Removal of Pharmaceuticals from Water by Tomato Waste as Novel Promising Biosorbent: Equilibrium, Kinetics, and Thermodynamics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Pharmaceutical Standards and Reagents
2.2. Preparation and Characterization of Tomato Waste as Biosorbent
2.3. Batch Adsorption Experiments
2.4. Adsorption Analysis and Calculations
2.5. Sorption Kinetics
3. Results
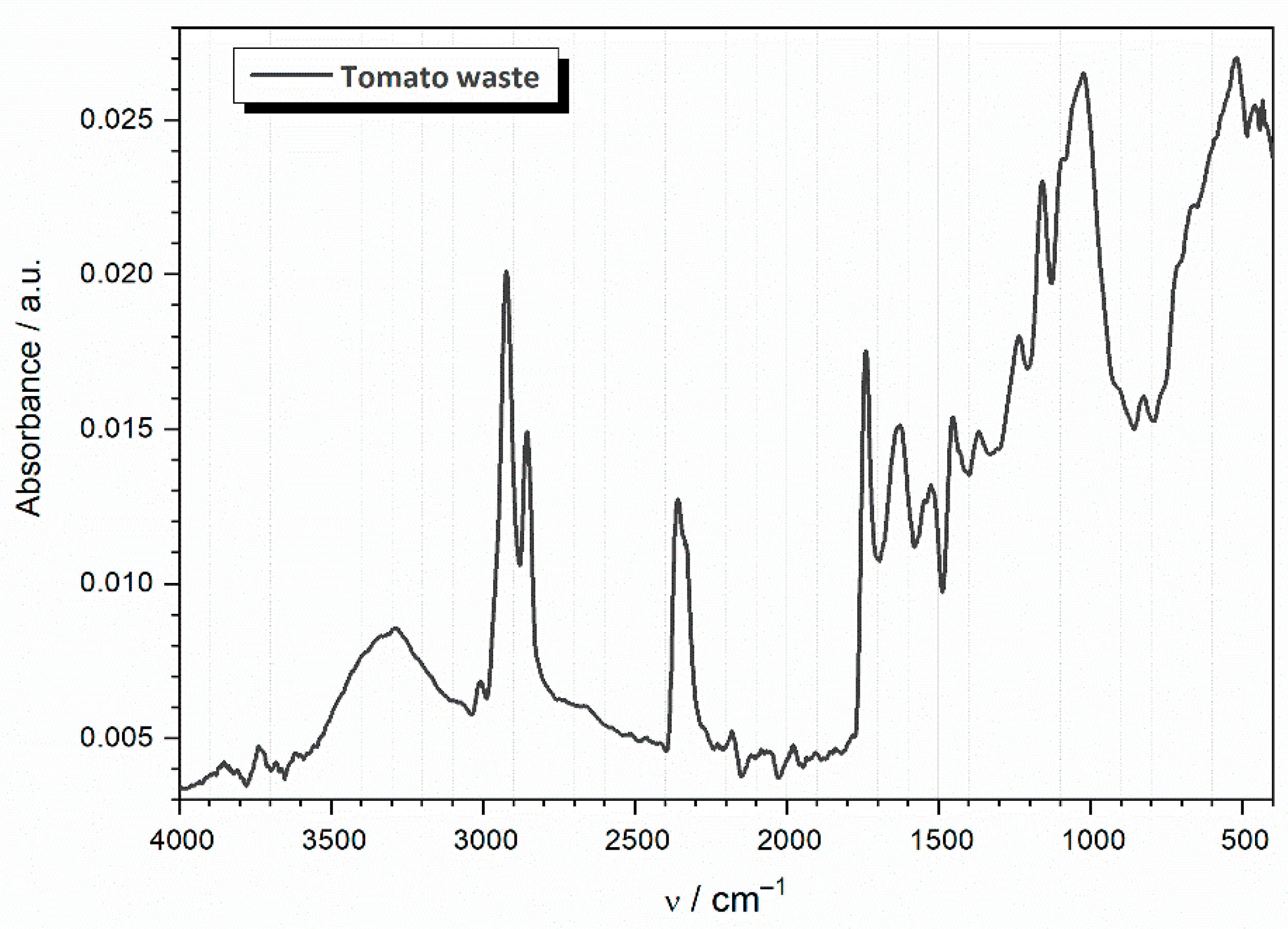
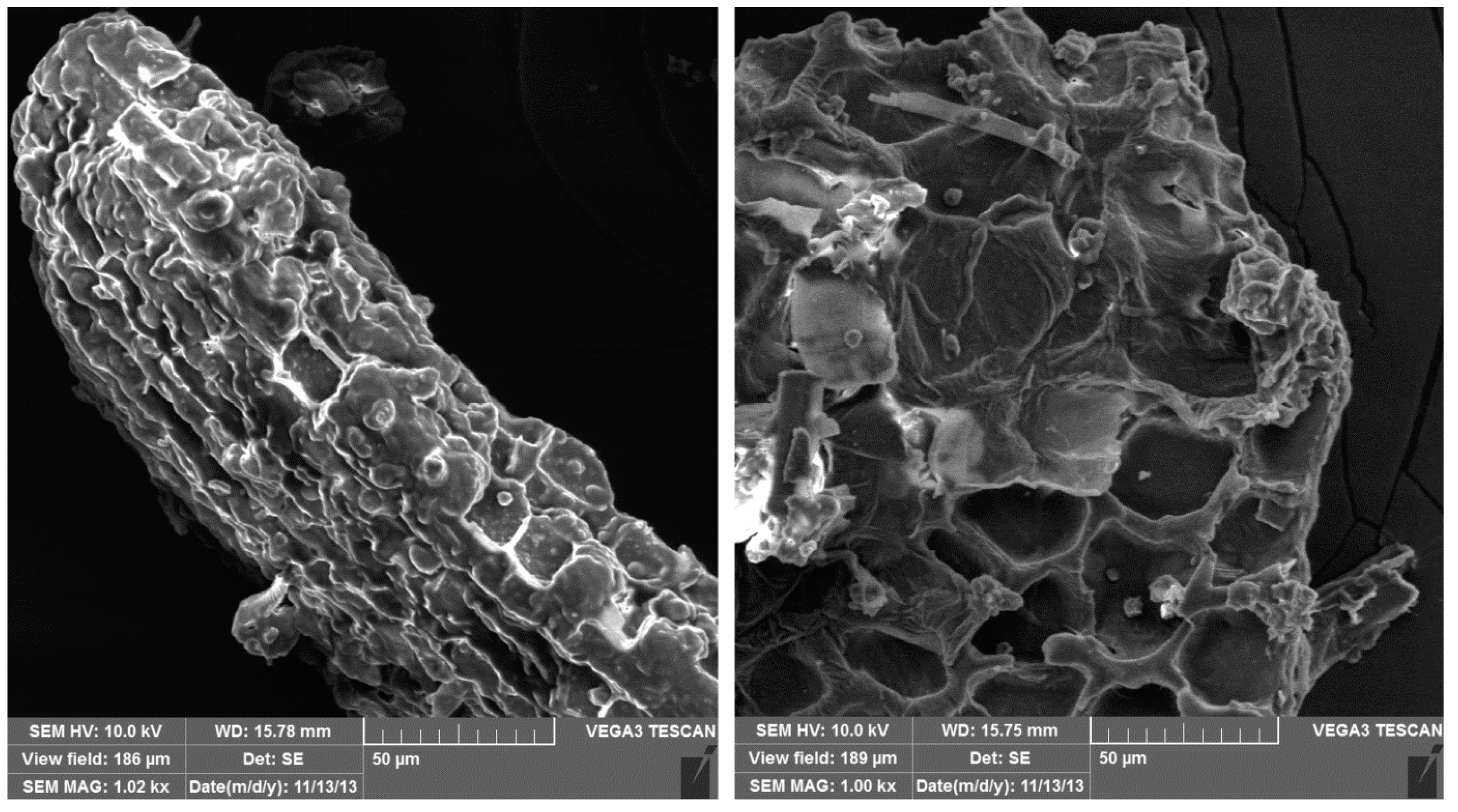
3.1. Characterization of the TW Biosorbent
3.2. Biosorption Isotherm
3.3. Adsorption Kinetic Studies
3.4. Adsorption Thermodynamics
3.5. Tomato Waste Biosorption Activity and Efficiency
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Christensen, F.M. Pharmaceuticals in the environment—A human risk? Regul. Toxicol. Pharmacol. 1998, 28, 212–221. [Google Scholar] [CrossRef] [PubMed]
- Hernando, M.D.; Mezcua, M.; Ferńandez-Alba, A.R.; Barceló, D. Environmental risk assessment of pharmaceutical residues in wastewater effluents, surface waters and sediments. Talanta 2006, 69, 334–342. [Google Scholar] [CrossRef] [PubMed]
- Petrović, M.; Hernando, M.D.; Díaz-Cruz, M.S.; Barceló, D. Liquid chromatography–tandem mass spectrometry for the analysis of pharmaceutical residues in environmental samples: A review. J. Chromatogr. A 2005, 1067, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Aksu, Z. Application of biosorption for the removal of organic pollutants: A review. Process Biochem. 2005, 40, 997–1026. [Google Scholar] [CrossRef]
- Kumar, K.V. Optimum sorption isotherm by linear and non-linear methods for malachite green onto lemon peel. Dye. Pigment. 2007, 74, 595–597. [Google Scholar] [CrossRef]
- Namasivayam, C.; Kavitha, D. Removal of Congo Red from water by adsorption onto activated carbon prepared from coir pith, an agricultural solid waste. Dye. Pigment. 2002, 54, 47–58. [Google Scholar] [CrossRef]
- Hamdaoui, O.; Saoudi, F.; Chiha, M.; Naffrechoux, E. Sorption of malachite green by a novel sorbent, dead leaves of plane tree: Equilibrium and kinetic modelling. Chem. Eng. J. 2008, 143, 73–84. [Google Scholar] [CrossRef]
- Hamdaoui, O.; Naffrechoux, E. Modeling of adsorption isotherms of phenol and chlorophenols onto granular activated carbon Part, I. Two-parameter models and equations allowing determination of thermodynamic parameters. J. Hazard. Mater. 2007, 147, 381–394. [Google Scholar] [CrossRef]
- Hamdaoui, O.; Naffrechoux, E. Modeling of adsorption isotherms of phenol and chlorophenols onto granular activated carbon Part II. Models with more than two parameters. J. Hazard. Mater. 2007, 147, 401–411. [Google Scholar] [CrossRef]
