Plant Functional Diversity Is Linked to Carbon Storage in Deciduous Dipterocarp Forest Edges in Northern Thailand
Abstract
:1. Introduction
2. Materials and Methods
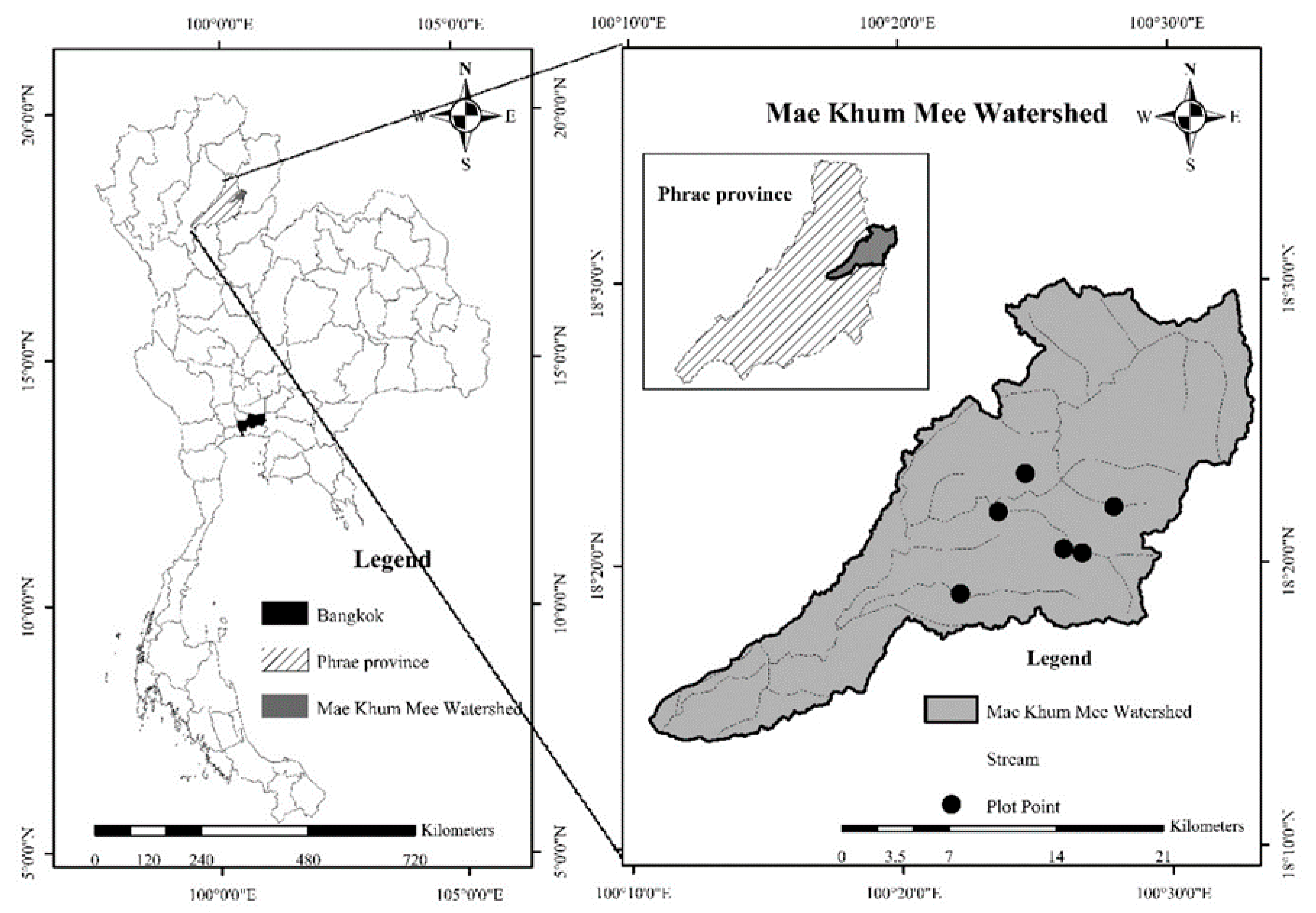
2.1. Study Area
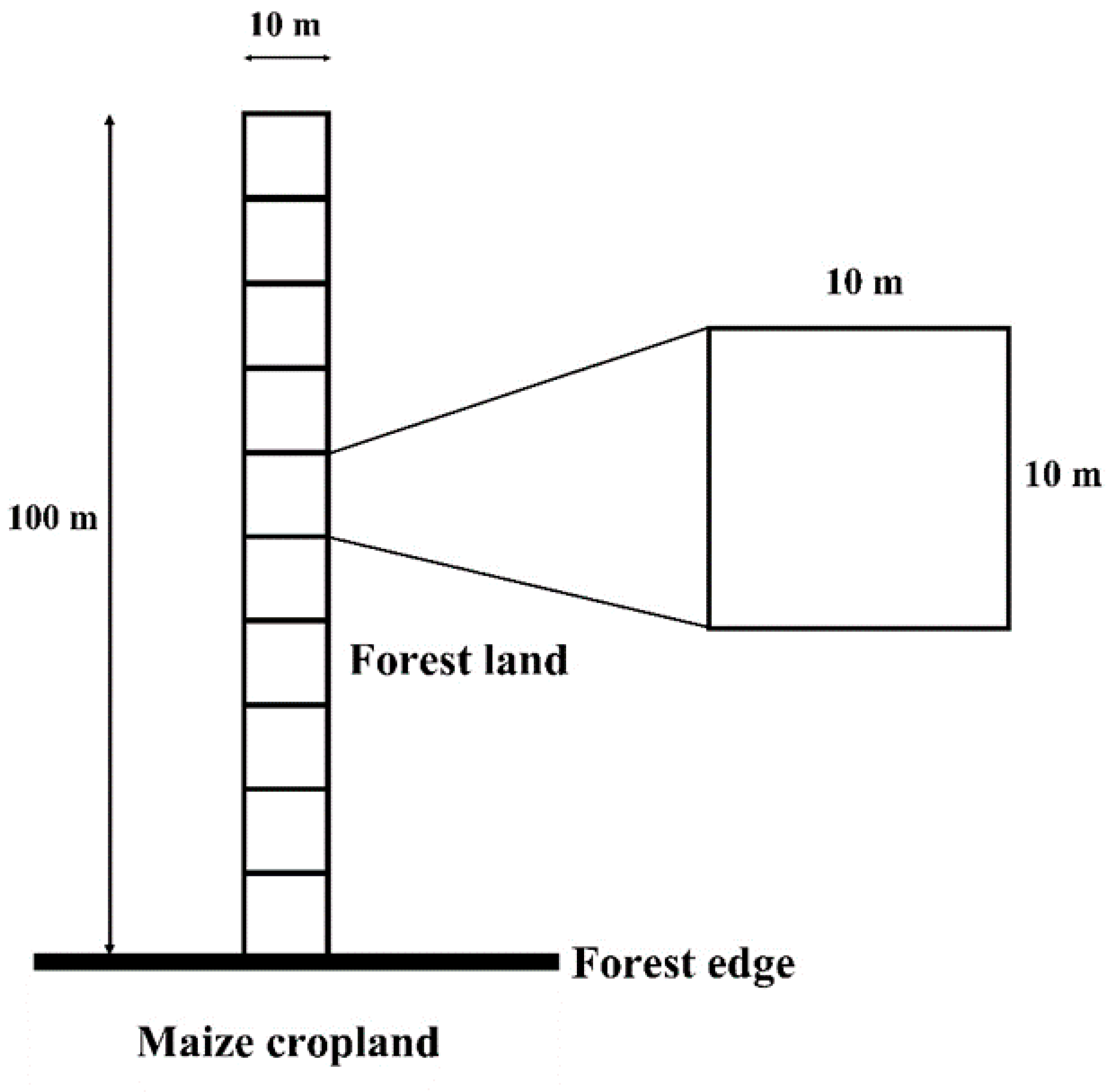
2.2. Sampling Plot Selection and Tree Data
2.3. Carbon Storage Assessment
2.4. Plant Trait Measurements
2.5. Functional Diversity Assessment
2.6. Statistical Analyses
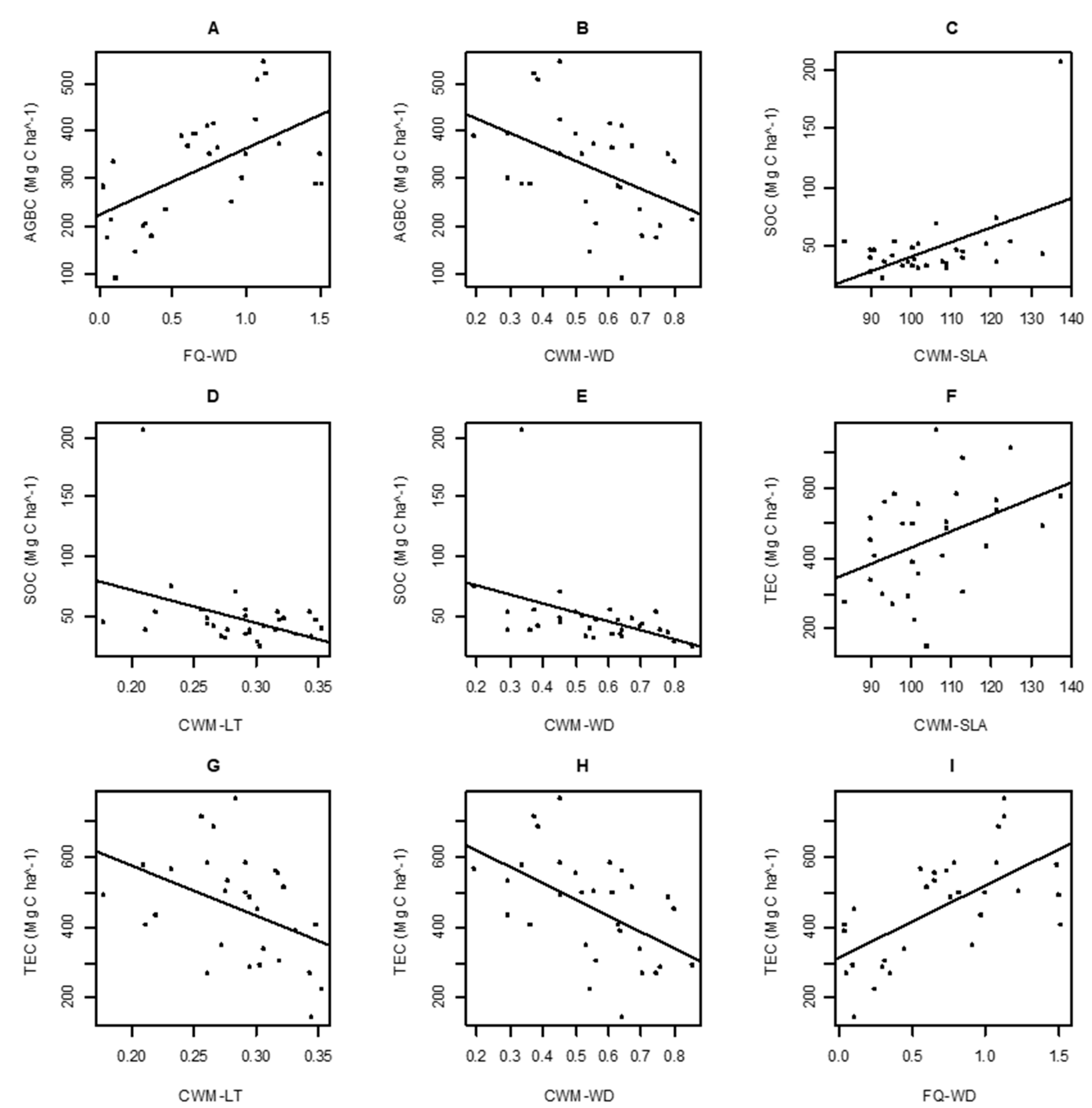
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- IPPC. Climate Change 2014: Synthesis Report; IPPC: Geneva, Switzerland, 2014. [Google Scholar]
- Nunes, L.J.R.; Meireles, C.I.R.; Gomes, C.J.P.; Ribeiro, N.M.C.A. Forest Management and Climate Change Mitigation: A review on carbon cycle flow models for the sustainability of resources. Sustainability 2019, 11, 5276. [Google Scholar] [CrossRef] [Green Version]
- Dusenge, M.E.; Duarte, A.G.; Way, D.A. Plant carbon metabolism and climate change: Elevated CO2 and temperature impacts on photosynthesis, photorespiration and respiration. New Phytol. 2019, 221, 32–49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vries, W.D.; Du, E.; Butterbach-bahl, K. Short and long-term impacts of nitrogen deposition on carbon sequestration by forest ecosystems. Curr. Opin. Environ. Sustain. 2014, 10, 90–104. [Google Scholar] [CrossRef]
- Conti, G.; Díaz, S. Plant functional diversity and carbon storage-an empirical test in semi-arid forest ecosystems. J. Ecol. 2013, 101, 18–28. [Google Scholar] [CrossRef]
- Rawat, M.; Arunachalam, K.; Arunachalam, A.; Alatalo, J.; Pandey, R. Associations of plant functional diversity with carbon accumulation in a temperate forest ecosystem in the Indian Himalayas. Ecol. Indic. 2019, 98, 861–868. [Google Scholar] [CrossRef]
- Zhu, J.; Jiang, L.; Zhang, Y. Relationships between functional diversity and aboveground biomass production in the Northern Tibetan alpine grasslands. Sci. Rep. 2016, 6, 34105. [Google Scholar] [CrossRef] [Green Version]
