Simultaneous Adsorption and Reduction of Cr(VI) to Cr(III) in Aqueous Solution Using Nitrogen-Rich Aminal Linked Porous Organic Polymers
Abstract
1. Introduction
2. Materials, Methods, and Instrumentations
2.1. Materials
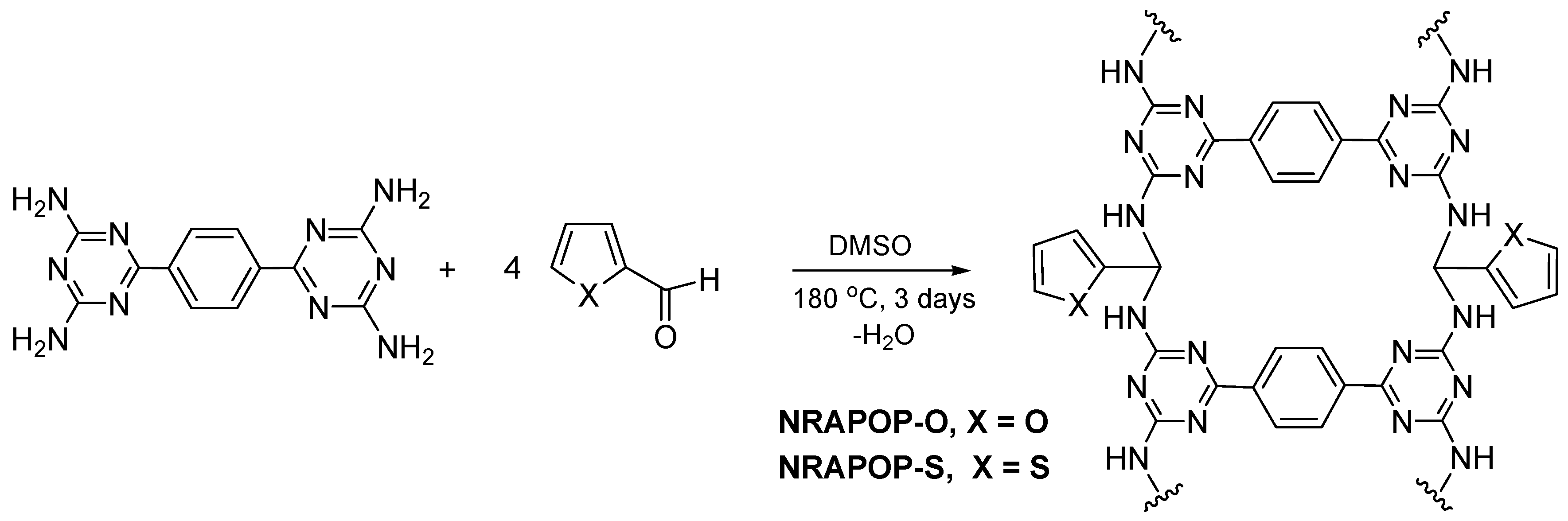
2.2. Synthesis of NRAPOPs
2.2.1. NRAPOP-O
2.2.2. NRAPOP-S
2.3. Adsorption and Reduction of Cr(VI) by NRAPOPs
2.4. Instrumentations
3. Results and Discussion
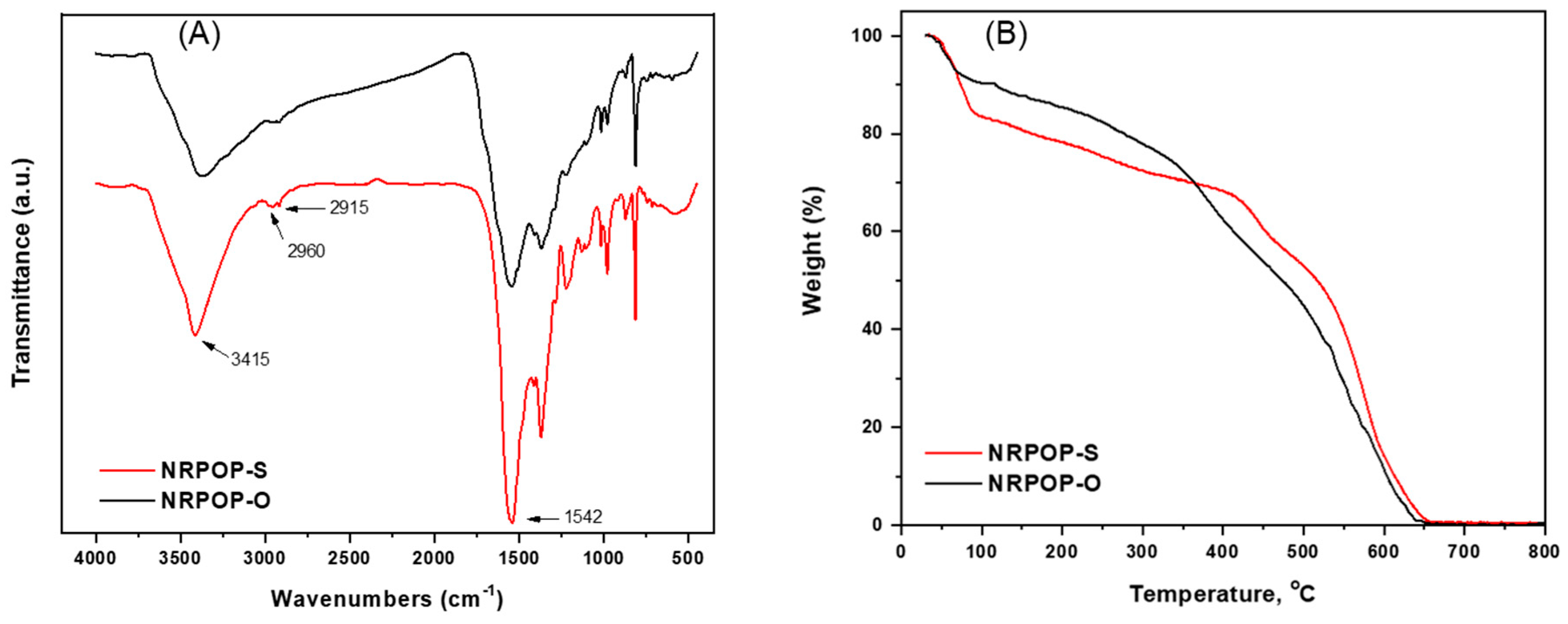
3.1. Synthesis and Characterization
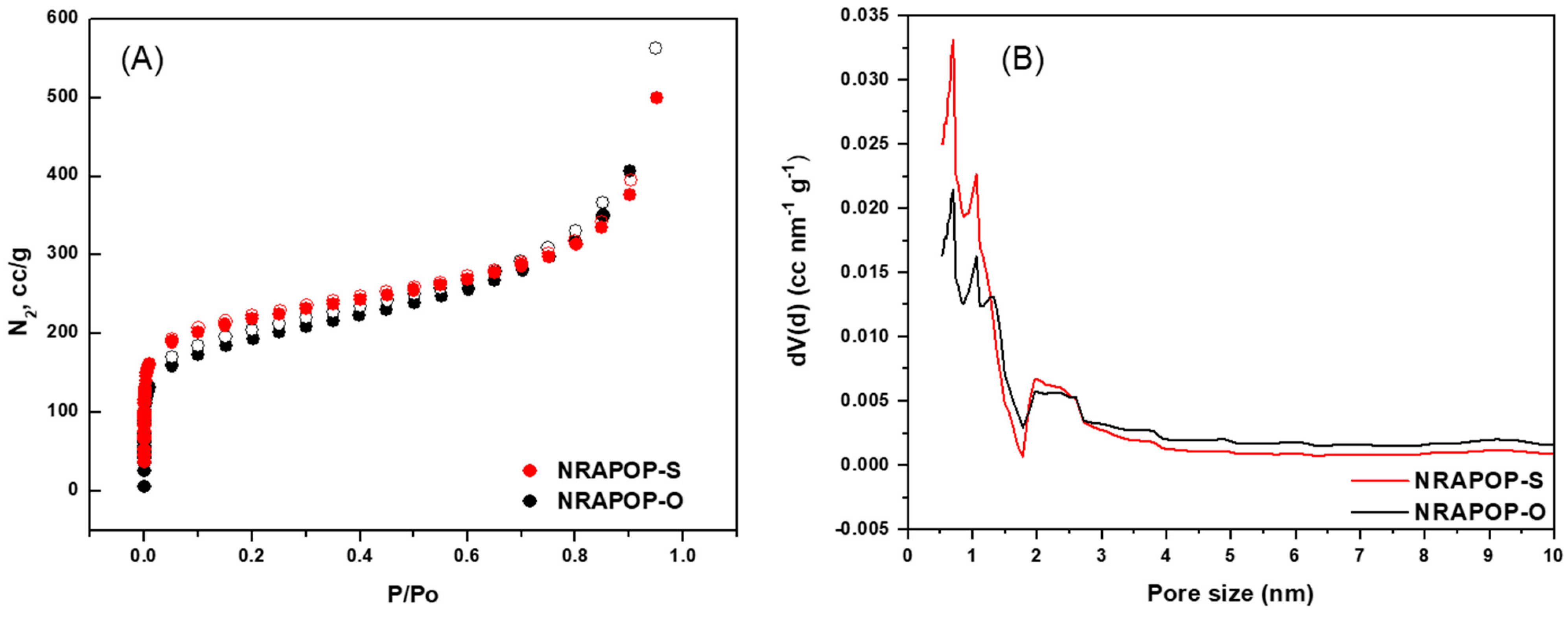
3.2. Porosity Study
3.3. Adsorption and Reduction of Cr(VI) to Cr(III)
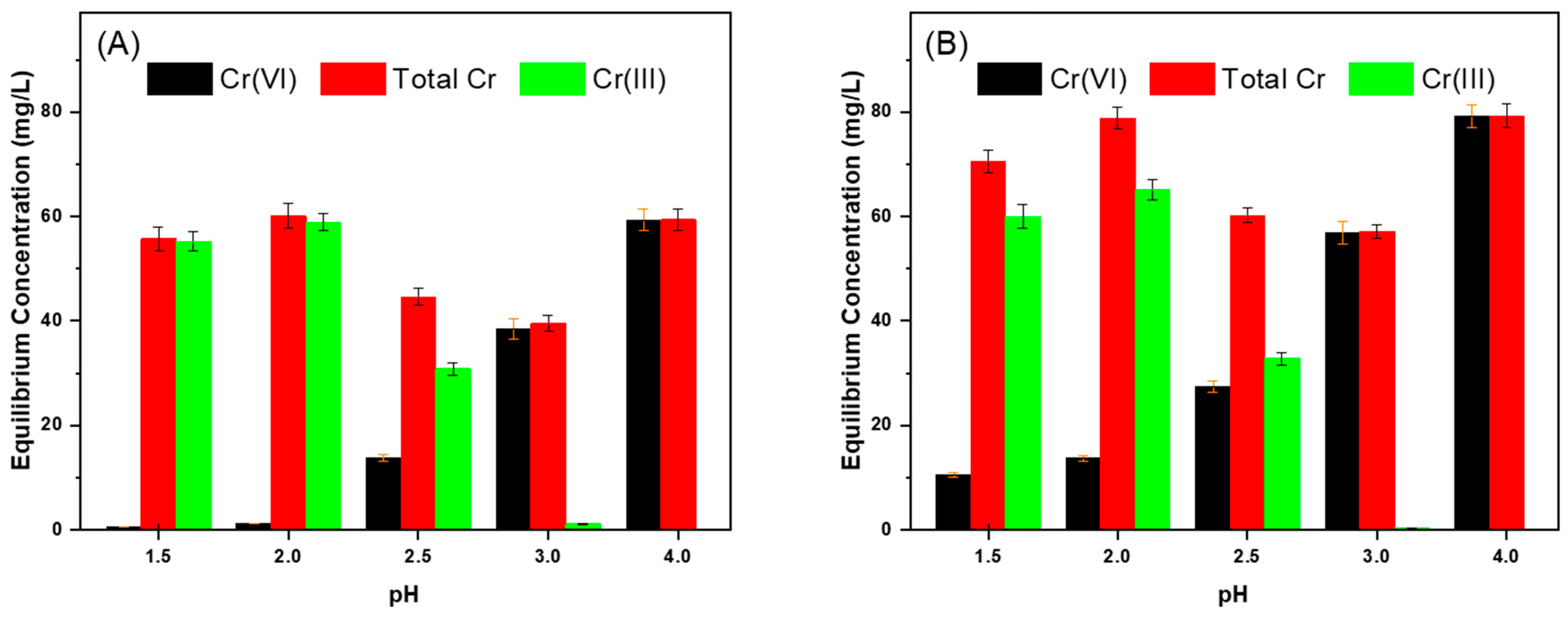
3.3.1. Effect of Initial pH
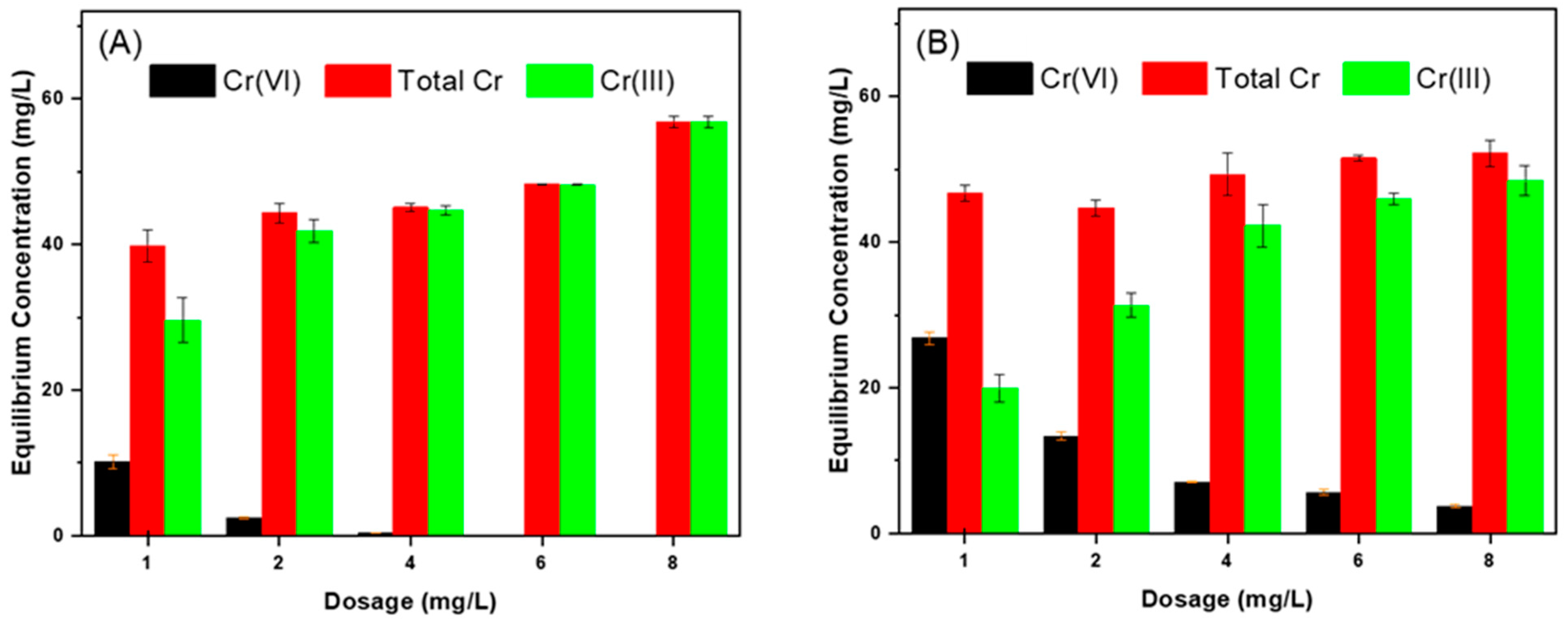
3.3.2. Effect of Dosage
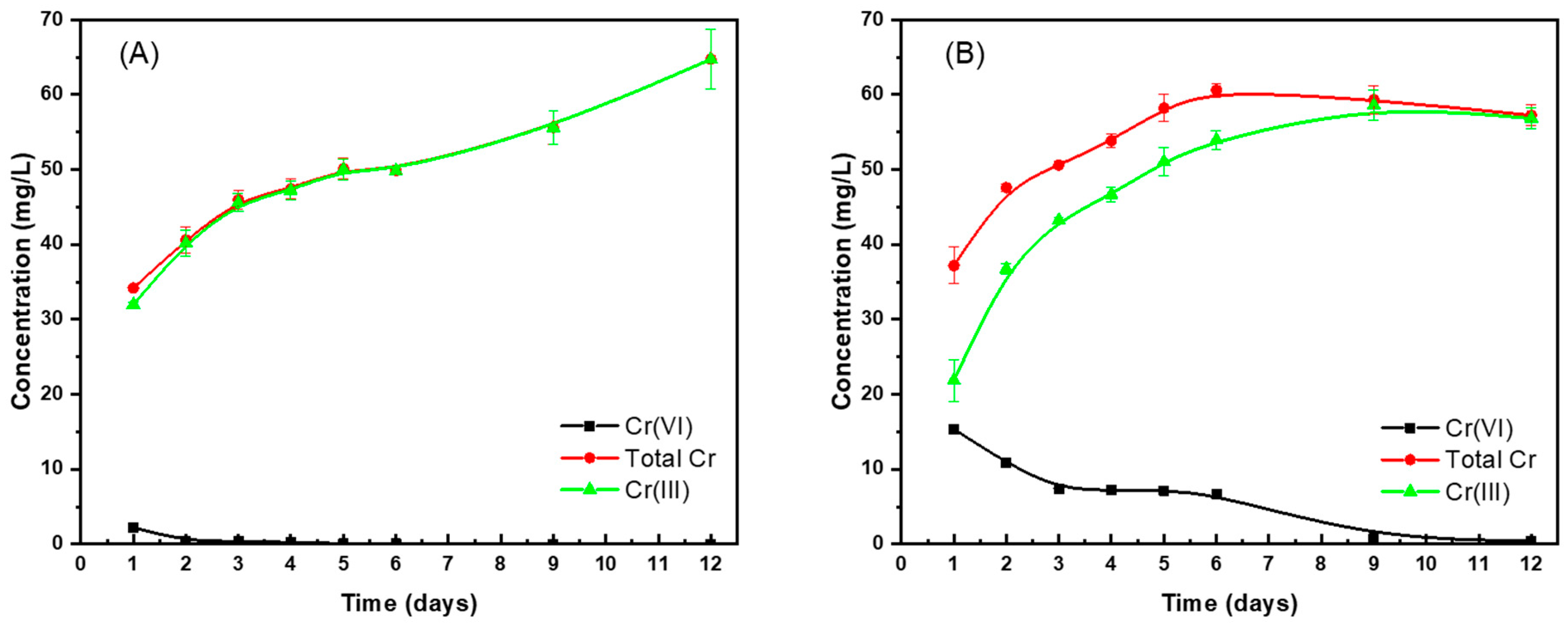
3.3.3. Effect of Contact Time
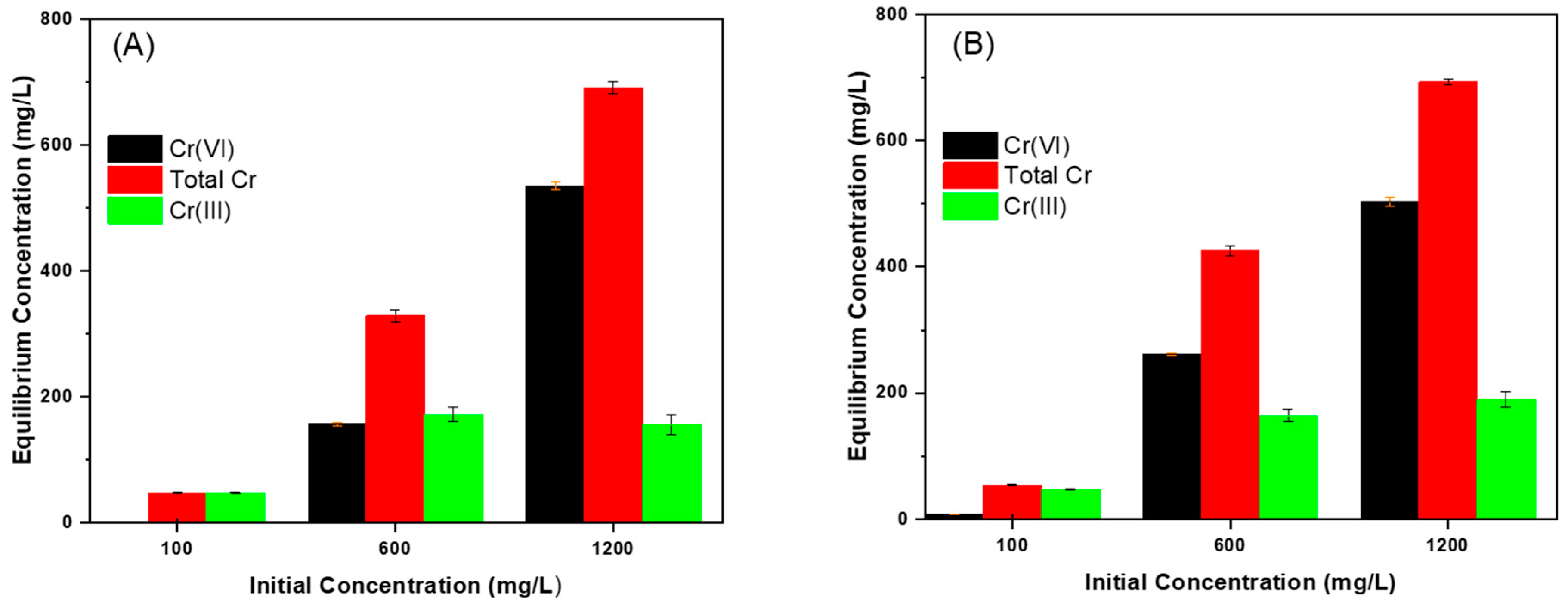
3.3.4. Effect of Initial Concentration
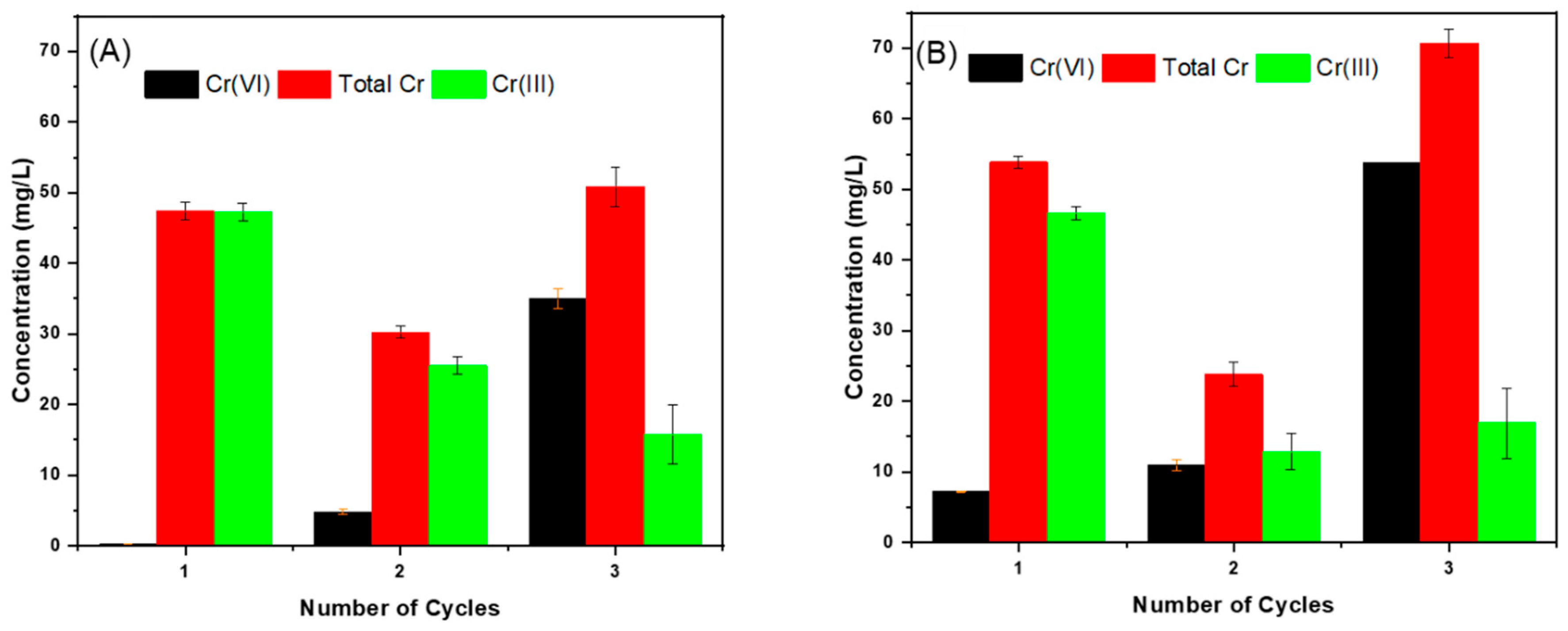
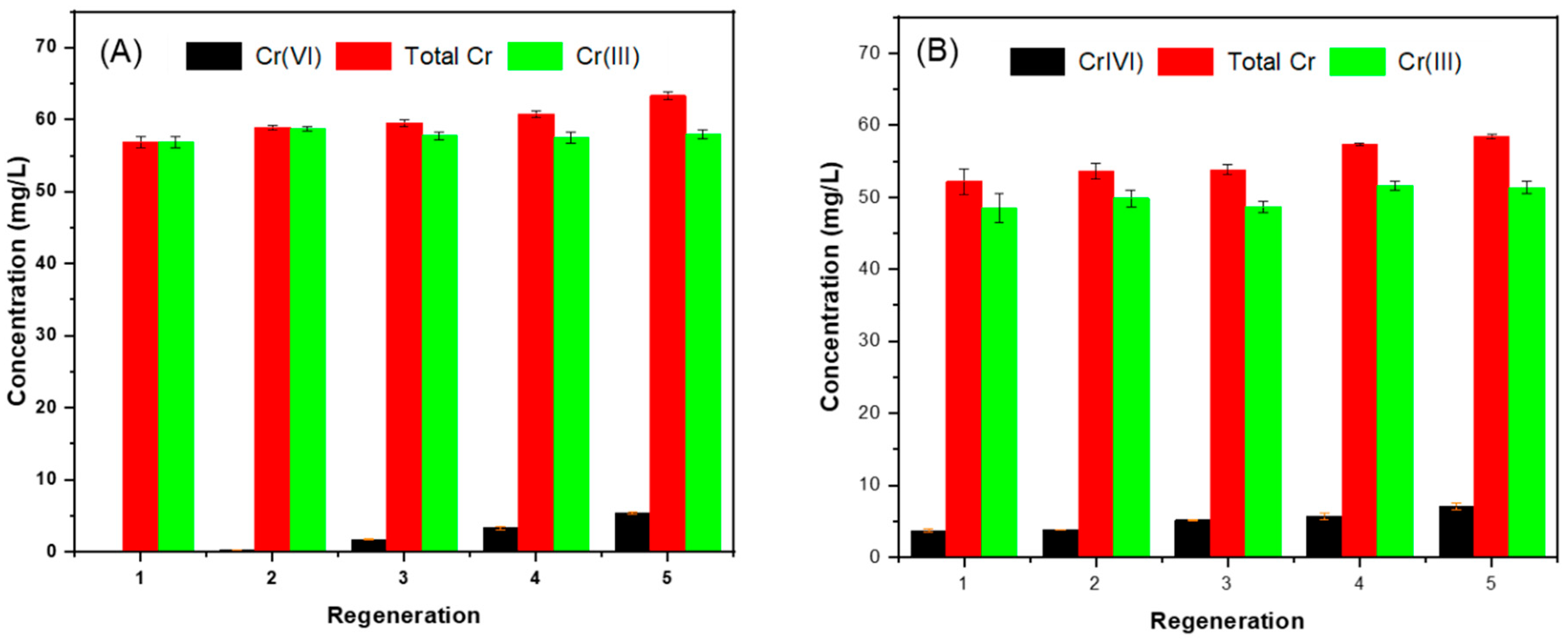
3.4. Reuse and Regeneration of NRAPOPs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Malaviya, P.; Singh, A. Physicochemical technologies for remediation of chromium-containing waters and wastewaters. Crit. Rev. Environ. Sci. Technol. 2011, 41, 1111–1172. [Google Scholar] [CrossRef]
- Khezami, L.; Capart, R. Removal of chromium(VI) from aqueous solution by activated carbons: Kinetic and equilibrium studies. J. Hazard. Mater. 2005, 123, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Gode, F.; Pehlivan, E. Sorption of Cr(III) onto chelating b-DAEG-sporopollenin and CEP-sporopollenin resins. Bioresour. Technol. 2007, 98, 904–911. [Google Scholar] [CrossRef] [PubMed]
- Costa, M. Potential hazards of hexavalent chromate in our drinking water. Toxicol. Appl. Pharmacol. 2003, 188, 1–5. [Google Scholar] [CrossRef]
- Kotaś, J.; Stasicka, Z. Chromium occurrence in the environment and methods of its speciation. Environ. Pollut. 2000, 107, 263–283. [Google Scholar] [CrossRef]