- Zhao, X.; Ouyang, W.; Hao, F.; Lin, C.; Wang, F.; Han, S.; Geng, X. Properties comparison of biochars from corn straw with different pretreatment and sorption behaviour of atrazine. Bioresour. Technol. 2013, 147, 338–344. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, L.; Cheng, Z. Removal of organic pollutants from aqueous solution using agricultural wastes: A review. J. Mol. Liq. 2015, 212, 739–762. [Google Scholar] [CrossRef]
- Mirabella, N.; Castellani, V.; Sala, S. Current options for the valorization of food manufacturing waste: A review. J. Clean. Prod. 2014, 65, 28–41. [Google Scholar] [CrossRef] [Green Version]
- Abia, A.A.; Horsfall, M.; Didi, O. The use of chemically modified and unmodified cassava waste for the removal of Cd, Cu and Zn ions from aqueous solution. Bioresour. Technol. 2003, 90, 345–348. [Google Scholar] [CrossRef]
- Israel, U.; Eduok, U.M. Biosorption of zinc from aqueous solution using coconut. Arch. Appl. Sci. Res. 2012, 4, 809–819. [Google Scholar]
- Horsfall, M.; Spiff, A.I. Equilibrium Sorption Study of Al3+, Co2+ and Ag+ in Aqueous Solutions by Fluted Pumpkin (Telfairia Occidentalis HOOK f) Waste Biomass. Acta Chim. Slov. 2005, 52, 174–181. [Google Scholar]
- Randall, J.M.; Hautala, E.; Waiss, A.C. Removal and recycling of heavy metal ions from agricultural byproducts. In Proceedings of the 4th Mineral Waste Utilization Symposium, Chicago, IL, USA, 7–8 May 1974. [Google Scholar]
- Iqbal, M.; Saeed, A.; Iqbal Zafar, S. FTIR spectrophotometry, kinetics and adsorption isotherms modelling, ion exchange, and EDX analysis for understanding the mechanism of Cd2+ and Pb2+ removal by mango peel waste. J. Hazard. Mater. 2009, 164, 161–171. [Google Scholar] [CrossRef]
- Hossain, M.A.; Ngo, H.H.; Guo, W.S.; Nguyen, T.V. Biosorption of Cu(II) From Water by Banana Peel Based Biosorbent: Experiments and Models of Adsorption and Desorption. J. Water Sustain. 2012, 2, 87–104. [Google Scholar]
- Anwar, J.; Shafique, U.; Zaman, W.; Salman, M.; Dar, A.; Anwar, S. Removal of Pb(II) and Cd(II) from water by adsorption on peels of banana. Bioresour. Technol. 2010, 101, 1752–1755. [Google Scholar] [CrossRef] [PubMed]
- Ho, Y.S.; Wase, D.A.J.; Forster, C.F. Removal of lead ions from aqueous solution using sphagnum moss peat as absorbent. Water SA 1996, 22, 219–224. [Google Scholar]
- Anastopoulos, I.; Kyzas, G.Z. Agricultural peels for dye adsorption: A review of recent literature. J. Mol. Liq. 2014, 200, 381–389. [Google Scholar] [CrossRef]
- Adeniyi, A.G.; Ighalo, J.O. Biosorption of pollutants by plant leaves: An empirical review. J. Environ. Chem. Eng. 2019, 7, 103100. [Google Scholar] [CrossRef]
- Kalogeropoulos, N.; Chiou, A.; Pyriochou, V.; Peristeraki, A.; Karathanos, V.T. Bioactive phytochemicals in industrial tomatoes and their processing byproducts. LWT-Food Sci. Technol. 2012, 49, 213–216. [Google Scholar] [CrossRef]