- Diaz, S.; Hodgson, J.G.; Thompson, K.; Cabido, M.; Cornelissen, J.H.C.; Jalili, A.; Montserrat-Marti, G.; Grime, J.P.; Zarrinkamar, F.; Asri, Y.; et al. The plant traits that drive ecosystems: Evidence from three continents. J. Veg. Sci. 2004, 15, 295–304. [Google Scholar] [CrossRef] [Green Version]
- De Deyn, G.B.; Cornelissen, J.H.C.; Bardgett, R.D. Plant functional traits and soil carbon sequestration in contrasting biomes. Ecol. Lett. 2008, 11, 516–531. [Google Scholar] [CrossRef] [PubMed]
- Díaz, S.; Hector, A.; Wardle, D.A. Biodiversity in forest carbon sequestration initiatives: Not just a side benefit. Curr. Opin. Environ. Sustain. 2009, 1, 55–60. [Google Scholar] [CrossRef] [Green Version]
- Bu, W.; Huang, J.; Xu, H.; Zang, R.; Ding, Y.; Li, Y.; Lin, M.; Wang, J.; Zhang, C. Plant functional traits are the mediators in regulating effects of abiotic site conditions on aboveground carbon stock-evidence from a 30 ha. tropical forest plot. Front. Plant Sci. 2019, 9, 1958. [Google Scholar] [CrossRef]
- Díaz, S.; Lavorel, S.; De Bello, F.; Quétier, F.; Grigulis, K.; Robson, T.M. Incorporating plant functional diversity effects in ecosystem service assessments. Proc. Natl. Acad. Sci. USA 2007, 104, 20684–20689. [Google Scholar] [CrossRef] [Green Version]
- Cadotte, M.W. Functional traits explain ecosystem function through opposing mechanisms. Ecol. Lett. 2017, 20, 989–996. [Google Scholar] [CrossRef]
- Cardinale, B.J.; Wright, J.P.; Cadotte, M.W.; Carroll, I.T.; Hector, A.; Srivastava, D.S.; Loreau, M.; Weis, J.J. Impacts of plant diversity on biomass production increase through time because of species complementarity. Proc. Natl. Acad. Sci. USA 2007, 104, 18123–18128. [Google Scholar] [CrossRef] [Green Version]
- Fearnside, P.M.; Laurance, W.F. Tropical deforestation and greenhouse-gas emissions. Ecol. Appl. 2004, 14, 982–986. [Google Scholar] [CrossRef]
- Nogueira, E.M.; Yanai, A.M.; de Vasconcelos, S.S.; de Alencastro Graça, P.M.L.; Fearnside, P.M. Carbon stocks and losses to deforestation in protected areas in Brazilian Amazonia. Reg. Environ. Chang. 2018, 18, 261–270. [Google Scholar] [CrossRef]