- Dakiky, M.; Khamis, M.; Manassra, A.; Mer’eb, M. Selective adsorption of chromium(VI) in industrial wastewater using low-cost abundantly available adsorbents. Adv. Environ. Res. 2002, 6, 533–540. [Google Scholar] [CrossRef]
- McNeill, L.S.; McLean, J.E.; Parks, J.L.; Edwards, M.A. Hexavalent chromium review, part 2: Chemistry, occurrence, and treatment. J. Am. Water Works Assoc. 2012, 104, E395–E405. [Google Scholar] [CrossRef]
- McLean, J.E.; McNeill, L.S.; Edwards, M.A.; Parks, J.L. Hexavalent chromium review, part 1: Health effects, regulations, and analysis. J. Am. Water Works Assoc. 2012, 104, E348–E357. [Google Scholar] [CrossRef]
- Wilbur, S.; Abadin, H.; Fay, M.; Yu, D.; Tencza, B.; Ingerman, L.; Klotzbach, J.; James, S. Toxicological Profile for Chromium; Agency for Toxic Substances and Disease Registry: Atlanta, GA, USA, 2012. [Google Scholar]
- Owlad, M.; Aroua, M.K.; Daud, W.A.W.; Baroutian, S. Removal of hexavalent chromium-contaminated water and wastewater: A review. Water Air Soil Pollut. 2009, 200, 59–77. [Google Scholar] [CrossRef]
- Zhu, J.; Wei, S.; Gu, H.; Rapole, S.B.; Wang, Q.; Luo, Z.; Haldolaarachchige, N.; Young, D.P.; Guo, Z. One-pot synthesis of magnetic graphene nanocomposites decorated with core@double-shell nanoparticles for fast chromium removal. Environ. Sci. Technol. 2012, 46, 977–985. [Google Scholar] [CrossRef]
- Orozco, A.M.F.; Contreras, E.M.; Zaritzky, N.E. Cr(VI) reduction capacity of activated sludge as affected by nitrogen and carbon sources, microbial acclimation and cell multiplication. J. Hazard. Mater. 2010, 176, 657–665. [Google Scholar] [CrossRef] [PubMed]
- Qiu, B.; Wang, Y.; Sun, D.; Wang, Q.; Zhang, X.; Weeks, B.L.; O’Connor, R.; Huang, X.; Wei, S.; Guo, Z. Cr(VI) removal by magnetic carbon nanocomposites derived from cellulose at different carbonization temperatures. J. Mater. Chem. A 2015, 3, 9817–9825. [Google Scholar] [CrossRef]
