Abstract
Gunung Dahu Research Forest (GDRF) is a 250 ha tropical degraded land reforested by native dipterocarps species. The reforestation success was valued by evaluating the planted trees’ growth performance, their potential timber stock, natural regeneration capacity, soil improvement, biological interdependence, and environmental services. This scientific report used a combination of literature review and also primary data processing to describe the reforestation success within the area. A hilly species of Shorea platyclados showed the best growth performance with its average diameter and height of 43 cm and 23 m, respectively, with its mean diameter annual increment of 2.1 cm/year and the predicted standing stock at 220 m3/ha. Six Shorea species were identified to show their natural regeneration capacity and the occurrence of ectomycorrhizal fruiting bodies, predominantly by the genus Rusula, determined the establishment of biological interdependency at the site. Reforestation improved soil organic matters as revealed by high soil porosity (51.06–52.32%) and infiltration rate (120–155.33 mm/h). The reforested landscape also ensures a continuous water supply and provides an economic benefit for the community. Thus, planting native trees for reforesting degraded tropical landscapes is prospective and may deliver multiple benefits in an ecological and economic view.
1. Introduction
Land degradation and deforestation act as a serious threat to both environment and human wellbeing due to the significant effect of losing biodiversity, causing soil degradation, and contributing to significant greenhouse gas emissions [1]. Among the main direct drivers of land degradation and associated biodiversity loss are the expansion of agricultural activities into the forest area and/or native vegetation, unsustainable agricultural and forestry practice, extractive industry, and climate change [2]. At the global scale, the area of pristine land is shrinking [3] while land requirements for various economic uses keep growing. This imbalanced condition harms the product of nature such as food, water, energy, and livelihood security. Those will directly affect the quality of physical and mental health of humans as individuals and/or society [4]. There has also been an escalating issue both at the regional and global scale to rehabilitate the degraded tropical forest [5,6] using native trees. They are considered to have multiple benefit such as timber source, medicinal use, food source, and deliver ecosystem services [7,8,9,10,11]. Forest rehabilitation and restoration was determined to be successful when planted vegetation could survive and grow, as well as having reproductive ability at a sufficient rate from the self-sustaining population in the long term [12].
The forestry sector has also been the focus in mitigating global climate change since the forests are well known for their role in absorbing carbon dioxide and alleviating global warming [13,14]. Forests are known to be a big natural carbon store. There is at least about 80% terrestrial above-ground and 40% below-ground biomass from forests [15]. Forests reduce ambient CO2 by sequestration of atmospheric C into the growth of their biomass and increase the soil organic carbon content [16,17]. Thus, conserving and restoring the remnant forest landscape and establishing the new ones is an urgent task.
Increasing concern for ecology has triggered the higher use of native tree species in reforestation activities around the globe. There has been a significant increase in the numbers of native species planted at various reforestation sites [3,18,19,20,21,22,23]. Yet, for several reasons, the use of exotic species is still dominant as they are relatively easy to produce in the nursery, overvalued, more adaptive, and grow faster in degraded lands [24,25,26,27,28,29,30,31]. Lack of knowledge on seed supply and silvicultural aspects are among the difficulties for native species to be used widely in reforestation activities. However, recent studies showed that good nursery management, post-nursery care, planting techniques, and post-planting treatment could support higher use of native species in reforestation and revegetation activities [32,33,34,35,36,37,38].
Among those highly valuable native tree species are dipterocarps, which dominate the tropical forest of Southeast Asia [39,40]. Dipterocarp is a dominant family growing naturally in Indonesia which spreads throughout the islands of Sumatera, Kalimantan, Java, Sulawesi, Nusa Tenggara, Moluccas, and Papua, and it also consists of at least 8 genera and 155 species [41]. The trees are ecologically important and significant commodities of tropical economies. On a global scale, dipterocarp forests are of huge concern because their wood is highly favored for a variety of uses, leading to a high risk of tropical forest degradation [42].
Planting dipterocarps for forest rehabilitation and restoration has been widely adopted in Indonesia and can be a form of ex situ conservation [43,44,45]. Dipterocarp seedlings can be planted on open grassland with high light intensity as the major limiting factor for species survival. Thus, planting under nurse trees has been recommended to yield higher survival in such open conditions [46]. Dipterocarps have also been the common species used for enrichment planting of commercially logged dipterocarp forests. The main factors that contributed to the success of the restoration program using dipterocarps species were the selection of species site-matching, mycorrhizal fungi symbiosis, and post-planting maintenance [3,47,48]. Several dipterocarps species have been determined to have faster mean annual diameter increment in the range of 1.16–1.3 cm/year and identified as high potential for broader rehabilitation scale of the logged-over area, including Shorea leprosula, S. parvivolia, S. johorensis, and S. platyclados [47,48,49].
This study aimed to describe the result of reforestation activities by planting native dipterocarp trees, which transformed degraded land into a forested landscape. The successful reforestation activities were observed in several aspects. These include the presence of timber stock potential, forest cover increment, the natural regeneration capacity of the planted species, soil characteristic, land productivity, potential hydrological value, biological interdependence, environmental service, and community livelihood.
2. Methodology
2.1. Study Area
The Gunung Dahu Research Forest (GDRF) is located in West Java, Indonesia (Figure 1). It lies on a submontane area (550–900 m asl) with a total area of 250 ha, to which ±160.7 ha is currently planted with dipterocarp species. The remaining 89.3 ha is covered with pine stands, mixed gardens, and rice fields. The GDRF has hilly, steep topography, and inceptisol soil type, predominated by clay (>60%). The annual rainfall is 2500–2700 mm, and the soil is prone to landslides. The area was previously a stretch of degraded land that used to be an abandoned tea plantation where unproductive shrubs, a small portion of old pine, and wild bamboo trees grew (Forest Research and Development Center, 2020). A land rehabilitation program using dipterocarps species was introduced in this area in 1997–1999 to increase land productivity and prevent landslide hazards.
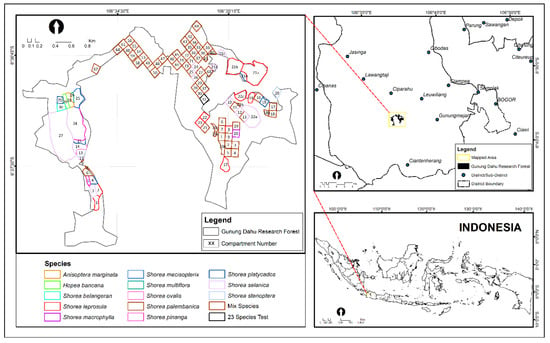
Figure 1.
Map of study area in Gunung Dahu Research Forest.
2.2. Measurement of the Impact Generated by Reforestation
To identify the impacts of a successful reforestation effort in a tropical region, we collected data from primary and secondary sources. Data collected from the secondary source include peer-reviewed publications (i.e., guidebooks, conference proceedings) and non-peer-reviewed articles related to GDRF (unpublished data, village or other similar data records, institution reports, technical reports, project reports, statistical documents, modules). Other kinds of literature on similar works were also added to the list to enrich the discussion. Several examples from a similar tropical project site were also included to deliver a broader context of comparison and discussion. The primary data collection and analysis were also carried out to present broader and deeper results. Both data sources were compiled and selected according to their relevancy on a specific section of discussion as follows:
- (a)
- How reforestation by native trees yield timber stock and increase forest cover;
- (b)
- To what extent planted native trees can support a self-sustaining ecosystem through their natural regeneration capacity;
- (c)
- How reforestation can enrich soil characteristics, and increase land productivity and potential hydrological value;
- (d)
- How biological interdependent has been established from successful reforestation using native trees;
- (e)
- To what extend reforestation success can also provide economic value to the surrounding community.
The following methods were used in this study to describe how reforestation affects each of the above topics from (a) to (e).
2.2.1. Measurement on Growth Performance, Timber Stock, and Forest Cover Dynamic
The timber yield stock is indicated through the growth performance of the species planted in the GDRF. The parameters used to indicate the growth performance and timber stock were diameters at breast height (DBH), diameter mean annual increment (DMAI), average height, and volume/ha of Shorea leprosula and S. selanica previously reported by Subiakto et al. [50] and Rachmat et al. [51]. The data of other species were observed directly in the field through periodic measurement to the sample of 100 individuals per species in different plots. In the first to fifth year, the observations were carried out once a year. The following year, the measurement was carried out once in three years. Data were analyzed by applying the same formula as previous studies [50,51].
The dynamics of land cover changes were examined to determine the impact of a reforestation activity. An ArcGIS 10.5 software was used to analyze Landsat TM 5 1997 and 2007 images (band 5, band 4, and band 3) and Landsat 8 OLI 2017 (band 6, band 5, and band 4), which is a combination of bands for vegetation analysis. The Landsat image data are downloaded from earth explorer as a data source (https://earthexplorer.usgs.gov, accessed on 21 March 2021). An unsupervised classification approached was used to classify the landscape’s coverage, i.e., forest and non-forest. Unsupervised classification is selected when field data or prior knowledge of the study area are not available [52].
2.2.2. Review and Measurement on Natural Regeneration Capacity
Assessment of natural regeneration capacity was conducted on three model species, i.e., Shorea leprosula, S. pinanga, and S. platyclados, through regular monitoring on the phenology, observations on micro-habitat, and measurements of the offsprings’ growth. The first record was obtained in 2018, twenty years after reforestation took place [53,54]. The follow-up monitoring was carried out in early 2020 by applying the same method. Other recorded flowering and fruiting dipterocarp in the area included S. selanica, S. macrophylla, and S. stenoptera. Measured data and parameters included: (i) year of the first flowering and flowering frequency, obtained from the field notebook; (ii) the number of potential parent trees obtained from field notebook; (iii) the number of offspring, obtained from direct measurement at observed plots by the census; (iv) soil texture, analyzed using disturbed soil samples [55]; (v) canopy coverage, analyzed using canopy closure measurement [56]; (vi) slope, obtained by direct measurement using a clinometer.
2.2.3. Measurement of Soil Physicochemical Characteristics and Potential Hydrological Value
Data on soil characteristics and hydrological potential were obtained from previous studies examining soil macro- and mesofauna in reforestation areas [57]. This includes assessment of the growth performance of S. platyclados planted on five different slope gradients, namely flat (0 ≤ 8%), gentle (8 ≤ 15%), moderately steep (15 ≤ 25%), steep (25 ≤ 45%), and very steep (≥45%) [58]. In addition, observation was made on how reforestation improved soil physical properties (i.e., bulk density, porosity, moisture content, drainage pore, and permeability) at reforested sites planted with S. leprosula and S. selanica trees [59].