- Pennington, R.T.; Lavin, M.; Oliveira-filho, A. Woody plant diversity, evolution, and ecology in the tropics: Perspectives from Seasonally Dry Tropical Forests. Annu. Rev. Ecol. Evol. Syst. 2009, 40, 437–457. [Google Scholar] [CrossRef] [Green Version]
- Dexter, A.K.G.; Smart, B.; Baldauf, C.; Baker, T.R.; Balinga, M.P.B.; Brienen, R.J.W.; Fauset, S.; Feldpausch, T.R.; Silva, L.F.; Muledi, J.I. Floristics and biogeography of vegetation in seasonally dry tropical regions. Int. For. Rev. 2015, 17, 10–32. [Google Scholar] [CrossRef]
- Wanthongchai, K.; Bauhus, J.; Goldammer, J.G. Nutrient losses through prescribed burning of aboveground litter and understorey in dry dipterocarp forests of different fire history. Catena 2008, 74, 321–332. [Google Scholar] [CrossRef]
- Delang, C.O. Deforestation in Northern Thailand: The result of Hmong farming practices or Thai development strategies? Soc. Nat. Resour. 2002, 15, 483–501. [Google Scholar] [CrossRef]
- Sloan, S.; Sayer, J.A. Forest resources assessment of 2015 shows positive global trends but forest loss and degradation persist in poor tropical countries. For. Ecol. Manage. 2015, 352, 134–145. [Google Scholar] [CrossRef] [Green Version]
- Marklund, L.G.; Schoene, D. Global Assessment of Growing Stock, Biomass and Carbon Stock; Forestry Department, Food and Agriculture Organization of the United Nations: Rome, Italy, 2006. [Google Scholar]
- Magnago, L.F.S.; Magrach, A.; Barlow, J.; Ernesto, C.; Schaefer, G.R.; Laurance, W.F.; Martins, S.V.; Edwards, D.P. Do fragment size and edge effects predict carbon stocks in trees and lianas in tropical forests? Funct. Ecol. 2017, 31, 542–552. [Google Scholar] [CrossRef] [Green Version]
- Chaplin-Kramer, R.; Ramler, I.; Sharp, R.; Haddad, N.M.; Gerber, J.S.; West, P.C.; Mandle, L.; Engstrom, P.; Baccini, A.; Sim, S.; et al. Degradation in carbon stocks near tropical forest edges. Nat. Commun. 2015, 6, 10158. [Google Scholar] [CrossRef]