- Yu, Z.; Zhang, X.; Huang, Y. Magnetic chitosan-iron(III) hydrogel as a fast and reusable adsorbent for chromium(VI) removal. Ind. Eng. Chem. Res. 2013, 52, 11956–11966. [Google Scholar] [CrossRef]
- Krishna Kumar, A.S.; Jiang, S.J.; Tseng, W.L. Effective adsorption of chromium(VI)/Cr(III) from aqueous solution using ionic liquid functionalized multiwalled carbon nanotubes as a super sorbent. J. Mater. Chem. A 2015, 3, 7044–7057. [Google Scholar] [CrossRef]
- Mohan, D.; Pittman, C.U. Activated carbons and low cost adsorbents for remediation of tri- and hexavalent chromium from water. J. Hazard. Mater. 2006, 137, 762–811. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Korenaga, T.; Takahashi, T.; Moriwake, T.; Shinoda, S. A process monitoring/controlling system for the treatment of wastewater containing chromium(VI). Water Res. 1993, 27, 1049–1054. [Google Scholar] [CrossRef]
- Tiravanti, G.; Petruzzelli, D.; Passino, R. Pretreatment of tannery wastewaters by an ion exchange process for Cr(III) removal and recovery. Water Sci. Technol. 1997, 36, 197–207. [Google Scholar] [CrossRef]
- Khamis, M.; Jumean, F.; Abdo, N. Speciation and removal of chromium from aqueous solution by white, yellow and red UAE sand. J. Hazard. Mater. 2009, 169, 948–952. [Google Scholar] [CrossRef]
- Dahbi, S.; Azzi, M.; De La Guardia, M. Removal of hexavalent chromium from wastewaters by bone charcoal. Fresenius J. Anal. Chem. 1999, 363, 404–407. [Google Scholar] [CrossRef]
- Kongsricharoern, N.; Polprasert, C. Chromium removal by a bipolar electro-chemical precipitation process. Water Sci. Technol. 1996, 34, 109–116. [Google Scholar] [CrossRef]
- Pagilla, K.R.; Canter, L.W. Laboratory studies on remediation of chromium-contaminated soils. J. Environ. Eng. 1999, 125, 243–248. [Google Scholar] [CrossRef]
- Almeida, J.C.; Cardoso, C.E.D.; Tavares, D.S.; Freitas, R.; Trindade, T.; Vale, C.; Pereira, E. Chromium removal from contaminated waters using nanomaterials—A review. TrAC Trends Anal. Chem. 2019, 118, 277–291. [Google Scholar] [CrossRef]
