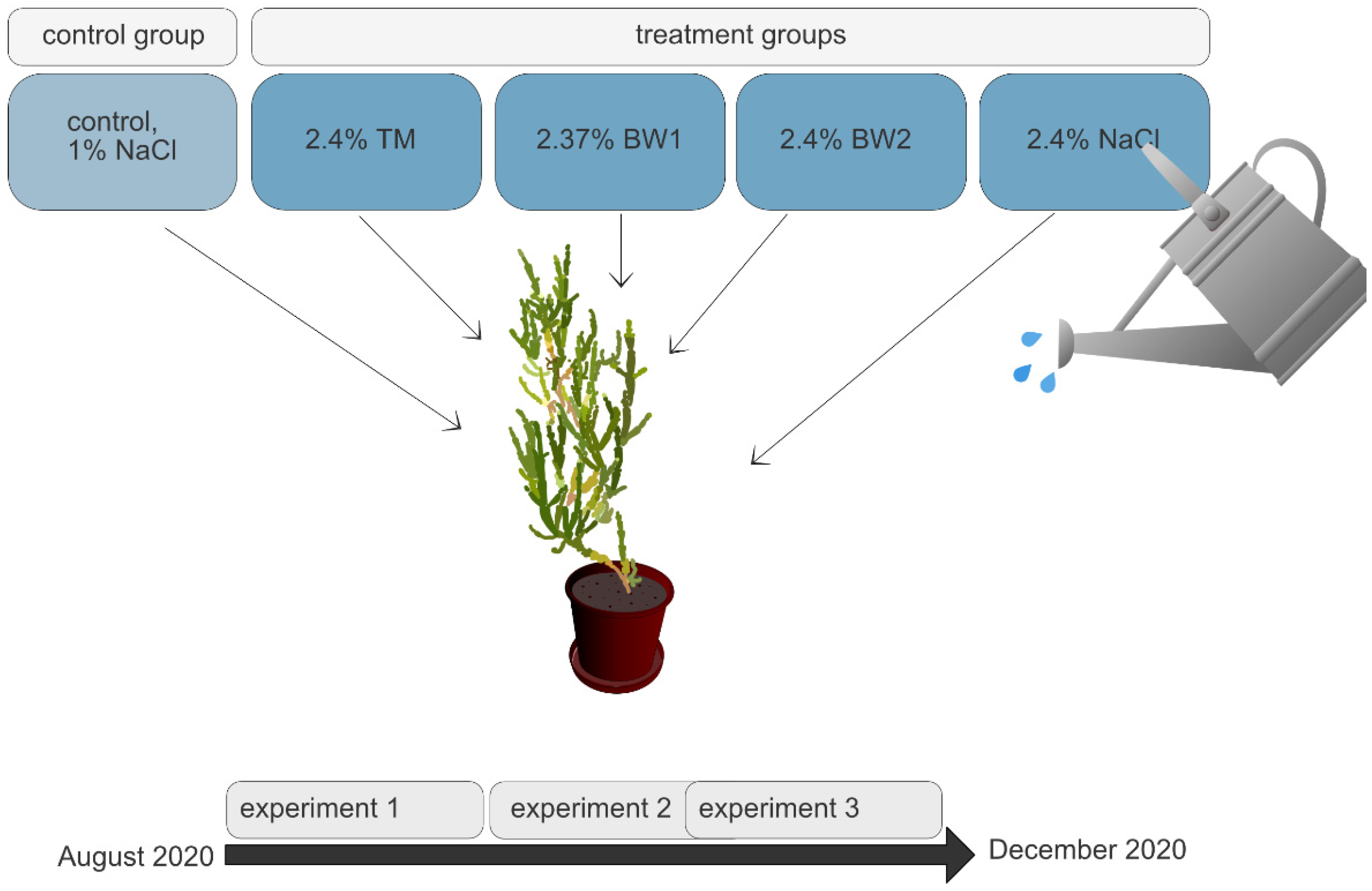
2.4. Experimental Design
2.5. Experimental Procedure
2.6. Purchased Salicornia Product
2.7. Determination of Anion Concentrations in the Saline Solutions
2.8. Determination of Chlorophylls and Carotenoids in the Plants
2.9. Statistical Analysis
3. Results
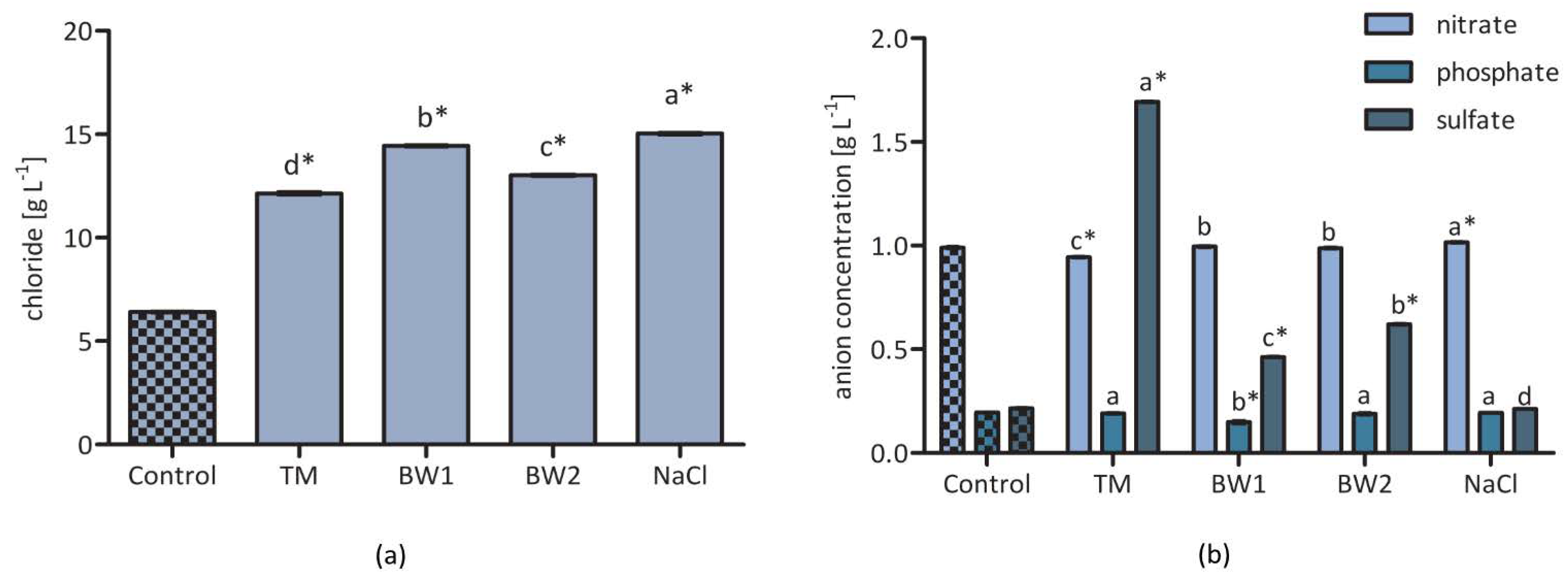
3.1. Composition of the Different Saline Solutions
3.2. Impact of Treatment on Yield and Phytochemicals
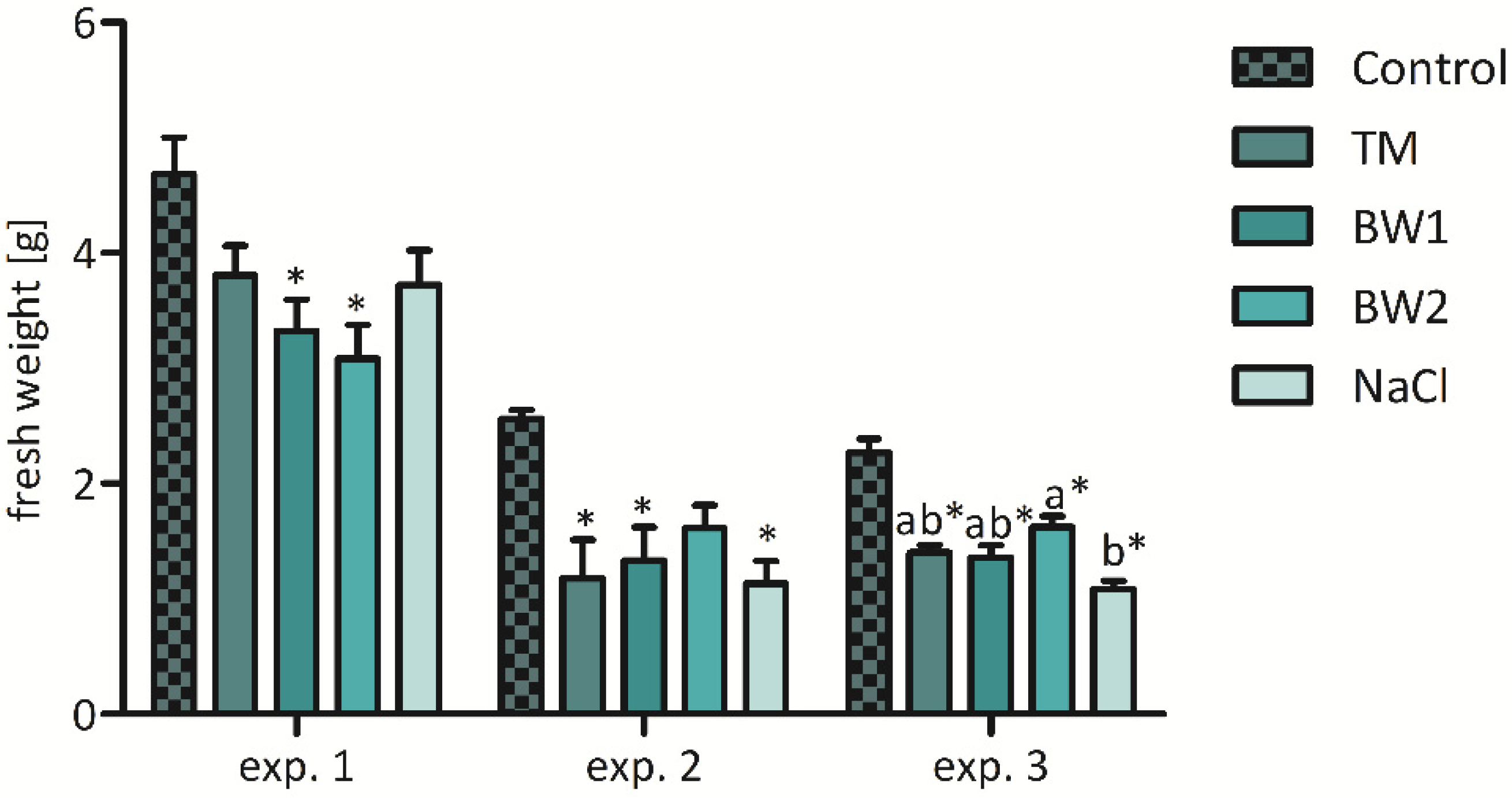
3.2.1. Impact of Treatment on Yield
3.2.2. Impact of Treatment on the Phytochemical Content
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gosling, S.N.; Arnell, N.W. A global assessment of the impact of climate change on water scarcity. Clim. Chang. 2016, 134, 371–385. [Google Scholar] [CrossRef] [Green Version]
- FAO. Overcoming water challanges in agriculture. In The State of Food and Agriculture; FAO: Rome, Italy, 2020. [Google Scholar]
- FAO. Overcoming water scarcity with sustainable irrigation. In FAO Agricultural Development Economics Policy Brief; FAO: Rome, Italy, 2020; Volume 32. [Google Scholar]
- Asseng, S.; Guarin, J.R.; Raman, M.; Monje, O.; Kiss, G.; Despommier, D.D.; Meggers, F.M.; Gauthier, P.P.G. Wheat yield potential in controlled-environment vertical farms. Proc. Natl. Acad. Sci. USA 2020, 117, 19131–19135. [Google Scholar] [CrossRef]
- von Braun, J.; Afsana, K.; Fresco, L.O.; Hassan, M. Food systems: Seven priorities to end hunger and protect the planet. Nature 2021, 597, 29–30. [Google Scholar] [CrossRef]
- FAO. The global status of seaweed production, trade and utilization. In Globefish Research Programme; FAO: Rome, Italy, 2018; Volume 124, p. 120. [Google Scholar]
- Gleick, P.H. (Ed.) Water in Crisis: A Guide to the World’s Fresh Water Resources; Oxford University Press: New York, NY, USA, 1993. [Google Scholar]
- Ventura, Y.; Sagi, M. Halophyte crop cultivation: The case for Salicornia and Sarcocornia. Environ. Exp. Bot. 2013, 92, 144–153. [Google Scholar] [CrossRef]