- Asanok, L.; Taweesuk, R.; Papakjan, N. Woody species colonization along edge-interior gradients of deciduous forest remnants in the Mae Khum Mee Watershed, Northern Thailand. Int. J. For. Res. 2020, 2020, 5867376. [Google Scholar] [CrossRef]
- Forest Herbarium. Thai Plant Names: Tem Smitinand; Department of National Parks, Wldlife and Plant Conservation: Bangkok, Thailand, 2014. [Google Scholar]
- Ogawa, H.; Yoda, K.; Ogino, K.; Kira, T. Comparative ecological studies on three main types of forest vegetation in Thailand II plant biomass. Nat. Life Southeast Asia 1965, 4, 49–80. [Google Scholar]
- IPCC. IPCC Guidelines for National Greenhouse Gas Inventories; The National Greenhouse Gas Inventories Program, IGES: Kanagawa, Japan, 2006. [Google Scholar]
- Walkley, A.; Black, I.A. An examination of the degtjareff method for determining soil organic matter, and a proposed modification of the chromic acid titration method. Soil Sci. 1934, 37, 29–38. [Google Scholar] [CrossRef]
- Nelson, D.W.; Sommers, L.E. Total carbon, organic carbon, and organic matter. Methods Soil Anal. 2018, 3, 961–1010. [Google Scholar] [CrossRef]
- Pérez-Harguindeguy, N.; Diaz, S.; Garnier, E.; Lavorel, S.; Poorter, H.; Jaureguiberry, P.; Bret-Harte, M.S.; Cornwell, W.K.; Craine, J.M.; Gurvich, D.E. New handbook for standardized measurement of plant functional traits worldwide. Aust. J. Bot. 2013, 61, 167–234. [Google Scholar] [CrossRef]
- Ricotta, C.; Moretti, M. CWM and Rao’s quadratic diversity: A unified framework for functional ecology. Oecologia 2011, 167, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Loiola, P.P.; Scherer-lorenzen, M.; Batalha, A.M. The role of environmental filters and functional traits in predicting the root biomass and productivity in savannas and tropical seasonal forests. For. Ecol. Manag. 2015, 342, 49–55. [Google Scholar] [CrossRef]
- Laliberte, E.; Legendre, P. A distance-based framework for measuring functional diversity from multiple traits. Ecology 2010, 91, 299–305. [Google Scholar] [CrossRef]
- Burnham, K.P.; Anderson, D.R. Model Selection and Inference: A practical Information-Theoretic Approach; Springer: New York, NY, USA, 2001. [Google Scholar]