- GracePavithra, K.; Jaikumar, V.; Kumar, P.S.; SundarRajan, P.S. A review on cleaner strategies for chromium industrial wastewater: Present research and future perspective. J. Clean. Prod. 2019, 228, 580–593. [Google Scholar] [CrossRef]
- Kim, W.; Park, J.Y.; Kim, Y. Fabrication of branched-TiO2 microrods on the FTO glass for photocatalytic reduction of Cr(VI) under visible-light irradiation. J. Ind. Eng. Chem. 2019, 73, 248–253. [Google Scholar] [CrossRef]
- Jiang, B.; Gong, Y.; Gao, J.; Sun, T.; Liu, Y.; Oturan, N.; Oturan, M.A. The reduction of Cr(VI) to Cr(III) mediated by environmentally relevant carboxylic acids: State-of-the-art and perspectives. J. Hazard. Mater. 2019, 365, 205–226. [Google Scholar] [CrossRef]
- Qian, A.; Liao, P.; Yuan, S.; Luo, M. Efficient reduction of Cr(VI) in groundwater by a hybrid electro-Pd process. Water Res. 2014, 48, 326–334. [Google Scholar] [CrossRef]
- Wani, P.A.; Wahid, S.; Khan, M.S.A.; Rafi, N.; Wahid, N. Investigation of the role of chromium reductase for Cr(VI) reduction by Pseudomonas species isolated from Cr(VI) contaminated effluent. Biotechnol. Res. Innov. 2019, 3, 38–46. [Google Scholar] [CrossRef]
- Ding, J.; Pu, L.; Wang, Y.; Wu, B.; Yu, A.; Zhang, X.; Pan, B.; Zhang, Q.; Gao, G. Adsorption and Reduction of Cr(VI) together with Cr(III) sequestration by polyaniline confined in pores of polystyrene beads. Environ. Sci. Technol. 2018, 52, 12602–12611. [Google Scholar] [CrossRef]
- Lu, Y.Z.; Fu, L.; Ding, J.; Ding, Z.W.; Li, N.; Zeng, R.J. Cr(VI) reduction coupled with anaerobic oxidation of methane in a laboratory reactor. Water Res. 2016, 102, 445–452. [Google Scholar] [CrossRef]
- Wang, L.; Wang, N.; Zhu, L.; Yu, H.; Tang, H. Photocatalytic reduction of Cr(VI) over different TiO2 photocatalysts and the effects of dissolved organic species. J. Hazard. Mater. 2008, 152, 93–99. [Google Scholar] [CrossRef]