- Lazos, E.S.; Kalathenos, P. Technical Note: Composition of tomato processing wastes. Int. J. Food Sci. Tech. 1988, 23, 649–652. [Google Scholar] [CrossRef]
- Pehlivan, E.; Yanık, B.H.; Ahmetli, G.; Pehlivan, M. Equilibrium isotherm studies for the uptake of cadmium and lead ions onto sugar beet pulp. Bioresour. Technol. 2008, 99, 3520–3527. [Google Scholar] [CrossRef]
- Shukla, S.R.; Pai, R.S. Removal of Pb(II) from solution using cellulose containing materials. J. Chem. Technol. Biotechnol. 2005, 80, 176–183. [Google Scholar] [CrossRef]
- Xuan, Z.X.; Tang, Y.R.; Li, X.M.; Liu, Y.H.; Luo, F. Study on the equilibrium, kinetics and isotherm of biosorption of lead ions onto pretreated chemically modified orange peel. Biochem. Eng. J. 2006, 31, 160–164. [Google Scholar] [CrossRef]
- Ribeiro, G.C.; Coelho, L.M.; Oliveira, E.; Coelho, N.M.M. Removal of Cu(II) from Ethanol fuel using mandarin peel as biosorbent. BioResources 2013, 8, 3309–3321. [Google Scholar] [CrossRef] [Green Version]
- Tolić, K.; Mutavdžić Pavlović, D.; Stankir, N.; Runje, M. Biosorbents from Tomato, Tangerine, and Maple Leaves for the Removal of Ciprofloxacin from Aqueous Media. Water Air Soil Pollut. 2021, 232, 218. [Google Scholar] [CrossRef]
- Gutha, Y.; Munagapati, V.S.; Naushad, M.; Abburi, K. Removal of Ni (II) from aqueous solution by Lycopersicum esculentum (Tomato) leaf powder as a low-cost biosorbent. Desalination Water Treat. 2015, 54, 200–208. [Google Scholar] [CrossRef]
- Babić, S.; Horvat, A.J.M.; Mutavdžić Pavlović, D.; Kaštelan-Macan, M. Determination of pKa values of active pharmaceutical ingredients. Trends Anal. Chem. 2007, 26, 1043–1061. [Google Scholar] [CrossRef]
- EPIweb 4.0. Available online: http://www.epa.gov/oppt/exposure/pubs/episuitedl.htm (accessed on 14 September 2020).
- Mutavdžić Pavlović, D.; Ćurković, L.; Macan, J.; Žižek, K. Eggshell as a new biosorbent for the removal of pharmaceuticals from aqueous solutions. CLEAN—Soil Air Water 2017, 45, 1–14. [Google Scholar] [CrossRef]
- Hörsing, M.; Ledin, A.; Grabic, R.; Fick, J.; Tysklind, M.; Cour Jansen, J.; Andersen, H.R. Determination of sorption of seventy-five pharmaceuticals in sewage sludge. Water Res. 2011, 45, 4470–4482. [Google Scholar] [CrossRef] [Green Version]
- Freundlich, H.M.F. Über die Adsorption in Lösungen. Zeitschrift Physikalische Chemie 1906, 57, 385–470. [Google Scholar] [CrossRef]
- Dubinin, M.M.; Radushkevich, L.V. Equation of the characteristic curve of activated charcoal. Chem. Zent. 1947, 1, 875–890. [Google Scholar]
- Lagergren, S.; Svenska, B.K. Zur theorie der sogenannten adsorption geloester stoffe. Veternskapsakad Handlingar 1898, 24, 1–39. [Google Scholar]
- Ho, Y.S.; McKay, G. The kinetics of sorption of basic dyes from aqueous solutions by sphagnum moss peat. Can. J. Chem. Eng. 1998, 76, 822–827. [Google Scholar] [CrossRef]