2.2.4. Review on Ectomycorrhizal Association
The success of reforested GRDF is associated with biological processes and overviewed by the occurrence of mutual symbiosis such as ectomycorrhiza (ECM) fungi. The occurrence and diversity of the ECM fungal body in the GDRF delivered in this paper were compiled from previous studies [57,60], including: (i) direct collection of the sample of EMC’s fungal bodies and its documentation picture; (ii) the number of EMC fungal body, observed by the census [61,62]; and (iii) the morphology identification of fresh EMC’s fungal body [63].
2.2.5. Assessment of the Impact of Reforestation on Environmental Services and the Community Livelihood
Information on environmental services and impact on community livelihood provided by this revegetated GDRF included assessment of carbon sequestration capacity, ecotourism, and hydrological regime. The potential carbon sequestration of the revegetated landscape was reviewed from a report carried out on the experimental plots planted by S. leprosula and S. selanica at different planting distances [64]. The ecotourism development and economic value to its surrounding community were analyzed from internal records of tourist ticket sales to determine the number of visitors [65] and direct interviews with all shop owners to find out their daily income. The occurrence of existing spring water provides a new hydrological regime that benefits the revegetated landscape; such information was obtained from the previous record of spring waters [66], which was further reconfirmed by field checking at the end of the year 2020 to ensure the existence of the springs and their discharge.
3. Results and Discussion
3.1. Reforestation Yields Timber Stock and Increases Forest Cover
Thirty-one dipterocarp species have been planted in GDRF. We presented our data analysis on their growth performance in Table 1. The table also shows the DMAI, which indicates the growth of stem diameter each year at a specific time. Based on the calculation of DMAI, S. platyclados has the highest value and was similar to those observed in Kalimantan [67]. S. platyclados are commonly found on hilly and mountain ranges from 700 to 1350 above sea level [68]. Hence, the reforested area of GDRF may have similar habitat conditions to the natural forest, thus supporting the growth of S. platyclados. However, the DMAI value of other species, such as S. leprosula and S. johorensis were lower compared with their DMAI value than those in Kalimantan [69,70] that recorded DMAI of 0.75–1.2 cm/year for S. leprosula. The species is one of the fast-growing dipterocarps that could grow rapidly in its early 20 years [71] within 10 m × 100 m lane planting technique at the studied site. The lowest DMAI value (0.64 cm/year) was recorded in S. seminis. This species is usually found in the lowland forest near the river [72], where the water is available throughout the year and categorized as a moderately slow-growing dipterocarp [73]. Hence, the GDRF landscape is located in a higher altitude with drier and limited water availability and thus may become the limiting factor for the growth of S. seminis. The low value of DMAI was an indication of a species less adaptability to their environment [74].

Table 1.
Growth performance of dipterocarps species at different spacing distance and planting technique.
The calculations of standing stock volume indicated that S. platyclados has the highest volume with 220.8 m3/ha per hectare observed from 4 m × 8 m spacing distance, followed by S. leprosula with 3 m × 3 m of spacing distance (215.4 m3/ha) (Table 1). A wider spacing distance yields a higher DBH and height growth but results in a lower volume per hectare due to fewer trees/ha [50]. In accordance with the result of S. leprosula, the higher number of individual trees resulted in a high value of volume per ha. The standing stock volume for many species in the reforested study area was relatively high compared with the standing stock potency in the logged-over area (LOA) in Indonesia that ranged between 35 to 40 m3/ha [75].
Each plant species, including dipterocarps, has different growth performance affected by the physiological and environmental conditions, including competition among individuals [76]. This competition would increase in the later developmental stages, with a denser population has higher competition and affected the availability of nutrients, light, and water [50]. Different growth performances were also observed within similar dipterocarps species affected by slopes and spacing distance [44]. Table 1 shows the performance of dipterocarps species planted at the reforested landscape with various initial conditions. The increment value of the same species may differ [76] since the increment value is more site-specific, depending on the habitat where they grow [77] and also different silvicultural techniques [78].
The restoration program in the man-made dipterocarps forest of GDRF focused more on research and conservation aspects with no post-planting silvicultural technique applied. On the other hand, the elevation of GDRF is higher than the dipterocarps’ growth requirement. However, the growth of dipterocarps species on this reforested area was as good as the growth of other dipterocarps of S. stenoptera and S. pinanga in the research trial in Kuching, Serawak, that reached the DMAI of 1.10 cm/year on the age of 33 years [79]. The result showed that dipterocarps in GDRF could reach the same value at a younger age and indicated that native trees are very likely to be planted as the restoration commodity. The transformation of vegetation cover before and after reforestation is shown in Figure 2.

Figure 2.
The initial condition (a,b) of GDRF before reforestation, and after reforestation (c).
Planting activities at GDRF increased the forested area as well as environmental quality. We presented our analysis result related to a significant increase in forest cover by the reforestation effort, as shown in Figure 3. The average forest cover change increased at around 26% for every ten years, from 79.2 ha (29.5%) to 219.1 ha (81.8%) within 20 years.
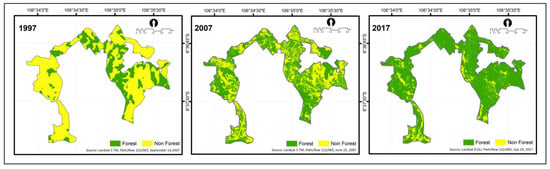
Figure 3.
Forest cover classified images of 1997, 2007, and 2017 in Gunung Dahu Research Forest.
3.2. The Capacity of Natural Regeneration of the Planted Trees Plays a Key Role in the Self-Sustaining Forest
The success of natural regeneration is strongly related to forest sustainability. Forest regeneration is how the tree crop regenerates either naturally or artificially [80]. Natural forest regeneration involves the colonization process, stand establishment, and the process to grow and survive. Forest regeneration is the dynamic vegetation process that starts from vegetation’s occurrence until it reaches the reproductive stage. Many factors influence the process of forest regeneration both from natural and anthropogenic aspects [81]. Man-made forests can also perform their natural regeneration function, as shown here for reforested GDRF. Artificial interventions such as providing growing space and disturbances monitoring are crucial for the success of the natural regeneration in a man-made forest.
In their natural habitat, dipterocarp species reproduced when they reached the top layer of the forest canopy. This may take many years to reach flowering age under natural forest conditions [82], generally at 30 years [83]. However, they may reproduce earlier in the secondary and plantation forest. For example, Dipterocarpus oblongifolius has flowered at only seven months in Kepong cultivated saplings [82], and 2 to 7 years for red meranti [84,85]. In the GDRF, our observation recorded the flowering age of dipterocarp species is relatively late compared with the previously mentioned plantation. S. leprosula and S. pinanga start to flower at 16 years, and S. platyclados at the age of 15 years (Table 2). Hence, the first flowering age of the studied species in GDRF is in between the common first flowering age of dipterocarp species in plantation and natural forest. The difference in age of first reproduced may occur due to the reforested GDRF at relatively high altitude, soil type, and texture.

Table 2.
Summary of planting, flowering times and offspring of Shorea leprosula, S. pinanga, S. platyclados, S. selanica, S. macrophylla and S. stenoptera at reforested landscape of GDRF.
In the reforested landscape of GDRF, dipterocarps species were recognized to have irregular flowering times every 2–7 years, and the species may flourish more frequently in the plantation forest [82]. Records from our study based on three model species of S. leprosula, S. pinanga, and S. platyclados, follow the common phenomena except for S. platyclados, which flowers annually (Table 2). S. platyclados located at plots 4, 20, and 21e (Figure 1) flowered almost every year since they were 16 years old, while those at plots 15 had flowered three times since they first reproduced when they reached 15 years. Different reports were recorded for S. platyclados that show irregular flowering times between 3–4 years with a flowering period of 4 months, usually in April until July [83]. The difference in frequency of flowering time on S. platyclados may occur due to different dry seasons in different sites that stimulate the flowering behavior of the species.
Meanwhile, S. leprosula and S. pinanga showed a similar pattern of flowering behavior. S. leprosula was known to flower when they were 16 to 23 years, and S. pinanga reached 16 and 18 years when the first flowering occurred (Table 2). The trees of S. leprosula that flowers at an older age (in this case 19 and 23 years) were found to reproduce only once, compared with those of flowerers at an earlier age (16, 17, and 18 years) (Table 2). A similar finding was noted from S. leprosula grown at PT. Arara Abadi Riau (Sumatera) has been flowered three times at the age of 15 [50]. The differences in the longer flowering period may occur due to the higher altitude of this reforested landscape. This may become the limiting factor of S. leprosula development so that only individuals with good adaptation can reach the flowering stage.
The frequency of flowering times of S. leprosula at this reforested landscape is also in accordance with a common pattern in the seasonal forest. Previous studies showed that the flowering time of S. leprosula in natural forests occurs every 2–4 years [83,86]. The irregular flowering times observed in the seasonal dipterocarp forests that grow from South Asia to the Malesian region are vital for seed procurement strategy [82].
Natural regeneration will be optimal if natural saplings’ production is abundant without any disturbance, either pests, diseases, or humans [87]. Natural regeneration for each model species is represented in Figure 4. Observation on offspring density revealed that the amount of sapling was less than that of seedlings (Table 2). Among the three model species, S. pinanga has the least number of offspring (Table 2), and was only found in plot 24. This was incomparable with the relatively high number of potential parent trees in the two plots. The parent trees have been known to reproduce four times for twenty years, and only two saplings have survived from the first two or three reproductive cycles, while seedlings from the last reproductive cycle were abundant. Closed observation in the plots revealed that ferns dominated the plots as understorey with a height of more than 1 m. The seedlings of S. pinanga may have struggled in competing for space and nutrition with the understorey plants. Hence, the seedlings may not have survived. The presence of understorey plays a role in inhibiting the growth of tree regeneration [88]. It may prevent the intensity of light entering the forest floor required by the offspring to grow [89]. The canopy coverage in plot 24 was relatively high (62.28%). Hence, clearing of understorey surrounding the parent trees was urgently carried out to germinate the fallen fruit [90]. Seed germination of Shorea sp. needs shade from direct sunlight [68]. Meanwhile, no offspring of S. pinanga were found in plot five with 40 potential mother trees that flowered and produced abundant fruits, but the fruits failed to germinate and survive. The major cause for the failure of natural reproduction of the species at this plot might be an anthropogenic factor. The beautiful view on the peak of the hilly landscape and the availability of clear springs in the lower part have become a tourism spot that was intensively managed. The forest floor was regularly cleared using a grass cutter, and an intensive visit might lead to high soil compactness that was impenetrable for seed roots to germinate. However, with very high anthropogenic constraints and differences in habitat characteristics, S. pinanga seemed to adapt well by showing their natural reproductive ability.