- Pan, X.; Liu, Z.; Chen, Z.; Cheng, Y.; Pan, D.; Shao, J.; Lin, Z.; Guan, X. Investigation of Cr(VI) reduction and Cr(III) immobilization mechanism by planktonic cells and biofilms of Bacillus subtilis ATCC-6633. Water Res. 2014, 55, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Pakade, V.E.; Tavengwa, N.T.; Madikizela, L.M. Recent advances in hexavalent chromium removal from aqueous solutions by adsorptive methods. RSC Adv. 2019, 9, 26142–26164. [Google Scholar] [CrossRef]
- Xie, A.; Ji, L.; Luo, S.; Wang, Z.; Xu, Y.; Kong, Y. Synthesis, characterization of poly(m-phenylenediamine)/palygorskite and its unusual and reactive adsorbability to chromium(VI). New J. Chem. 2014, 38, 777–783. [Google Scholar] [CrossRef]
- Saha, B.; Orvig, C. Biosorbents for hexavalent chromium elimination from industrial and municipal effluents. Coord. Chem. Rev. 2010, 254, 2959–2972. [Google Scholar] [CrossRef]
- Solgi, M.; Najib, T.; Ahmadnejad, S.; Nasernejad, B. Synthesis and characterization of novel activated carbon from Medlar seed for chromium removal: Experimental analysis and modeling with artificial neural network and support vector regression. Resour. Technol. 2017, 3, 236–248. [Google Scholar] [CrossRef]
- Kan, C.C.; Ibe, A.H.; Rivera, K.K.P.; Arazo, R.O.; de Luna, M.D.G. Hexavalent chromium removal from aqueous solution by adsorbents synthesized from groundwater treatment residuals. Sustain. Environ. Res. 2017, 27, 163–171. [Google Scholar] [CrossRef]
- Ray, P.; Sabri, M.A.; Ibrahim, T.H.; Khamis, M.I.; Jumean, F.H. Design and optimization of a batch sequential contactor for the removal of chromium(VI) from industrial wastewater using sheep wool as a low-cost adsorbent. Desalin. Water Treat. 2018, 113, 109–113. [Google Scholar] [CrossRef]
- Jumean, F.H.; Khamis, M.I.; Sara, Z.A.; AbouRich, M.S. Concurrent removal and reduction of Cr(VI) by wool: Short and long term equilibration studies. Am. J. Anal. Chem. 2015, 6, 47–57. [Google Scholar] [CrossRef]