- He, Q.; Silliman, B.R.; Cui, B. Incorporating thresholds into understanding salinity tolerance: A study using salt-tolerant plants in salt marshes. Ecol. Evol. 2017, 7, 6326–6333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patel, S. Salicornia: Evaluating the halophytic extremophile as a food and a pharmaceutical candidate. 3 Biotech 2016, 6, 104. [Google Scholar] [CrossRef] [Green Version]
- Panta, S.; Flowers, T.; Lane, P.; Doyle, R.; Haros, G.; Shabala, S. Halophyte agriculture: Success stories. Environ. Exp. Bot. 2014, 107, 71–83. [Google Scholar] [CrossRef]
- Kc, K.B.; Dias, G.M.; Veeramani, A.; Swanton, C.J.; Fraser, D.; Steinke, D.; Lee, E.; Wittman, H.; Farber, J.M.; Dunfield, K.; et al. When too much isn’t enough: Does current food production meet global nutritional needs? PLoS ONE 2018, 13, e0205683. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marschner, H.; Kylin, A.; Kuiper, P.J.C. Differences in salt tolerance of three sugar beet genotypes. Physiol. Plant. 1981, 51, 234–238. [Google Scholar] [CrossRef]
- Hariadi, Y.; Marandon, K.; Tian, Y.; Jacobsen, S.E.; Shabala, S. Ionic and osmotic relations in quinoa (Chenopodium quinoa Willd.) plants grown at various salinity levels. J. Exp. Bot. 2011, 62, 185–193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flowers, T.J.; Hajibagheri, M.A. Salinity tolerance in Hordeum vulgare: Ion concentrations in root cells of cultivars differing in salt tolerance. Plant Soil 2001, 231, 1–9. [Google Scholar] [CrossRef]
- O’leary, J.W.; Glenn, E.P.; Watson, M.C. Agricultural production of halophytes irrigated with seawater. Plant Soil 1985, 89, 311–321. [Google Scholar] [CrossRef]
- Ebadi, A.; Sima, N.; Olamaee, M.; Hashemi, M.; Nasrabadi, R. Remediation of saline soils contaminated with crude oil using the halophyte Salicornia persica in conjunction with hydrocarbon-degrading bacteria. J. Environ. Manag. 2018, 219, 260–268. [Google Scholar] [CrossRef] [PubMed]
- Aronson, J. Economic halophytes—A global review. In Plants for Arid Lands; Wickens, G.E., Goodin, J.R., Field, D.V., Eds.; Springer: Dordrecht, The Netherlands, 1985; pp. 177–188. [Google Scholar] [CrossRef]