- Villéger, S.; Mason, N.W.H.; Mouillot, D. New multidimensional functional diversity indices for a multifaceted framework in functional ecology. Ecology 2008, 89, 2290–2301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biswas, S.R.; Mallik, A.U.; Braithwaite, N.T.; Wagner, H.H. A conceptual framework for the spatial analysis of functional trait diversity. Oikos 2016, 125, 192–200. [Google Scholar] [CrossRef]
- Yuan, Z.; Wang, S.; Ali, A.; Gazol, A.; Ruiz-Benito, P.; Wang, X.; Lin, F.; Ye, J.; Hao, Z.; Loreau, M. Aboveground carbon storage is driven by functional trait composition and stand structural attributes rather than biodiversity in temperate mixed forests recovering from disturbances. Ann. For. Sci. 2018, 75, 67. [Google Scholar] [CrossRef] [Green Version]
- Razafindratsima, O.H.; Brown, K.A.; Carvalho, F.; Johnson, S.E.; Wright, P.C.; Dunham, A.E. Edge effects on components of diversity and above-ground biomass in a tropical rainforest. J. Appl. Ecol. 2018, 55, 977–985. [Google Scholar] [CrossRef]
- Mensah, S.; Veldtman, R.; Assogbadjo, A.E.; Kakai, G.R.; Seifert, T. Tree species diversity promotes aboveground carbon storage through functional diversity and functional dominance. Ecol. Evol. 2016, 6, 7546–7557. [Google Scholar] [CrossRef]
- Asanok, L.; Taweesuk, R. Using Plant Functional Trait for Predicting the Ability of Carbon Dioxide (CO2) Capturing of Tree Species in the Deciduous Forest Edge Caused by Highland Mize Corpping at Mae Khum Mee Watershed, Phrae Province; Maejo University: Phrae, Thailand, 2019. [Google Scholar]
- Chave, J.; Coomes, D.; Jansen, S.; Lewis, S.L.; Swenson, N.G.; Zanne, A.E. Towards a worldwide wood economics spectrum. Ecol. Lett. 2009, 12, 351–366. [Google Scholar] [CrossRef]
- Phillips, O.L.; Sullivan, M.J.P.; Baker, T.R.; Mendoza, M.A.; Vargas, N.P.; Vásquez, R. Species matter: Wood density influences tropical forest biomass at multiple scales. Surv. Geophys. 2019, 40, 913–935. [Google Scholar] [CrossRef] [Green Version]