- Das, S.; Heasman, P.; Ben, T.; Qiu, S. Porous organic materials: Strategic design and structure-function correlation. Chem. Rev. 2017, 117, 1515–1563. [Google Scholar] [CrossRef]
- Zhang, T.; Xing, G.; Chen, W.; Chen, L. Porous organic polymers: A promising platform for efficient photocatalysis. Mater. Chem. Front. 2020, 4, 332–353. [Google Scholar] [CrossRef]
- Enjamuri, N.; Sarkar, S.; Reddy, B.M.; Mondal, J. Design and catalytic application of functional porous organic polymers: Opportunities and challenges. Chem. Rec. 2019, 19, 1782–1792. [Google Scholar] [CrossRef] [PubMed]
- Arab, P.; El-Kadri, O.M.; El-Kaderi, H.M. Designing functional porous organic frameworks for gas storage and separation. In Monographs in Supramolecular Chemistry; RSC Publishing: Cambridge, UK, 2017; pp. 388–411. ISBN 9781782624172. [Google Scholar]
- Kassab, R.M.; Jackson, K.T.; El-Kadri, O.M.; El-Kaderi, H.M. Nickel-catalyzed synthesis of nanoporous organic frameworks and their potential use in gas storage applications. Res. Chem. Intermed. 2011, 37, 747–757. [Google Scholar] [CrossRef]
- Rabbani, M.G.; Sekizkardes, A.K.; El-Kadri, O.M.; Kaafarani, B.R.; El-Kaderi, H.M. Pyrene-directed growth of nanoporous benzimidazole-linked nanofibers and their application to selective CO2 capture and separation. J. Mater. Chem. 2012, 22, 25409–25417. [Google Scholar] [CrossRef]
- El-Kadri, O.M.; Tessema, T.D.; Almotawa, R.M.; Arvapally, R.K.; Al-Sayah, M.H.; Omary, M.A.; El-Kaderi, H.M. Pyrene bearing azo-functionalized porous nanofibers for CO2 separation and toxic metal cation sensing. ACS Omega 2018, 3, 15510–15518. [Google Scholar] [CrossRef] [PubMed]
- Cui, F.; Liang, R.; Qi, Q.; Jiang, G.; Zhao, X. Efficient Removal of Cr(VI) from aqueous solutions by a dual-pore covalent organic framework. Adv. Sustain. Syst. 2019, 3, 1800150. [Google Scholar] [CrossRef]