- Khalilzadeh, R.; Pirzad, A.; Sepehr, E.; Khan, S.; Anwar, S. The Salicornia europaea potential for phytoremediation of heavy metals in the soils under different times of wastewater irrigation in northwestern Iran. Environ. Sci. Pollut. Res. 2021, 28, 47605–47618. [Google Scholar] [CrossRef]
- Kim, S.; Lee, E.-Y.; Hillman, P.F.; Ko, J.; Yang, I.; Nam, S.-J. Chemical structure and biological activities of secondary metabolites from Salicornia europaea L. Molecules 2021, 26, 2252. [Google Scholar] [CrossRef]
- Mitra, S.; Rauf, A.; Tareq, A.M.; Jahan, S.; Emran, T.B.; Shahriar, T.G.; Dhama, K.; Alhumaydhi, F.A.; Aljohani, A.S.M.; Rebezov, M.; et al. Potential health benefits of carotenoid lutein: An updated review. Food Chem. Toxicol. 2021, 154, 112328. [Google Scholar] [CrossRef] [PubMed]
- Fiedor, J.; Burda, K. Potential role of carotenoids as antioxidants in human health and disease. Nutrients 2014, 6, 466–488. [Google Scholar] [CrossRef] [Green Version]
- Giordano, R.; Saii, Z.; Fredsgaard, M.; Hulkko, L.S.S.; Poulsen, T.B.G.; Thomsen, M.E.; Henneberg, N.; Zucolotto, S.M.; Arendt-Nielsen, L.; Papenbrock, J.; et al. Pharmacological insights into halophyte bioactive extract action on anti-inflammatory, pain relief and antibiotics-type mechanisms. Molecules 2021, 26, 3140. [Google Scholar] [CrossRef] [PubMed]
- Eisenhauer, B.; Natoli, S.; Liew, G.; Flood, V.M. Lutein and zeaxanthin—Food sources, bioavailability and dietary variety in age-related macular degeneration protection. Nutrients 2017, 9, 120. [Google Scholar] [CrossRef] [PubMed]
- Boon, C.S.; McClements, D.J.; Weiss, J.; Decker, E.A. Factors influencing the chemical stability of carotenoids in foods. Crit. Rev. Food Sci. Nutr. 2010, 50, 515–532. [Google Scholar] [CrossRef]
- Norton, S.C. Commercial Feasibility of Indoor Saltwater Agriculture Using Saliconia europaea. Master’s Thesis, College of Charleston, Charleston, SC, USA, 2021. [Google Scholar]
- Gunning, D. Cultivating Salicornia europaea (Marsh Samphire); Daithi O’ Murchu Marine Research Station & Univerity College Cork: Dublin, Ireland, 2016; pp. 1–50. [Google Scholar]
- Glenn, E.P.; O’Leary, J.W. Relationship between salt accumulation and water content of dicotyledonous halophytes. Plant Cell Environ. 1984, 7, 253–261. [Google Scholar] [CrossRef]