Figure 4.
Forest floor with seedlings (a) and saplings stage (b) of S. platyclados and seedlings of S. leprosula (c) and S. pinanga (d).
The number of seedlings of S. platyclados was much higher than that of S. pinanga, amounting to >12,000 seedlings (Table 2). S. platyclados is adaptive in highland areas, which could grow on the elevation of 300–1200 m asl, but it has optimum growth at 750–1000 m asl [91]. Hence, this reforested landscape has a similar environmental condition to the natural habitat of the species. The species that flowers annually have a much higher number of offspring than those of irregular flowering. The highest number of offspring was recorded in plot 20. The plot has a soil texture of sandy loam, indicating that a lot of soil contains a sand fraction (40–90%) that has the property of easily escaping water, resulting in good aeration and rapid decomposition of organic matter [92]. Thus, it is suitable for S. platyclados, which prefers well-drained land. The topography of this plot is also suitable for the growth of S. platyclados, as reported by a previous study [90]: topography with 20–40% slopes has good drainage that supports most of Shorea spp growth. Meanwhile, there was no sapling on Plot 21e, and the number of seedlings in this plot was the least compared with the other plots. Several authors [93,94] have reported that there was no regeneration found, particularly on the slopes of hill forests due to the presence of understorey competition. Among other plots where S. platyclados were planted, plot 21 was located at a hill with a slope of 70%.
The natural regeneration of the S. leprosula was recorded in seven plots with abundant seedlings in plots 1, 2, and 5, but only one sapling in each of plots 5 and 7 (Table 2). These saplings were likely to have survived from the previous flowering cycles as the shade-tolerant dipterocarps seedlings prefer to grow below closed-canopy forest in quite a while of more than ten years [82]. No saplings were observed in the remaining plots, and there was no offspring in plot 21a. The difference in the amount of natural regeneration on each plot may vary because of the flowering intensity. The parent trees in plots 1, 2, 5, 6, and 7 had more intense flowering time, while the other two plots were only flowering once. The intensity of dense flowering produced more abundant fruits resulting in a higher germination rate and a higher probability to produce viable offspring. This can be achieved when the parent trees are selected to have good phenotypic performance [95]. The presence of thick understorey plants in plot 21a may have been limiting factors for the seedling growth as their presence has prevented light from reaching the forest floor to stimulate seed germination as well as causing nutrient competition for the seedlings. The absence of saplings in plots with a high number of seedlings (Table 2) was a critical event as an early survival for this species. Many factors affect the seedlings’ survivorships, but their survival and establishment are usually influenced by site-specific, particular biotic, conditions, edaphic characteristics, and microclimate [82].
The other three Shorea species, namely S. selanica, S. macrophylla, and S. stenoptera, indicated less reproductive capacity comparing with the other three previously studied species. It is indicated by the absence of survived seedlings of S. macrophylla and S. stenoptera, and even only five survived seedlings found for S. selanica. In terms of the first flowering age, these Shorea species showed late flowering age compared with others. However, they have a relatively successful reproductive capacity as many others species in the area have not yet shown a similar ability.
During the twenty years of the reforestation process, the three studied species planted in the GDRF landscape have a different capacity to regenerate naturally. S. platyclados has the best performance in terms of natural regeneration. It is flowering in two patterns, annual and irregular, with an abundant number of survived offspring, and it is rarely found in nature. S. pinanga and S. leprosula showed the relatively low natural regeneration capacity that requires human intervention to stimulate the growth of the offspring, particularly during early survival. Hence, artificial interventions to ensure seedlings’ survival are required. These are to enhance the natural regeneration capacity in the GDRF.
3.3. Reforestation Improves the Productivity of Land, Soil Characteristic, and the Potential Hydrological Value
The reforested GDRF landscape was characterized by its acidic soil with a high clay content of more than 60% [96], high soil adhesiveness, dominant plasticity [59], low content of soil phosphorus (P), potassium (K), calcium (Ca), and magnesium (Mg), and high aluminum (Al) and iron (Fe) contents [97]. Based on the field study conducted by the Center for Land Resource Research and Development, the initial condition at the reforested landscape was categorized as potentially degraded lands with a decreasing number of permanent vegetation and dominated by a very steep slope (>25%). The continuous decline in vegetation number will trigger severe erosion and landslide of the area.
Vegetation has a major role in soil formation; different vegetation will form different soil characteristics. Physically, the vegetation becomes a determining factor for the amount of infiltration capacity. The greater number and size of the vegetation will increase their infiltration capacity [98]. Infiltration capacity will generally increase with the increasing age of the stands or plants [99], and the effect of vegetation on infiltration is determined by distinct root systems between plants [100]. The infiltration capacity of grass and moorlands tends to be low because both vegetation types have fibrous roots with a very limited depth to support the optimum infiltration process, while in shrublands, the vegetation composition is quite varied, consisting of weeds, shrubs, and woody plants, which can support the higher infiltration process. Thus, infiltration in perennial plantations such as forest plantations will be higher than in seasonal crops [101].
Forest vegetation will also influence soil’s biochemical characteristics through the generated litter. Litter is dead materials consisting of twigs, leaves, small branches, bark, flowers, and fruit, located above the soil surface that has undergone decomposition and mineralization [102]. Its function is essential to the forest floor because most of the returned nutrients into the forest floor are derived from the litter. The mineralization process of litter will change it into organic material, which increases nutrient content in the soil. This mineralization process is assisted by soil organisms, including soil fauna and flora. The value of C-organic as a reflection of organic matter tends to increase as plants age [34,103,104,105], and organic matter will affect plant growth through its influence on soil physical and biochemical properties [106,107]. Previous research findings [108] asserted that soil’s physical and chemical properties (C-organic, P-availability, bulk density, sand, and clay content) affect the growth of diameter and height of S. palembanica.
Various microorganisms inhabit the soil. The number of microorganisms is beneficial in determining their location in relation to the root system, residual organic matter, soil depth, and soil fertility [109,110]. Soil microorganisms are responsible for most biological processes (60–80%) associated with nutrient cycling and organic matter decomposition [111]. There is a close interaction between plant diversity and soil microorganism diversity, in which plants are assumed to become mediators of changes in soil microorganism communities that affect ecosystem function [112].
Research conducted at GDRF [57] found that ants and worms were soil fauna that dominated S. leprosula stands. The ant species identified in this area were Monomorium pharaonic, Anoplolepis gracilipes, Pheidole dentata, and Odontomachus denticulata. Ants in the ecosystem are crucial as detritivores, pollinators, soil aerators, and predators [113]. At the same time, ants themselves are classified as soil macrofauna [114]. They are generally secondary consumers that cannot utilize coarse organic matter/litter directly. Instead, they use matters that have been destroyed by soil microorganisms [115]. Therefore, ants prefer to live in organic matter, which has been further decomposed (low C/N ratio). Earthworms of Lumbricus terrestris were also observed below the stands of S. leprosula. They are known to have a crucial part in the energy cycle of the ecosystem [88]. The best source of food for earthworms is litter because it contains relatively high carbohydrates and low lignocellulose.
Among important land characteristics that contributed to land productivity was slope gradient. Different slope gradients will affect stand performance in general and tree growth in particular [44]. The growth of S. platyclados in different slope gradients at reforested areas has been observed [58], and we compiled the results as shown in Table 3.

Table 3.
Average growth of S. platyclados on various slope classes in reforested GDRF.
S. platyclados in the reforested site is categorized [73] as fast-growing dipterocarp. The gentle-sloping area has thicker litter (11.7 cm) compared with the other slope classes (6.3–7.9 cm). Litter thickness is considered the main factor in generating higher diameter growth and tree height since litter affects soil organic matter content and increase soil fertility [116]. Vegetation in more fertile soil will show better growth performance. The growth of S. platyclados at the reforested site in GDRF is affected by the lower stand density at the gentle-sloping area, as denser stands will trigger higher competition for nutrients and light.
Soil solum depth is the main aspect determining land productivity. In the revegetated area in GDRF, the soil solum depth was >100 cm and was categorized as deep, and the effective root depth was more or less 90 cm, optimizing the growth of plants. A deep soil solum (>70 cm) enables nutrients and minerals for plant growth as the nutrient recycling process occurs. The effective root depth generally follows the depth of the soil solum as roots will not develop when facing mechanical obstacles in the form of rocks or soil volume weight [117].
Improving soil physical properties is one of the benefits of more than 20 years of reforestation activities in GDRF. Soil microorganisms help fallen litter from planted trees transform into obtainable nutrients and minerals to establish the nutrient cycle in the reforested site. Furthermore, the soil under the canopy also provided the optimum habitat for various soil fauna. Earthworms are best known as an agent to improve soil aggregation and porosity, thus improving soil structure and supporting plant growth and soil hydrology [118].
Observations of soil physical properties [59], i.e., bulk density, porosity, moisture content, drainage pore, and permeability in S. leprosula and S. selanica stands in GDRF, aimed to characterize whether those characteristics influenced the rate of soil infiltration, and the results of our analysis are shown in Table 4.

Table 4.
Index of soil physical properties under S. leprosula and S. selanica stands in reforested GDRF.
The high bulk density value was characterized by high clay content. The high clay content soil means the soil has high cohesiveness, allowing the improvement in soil density [120]. The total pore value was classified as good. Thus, in turn, it will directly affect the infiltration rate that categorized [121] as fast (155.33 mm/hour) in S. selanica stand and rather fast (120 mm/hour) in S. leprosula stand. Soil physical properties such as high porosity and the soil cover (understorey plants and litter) influence the higher infiltration rate in S. selanica stand. Forest litter serves as a temporary storage area for water and slowly releases it into the soil alongside the dispersed organic matter. In the end, it will increase the soil absorption ability [122]. The existence of understorey vegetation can boost soil porosity and decrease the raindrop effect. The correlation between infiltration rate and the value of slow drainage pores in S. selanica stands was positive (0.643).
3.4. The Formation of the Ectomycorrhizal Association in Reforested GDRF Landscape
The mycorrhizae are a form of the symbiotic association between certain soil fungi and plant roots. Dipterocarp trees are known to have an association with ectomycorrhizal fungi (ECM). ECM will modify the host tree’s lateral root system and give several benefit in turn, such as increased nutrient uptake, protecting against root pathogen, improving the growth of seedling in the nursery, and increasing drought tolerance [63,123,124,125,126,127,128,129,130]. Ectomycorrhizal macroscopic fruit bodies generally appear above the soil surface [63]. Several ECM families can be associated with Dipterocarpaceae, including Thelephoraceae, Russulaceae, Amanitaceae, Cortinariaceae, Sclerodermataceae, Agaricaceae, Pisolitaceae, and Boletaceae [63,131], Cantharellus [132], Pisolithus [133], Boletus enodensis, Lactarius spp. [134], and Lactarius [132,135].