- Yeddou-Mezenner, N. Kinetics and Mechanism of Dye Biosorption onto an Untreated Antibiotic Waste. Desalination 2010, 262, 251–259. [Google Scholar] [CrossRef]
- Weber, W.J.; Moris, J.C. Kinetics of adsorption on carbon from solution. J. Sanit. Eng. Div. Am. Soc. Civ. Eng. 1963, 89, 31–60. [Google Scholar] [CrossRef]
- Boyd, G.E.; Adamson, A.W.; Myers Jr, L.S. The Exchange Adsorption of Ions from Aqueous Solutions by Organic Zeolites. II. Kinetics. J. Am. Chem. Soc. 1947, 69, 2836–2848. [Google Scholar] [CrossRef]
- Yargıç, A.Ş.; Sahin, R.Z.Y.; Özbay, N.; Önal, E. Assessment of toxic copper(II) biosorption from aqueous solution by chemically-treated tomato waste. J. Clean. Prod. 2015, 88, 152–159. [Google Scholar] [CrossRef]
- Kamil, M.M.; Mohamed, G.F.; Shaheen, M.S. Fourier Transformer Infrared Spectroscopy for Quality Assurance of Tomato Products. J. Am. Sci. 2011, 7, 559–572. [Google Scholar]
- Vermeir, S.; Beullens, K.; Mészáros, P.; Polshin, E.; Nicolad, B.M..; Lammertyn, J. Sequential injection ATR-FTIR spectroscopy for taste analysis in tomato. Sens. Actuators B Chem. 2009, 137, 715–721. [Google Scholar] [CrossRef]
- Gong, G.; Ye, S.; Tian, Y.; Wang, Q.; Ni, J.; Chen, Y. Preparation of a new sorbent with hydrated lime and blast furnace slag for phosphorus removal from aqueous solution. J. Hazard. Mater. 2009, 166, 714–719. [Google Scholar] [CrossRef]
- Zheng, Y.M.; Lim, S.F.; Chen, J.P. Preparation and characterization of zirconium-based magnetic sorbent for arsenate removal. J. Colloid Interface Sci. 2009, 338, 22–29. [Google Scholar] [CrossRef]
- Zaini, M.A.A.; Zakaria, M.; Alias, N.; Zakaria, Z.Y.; Johari, A.; Setapar, S.H.; Kamaruddin, J.; Yunus, A.C. Removal of heavy metals onto KOH-activated ash-rich sludge adsorbent. Energy Procedia 2014, 61, 2572–2575. [Google Scholar] [CrossRef] [Green Version]
- Bansal, R.C.; Donnet, J.B.; Stoeckli, F. Active Carbon; Marcel Dekker: New York, NY, USA, 1988; p. 121. [Google Scholar]
- Białk-Bielińska, A.; Maszkowska, J.; Mrozik, W.; Bielawska, A.; Kołodziejska, M.; Palavinskas, R.; Stepnowski, J.; Kumirska, J. Sulfadimethoxine and sulfaguanidine: Their sorption potential on natural soils. Chemosphere 2012, 86, 1059–1065. [Google Scholar] [CrossRef] [PubMed]
- Maszkowska, J.; Kołodziejska, M.; Białk-Bielińska, A.; Mrozik, W.; Kumirska, J.; Stepnowski, P.; Palavinskas, R.; Krüger, O.; Kalbe, U. Column and batch tests of sulfonamide leaching from different types of soil. J. Hazard. Mater. 2013, 260, 468–474. [Google Scholar] [CrossRef]