- Ventura, Y. Effect of seawater concentration on the productivity and nutrional value of annual Salicornia and perennial Sarcocornia halophytes as leafy vegetable crops. Sci. Hortic. 2011, 128, 189–196. [Google Scholar] [CrossRef]
- Orlovsky, N.; Japakova, U.; Zhang, H.; Volis, S. Effect of salinity on seed germination, growth and ion content in dimorphic seeds of Salicornia europaea L. (Chenopodiaceae). Plant Divers. 2016, 38, 183–189. [Google Scholar] [CrossRef] [Green Version]
- Araus, J.L.; Rezzouk, F.Z.; Thushar, S.; Shahid, M.; Elouafi, I.A.; Bort, J.; Serret, M.D. Effect of irrigation salinity and ecotype on the growth, physiological indicators and seed yield and quality of Salicornia europaea. Plant Sci. 2021, 304, 110819. [Google Scholar] [CrossRef]
- Díaz, F.J.; Benes, S.E.; Grattan, S.R. Field performance of halophytic species under irrigation with saline drainage water in the San Joaquin Valley of California. Agric. Water Manag. 2013, 118, 59–69. [Google Scholar] [CrossRef]
- Wawrzyniak, M.K.; Matas Serrato, L.A.; Blanchoud, S. Artificial seawater based long-term culture of colonial ascidians. Dev. Biol. 2021, 480, 91–104. [Google Scholar] [CrossRef] [PubMed]
- Frede, K.; Schreiner, M.; Baldermann, S. Light quality-induced changes of carotenoid composition in pak choi Brassica rapa ssp. chinensis. J. Photochem. Photobiol. B 2019, 193, 18–30. [Google Scholar] [CrossRef]
- White, D.E. Saline waters in sedimentary rocks. In Fluids in Subsurface Environments; Young, A., Galley, J.E., Eds.; American Association of Petroleu Geologists: Tulsa, OK, USA, 1965; Volume 4. [Google Scholar]
- Shpak, N.; Kulyniak, I.; Gvozd, M.; Vveinhardt, J.; Horbal, N. Formulation of development strategies for regional agricultural resource potential: The ukrainian case. Resources 2021, 10, 57. [Google Scholar] [CrossRef]
- Götzfried, F. Die volkswirtschaftliche Bedeutung von Salz in Deutschland. Kali und Steinsalz 2017, 1, 6–15. [Google Scholar]
- Cacciapuoti, S.; Luciano, M.A.; Megna, M.; Annunziata, M.C.; Napolitano, M.; Patruno, C.; Scala, E.; Colicchio, R.; Pagliuca, C.; Salvatore, P.; et al. The role of thermal water in chronic skin diseases management: A review of the literature. J. Clin. Med. 2020, 9, 3047. [Google Scholar] [CrossRef]