Reforestation activities were carried out by planting uninoculated dipterocarps seedlings, and the degraded landscape has been transformed into lush and productive man-made dipterocarp forest. Yet, the establishment of this man-made dipterocarp forest was accompanied by its biological processes, such as ectomycorrhizal associations that resemble one of the main characteristics of natural dipterocarp forests.
In total, there were ten species of ECM fruit body collected from the S. leprosula and S. selanica stand. These ten species of ECM were from the family of Amanitaceae, Boletaceae, Hydnangiaceae, Russulaceae, and Schelodermataceae, with Russula and Boletus, were the most commonly found ECM. The ectomycorrhizal fungi diversity index in the observed plot planted by S. leprosula and S. selanica are classified as moderate, with low richness and evenness indices. The similarity of ECM found at the study site was due to a similarity in microclimate and soil characteristics in the observed plots. However, there was a slight difference in the number of ECM fungi found in S. selanica and S. leprosula. S. selanica (103 individuals/ha) has a denser canopy than S. leprosula (69 individuals/ha) stands, hence contributing different light intensity among both stands. Previous studies [136] determined that forest canopy affects the structure of the fungus by increasing the diversity of fungi, those included in ectomycorrhizas.
Not surprisingly, no ectomycorrhizal fruiting bodies were recorded on unplanted land adjacent to the dipterocarp trees planted in the site [137]. The study revealed that the presence of ectomycorrhiza is closely related to the dipterocarp forest establishment as a result of successful reforestation more than 20 years ago. The success of the reforestation proves it was followed by the development of the edaphic condition, which supports the ectomycorrhizal community at the reforested site.
Frequent and continuous observation and identification are needed to record the occurrence and diversity of the ECM associated with dipterocarps in certain forest landscapes [138,139,140]. Period of observation in different seasons and climatic differences will result in different ECM numbers found in terms of genera, species, and individuals [141,142,143]. The smaller number of species found in reforested GDRF may be contributed to the shorter and discontinuous observation period. Moreover, the number of ECM species found in the natural forest was higher than in plantation forests [144]. Longer observation may yield a greater number of associated ECM identified in the site. A 3-year ECM observation at tropical rainforests in Pasoh, Malaysia, found 296 ECM species, of which 8 of them were Sclerodermataceae [145].
3.5. Reforested Landscape Delivers Environmental Services Value and Supports Community Livelihood
Carbon sequestration capacity among landscapes with different vegetation cover is varied. Bareland, young secondary forest, mixed garden, plantation forest, old-growth forest, and other forms of vegetation cover types have been determined to have a different level of carbon sequestration [17,146,147]. GDRF has been transformed from almost bare land area with few old pine trees into a densely planted forest landscape. This 20 years old reforested area has brought many benefits in the form of socioeconomic and also environmental services. Aboveground tree biomass carbon stocks have been calculated and were varied depending on the silvicultural technique applied. The S. leprosula stand at a spacing distance of 2 m × 2 m, 3 m × 3 m, 4 m × 4 m, has aboveground carbon stocks of 73.4 tonnes C/ha, 85.6 tonnes C/ha, and 45.4 tonnes C/ha, respectively, while the potential carbon stock of S. selanica species at a spacing of 2 m × 2 m, 3 m × 3 m, 4 m × 4 m, respectively, is 66.9 tonnes C/ha, 49.4 tonnes C/ha, and 30.9 tonnes C/ha [64].
Not only do they increase the carbon stock, but reforestation activities have also changed the landscape characteristics. This reforested landscape provides a mosaic pattern of an intact lush forest standing side by side with a paddy field and blending with a waterfall scene (Figure 5 and Figure 6d). The beautiful scenery and vegetation structure diversity has made this man-made forest a new interesting ecotourism spot around the site and attracts tourists to enjoy the scenery (Figure 6a,b). Landscape restoration carried out 20 years ago has provided new benefits in providing environmental services to their society [148].

Figure 5.
Within the harmony: blended landscape of forest and paddy field.

Figure 6.
Reforested landscape which brings beautiful fresh scenery to enjoy (a,b) and provides water that are used widely by the community for household and agricultural needs (c–e).
Based on the actual visitor record [65], the visitor intensity is divided into three phases, namely: booming (June 2017–December 2018), steady (January 2019–March 2020), and pandemic or closure period (April 2020–present). In the two active boom and steady phases, peak visits occurred on weekends. The average number of visitors was 250–300 person/day and 40–70 person/day, respectively, while weekday visits for both phases were 30–50 person/day and 15–30 person/day. The outbreak of COVID-19 also significantly impacted this location due to the closure of public locations starting in April 2020. Many visitors who enjoy the scenery has created a multiplier effect for the surrounding community by generating new income from selling food, goods, and services. There were more than 30 food counters opened on-site during the booming period generating revenue for the seller of around IDR 600.000 (equal to about USD 42) and IDR 300.000 (equal to about USD 21) per day during the weekend and weekdays.
Other ecosystem services of the reforestation product are the availability of clean water. Reforested landscapes with a variety of vegetation planted together are known to have functions in the water regulator system. Trees can act like sponges that absorb and filter rainfall and release it slowly back into streams or rivers. The ability of forests to filter water is very important because it directly relates to human and ecosystem health. Forested landscape plays an important role in filtering clean water. More vegetation cover in a water catchment area will result in the cheaper cost of water treatment for consumption purposes as well as vegetation is highly recognized as an effective pollutant filter [149,150,151].
Five springs have been identified within the GDRF reforestation landscape. The five sources are Cikutu, Gunung Menteng, Cilame, Pondok, and Legok Gintung springs [66]. The community surrounding the forest also gave a fairly positive response to the existence of the GDRF. They argue that the success of the reforestation program has positive implications to forest landscape provides such as more sustainable water supply and higher discharge from existing springs than before reforestation activities (Figure 6c,d). The plantation forests and the use of exotic tree species can disrupt the evapotranspiration balance due to its negative impact on water availability [152]. However, restoration using mixed native species in the tropics may increase infiltration capacity [153]. Soil structure and soil organic materials will be improved by tree roots, enhancing soil aggregate stability and promoting the soil fauna, thus leading to higher macro-porosity that will create more rapid water infiltration [154]. Shading and litter under trees can also further reduce losses on soil evaporation.
3.6. The Challenges and Opportunities of Restoration in the GDRF Landscape
Today, many benefits are gained from the present GDRF landscape. The process of restoration itself required persistence and perseverance actions from all the actors. Chazdon et al. [155] stated three cross-cutting challenges in prevailing forest and landscape restoration developed from the literature: insignificant government support across levels and sectors; the environmental and social divergency; and poor enabling environments and operation capacity. Species, habitat suitability, and society’s view of the restoration process were on the top list of challenges in the restoration implementation in GDRF. How the native species choice successfully improves the unproductive land and increases land cover in GDRF, bringing the community’s positive impact, and the long restoration process has finally reached the goal. On the other hand, the government and stakeholders’ support become the opportunities, especially their support in enabling conditions and operational capacity in the restoration program. As many areas around the globe face many disasters suspected of any relations with climate change, landscape restoration becomes one of the answers. Then, we should be able to see the opportunities to overcome the challenges in implementing restoration programs.
4. Conclusions
Reforestation activities during 20 years using 31 Dipterocarpaceae species of native Indonesia in the GDRF have shown to deliver a wide spectrum of benefits. This includes timber stock, increasing the vegetation cover and carbon storage, establishing a biological process that supports land productivity, and delivering environmental services vital for the surrounding community. Several planted dipterocarps species in GDRF produced relatively higher standing stock volume (>35 to 220 m3/ha) compared with the standing stock potency of the logged-over area (LOA) in Indonesia. Forest cover has also been increased by 72% from the initial condition. The three studied species planted in the GDRF landscape have a different capacity to regenerate naturally. S. platyclados has the best performance in terms of natural regeneration. S. pinanga and S. leprosula showed the relatively low natural regeneration capacity that requires human intervention to stimulate the growth of the offspring, particularly during early survival. Successful reforestation has also created mutual biological interaction that improves soil physical properties, and increases infiltration and water regulatory function. The obvious environmental service that came to benefit the surrounding community was the establishment of livelihood generated from the transformation of degraded land into the recreational forest that attracts huge numbers of visitors. The landscape has been developed into high aesthetic value. A sincere peace and its gentle fresh air are not beneficial only for human physical health but also for psychological health. Yet, it is no wonder that this reforested landscape has been established as a popular spot for eco-tourism. Considering the obvious success of reforestation using native Dipterocarp species, future rehabilitation activities that are mainstreaming native species should be a priority in order to provide more benefits.