- Häger, A.; Avalos, G. Do functional diversity and trait dominance determine carbon storage in an altered tropical landscape? Oecologia 2017, 184, 569–581. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Epstein, H.E.; Wen, Z.; Zhao, J.; Jin, J.; Jing, G.; Cheng, J.; Du, G. Community-weighted mean traits but not functional diversity determine the changes in soil properties during wetland drying on the Tibetan Plateau. Solid Earth 2017, 8, 137–147. [Google Scholar] [CrossRef] [Green Version]
- He, M.; Zhang, K.; Tan, H.; Hu, R.; Su, J.; Wang, J.; Huang, L.; Zhang, Y.; Li, X. Nutrient levels within leaves, stems, and roots of the xeric species Reaumuria soongorica in relation to geographical, climatic, and soil conditions. Ecol. Evol. 2015, 5, 1494–1503. [Google Scholar] [CrossRef]
- Harper, K.A.; Macdonald, S.E.; Burton, P.J.; Chen, J.; Brosofske, K.D.; Saunders, S.C.; Euskirchen, E.S.; Roberts, D.; Jaiteh, M.S.; Esseen, P. Edge influence on forest structure and composition in fragmented landscapes. Conserv. Biol. 2005, 19, 768–782. [Google Scholar] [CrossRef]
- Asanok, L.; Marod, D.; Duengkae, P.; Pranmongkol, U.; Kurokawa, H.; Aiba, M.; Katabuchi, M.; Nakashizuka, T. Relationships between functional traits and the ability of forest tree species to reestablish in secondary forest and enrichment plantations in the uplands of northern Thailand. For. Ecol. Manag. 2013, 296, 9–23. [Google Scholar] [CrossRef]
- Wright, I.J.; Reich, P.B.; Westoby, M.; Ackerly, D.D.; Baruch, Z.; Bongers, F.; Cavender-bares, J.; Chapin, T.; Cornelissen, J.H.C.; Diemer, M. The worldwide leaf economics spectrum. Nature 2004, 428, 821–827. [Google Scholar] [CrossRef] [PubMed]
- Bakker, M.A.; Carreño-Rocabado, G.; Poorter, L. Leaf economics traits predict litter decomposition of tropical plants and differ among land use types. Funct. Ecol. 2011, 25, 473–483. [Google Scholar] [CrossRef]
- Manning, P.; de Vries, F.T.; Tallowin, J.R.B.; Smith, R.; Mortimer, S.R.; Pilgrim, E.S.; Harrison, K.A.; Wright, D.G.; Quirk, H.; Benson, J. Simple measures of climate, soil properties and plant traits predict national-scale grassland soil carbon stocks. J. Appl. Ecol. 2015, 52, 1188–1196. [Google Scholar] [CrossRef] [Green Version]