- Zhu, D.; Zhou, S.; Zhou, Z.; Li, R.; Ye, J.; Ziyu, X.; Lan, S.; Zhang, Y.; Miao, S.; Wang, W. Highly efficient and selective removal of Cr(VI) by covalent organic frameworks: Structure, performance and mechanism. Colloids Surfaces A Physicochem. Eng. Asp. 2020, 600. [Google Scholar] [CrossRef]
- Chen, J.; Wang, Y.; Ye, C.; Lyu, W.; Zhu, J.; Yan, W.; Qiu, T. Self-reducible conjugated microporous polyaniline for long-term selective Cr(VI) detoxication driven by tunable pore dimension. ACS Appl. Mater. Interfaces 2020, 12, 28681–28691. [Google Scholar] [CrossRef]
- Sen, S.; Al-Sayah, M.H.; Mohammed, M.S.; Abu-Abdoun, I.I.; El-Kadri, O.M. Multifunctional nitrogen-rich aminal-linked luminescent porous organic polymers for iodine enrichment and selective detection of Fe3+ ions. J. Mater. Sci. 2020, 55, 10896–10909. [Google Scholar] [CrossRef]
- Abdelmoaty, Y.H.; Tessema, T.D.; Choudhury, F.A.; El-Kadri, O.M.; El-Kaderi, H.M. Nitrogen-rich porous polymers for carbon dioxide and iodine sequestration for environmental remediation. ACS Appl. Mater. Interfaces 2018, 10, 16049–16058. [Google Scholar] [CrossRef]
- Sabri, M.A.; Al-Sayah, M.H.; Sen, S.; Ibrahim, T.H.; El-Kadri, O.M. Fluorescent aminal linked porous organic polymer for reversible iodine capture and sensing. Sci. Rep. 2020, 10, 15943. [Google Scholar] [CrossRef]
- Cañas-Ventura, M.E.; Xiao, W.; Wasserfallen, D.; Müllen, K.; Brune, H.; Barth, J.V.; Fasel, R. Self-assembly of periodic bicomponent wires and ribbons. Angewandte Chemie Int. Ed. 2007, 46, 1814–1818. [Google Scholar] [CrossRef] [PubMed]
- Song, W.C.; Xu, X.K.; Chen, Q.; Zhuang, Z.Z.; Bu, X.H. Nitrogen-rich diaminotriazine-based porous organic polymers for small gas storage and selective uptake. Polym. Chem. 2013, 4, 4690–4696. [Google Scholar] [CrossRef]
- Schwab, M.G.; Fassbender, B.; Spiess, H.W.; Thomas, A.; Feng, X.; Müllen, K. Catalyst-free preparation of melamine-based microporous polymer networks through Schiff base chemistry. J. Am. Chem. Soc. 2009, 131, 7216–7217. [Google Scholar] [CrossRef] [PubMed]