Author Contributions
Each author (H.H.R., K.L.G., Y.L., A.H., R.I., R.A.F., K.S.Y. and A.S.) has an equal work as the main contributor who equally conceived and designed the outline of the manuscript, collected and contributed data, performed the analysis, provided constructive feedback for each section, wrote and edited the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
The authors received no specific funding for this review.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Acknowledgments
We are grateful to the anonymous Reviewers and Academic Editors for helpful comments of the manuscript. We also thank to project collaboration between Forest Research and Development Centre (Indonesia Ministry of Environment and Forestry) and Komatsu Marketing Support Indonesia for their historical research collaboration that enabled the establishment of Gunung Dahu Research Forest (GDRF) in Bogor-Indonesia. We thank Atok Subiakto as the founder of GDRF in giving us valuable comments and information during the construction of this manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Yirdaw, E.; Tigabu, M.; Monge, A. Rehabilitation of degraded dryland ecosystems—Review. Silva Fenn. 2017, 50, 32. [Google Scholar] [CrossRef]
- Scholes, R.; Montanarella, L.; Brainich, A.; Barge, N.; ten Brink, B.; Cantele, M.; Erasmus, B.; Fisher, J.; Gardner, T.; Holland, T.G.; et al. Summary for Policymakers of the Assessment Report on Land Degradation and Restoration of the Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services; IPBES Secretariat: Bonn, Germany, 2018. [Google Scholar]
- Kettle, C.J. Ecological considerations for using dipterocarps for restoration of lowland rainforest in southeast asia. Biodivers. Conserv. 2010, 19, 1137–1151. [Google Scholar] [CrossRef]
- Potapov, P.; Hansen, M.C.; Laestadius, L.; Turubanova, S.; Yaroshenko, A.; Thies, C.; Smith, W.; Zhuravleva, I.; Komarova, A.; Minnemeyer, S.; et al. Esipova E. The last frontiers of wilderness: Tracking loss of intact forest landscapes from 2000 to 2013. Sci. Adv. 2017, 3, e1600821. [Google Scholar] [CrossRef] [PubMed]
- Tidwell, T.L. Nexus between food, energy, water, and forest ecosystems in the USA. J. Environ. Stud. Sci. 2016, 6, 214–224. [Google Scholar] [CrossRef]
- Lamb, D.; Erskine, P.D.; Parrotta, J.A. Restoration of degraded tropical forest landscapes. Science 2005, 5754, 1628–1632. [Google Scholar] [CrossRef]
- Fisher, R.F. Amelioration of degraded rain forest soils by plantations of native trees. Soil Sci. Soc. Am. J. 2006, 59, 544–549. [Google Scholar] [CrossRef]
- Castro, J.; Zamora, R.; Hódar, J.A.; Gómez, J.M. Use of shrubs as nurse plants: A new technique for reforestation in mediterranean mountains. Restor. Ecol. 2002, 10, 297–305. [Google Scholar] [CrossRef]
- Elliott, S.; Navakitbumrung, P.; Kuarak, C.; Zangkum, S.; Anusarnsunthorn, V.; Blakesley, D. Selecting framework tree species for restoring seasonally dry tropical forests in northern thailand based on field performance. For. Ecol. Manag. 2003, 184, 177–191. [Google Scholar] [CrossRef]
- Kitao, M.; Yoneda, R.; Tobita, H.; Matsumoto, Y.; Maruyama, Y.; Arifin, A.; Azani, A.M.; Muhamad, M.N. Susceptibility to photoinhibition in seedlings of six tropical fruit tree species native to malaysia following transplantation to a degraded land. Trees-Struct. Funct. 2006, 20, 601–610. [Google Scholar] [CrossRef]
- Kenzo, T.; Yoneda, R.; Matsumoto, Y.; Azani, M.A.; Majid, N.M. Leaf photosynthetic and growth responses on four tropical tree species to different light conditions in degraded tropical secondary forest, Peninsular Malaysia. Jpn. Agric. Res. Q. 2008, 42, 299–306. [Google Scholar] [CrossRef]
- Lamb, D. Regreening the Bare Hills; Springer, Business Media: New York, NY, USA, 2011. [Google Scholar]
- Berndes, G.; Abts, B.; Asikainen, A.; Cowie, A.L.; Dale, V.; Egnell, G.; Lindner, M.; Marelli, L.; Paré, D.; Pingoud, K.; et al. Forest biomass, carbon neutrality and climate change mitigation. Sci. Policy 2016, 3, 3–27. [Google Scholar]
- Nunes, L.J.R.; Meireles, C.I.R.; Gomes, C.J.P.; Ribeiro, N.M.C.A. Forest management and climate change mitigation: A review on carbon cycle flow models for the sustainability of resources. Sustainability 2019, 11, 5276. [Google Scholar] [CrossRef]
- Kirschbaum, M.U.F. The Carbon Sequestration Potential of Tree Plantations in Australia. In Environmental Management: The Role of Eucalypts and Other Fast Growing Species; Eldridge, K.G., Crowe, M.P., Old, K.M., Eds.; CSIRO Forestry and Forest Products: Canberra, Australia, 1996; pp. 77–89. [Google Scholar]
- Nejat, P.; Jomehzadeh, F.; Taheri, M.M.; Gohari, M.; Muhd, M.Z.A. A Global review of energy consumption, CO2 emissions and policy in the residential sector (with an overview of the top ten co2 emitting countries). Renew. Sustain. Energy Rev. 2015, 43, 843–862. [Google Scholar] [CrossRef]
- Rahman, F.A.; Aziz, M.M.A.; Saidur, R.; Bakar, W.A.W.A.; Hainin, M.R.; Putrajaya, R.; Hassan, N.A. Pollution to solution: Capture and sequestration of carbon dioxide (CO2) and its utilization as a renewable energy source for a sustainable future. Renew. Sustain. Energy Rev. 2017, 71, 112–126. [Google Scholar] [CrossRef]
- Wang, J.; Meng, J. Identifying indigenous tree species for land reforestation, forest restoration, and plantation transformation on Hainan Island, China. J. Mt. Sci. 2018, 15, 2433–2444. [Google Scholar] [CrossRef]
- Di Sacco, A.; Hardwick, K.A.; Blakesley, D.; Brancalion, P.H.S.; Breman, E.; Cecilio Rebola, L.; Chomba, S.; Dixon, K.; Elliott, S.; Ruyonga, G.; et al. Ten golden rules for reforestation to optimize carbon sequestration, biodiversity recovery and livelihood benefits. Glob. Chang. Biol. 2021, 27, 1328–1348. [Google Scholar] [CrossRef]
- MacKenzie, W.H.; Mahony, C.R. An Ecological approach to climate change-informed tree species selection for reforestation. For. Ecol. Manag. 2021, 481, 118705. [Google Scholar] [CrossRef]
- Lampela, M.; Jauhiainen, J.; Sarkkola, S.; Vasander, H. Promising native tree species for reforestation of degraded tropical peatlands. For. Ecol. Manag. 2017, 394, 52–63. [Google Scholar] [CrossRef]
- Jensen, D.A.; Rao, M.; Zhang, J.; Grøn, M.; Tian, S.; Ma, K.; Svenning, J.-C. The potential for using rare, native species in reforestation—A case study of yews (Taxaceae) in China. For. Ecol. Manag. 2021, 482, 118816. [Google Scholar] [CrossRef]
- Nunes, S.; Gastauer, M.; Cavalcante, R.B.L.; Ramos, S.J.; Caldeira, C.F.; Silva, D.; Rodrigues, R.R.; Salomão, R.; Oliveira, M.; Souza-Filho, P.W.M.; et al. Challenges and opportunities for large-scale reforestation in the Eastern Amazon using native species. For. Ecol. Manag. 2020, 466, 118120. [Google Scholar] [CrossRef]
- Sudrajat, D.J.; Rustam, E. Reforestation by direct seeding of Gmelina arborea using seed briquettes: Composition, size and site preparation, and sowing date. IOP Conf. Ser. Earth Environ. Sci. 2020, 533, 12014. [Google Scholar] [CrossRef]
- Casas, J.V.; Tulod, A.M.; Aribal, L.G.; Patricio, J.H.P. Exotic Gmelina arborea Roxb. plantation supports better understory plant diversity than native Nauclea orientalis (L.) plantation 30 years after their establishment in a watershed area in Southern Philippines. J. Biodivers. Environ. Sci. 2017, 11, 134–147. [Google Scholar]
- Tang, L.; Ang, L.; Ho, W. The potential of Acacia mangiun × Acacia auriculiformis hybrid as an afforestation species for impoverished sand tailings. Int. J. Agric. For. Plant. 2016, 4, 68–71. [Google Scholar]
- Permadi, D.B.; Burton, M.; Pandit, R.; Walker, I.; Race, D. Which smallholders are willing to adopt Acacia mangium under long-term contracts? Evidence from a choice experiment study in Indonesia. Land Use Policy 2017, 65, 211–223. [Google Scholar] [CrossRef]
- Moro, M.F.; Castro, A.S.F. A check list of plant species in the urban forestry of Fortaleza, Brazil: Where are the native species in the country of megadiversity? Urban Ecosyst. 2015, 18, 47–71. [Google Scholar] [CrossRef]
- Richardson, D.M.; Le Roux, J.J.; Wilson, J.R.U. Australian acacias as invasive species: Lessons to be learnt from regions with long planting histories. South. For. J. For. Sci. 2015, 77, 31–39. [Google Scholar] [CrossRef]
- Silva, L.D.D.; Lima, A.P.L.; de Lima, S.F.; Silva, R.C.; Paniago, G.F.; Silva, L.D.D.; Lima, A.P.L.; de Lima, S.F.; Silva, R.C.; Paniago, G.F. Controlled-release Fertilizer in the Production and Quality of Acacia mangium Seedlings. Floresta Ambiente 2019, 26. [Google Scholar] [CrossRef]
- Liu, T.; Jiang, K.; Tan, Z.; He, Q.; Zhang, H.; Wang, C. A Method for Performing Reforestation to Effectively Recover Soil Water Content in Extremely Degraded Tropical Rain Forests. Front. Ecol. Evol. 2021, 9, 19. [Google Scholar] [CrossRef]