- Niklas, K.J.; Spatz, H.C. Worldwide correlations of mechanical properties and green wood density. Am. J. Bot. 2010, 97, 1587–1594. [Google Scholar] [CrossRef] [PubMed]
- Kazakou, E.; Violle, C.; Roumet, C.; Pintor, C.; Gimenez, O.; Garnier, E. Litter quality and decomposability of species from a Mediterranean succession depend on leaf traits but not on nitrogen supply. Ann. Bot. 2009, 104, 1151–1161. [Google Scholar] [CrossRef] [Green Version]
| Component of Trees, Carbon and FD | Minimum | Maximum | Mean ± SD |
|---|---|---|---|
| Tree dimension | |||
| DBH (cm) | 4.46 | 36.46 | 11.93 ± 5.48 |
| Tree height (m) | 2.00 | 14.00 | 8.29 ± 2.39 |
| Biomass (kg stem−1) | 14.65 | 3398.73 | 4.10.69 ± 473.11 |
| Carbon component | |||
| AGBC (Mg C ha−1) | 90.04 | 545.90 | 320.6 ± 20.34 |
| SOC (Mg C ha−1) | 23.35 | 206.64 | 48.67 ± 5.83 |
| TEC (Mg C ha−1) | 147.33 | 762.84 | 455.84 ± 27.04 |
| FQ | |||
| FQ-LA | 0.02 | 2.05 | 0.67 ± 0.11 |
| FQ-SLA | 0.03 | 1.49 | 0.42 ± 0.06 |
| FQ-LT | 0.12 | 1.55 | 0.83 ± 0.07 |
| FQ-LDMC | 0.01 | 1.40 | 0.47 ± 0.07 |
| FQ-WD | 0.03 | 1.52 | 0.69 ± 0.08 |
| CWM | |||
| CWM-LA | 99.07 | 586.08 | 258.61 ± 22.33 |
| CWM-SLA | 83.18 | 137.88 | 105.25 ± 2.45 |
| CWM-LT | 0.18 | 0.35 | 0.29 ± 0.01 |
| CWM-LDMC | 405.71 | 573.46 | 469.21 ± 6.55 |
| CWM-WD | 0.19 | 0.85 | 0.55 ± 0.03 |
| Functional Diversity Component | AGBC | SOC | TEC | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Estimate | R2 | p-Value | Estimate | R2 | p-Value | Estimate | R2 | p-Value | |
| FQ of LA | 2.401 | 0.013 | 0.945 | −7.136 | 0.019 | 0.469 | −4.086 | <0.001 | 0.925 |
| FQ of SLA | 85.556 | 0.260 | 0.166 | 12.223 | 0.017 | 0.495 | 120.879 | 0.076 | 0.140 |
| FQ of LT | 80.431 | 0.077 | 0.139 | −28.9 | 0.121 | 0.061 | 73.247 | 0.036 | 0.316 |
| FQ of LDMC | 10.533 | <0.001 | 0.857 | 14.431 | 0.027 | 0.385 | 27.809 | 0.005 | 0.720 |