- Mutavdžić Pavlović, D.; Ćurković, L.; Blažek, D.; Župan, J. The sorption of sulfamethazine on soil samples: Isotherms and error analysis. Sci. Total. Environ. 2014, 497–498, 543–552. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aksu, Z.; Tatlı, A.İ.; Tunç, Ö. A comparative adsorption/biosorption study of Acid Blue 161: Effect of temperature on equilibrium and kinetic parameters. Chem. Eng. J. 2008, 142, 23–39. [Google Scholar] [CrossRef]
- Treybal, R.E. Mass Transfer Operations, 3rd ed.; McGraw-Hill: New York, NY, USA, 1980. [Google Scholar]
- Hao, Y.M.; Chen, M.; Hu, Z.B. Effective removal of Cu (II) ions from aqueous solution by amino-functionalized magnetic nanoparticles. J. Hazard. Mater. 2010, 184, 392–399. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, S. Equations and Models Describing Adsorption Processes in Soils; Chemical Processes in Soils SSSA Book Series; Soil Science Society of America Inc.: Madison, WI, USA, 2005; Volume 8, pp. 489–517. [Google Scholar]
- Liao, X.; Zhang, C.; Yao, L.; Li, J.; Liu, M.; Xu, L.; Evalde, M. Sorption behavior of nonylphenol (NP) on sewage-irrigated soil: Kinetic and thermodynamic studies. Sci. Total Environ. 2014, 473–474, 530–536. [Google Scholar] [CrossRef]
- Bilgili, M.S. Adsorption of 4-chlorophenol from aqueous solutions by xad-4 resin: Isotherm, kinetic, and thermodynamic analysis. J. Hazard. Mater. 2006, 137, 157–164. [Google Scholar] [CrossRef]
- Khattri, S.D.; Singh, M.K. Removal of malachite green from dye wastewater using neem sawdust by adsorption. J. Hazard. Mater. 2009, 167, 1089–1094. [Google Scholar] [CrossRef]
- Brito, S.M.O.; Andrade, H.M.C.; Soares, L.F.; Azevedo, R.P. Brazil nut shells as a new biosorbent to remove methylene blue and indigo carmine from aqueous solutions. J. Hazard. Mater. 2010, 174, 84–92. [Google Scholar] [CrossRef]
- Hu, Z.; Chen, H.; Ji, F.; Yuan, S. Removal of Congo Red from aqueous solution by cattail root. J. Hazard. Mater. 2010, 173, 292–297. [Google Scholar] [CrossRef] [PubMed]
- Heraldy, E.; Wahyu Lestari, W.; Permatasaria, D.; Dwi Arimurtia, D. Biosorbent from tomato waste and apple juice residue for lead removal. J. Env. Chem. Eng. 2018, 6, 1201–1208. [Google Scholar] [CrossRef]
- Changmai, M.; Banerjee, P.; Nahar, K.; Purkait, M.K. A novel adsorbent from carrot, tomato and polyethylene terephthalate waste as a potential adsorbent for Co (II) from aqueous solution: Kinetic and Equilibrium studies. J. Env. Chem. Eng. 2018, 6, 246–257. [Google Scholar] [CrossRef]
- Pereira Leal, R.M.; Ferracciú Alleoni, L.R.; Tornisielo, V.L.; Borges Regitano, J. Sorption of fluoroquinolones and sulfonamides in 13 Brazilian soils. Chemosphere 2013, 92, 979–985. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peruchi, L.M.; Fostier, A.H.; Rath, S. Sorption of norfloxacin in soils: Analytical method, kinetics and Freundlich isotherms. Chemosphere 2015, 119, 310–317. [Google Scholar] [CrossRef]