- Moreira da Silva, A.P.; Schweizer, D.; Rodrigues Marques, H.; Cordeiro Teixeira, A.M.; Nascente dos Santos, T.V.M.; Sambuichi, R.H.R.; Badari, C.G.; Gaudare, U.; Brancalion, P.H.S. Can current native tree seedling production and infrastructure meet an increasing forest restoration demand in Brazil? Restor. Ecol. 2017, 25, 509–515. [Google Scholar] [CrossRef]
- Jalonen, R.; Valette, M.; Boshier, D.; Duminil, J.; Thomas, E. Forest and landscape restoration severely constrained by a lack of attention to the quantity and quality of tree seed: Insights from a global survey. Conserv. Lett. 2018, 11, e12424. [Google Scholar] [CrossRef]
- Rachmat, H.H.; Subiakto, A.; Susilowati, A. Mass vegetative propagation of rare and endangered tree species of Indonesia by shoot cuttings by KOFFCO method and effect of container type on nursery storage of rooted cuttings. Biodiversitas 2018, 19, 2353–2358. [Google Scholar] [CrossRef]
- Lu, Y.; Ranjitkar, S.; Xu, J.; Ou, X.; Zhou, Y.; Ye, J.-F.; Wu, X.-F.; Weyerhaeurser, H.; He, J. Propagation of Native Tree Species to Restore Subtropical Evergreen Broad-Leaved Forests in SW China. Forest 2016, 7, 12. [Google Scholar] [CrossRef]
- Nichols, J.; Vanclay, J. Domestication of native tree species for timber plantations: Key insights for tropical island nations. Int. For. Rev. 2012, 14, 402–413. [Google Scholar] [CrossRef][Green Version]
- Luna-Nieves, A.L.; Meave, J.A.; Morellato, L.P.C.; Ibarra-Manríquez, G. Reproductive phenology of useful Seasonally Dry Tropical Forest trees: Guiding patterns for seed collection and plant propagation in nurseries. For. Ecol. Manag. 2017, 393, 52–62. [Google Scholar] [CrossRef]
- Schmidt, I.B.; de Urzedo, D.I.; Piña-Rodrigues, F.C.M.; Vieira, D.L.M.; de Rezende, G.M.; Sampaio, A.B.; Junqueira, R.G.P. Community-based native seed production for restoration in Brazil—The role of science and policy. Plant Biol. 2019, 21, 389–397. [Google Scholar] [CrossRef]
- Ashton, P.; Kettle, C.J. Dipterocarp Biology as a Window to the Understanding of Tropical Forest Structure: Where are we Looking Now? Biotropica 2012, 44, 575–576. [Google Scholar] [CrossRef]
- Hiroshi, A.; Nakai, Y.; Hasegawa, G.P. Economic importance of the endemic Sumatran lowland dipterocarp tree species (Shorea javanica). Afr. J. Trop. Agric. 2015, 3, 163–172. [Google Scholar]
- Symington, C.F. Malayan Forest Records No. 16, Foresters’ Manual of Dipterocarps; Universiti Malaya: Kuala Lumpur, Malysia, 1974. [Google Scholar]
- Brearley, F.Q.; Banin, L.F.; Saner, P. The ecology of the Asian dipterocarps. Plant Ecol. Divers. 2016, 9, 429–436. [Google Scholar] [CrossRef]
- Lestari, D.A.; Fiqa, A.P.; Fauziah, F.; Budiharta, S. Growth evaluation of native tree species planted on post coal mining reclamation site in East Kalimantan, Indonesia. Biodiversitas 2019, 20, 134–143. [Google Scholar] [CrossRef]
- Pamoengkas, P.; Rachmat, H.H.; Khalifa, N. The growth of shorea leprosula at various planting distances and slopes in gunung dahu research forest, Bogor, Indonesia. Biodiversitas 2020, 21, 4396–4404. [Google Scholar] [CrossRef]
- Yuwati, T.W.; Rachmanadi, D.; Qirom, M.A.; Santosa, P.B.; Kusin, K.; Tata, H.L. Peatland Restoration in Central Kalimantan by Rewetting and Rehabilitation with Shorea balangeran. In Tropical Peatland Eco-management; Osaki, M., Tsuji, N., Foead, N., Rieley, J., Eds.; Springer: Singapore, 2021; pp. 595–611. ISBN 978-981-33-4654-3. [Google Scholar]
- Daisuke, H.; Tanaka, K.; Joseph Jawa, K.; Ikuo, N.; Katsutoshi, S. Rehabilitation of degraded tropical rainforest using dipterocarp trees in Sarawak, Malaysia. Int. J. For. Res. 2013, 2013, 683017. [Google Scholar] [CrossRef]
- Ådjers, G.; Hadengganan, S.; Kuusipalo, J.; Nuryanto, K.; Vesa, L. Enrichment planting of dipterocarps in logged-over secondary forests: Effect of width, direction and maintenance method of planting line on selected Shorea species. For. Ecol. Manag. 1995, 73, 259–270. [Google Scholar] [CrossRef]
- Shono, K.; Davies, S.J.; Chua, Y.K. performance of 45 native tree species on degraded lands in Singapore. J. Trop. For. Sci. 2007, 19, 25–34. [Google Scholar]
- Hassan, A.; Wahab, R.; Alias, M.A.; Salim, R.M. Growth performance of 9-years-old selected 5 indigenous wood species planted on degraded forest land. Int. J. Agric. Res. 2007, 2, 302–306. [Google Scholar] [CrossRef]
- Subiakto, A.; Rachmat, H.H.; Sakai, C. Choosing native tree species for establishing man-made forest: A new perspective for sustainable forest management in changing world. Biodiversitas 2016, 17, 620–625. [Google Scholar] [CrossRef]
- Rachmat, H.H.; Pamoengkas, P.; Sholihah, L.; Fambayun, R.A.; Susilowati, A. The effect of planting technique on the growth of two Shorea species in Gunung Dahu, Bogor, Indonesia. Biodiversitas 2020, 21, 4131–4138. [Google Scholar] [CrossRef]
- Wu, Q. GIS and Remote Sensing Applications in Wetland Mapping and Monitoring. In Comprehensive Geographic Information Systems; Huang, B., Ed.; Elsevier: Oxford, UK, 2018; pp. 140–157. [Google Scholar]
- Maulidah, I.; Pamoengkas, P.; Rachmat, H.H. Natural Regeneration Potential of Shorea Pinanga Scheff and Shorea Platyclados Slooten Ex Endert. in Gunung Dahu Research Forest, Bogor-West Java. IOP Conf. Ser. Earth Environ. Sci. 2019, 394, 012063. [Google Scholar] [CrossRef]
- Nurkhaeni, W. Potensi Regenerasi Alami Shorea leprosula Miq. Di Hutan Penelitian Gunung Dahu, Leuwiliang, Kabupaten Bogoritle, Bogor; IPB University: Bogor, Indonesia, 2019. [Google Scholar]
- Wang, W.; Zhong, Z.; Wang, Q.; Wang, H.; Fu, Y.; He, X. Glomalin contributed more to carbon, nutrients in deeper soils, and differently associated with climates and soil properties in vertical profiles. Sci. Rep. 2017, 7, 13003. [Google Scholar] [CrossRef]
- Supriyanto, I.U. Teknik Pengukuran Penutupan Tajuk dan Pembukaan Tajuk Tegakan Dengan Menggunakan Spherical Densiometer; SEAMEO-BIOTROP: Bogor, Indonesia, 2001. [Google Scholar]
- Chotimah, T. Pertumbuhan Meranti (Shorea leprosula Miq) di Hutan Penelitian Gunung Dahu, Leuwiliang, Kabupaten Bogor; Bogor Agricultural University: Bogor, Indonesia, 2020. [Google Scholar]
- Pertiwi, P.R. Pengaruh Kelerengan Terhadap Pertumbuhan Shorea platyclados di Hutan Penelitian Gunung Dahu, Leuwiliang, Kabupaten Bogor; Bogor Agricultural University: Bogor, Indonesia, 2020. [Google Scholar]
- Saputra, N.E. Analisis Sifat Fisik Tanah dan Laju Infiltrasi pada Berbagai Penggunaan Lahan di Hutan Penelitian Gunung Dahu; Bogor Agricultural University: Bogor, Indonesia, 2020. [Google Scholar]
- Nuraini, T. Keanekaragaman Fungi Ektomikoriza di Hutan Penelitian Meranti Gunung Dahu Leuwiliang Kabupaten Bogor; Bogor Agicultural University: Bogor, Indonesia, 2017. [Google Scholar]
- Kasongat, H.; Gofur, M.A. Ponisri Identifikasi dan kenaekargaman jenis jamur ektomikoriza pada hutan jati di Seram bagian timur. Median 2019, 11, 39–46. [Google Scholar] [CrossRef]
- Karmilasanti, K.; Maharani, R. Keanekaragaman Jenis Jamur Ektomikoriza Pada Ekosistem Hutan Dipterokarpa Di Khdtk Labanan, Berau, Kalimantan Timur. J. Penelit. Ekosist. Dipterokarpa 2016, 2, 57–66. [Google Scholar] [CrossRef]
- Brundrett, M.; Bougher, N.; Dell, B.; Grove, T.; Malajczuk, N. Working with Mycorrhizae in Forestry and Agriculture; ACIAR: Canberra, Australia, 1995. [Google Scholar]
- Rachmat, H.H.; Fambayun, R.A. Restorasi Bentang Alam Terdegradasi: Pembelajaran dari Beberapa Kisah Pemulihan Lahan dengan Pengembangan Teknik KOFFCO. In Konservasi KEHATI Skala Demo Plot; Sawitri, R., Ed.; IPB Press: Bogor, Indonesia, 2019; pp. 269–300. [Google Scholar]
- LMDH. Association of Forest Village Community. 2020; Pabangbon Village: Bogor, Indonesia, 2020. [Google Scholar]
- P3H. Gunung Dahu Long-Term Management Plan; Pusat Litbang Hutan: Bogor, Indonesia, 2020. [Google Scholar]
- Widiyatno, W.; Na’iem, M.; Kanzaki, M.; Purnomo, S.; Jatmiko, J. Application of silviculture treatment to support rehabilitation on logged over area (LOA) of tropical rainforest, Central Kalimantan, Indonesia. Int. J. Sustain. Futur. Hum. Secur. 2013, 1, 50–55. [Google Scholar] [CrossRef]
- Soerianegara, I.; Lemmens, R.H.M.J. Plant Resources of South-East Asia No 5(1) Timber Trees: Major Commercial Timbe; Backhuys Publishers: Leiden, The Netherlands, 1995; ISBN 9022010333. [Google Scholar]
- Widiyatno, W.; Soekotjo, S.; Naiem, M.; Hardiwinoto, S.; Purnomo, S. Pertumbuhan meranti (Shorea spp.) pada sistem tebang pilih tanam jalur dengan teknik silvikultur intensif (TPTJ-SILIN). J. Penelit. Hutan Dan Konserv. Alam 2011, 8, 373–383. [Google Scholar] [CrossRef][Green Version]
- Na’iem, M.; Faridah, E. Model of Intensive Enrichment Planting (TPTII), Silviculture System of 93 Evaluation of Silvicultural System Application Activities. In Indonesia’s Dipterocarps Forest Management: A Lesson Learned; Faculty of Forestry GMU and International Tropical Timber Organization: Yogyakarta, Indonesia, 2006; pp. 25–36. [Google Scholar]