- Weng, J.Y.; Xu, Y.L.; Song, W.C.; Zhang, Y.H. Tuning the adsorption and fluorescence properties of aminal-linked porous organic polymers through N-heterocyclic group decoration. J. Polym. Sci. Part A Polym. Chem. 2016, 54, 1724–1730. [Google Scholar] [CrossRef]
- Pan, X.; Qin, X.; Zhang, Q.; Ge, Y.; Ke, H.; Cheng, G. N- and S-rich covalent organic framework for highly efficient removal of indigo carmine and reversible iodine capture. Microporous Mesoporous Mater. 2020, 296, 109990. [Google Scholar] [CrossRef]
- Odeh, L.; Odeh, I.; Khamis, M.; Khatib, M.; Qurie, M.; Shakhsher, Z.; Qutob, M. Hexavalent chromium removal and reduction to Cr(III) by polystyrene tris(2-aminoethyl)amine. Am. J. Anal. Chem. 2015, 6, 26–37. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sabri, M.A.; Sara, Z.; Al-Sayah, M.H.; Ibrahim, T.H.; Khamis, M.I.; El-Kadri, O.M. Simultaneous Adsorption and Reduction of Cr(VI) to Cr(III) in Aqueous Solution Using Nitrogen-Rich Aminal Linked Porous Organic Polymers. Sustainability 2021, 13, 923. https://doi.org/10.3390/su13020923
Sabri MA, Sara Z, Al-Sayah MH, Ibrahim TH, Khamis MI, El-Kadri OM. Simultaneous Adsorption and Reduction of Cr(VI) to Cr(III) in Aqueous Solution Using Nitrogen-Rich Aminal Linked Porous Organic Polymers. Sustainability. 2021; 13(2):923. https://doi.org/10.3390/su13020923
Chicago/Turabian StyleSabri, Muhammad A., Ziad Sara, Mohammad H. Al-Sayah, Taleb H. Ibrahim, Mustafa I. Khamis, and Oussama M. El-Kadri. 2021. "Simultaneous Adsorption and Reduction of Cr(VI) to Cr(III) in Aqueous Solution Using Nitrogen-Rich Aminal Linked Porous Organic Polymers" Sustainability 13, no. 2: 923. https://doi.org/10.3390/su13020923
APA StyleSabri, M. A., Sara, Z., Al-Sayah, M. H., Ibrahim, T. H., Khamis, M. I., & El-Kadri, O. M. (2021). Simultaneous Adsorption and Reduction of Cr(VI) to Cr(III) in Aqueous Solution Using Nitrogen-Rich Aminal Linked Porous Organic Polymers. Sustainability, 13(2), 923. https://doi.org/10.3390/su13020923