| FQ of WD | 139.979 | 0.337 | 0.001 | 25.962 | 0.142 | 0.061 | 203.735 | 0.405 | <0.001 |
| CWM of LA | −0.221 | 0.059 | 0.196 | −0.035 | 0.018 | 0.481 | −0.316 | 0.068 | 0.164 |
| CWM of SLA | 2.671 | 0.104 | 0.083 | 1.224 | 0.266 | 0.004 | 4.616 | 0.175 | 0.021 |
| CWM of LT | −879.284 | 0.119 | 0.061 | −276.472 | 0.144 | 0.039 | −1393.16 | 0.17 | 0.024 |
| CWM of LDMC | 0.060 | 0.019 | 0.919 | −0.027 | 0.001 | 0.875 | 0.050 | <0.001 | 0.965 |
| CWM of WD | −297.92 | 0.202 | 0.013 | −77.604 | 0.167 | 0.025 | −455.963 | 0.268 | 0.003 |
| Response Variable | Model Form | Predictor Variables | Slope | SE | p-Value | Adj. R2 |
|---|---|---|---|---|---|---|
| AGBC | AGBC = 224.10 + 139.98 × FQ_WD − 28.90 × CWM_LT | Model | 92.3 | 0.0007 | 0.313 | |
| FQ-WD | + | 0.0081 | ||||
| CWM-LT | – | 0.0038 | ||||
| SOC | SOC = 80.12 − 45.11 × FQ_LT + 3.22 × CWM_SLA | Model | 27.83 | 0.0035 | 0.542 | |
| FQ-LT | – | 0.0012 | ||||
| CWM-SLA | + | 0.0002 | ||||
| TEC | TEC = 110.03 + 232.57 × FQ_WD + 4.62 × CWM_SLA | Model | 90.40 | 0.0008 | 0.403 | |
| FQ-WD | + | 0.0059 | ||||
| CWM-SLA | + | 0.0035 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Asanok, L.; Taweesuk, R.; Kamyo, T. Plant Functional Diversity Is Linked to Carbon Storage in Deciduous Dipterocarp Forest Edges in Northern Thailand. Sustainability 2021, 13, 11416. https://doi.org/10.3390/su132011416
Asanok L, Taweesuk R, Kamyo T. Plant Functional Diversity Is Linked to Carbon Storage in Deciduous Dipterocarp Forest Edges in Northern Thailand. Sustainability. 2021; 13(20):11416. https://doi.org/10.3390/su132011416
Chicago/Turabian StyleAsanok, Lamthai, Rungrawee Taweesuk, and Torlarp Kamyo. 2021. "Plant Functional Diversity Is Linked to Carbon Storage in Deciduous Dipterocarp Forest Edges in Northern Thailand" Sustainability 13, no. 20: 11416. https://doi.org/10.3390/su132011416
APA StyleAsanok, L., Taweesuk, R., & Kamyo, T. (2021). Plant Functional Diversity Is Linked to Carbon Storage in Deciduous Dipterocarp Forest Edges in Northern Thailand. Sustainability, 13(20), 11416. https://doi.org/10.3390/su132011416