- Newman, M.F.; Burgess, P.F.; Whitmore, T.C. Manuals of Dipterocarps for Foresters: Borneo Island Light Hardwoods: Anisoptera, Parashorea, Shorea (Red, White and Yellow Meranti); Royal Botanic Garden Edinburgh and CIFOR: Edinburgh, UK, 1996; ISBN 1-872291-76-7. [Google Scholar]
- Tampubolon, S.; Manurung, T.F.; Latifah, S. Sebaran Tengkawang (Shorea spp.) berdasarkan Fitogeografi pada Hutan Adat Pengajit Desa Sahan Kecamatan Seluas Kabupaten Bengkayang. J. Hutan Lestari 2018, 6, 883–893. [Google Scholar]
- Mindawati, N.; Tiryana, T. Pertumbuhan Jenis Pohon Khaya anthotheca di Jawa Barat. Bull. Penelit. Hutan 2002, 632, 47–58. [Google Scholar]
- Pamoengkas, P.; Prasetia, R. Pertumbuhan Meranti Merah (Shorea leprosula Miq) Dalam Sistem. Silvikultur Tebang Pilih Tanam Jalur (Studi Kasus di Areal. IUPHHK-HA PT. Sari Bumi Kusuma, Kalimantan Tengah). J. Silvikultur Trop. 2014, 5, 174–180. [Google Scholar]
- Van Gardingen, P.R.; McLeish, M.J.; Phillips, P.D.; Fadilah, D.; Tyrie, G.; Yasman, I. Financial and ecological analysis of management options for logged-over Dipterocarp forests in Indonesian Borneo. For. Ecol. Manag. 2003, 183, 1–29. [Google Scholar] [CrossRef]
- Marsoem, S.N. Studi Mutu Kayu Jati di Hutan Rakyat Gunungkidul I. Pengukuran Laju Pertumbuhan. J. Ilmu Kehutan. 2015, 7, 108–122. [Google Scholar]
- Kuswandi, R. Julius Dwi Nugroho Diameter Increment of Remnant Stands in Logged-Over Forest in Papua. J. Wasian 2019, 6, 125–133. [Google Scholar] [CrossRef]
- Lal, A.B. Silvicultural Systems and Forest Management; Jugal Kishore and Co.: Dehradun, India, 1961. [Google Scholar]
- Anderson, J. Illipe Nuts (Shorea spp.) as Potential Agricultural Crops. In Proceedings of the Symposium on South East Asian Plant Genetic Resources; Williams, J.T., Lamoureux, C.H., Soetjipto, N.W., Eds.; International Board for Plant Genetic Resources: Bogor, Indonesia, 1975; pp. 217–230. [Google Scholar]
- Weinland, G. Plantation. In A Review of Dipterocarps: Taxonomy, Ecology and Silviculture; Appanah, S., Turnbull, J.W., Eds.; CIFOR: Bogor, Indonesia, 1998; pp. 151–186. [Google Scholar]
- Ordonez, J.C.; Luedeling, E.; Kindt, R.; Tata, H.L.; Harja, D.; Jamnadass, R.; van Noordwijk, M. Constraints and opportunities for tree diversity management along the forest transition curve to achieve multifunctional agriculture. Curr. Opin. Environ. Sustain. 2014, 6, 54–60. [Google Scholar] [CrossRef]
- Ashton, P.S. Dipterocarpaceae. In Flora Malesiana I; van Steenis, C.G.G.J., Ed.; Martinus Nijhoff: Hague, The Netherland, 1982; pp. 237–552. [Google Scholar]
- Krishnapillay, B.; Tompsett, P.B. Seed Handling. In A Review of Taxonomy, Ecology and Silviculture; Appanah, S., Turnbull, J., Eds.; CIFOR: Bogor, Indonesia, 1998; pp. 80–88. [Google Scholar]
- Numata, S.; Yasuda, M.; Okuda, T.; Kachi, N.; Noor, N.S.M. Temporal and spatial patterns of mass flowerings on the Malay Peninsula. Am. J. Bot. 2003, 90, 1025–1031. [Google Scholar] [CrossRef]
- Fitriasari, D.A. Evaluasi Hasil Studi Pengaruh Inokulasi Fungi Ektomikoriza Terhadap Respon Pertumbuhan Bibit Shorea spp. di Persemaian; Bogor Agricultural University: Bogor, Indonesia, 2011. [Google Scholar]
- Wahyudi, A.; Sari, N.; Saridan, A. Ekologi, Morfologi & Upaya Konservasi. In Shorea Leprosula Miq dan Shorea Johorensis Foxw: Ekologi, Silvikultur, Budidaya dan Pengembangan; Balai Besar Penelitian Dipterokarpa: Samarinda, Indonesia, 2014; pp. 3–13. ISBN 978-602-9096-13-2. [Google Scholar]
- Fajri, M.; Fernandes, A. Harvesting Patterns of Tengkawang (Shorea machrophylla) and Its Natural Regeneration in Community’s Garden. J. Penelit. Ekosist. Dipterokarpa 2015, 1, 81–88. [Google Scholar] [CrossRef][Green Version]
- Hilwan, I.; Handayani, E.P. Keanekaragaman Mesofauna dan Makrofauna Tanah pada Areal Bekas Tambang Timah di Kabupaten Belitung, Provinsi Kepulauan Bangka-Belitung. J. SIlvikultur Trop. 2013, 4, 35–41. [Google Scholar]
- Panjaitan, S. Pertumbuhan dan komposisi jenis permudaan alam pada rumpang tebangan di Kalimantan Selatan. J. Penelit. Dipterokarpa 2013, 7, 63–74. [Google Scholar] [CrossRef]
- Soekotjo. Teknik Silvikultur Intensif (SILIN); Gadjah Mada University Press: Yogyakarta, Indonesia, 2009. [Google Scholar]
- Hardiwinoto, S.; Adriana, A.; Hadi Nurjanto, H.; Widiyatno, W.; Dhina, F.; Priyo, E. Pengaruh sifat fisika media terhadap kemampuan berakar dan pembentukan akar stek pucuk S. platyclados di PT Sari Bumi Kusuma Kalimantan Tengah. J. Pemuliaan Tanam. Hutan 2010, 4, 37–47. [Google Scholar] [CrossRef]
- Holilullah, H.; Afandi, A.; Novpriansyah, H. Karakteristik sifat fisik tanah pada lahan produksi rendah dan tinggi di PT Great Giant Pinapple. J. Agrotek Trop. 2015, 3, 278–282. [Google Scholar]
- Wong, M. Impact of dipterocarp seedlings on the vegetative and reproductive characteristics of Labisia Pumila (Myrsinaceae) in the understorey. Malays. For. 1981, 44, 370–376. [Google Scholar]
- Kusneti, M. The effect of Phrynium parvum Ridley (Marantaceae) on the growth and density of Dipterocarpus sublamellatus seedling. In Forest Biology and Conservation in Borneo; Murtedza, M., Omar, S., Eds.; Yayasan Sabah: Kinabalu, Malaysia, 1992; pp. 339–347. [Google Scholar]
- Mashudi, M.; Pudjiono, S.; Rayan, R.; Sulaeman, M. Pengaruh asal populasi dan pohon induk terhadap pertumbuhan bibit meranti tembaga Shorea leprosula Miq. sebagai materi untuk perbanyakan klonal. J. Penelit. Dipterokarpa 2012, 6, 97–109. [Google Scholar] [CrossRef][Green Version]
- Hardjowigeno, H.S. Klasifikasi Tanah dan Pedogenesis; Akademika Pressindo: Jakarta, Indonesia, 2003; ISBN 979-8035-48-8. [Google Scholar]
- Darmawijaya, M.I. Klasifikasi Tanah: Dasar Teori Bagi Peneliti Tanah dan Pelaksana Pertanian di Indonesia; Gadjah Mada University Press: Yogyakarta, Indonesia, 2005; ISBN 9794201537. [Google Scholar]
- Stothoff, S.A.; Or, D.; Groeneveld, D.P.; Jones, S.B. The effect of vegetation on infiltration in shallow soils underlain by fissured bedrock. J. Hydrol. 1999, 218, 169–190. [Google Scholar] [CrossRef]
- Aini, Z.Z.; Khasanah, N.; Kusuma, Z. Degradasi sifat fisik tanah sebagai akibat alih guna lahan hutan menjadi sistem kopi monokultur: Kajian perubahan makroporositas tanah. Agrivita 2004, 26, 60–68. [Google Scholar]
- Winanti, T. Pekarangan Sebagai Media Peresapan Air Hujan Dalam Upaya PengelolaanSumberdaya Air. In Proceedings of the Konferensi Nasional Pusat Studi Lingkungan BKPSL; Udayana University: Bali, Indonesia, 1996. [Google Scholar]
- Li, X.; Li, J. Distribution and Conservation Strategy of Eight Threatened Gymnosperm Species in China; China Academic of Science: Wuhan, China, 2004; pp. 1–5. [Google Scholar]
- Mindawati, N. Pratiwi Produksi dan laju dekomposisi serasah Acacia mangium Willd. Bul. Penelit. Hutan 2008, 6, 65–77. [Google Scholar]
- Haron, K.; Brookes, P.C.; Anderson, J.M.; Zakaria, Z.Z. Microbial biomass and soil organic matter dynamics in oil palm (Elaeis guineensis jacq.) plantations, West Malaysia. Soil Biol. Biochem. 1998, 30, 547–552. [Google Scholar] [CrossRef]
- Khasanah, N.; van Noordwijk, M.; Ningsih, H.; Rahayu, S. Carbon neutral? No change in mineral soil carbon stock under oil palm plantations derived from forest or non-forest in Indonesia. Agric. Ecosyst. Environ. 2015, 211, 195–206. [Google Scholar] [CrossRef]
- Brahene, S.W.; Owusu-Bennoah, E.; Abekoe, M.K. Dynamics of soil carbon sequestration under oil palm plantations of different ages. In Proceedings of the Global Symposium on Soil Organic Carbon; FAO: Rome, Italy, 2017; pp. 1–4. [Google Scholar]
- Alexander, M. Introduction to Soil Microbiology, 2nd ed.; Wiley Eastern Limited: Calcutta, India, 1978. [Google Scholar]
- Stevenson, F. Humus Chemistry: Genesis, Composition, Reactions, 2nd ed.; Willey Interscience: New York, NY, USA, 1994; ISBN 978-0-471-59474-1. [Google Scholar]
- Erizilina, E.; Pamoengkas, P.; Darwo, D. Hubungan sifat fisik dan kimia tanah dengan pertumbuhan meranti merah di khdtk haurbentes. J. Nat. Resour. Environ. Manag. 2019, 9, 68–74. [Google Scholar] [CrossRef]
- Anas, I. Biologi Tanah Dalam Praktek; Pusat Antar Universitas Bioteknologi IPB: Bogor, Indonesia, 1989. [Google Scholar]
- Rao, S.N.S. Mikroorganisme Tanah dan Pertumbuhan Tanaman; UI Press: Jakarta, Indonesia, 1994; ISBN 979-456-124-0. [Google Scholar]
- Breure, A.M. Soil biodiversity: Measurements, Indicators, Threats and soil functions. In Proceedings of the International Conference on Soil and Compost Eco-Biology; SoilAce: Leon, Spain, 2004; pp. 83–96. [Google Scholar]
- Carney, K.M.; Matson, P.A. Plant communities, soil microorganisms, and soil carbon cycling: Does altering the world belowground matter to ecosystem functioning? Ecosystems 2005, 8, 928–940. [Google Scholar] [CrossRef]
- Hölldobler, B.; Wilson, E.O. The Ants; Harvard University Press: Cambridge, MA, USA, 1990. [Google Scholar]