- Carabineiro, S.A.C.; Thavorn-Amornsri, T.; Pereira, M.F.R.; Figueiredo, J.L. Adsorption of ciprofloxacin on surface-modified carbon materials. Water Res. 2011, 45, 4583–4591. [Google Scholar] [CrossRef]
- Kurniawan, T.A.; Chan, G.Y.S.; Lo, W.-H.; Babel, S. Comparisons of low-cost adsorbents for treating wastewaters laden with heavy metals. Sci. Total Environ. 2006, 366, 409–426. [Google Scholar] [CrossRef]
- Cuerda-Correa, E.M.; Domínguez-Vargas, J.R.; Olivares-Marín, F.J.; de Heredia, J.B. On the use of carbon blacks as potential low-cost adsorbents for the removal of non-steroidal anti-inflammatory drugs from river water. J. Hazard. Mater. 2010, 177, 1046–1053. [Google Scholar] [CrossRef]







| Chemical Structure | CAS No. | Mw | pKa [31] | log Kow [32] | |
|---|---|---|---|---|---|
| PRAZ | 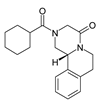 | 55268-74-1 | 312.4 | n.a. | 2.42 |
| TYL |  | 74610-55-2 | 1066.2 | 3.31; 7.50 | 1.63 |
| PROC | 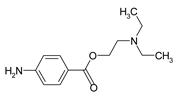 | 59-46-1 | 236.3 | 2.24; 8.84 | 2.14 |
| DEXA |  | 50-02-2 | 392.5 | n.a. | 1.94 |
| FEBA |  | 58306-30-2 | 446.5 | n.a. | 1.53 |
| pH | EC, μS cm−1 | CEC, mmol kg−1 |
|---|---|---|
| 4.58 | 1003 | 100.63 |
| Isotherm | Linear Form |
|---|---|
| Linear | |
| Freundlich | |
| Dubinin–Radushkevich |
| Pharmaceutical | Isotherm | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| T, K | Linear | Freundlich | Dubinin–Radushkevich | |||||||
| KD | R2 | n | KF | R2 | β | qm | E | R2 | ||
| DEXA | 298 | 45.4 | 0.9965 | 0.61 | 8.75 | 0.9791 | 5.6 × 10−6 | 461 | 0.299 | 0.9757 |
| 303 | 7.2 | 0.9964 | 0.98 | 5.49 | 0.9718 | 2.84 × 10−5 | 244 | 0.132 | 0.9657 | |
| 308 | 6.8 | 0.9912 | 0.44 | 24.5 | 0.992 | 5.11 × 10−5 | 215 | 0.099 | 0.9946 | |
| FEBA | 298 | 5.3 | 0.9929 | 1.74 | 10.51 | 0.9972 | 3.87 × 10−5 | 281 | 0.144 | 0.9649 |
| 303 | 8.5 | 0.9957 | 1.45 | 31.75 | 0.9999 | 1.48 × 10−5 | 370 | 0.184 | 0.9421 | |
| 308 | 21.7 | 0.9934 | 1.29 | 50.61 | 0.9987 | 5.9 × 10−6 | 524 | 0.291 | 0.9514 | |
| PRAZ | 298 | 12.7 | 0.8679 | 1.18 | 53.23 | 0.9046 | 4.62 ×10−6 | 449 | 0.329 | 0.9939 |
| 303 | 9.6 | 0.8729 | 1.73 | 63.96 | 0.9193 | 8.1 × 10−6 | 443 | 0.248 | 0.9948 | |
| 308 | 8.3 | 0.8945 | 1.74 | 60.17 | 0.8809 | 9.0 × 10−6 | 437 | 0.236 | 0.9999 | |
| PROC | 298 | 17.8 | 0.9848 | 0.35 | 3.79 | 0.9965 | 5.4 × 10−6 | 1230 | 0.304 | 0.9853 |
| 303 | 3.8 | 0.9851 | 0.50 | 1.27 | 0.9600 | 3.78 × 10−5 | 980 | 0.115 | 0.9993 | |
| 308 | 0.7 | 0.9980 | 1.10 | 10.50 | 0.9993 | 3.31 × 10−5 | 322 | 0.122 | 0.9572 | |
| TYL | 298 | 28.6 | 0.9963 | 0.57 | 1.87 | 0.9998 | 3.83 × 10−5 | 677 | 0.114 | 0.9774 |
| 303 | 27.0 | 0.9999 | 0.69 | 5.23 | 0.9987 | 2.54 × 10−5 | 628 | 0.140 | 0.9769 | |
| 308 | 25.1 | 0.9998 | 0.83 | 12.33 | 0.9986 | 1.66 × 10−5 | 581 | 0.173 | 0.9705 | |