- Aghaleh, M. Effect of salt stress on physiological and antioxidative responses in two species of Salicornia (S. persica and S. europaea). Acta Physiol. Plant. 2011, 33, 1261–1270. [Google Scholar] [CrossRef]
- Arjen, C.; de Vos, R.b. Developing and testing new halophyte crops: A case study of salt tolerance of two species of the Brassicaceae, Diplotaxis tenuifolia and Cochlearia officinalis. Environ. Exp. Bot. 2013, 92, 154–164. [Google Scholar] [CrossRef]
- Flowers, T.J.; Colmer, T.D. Salinity tolerance in halophytes. New Phytol. 2008, 179, 945–963. [Google Scholar] [CrossRef] [PubMed]
- ElSayed, A.I.; Rafudeen, M.S.; Gomaa, A.M.; Hasanuzzaman, M. Exogenous melatonin enhances the reactive oxygen species metabolism, antioxidant defense-related gene expression, and photosynthetic capacity of Phaseolus vulgaris L. to confer salt stress tolerance. Physiol. Plant. 2021, 171, 1–13. [Google Scholar] [CrossRef]
- Carillo, P. GABA shunt in durum wheat. Front. Plant Sci. 2018, 9, 100. [Google Scholar] [CrossRef]
- Semiz, G.D.; Suarez, D.L. Impact of grafting, salinity and irrigation water composition on eggplant fruit yield and ion relations. Sci. Rep. 2019, 9, 19373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ventura, Y.; Myrzabayeva, M.; Alikulov, Z.; Omarov, R.; Khozin-Goldberg, I.; Sagi, M. Effects of salinity on flowering, morphology, biomass accumulation and leaf metabolites in an edible halophyte. AoB Plants 2014, 6, plu053. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, H.-J.; Fonseca, J.M.; Choi, J.-H.; Kubota, C.; Kwon, D.Y. Salt in irrigation water affects the nutritional and visual properties of romaine lettuce (Lactuca sativa L.). J. Agric. Food Chem. 2008, 56, 3772–3776. [Google Scholar] [CrossRef]
- Abdel-Farid, I.B.; Marghany, M.R.; Rowezek, M.M.; Sheded, M.G. Effect of salinity stress on growth and metabolomic profiling of Cucumis sativus and Solanum lycopersicum. Plants 2020, 9, 1626. [Google Scholar] [CrossRef]