- Wallwork, J.A. Ecology of Soil Animals; Blower, J.G., Ed.; McGraw Hill: London, UK, 1971. [Google Scholar]
- Soepardi, G. Sifat dan Ciri Tanah; IPB Press: Bogor, Indonesia, 1983. [Google Scholar]
- Wezel, A.; Rajot, J.L.; Herbrig, C. Influence of shrubs on soil characteristics and their function in Sahelian agro-ecosystems in semi-arid Niger. J. Arid Environ. 2000, 44, 383–398. [Google Scholar] [CrossRef]
- Nugroho, Y. Analisis Kualitas Lahan Untuk Pengembangan Model Pertanaman Jati (Tectona Grandis L.F) Rakyat di Tropika Basah; Universitas Brawijaya: Kota Malang, Indonesia, 2015. [Google Scholar]
- Borror, D.J.; Triplehorn, C.A.; Ohnson, N.F. Pengenalan Pelajaran Serangga, 6th ed.; Gadjah Mada University Press: Yogyakarta, Indonesia, 1992; ISBN 979-420-237-1. [Google Scholar]
- Sutanto, R. Dasar-Dasar Ilmu Tanah: Konsep dan Kenyataan; Kanisius: Yogyakarta, Indonesia, 2005; ISBN 9792104674. [Google Scholar]
- Hakim, H.; Nyakpa, Y.; Lubis, A.M.; Nugroho, S.G.; Saul, M.R.; Diha, M.A.; Hong, G.B.; Bailey, H.H. Dasar-Dasar Ilmu Tanah; Universitas Lampung: Lampung, Indonesia, 1986. [Google Scholar]
- Kohnke, H. Soil Physics; McGraw-Hill Book Company: New York, NY, USA, 1968. [Google Scholar]
- Ginting, S. Pendugan Laju Infiltrasi Menggunakan Parameter Sifat Tanah Pada Kawasan Berlereng; University Sumatera Utara: Medan, Indonesia, 2009. [Google Scholar]
- Allen, M.F.; Swenson, W.; Querejeta, J.I.; Egerton-Warburton, L.M.; Treseder, K.K. Ecology of Mycorrhizae: A Conceptual Framework for Complex Interactions among Plants and Fungi. Annu. Rev. Phytopathol. 2003, 41, 271–303. [Google Scholar] [CrossRef] [PubMed]
- Baghel, R.K.; Sharma, R.; Pandey, A.K. Activity of acid phosphatase in the ectomycorrhizal fungus Cantharellus tropicalis under controlled conditions. J. Trop. For. Sci. 2009, 21, 218–222. [Google Scholar]
- Bechem, E.E.T.; Alexander, I.J. Phosphorus nutrition of ectomycorrhizal Gnetum africanum plantlets from Cameroon. Plant Soil 2012, 353, 379–393. [Google Scholar] [CrossRef]
- Onguene, N.A.; Kuyper, T.W. Importance of the ectomycorrhizal network for seedling survival and ectomycorrhiza formation in rain forests of south Cameroon. Mycorrhiza 2002, 12, 13–17. [Google Scholar] [CrossRef]
- Whipps, J.M. Prospects and limitations for mycorrhizas in biocontrol of root pathogens. Can. J. Bot. 2004, 82, 1198–1227. [Google Scholar] [CrossRef]
- Dell, B. Role of mycorrhiza fungi in ecosystems. CMU J. 2002, 1, 47–55. [Google Scholar]
- Duñabeitia, M.K.; Hormilla, S.; Garcia-Plazaola, J.I.; Txarterina, K.; Arteche, U.; Becerril, J.M. Differential responses of three fungal species to environmental factors and their role in the mycorrhization of Pinus radiata D. Don. Mycorrhiza 2004, 14, 11–18. [Google Scholar] [CrossRef]
- Ahonen-Jonnarth, U.; Roitto, M.; Markkola, A.M.; Ranta, H.; Neuvonen, S. Effects of nickel and copper on growth and mycorrhiza of Scots pine seedlings inoculated with Gremmeniella abietina. For. Pathol. 2004, 34, 337–348. [Google Scholar] [CrossRef]
- Amornpitak, T.Y.; Taweerat, V.; Morakot, T.; Supapon, C.; Sansnarak, R. Diversity of Ectomycorrhizal Fungi on Dipterocarpaceae in Thailand. J. Biol. Sci. 2006, 6, 1059–1064. [Google Scholar]
- Eyssartier, E.; Stubbe, D.; Walleyn, R.; Verbeken, A. New Records of Cantharellus Species (Basidiomycota, Cantharellaceae) from Malaysian Dipterocarp Rainforest. Fungal Divers. 2009, 36, 57–67. [Google Scholar]
- Reddy, M.S.; Singla, S.; Natarajan, K.; Senthilarasu, G. Pisolithus indicus, a new species of ectomycorrhizal fungus associated with Dipetrocarps in India. Mycologia 2005, 97, 839–843. [Google Scholar] [CrossRef]
- Noor, M.; Saridan, A. Keanekaragaman fungi makro pada tegakan benih Dipterocarpaceae di Taman Nasional Tanjung Puting dan Taman Nasional Sebangau Kalimantan Tengah. J. Penelit. Dipterokarpa 2013, 7, 53–62. [Google Scholar] [CrossRef][Green Version]
- Tata, H.L.; van Noordwijk, M.; Summerbell, R.; Werger, M.J.A. Limited response to nursery-stage mycorrhiza inoculation of Shorea seedlings planted in rubber agroforest in Jambi, Indonesia. New For. 2010, 39, 51–74. [Google Scholar] [CrossRef]
- Santos-Silva, C.; Goncalves, A.; Louro, R. Canopy cover influence on macrofungal richness and sporocarp production in montado ecosystems. Agrofor. Syst. 2011, 82, 149–159. [Google Scholar] [CrossRef]
- Chotimah, T.; Wasis, B.; Rachmat, H.H. Population of Macrofauna, Mesofauna, and Ectomycorrhizae’s Fruit Body at Shorea leprosula stand at Gunung Dahu Forest research. J. Penelit. Hutan Dan Konserv. Alam 2020, 17, 79–98. [Google Scholar] [CrossRef]
- Brearley, F.Q. Ectomycorrhizal associations of the Dipterocarpeace. Biotropica 2012, 44, 637–648. [Google Scholar] [CrossRef]
- Brearley, F.Q.; Saner, P.; Uchida, A.; Burslem, D.F.R.P.; Hector, A.; Nilus, R.; Scholes, J.D.; Egli, S. Testing the importance of a common ectomycorrhizal network for dipterocarp seedling growth and survival in tropical forests of Borneo. Plant Ecol. Divers. 2016, 9, 563–576. [Google Scholar] [CrossRef]
- Suwannasai, N.; Dokmai, P.; Yamada, A.; Watling, R.; Phosri, C. First ectomycorrhizal syntheses between astraeus sirindhorniae and dipterocarpus alatus (Dipterocarpaceae), pure culture characteristics, and molecular detection. Biodiversitas 2020, 21, 231–238. [Google Scholar]
- Weemstra, M.; Peay, K.G.; Davies, S.J.; Mohamad, M.; Itoh, A.; Tan, S.; Russo, S.E. Lithological constraints on resource economies shape the mycorrhizal composition of a Bornean rain forest. New Phytol. 2020, 228, 253–268. [Google Scholar] [CrossRef]
- Cuenca, G.; Lovera, M. Seasonal variation and distribution at different soil depths of arbuscular mycorrhizal fungi spores in a tropical sclerophyllous shrubland. Botany 2010, 88, 54–64. [Google Scholar] [CrossRef]
- Liu, J.; Kim, Y.S.; Richardson, C.E.; Tom, A.; Ramakrishnan, C.; Birey, F.; Katsumata, T.; Chen, S.; Wang, C.; Wang, X.; et al. Genetically targeted chemical assembly of functional materials in living cells, tissues, and animals. Science 2020, 367, 1372–1376. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Malajczuk, N.; Brundrett, M.; Dell, B. Fruiting of putative ectomycorrhizal fungi under blue gum [Eucalyptus globulus plantations of different ages in Western Australia. Mycorrhiza 1999, 8, 225–261. [Google Scholar] [CrossRef]
- Lee, S.; Watling, R.; Sikin, Y.N. Ectomycorrhizal basidiomata fruiting in lowland rain forests of Peninsular Malaysia. Bois Des Trop. 2002, 274, 33–43. [Google Scholar]
- Ontl, T.A.; Janowiak, M.K.; Swanston, C.W.; Daley, J.; Handler, S.; Cornett, M.; Hagenbuch, S.; Handrick, C.; McCarthy, L.; Patch, N. Forest management for carbon sequestration and climate adaptation. J. For. 2020, 118, 86–101. [Google Scholar] [CrossRef]
- Zam, S.I. In vitro Bioremediation of Dirtied Soil by Oil Refinery Waste in different pH Concentration) Syukria Ikhsan Zam. J. Agroteknologi 2011, 1, 1–7. [Google Scholar]
- Djaenudin, D. Laporan Hasil Kajian Kontribusi Sosial Ekonomi Hutan Penelitian Gunung Dahu; Pusat Litbang Hutan: Bogor, Indonesia, 2019. [Google Scholar]
- Vigiak, O.; Ribolzi, O.; Pierret, A.; Valentin, C.; Sengtaheuanghoung, O.; Noble, A. Filtering of water pollutants by riparian vegetation: Bamboo versus native grasses and rice in a Lao catchment. Unasylva 2007, 58, 11–16. [Google Scholar]
- Dosskey, M.G.; Vidon, P.; Gurwick, N.P.; Allan, C.J.; Duval, T.P.; Lowrance, R. The Role of Riparian Vegetation in Protecting and Improving Chemical Water Quality in Streams1. J. Am. Water Resour. Assoc. 2010, 46, 261–277. [Google Scholar] [CrossRef]
- Luqman, M.; Butt, T.M.; Tanvir, A.; Atiq, M.; Hussan, M.Z.Y.; Yaseen, M. Phytoremediation of polluted water by trees: A review. Afr. J. Agric. Res. 2013, 8, 1591–1595. [Google Scholar]
- Trabucco, A.; Zomer, R.J.; Bossio, D.A.; van Straaten, O.; Vercot, L.V. Climate change mitigation through afforestation/reforestation: Aglobal analysis of hydrologic impacts with four case studies. Agric. Ecosyst. Environ. 2008, 126, 81–97. [Google Scholar] [CrossRef]
- Ilstedt, U.; Malmer, A.; Verbeeten, E.; Murdiyarso, D. The effect of afforestation on water infiltration in the tropics: A systematic review and meta-analysis. For. Ecol. Manag. 2007, 251, 45–51. [Google Scholar] [CrossRef]
- Bargués Tobella, A.; Reese, H.; Almaw, A.; Bayala, J.; Malmer, A.; Laudon, H.; Ilstedt, U. The effect of trees on preferential flow and soil infiltrability in an agroforestry parkland in semiarid Burkina Faso. Water Resour. Res. 2014, 50, 3342–3354. [Google Scholar] [CrossRef]
- Chazdon, R.L.; Wilson, S.J.; Brondizio, E.; Guariguata, M.R.; Herbohn, J. Key challenges for governing forest and landscape restoration across different contexts. Land Use Policy 2021, 104, 104854. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).