| T, °C | C0, mg L−1 | qe, exp (µg g−1) | Kinetic Model | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Lagergren Pseudo-First Order | Ho’s Pseudo-Second Order | |||||||||
| k1 (min−1) × 10−3 | qe, cal (µg g−1) | R2 | k2 (g µg−1 min−1) × 10−5 | qe, cal (µg g−1) | h (µg g−1 min−1) | R2 | ||||
| DEXA | 25 | 25 | 190 | 4.6 | 87 | 0.9688 | 18.29 | 192 | 6.74 | 0.9997 |
| 75 | 393 | 4.9 | 231 | 0.9838 | 5.97 | 404 | 9.74 | 0.9997 | ||
| 30 | 25 | 99 | 3.7 | 40 | 0.9933 | 25.06 | 100 | 2.51 | 0.9998 | |
| 75 | 260 | 4.3 | 128 | 0.9715 | 7.35 | 265 | 5.16 | 0.9993 | ||
| 35 | 25 | 74 | 3.8 | 33 | 0.9744 | 43.82 | 75 | 2.46 | 0.9997 | |
| 75 | 163 | 3.7 | 73 | 0.9942 | 19.93 | 166 | 5.49 | 0.9997 | ||
| FEBA | 25 | 25 | 103 | 3.6 | 51 | 0.9940 | 14.94 | 107 | 1.71 | 0.9997 |
| 75 | 276 | 4.6 | 139 | 0.9969 | 5.33 | 280 | 4.18 | 0.9999 | ||
| 30 | 25 | 153 | 4.4 | 82 | 0.9927 | 17.06 | 153 | 3.99 | 0.9996 | |
| 75 | 383 | 5.0 | 207 | 0.9942 | 7.35 | 386 | 10.95 | 0.9996 | ||
| 35 | 25 | 193 | 3.4 | 67 | 0.9939 | 49.12 | 196 | 18.87 | 0.9995 | |
| 75 | 536 | 5.9 | 265 | 0.9442 | 9.03 | 540 | 26.33 | 0.9994 | ||
| PRAZ | 25 | 25 | 197 | 4.3 | 67 | 0.9675 | 26.05 | 199 | 10.32 | 0.9999 |
| 75 | 454 | 4.6 | 176 | 0.9619 | 9.42 | 456 | 19.59 | 0.9999 | ||
| 30 | 25 | 180 | 3.6 | 59 | 0.9450 | 24.43 | 182 | 8.09 | 0.9999 | |
| 75 | 438 | 5.1 | 192 | 0.9813 | 9.03 | 440 | 17.48 | 0.9998 | ||
| 35 | 25 | 174 | 3.8 | 64 | 0.9620 | 20.32 | 178 | 6.44 | 0.9998 | |
| 75 | 414 | 4.5 | 192 | 0.9769 | 8.13 | 420 | 14.34 | 0.9998 | ||
| PROC | 25 | 25 | 207 | 3.6 | 74 | 0.9383 | 21.18 | 208 | 9.16 | 0.9998 |
| 75 | 687 | 5.0 | 219 | 0.9675 | 8.21 | 690 | 39.09 | 0.9999 | ||
| 30 | 25 | 141 | 3.5 | 70 | 0.9698 | 20.80 | 144 | 4.31 | 0.9996 | |
| 75 | 538 | 4.2 | 224 | 0.9520 | 6.78 | 555 | 20.88 | 0.9996 | ||
| 35 | 25 | 112 | 3.5 | 45 | 0.9647 | 19.05 | 115 | 2.52 | 0.9997 | |
| 75 | 317 | 3.9 | 134 | 0.9637 | 6.35 | 320 | 6.50 | 0.9996 | ||
| TYL | 25 | 25 | 135 | 3.3 | 58 | 0.9421 | 0.76 | 138 | 0.14 | 0.9967 |
| 75 | 506 | 5.2 | 201 | 0.9689 | 0.56 | 510 | 1.46 | 0.9986 | ||
| 30 | 25 | 150 | 4.0 | 59 | 0.9572 | 0.53 | 149 | 0.03 | 0.9982 | |
| 75 | 515 | 5.2 | 212 | 0.9830 | 0.46 | 513 | 1.21 | 0.9997 | ||
| 35 | 25 | 163 | 3.5 | 59 | 0.9522 | 0.37 | 165 | 0.10 | 0.9999 | |
| 75 | 518 | 4.2 | 184 | 0.9577 | 0.45 | 520 | 1.22 | 0.9999 | ||
| T, °C | Webber’s Intraparticle Diffusion | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| First Stage of Sorption | Second Stage of Sorption | Third Stage of Sorption | ||||||||
| kp1 | C1 | R2 | kp2 | C2 | R2 | kp3 | C3 | R2 | ||
| DEXA | 25 | 32.83 | 6.78 | 0.9939 | 9.84 | 171.4 | 0.9253 | 3.05 | 284.89 | 0.9875 |
| 30 | 17.78 | 38.62 | 0.9390 | 5.10 | 140.5 | 0.9087 | 2.04 | 188.12 | 0.9590 | |
| 35 | 9.18 | 41.85 | 0.9829 | 2.95 | 90.92 | 0.9216 | 1.26 | 118.99 | 0.9314 | |