- Ebrahimzadeh, M.A.; Nabavi, S.M.; Nabavi, S.F.; Bahramian, F.; Bekhradnia, A.R. Antioxidant and free radical scavenging activity of H. officinalis L. var. angustifolius, V. odorata, B. hyrcana and C. speciosum. Pak. J. Pharm. Sci. 2010, 23, 29–34. [Google Scholar] [PubMed]
- Harttig, U.; Bailey, G.S. Chemoprotection by natural chlorophylls in vivo: Inhibition of dibenzo[a,l]pyrene-DNA adducts in rainbow trout liver. Carcinogenesis 1998, 19, 1323–1326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, X.; Hanschen, F.S.; Neugart, S.; Schreiner, M.; Vargas, S.A.; Gutschmann, B.; Baldermann, S. Boiling and steaming induced changes in secondary metabolites in three different cultivars of pak choi (Brassica rapa subsp. chinensis). J. Food Compos. Anal. 2019, 82, 103232. [Google Scholar] [CrossRef]
- Nemzer, B.; Al-Taher, F.; Abshiru, N. Extraction and natural bioactive molecules characterization in spinach, kale and purslane: A comparative study. Molecules 2021, 26, 2515. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Garcia, L.; Lunadei, L.; Barreiro, P.; Robla, I. A review of wireless sensor technologies and applications in agriculture and food industry: State of the art and current trends. Sensors 2009, 9, 4728–4750. [Google Scholar] [CrossRef] [Green Version]
- Souza, R.d.M.; Botrel, T. Development and evaluation of an automatized irrigation system for experimental areas. IRRIGA 2009, 14, 365–382. [Google Scholar] [CrossRef]
- Rigby, D.; Cáceres, D. Organic farming and the sustainability of agricultural systems. Agric. Syst. 2001, 68, 21–40. [Google Scholar] [CrossRef]
| Total Chlorophyll (ng mg−1 DW) | ||||||||||||
| exp. 1 | exp. 2 | exp. 3 | ||||||||||
| Control | 2843.01 | ± | 156.23 | 3477.02 | ± | 116.65 | 2592.50 | ± | 100.07 | ns | ||
| TM | 2430.70 | ± | 161.34 | 2876.78 | ± | 348.73 | 2310.38 | ± | 278.75 | ns | ||
| BW1 | 2365.12 | ± | 322.58 | * | 2706.29 | ± | 113.64 | * | 1853.27 | ± | 188.28 | ns |
| BW2 | 2251.70 | ± | 210.14 | * | 2722.68 | ± | 154.77 | * | 2248.02 | ± | 428.07 | ns |
| NaCl | 2499.83 | ± | 313.34 | 2813.55 | ± | 97.74 | 2106.85 | ± | 616.33 | ns | ||
| Chlorophyll a (ng mg−1 DW) | ||||||||||||