| FEBA | 25 | 29.19 | −75.31 | 0.9936 | 10.79 | 66.35 | 0.9655 | 1.88 | 209.17 | 0.8853 |
| 30 | 8.76 | −23.26 | 0.9797 | 8.30 | 196.15 | 0.9063 | 2.80 | 282.98 | 0.9322 | |
| 35 | 41.56 | 69.92 | 0.9996 | 9.98 | 313.1 | 0.8592 | 3.21 | 422.09 | 0.9140 | |
| PRAZ | 25 | 34.64 | 73.59 | 0.9935 | 9.18 | 270.19 | 0.9089 | 1.99 | 388.44 | 0.9795 |
| 30 | 8.76 | −23.26 | 0.9797 | 8.42 | 251.11 | 0.9114 | 0.59 | 10.13 | 0.9240 | |
| 35 | 15.17 | −50.74 | 0.9753 | 7.57 | 228.56 | 0.9187 | 3.03 | 306.89 | 0.9034 | |
| PROC | 25 | 42.94 | 216.84 | 0.9952 | 11.47 | 455.51 | 0.9407 | 2.27 | 606.01 | 0.8134 |
| 30 | 33.46 | 118.46 | 0.9637 | 11.79 | 291.97 | 0.9326 | 2.89 | 435.58 | 0.9084 | |
| 35 | 26.13 | −21.28 | 0.9562 | 9.10 | 120.65 | 0.8744 | 2.51 | 228.67 | 0.8587 | |
| TYL | 25 | 2.36 | −10.18 | 0.9520 | 9.55 | 308.68 | 0.8872 | 0.73 | 30.40 | 0.9428 |
| 30 | 8.76 | −23.26 | 0.9797 | 10.10 | 312.01 | 0.9324 | 0.59 | 10.13 | 0.9420 | |
| 35 | 15.17 | −50.74 | 0.9753 | 10.04 | 298.46 | 0.9361 | 0.14 | 6.53 | 0.9077 | |
| Pharmaceutical | KD (mL g−1) | ΔG° (kJ mol−1) | ΔH° (kJ mol−1) | ΔS° (kJ mol−1) | ||||
|---|---|---|---|---|---|---|---|---|
| 298 K | 303 K | 308 K | 298 K | 303 K | 308 K | |||
| DEXA | 15.4 | 8.9 | 6.8 | −6.77 | −5.51 | −4.91 | −62.5 | −0.19 |
| FEBA | 5.3 | 8.5 | 21.7 | −4.13 | −5.39 | −7.88 | 107.4 | 0.37 |
| PRAZ | 12.7 | 9.6 | 8.3 | −6.30 | −5.70 | −5.42 | −32.5 | −0.09 |
| PROC | 7.8 | 3.8 | 1.1 | −5.09 | −3.36 | −0.24 | −149.3 | −0.48 |
| TYL | 29.6 | 27 | 25.1 | −8.39 | −8.30 | −8.25 | −12.6 | −0.02 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mutavdžić Pavlović, D.; Ćurković, L.; Mandić, V.; Macan, J.; Šimić, I.; Blažek, D. Removal of Pharmaceuticals from Water by Tomato Waste as Novel Promising Biosorbent: Equilibrium, Kinetics, and Thermodynamics. Sustainability 2021, 13, 11560. https://doi.org/10.3390/su132111560
Mutavdžić Pavlović D, Ćurković L, Mandić V, Macan J, Šimić I, Blažek D. Removal of Pharmaceuticals from Water by Tomato Waste as Novel Promising Biosorbent: Equilibrium, Kinetics, and Thermodynamics. Sustainability. 2021; 13(21):11560. https://doi.org/10.3390/su132111560
Chicago/Turabian StyleMutavdžić Pavlović, Dragana, Lidija Ćurković, Vilko Mandić, Jelena Macan, Iva Šimić, and Dijana Blažek. 2021. "Removal of Pharmaceuticals from Water by Tomato Waste as Novel Promising Biosorbent: Equilibrium, Kinetics, and Thermodynamics" Sustainability 13, no. 21: 11560. https://doi.org/10.3390/su132111560
APA StyleMutavdžić Pavlović, D., Ćurković, L., Mandić, V., Macan, J., Šimić, I., & Blažek, D. (2021). Removal of Pharmaceuticals from Water by Tomato Waste as Novel Promising Biosorbent: Equilibrium, Kinetics, and Thermodynamics. Sustainability, 13(21), 11560. https://doi.org/10.3390/su132111560