| exp. 1 | exp. 2 | exp. 3 | ||||||||||
| Control | 2229.06 | ± | 131.31 | 2755.64 | ± | 85.36 | 1978.65 | ± | 124.72 | ns | ||
| TM | 1905.73 | ± | 145.10 | 2299.47 | ± | 274.90 | * | 1836.28 | ± | 221.58 | ns | |
| BW1 | 1853.07 | ± | 267.48 | 2149.04 | ± | 106.27 | * | 1404.60 | ± | 178.87 | ns | |
| BW2 | 1748.37 | ± | 169.81 | * | 2158.34 | ± | 114.23 | * | 1725.75 | ± | 384.16 | ns |
| NaCl | 1966.04 | ± | 258.95 | 2225.54 | ± | 69.43 | * | 1648.19 | ± | 493.74 | ns | |
| Chlorophyll b (ng mg−1 DW) | ||||||||||||
| exp. 1 | exp. 2 | exp. 3 | ||||||||||
| Control | 613.95 | ± | 35.82 | 721.38 | ± | 31.52 | 613.85 | ± | 49.17 | |||
| TM | 524.96 | ± | 21.17 | * | 577.32 | ± | 76.08 | 474.10 | ± | 59.31 | * | |
| BW1 | 512.04 | ± | 56.68 | * | 557.25 | ± | 16.38 | * | 448.67 | ± | 20.07 | * |
| BW2 | 503.33 | ± | 41.23 | * | 564.34 | ± | 41.09 | * | 522.27 | ± | 48.43 | |
| NaCl | 533.79 | ± | 56.44 | 588.00 | ± | 28.89 | 458.66 | ± | 124.38 | * | ||
| Chlorophyll a/b Ratio | ||||||||||||
| exp. 1 | exp. 2 | exp. 3 | ||||||||||
| Control | 3.63 | ± | 0.18 | ns | 3.82 | ± | 0.05 | ns | 3.25 | ± | 0.41 | ns |
| TM | 3.63 | ± | 0.20 | ns | 3.99 | ± | 0.15 | ns | 3.88 | ± | 0.15 | ns |
| BW1 | 3.61 | ± | 0.18 | ns | 3.86 | ± | 0.18 | ns | 3.13 | ± | 0.37 | ns |
| BW2 | 3.47 | ± | 0.09 | ns | 3.83 | ± | 0.09 | ns | 3.28 | ± | 0.50 | ns |
| NaCl | 3.68 | ± | 0.17 | ns | 3.79 | ± | 0.07 | ns | 3.58 | ± | 0.23 | ns |
| Total Carotenoids (ng mg−1 DW) | ||||||||||||
| exp. 1 | exp. 2 | exp. 3 | ||||||||||
| Control | 480.7 | ± | 26.39 | 551.23 | ± | 17.64 | 439.7 | ± | 17.38 | ns | ||
| TM | 425.6 | ± | 25.21 | 488.78 | ± | 67 | 403.66 | ± | 47.53 | ns | ||
| BW1 | 418.17 | ± | 44.57 | 458.16 | ± | 21.41 | 356.89 | ± | 11.03 | ns | ||
| BW2 | 398.87 | ± | 31.96 | * | 459.23 | ± | 25.97 | * | 409.66 | ± | 45.02 | ns |
| NaCl | 441.16 | ± | 34.31 | 468.53 | ± | 20.91 | 373.19 | ± | 99.08 | ns | ||
| Lutein | ||||||||||||
| exp. 1 | exp. 2 | exp. 3 | ||||||||||
| Control | 179.23 | ± | 14.58 | 193.68 | ± | 12.66 | 170.25 | ± | 17.16 | |||
| TM | 174.76 | ± | 6.628 | a | 197.62 | ± | 15.88 | a | 169.18 | ± | 16.85 | |
| BW1 | 167.25 | ± | 19.17 | a | 173.47 | ± | 9.341 | ab | 144.52 | ± | 12.83 | |
| BW2 | 140.49 | ± | 9.456 | b* | 161.62 | ± | 11.59 | b* | 157.35 | ± | 8.80 | |
| NaCl | 156.97 | ± | 7.494 | ab | 157.5 | ± | 5.648 | b | 130.87 | ± | 32.07 | * |
| β-Carotene | ||||||||||||
| exp. 1 | exp. 2 | exp. 3 | ||||||||||
| Control | 136.31 | ± | 8.777 | 158.5 | ± | 6.431 | 144.66 | ± | 15.72 | |||
| TM | 123.77 | ± | 6.796 | 145.89 | ± | 14.67 | a | 125.31 | ± | 12.11 | ||
| BW1 | 120.02 | ± | 11.08 | 128.34 | ± | 5.715 | * | 110.76 | ± | 5.68 | ||
| BW2 | 105.36 | ± | 8.281 | * | 126.86 | ± | 7.95 | *b | 124.74 | ± | 12.08 | |
| NaCl | 120.4 | ± | 12.88 | 131.3 | ± | 7.865 | *ab | 105.1 | ± | 30.47 | * | |
| Zeaxanthin | ||||||||||||
| exp. 1 | exp. 2 | exp. 3 | ||||||||||
| Control | 13.532 | ± | 3.347 | 8.0821 | ± | 1.1 | ns | 9.116 | ± | 4.29 | ns | |
| TM | 12.147 | ± | 2.435 | 21.509 | ± | 6.484 | ns | 15.683 | ± | 5.28 | ns | |
| BW1 | 15.524 | ± | 3.835 | 13.795 | ± | 4.066 | ns | 19.581 | ± | 0.73 | ns | |
| BW2 | 13.854 | ± | 1.839 | 12.484 | ± | 3.361 | ns | 16.076 | ± | 2.27 | ns | |
| NaCl | 21.027 | ± | 2.194 | * | 22.356 | ± | 15.45 | ns | 19.746 | ± | 5.24 | ns |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fitzner, M.; Fricke, A.; Schreiner, M.; Baldermann, S. Utilization of Regional Natural Brines for the Indoor Cultivation of Salicornia europaea. Sustainability 2021, 13, 12105. https://doi.org/10.3390/su132112105
Fitzner M, Fricke A, Schreiner M, Baldermann S. Utilization of Regional Natural Brines for the Indoor Cultivation of Salicornia europaea. Sustainability. 2021; 13(21):12105. https://doi.org/10.3390/su132112105
Chicago/Turabian StyleFitzner, Maria, Anna Fricke, Monika Schreiner, and Susanne Baldermann. 2021. "Utilization of Regional Natural Brines for the Indoor Cultivation of Salicornia europaea" Sustainability 13, no. 21: 12105. https://doi.org/10.3390/su132112105
APA StyleFitzner, M., Fricke, A., Schreiner, M., & Baldermann, S. (2021). Utilization of Regional Natural Brines for the Indoor Cultivation of Salicornia europaea. Sustainability, 13(21), 12105. https://doi.org/10.3390/su132112105







