Housefly Maggot Meal as a Potent Bioresource for Fish Feed to Facilitate Early Gonadal Development in Clarias gariepinus (Burchell,1822)
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Care and Acclimation
2.2. Experimental Diet Formulation
2.3. Experimental Procedure
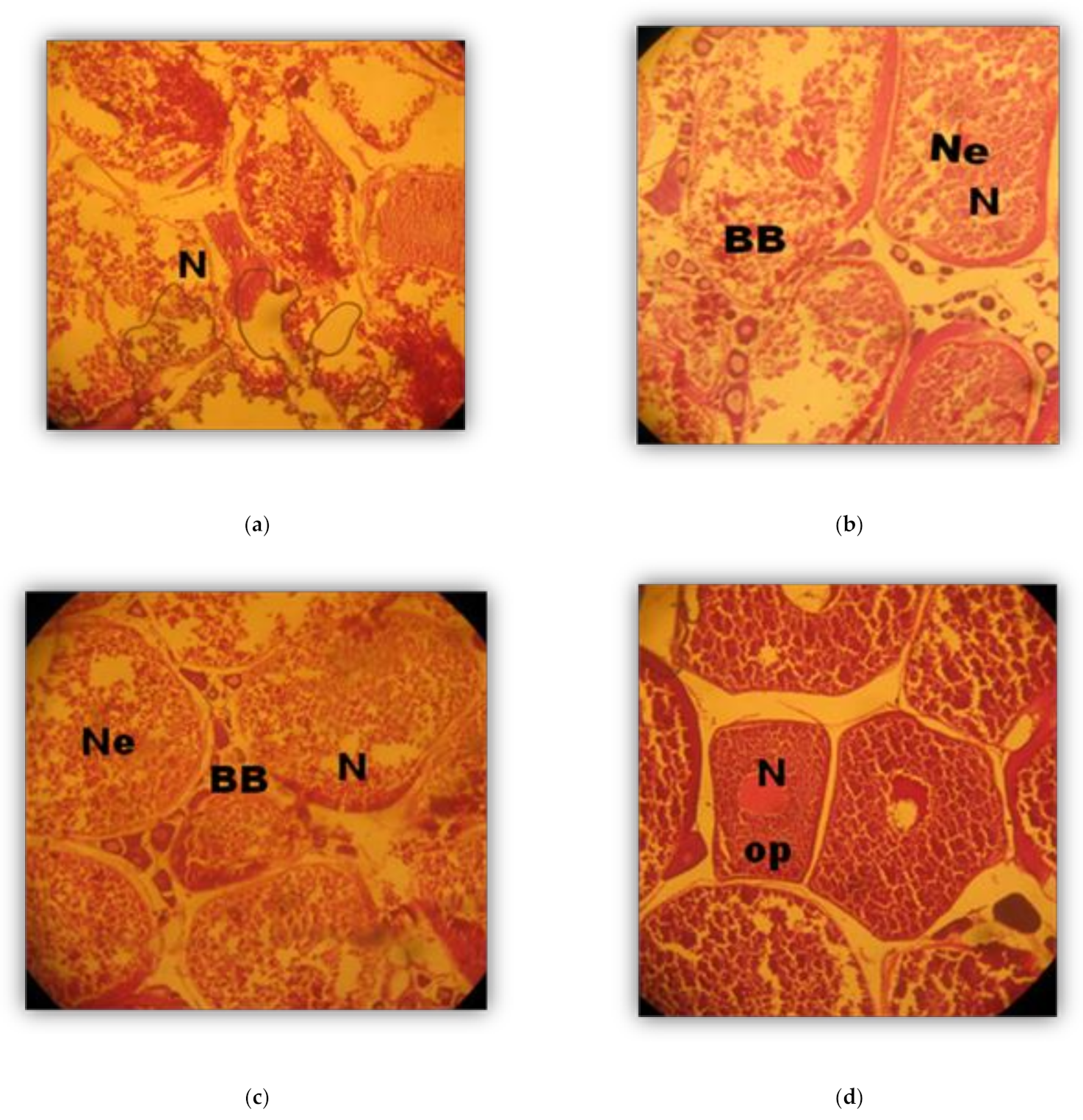
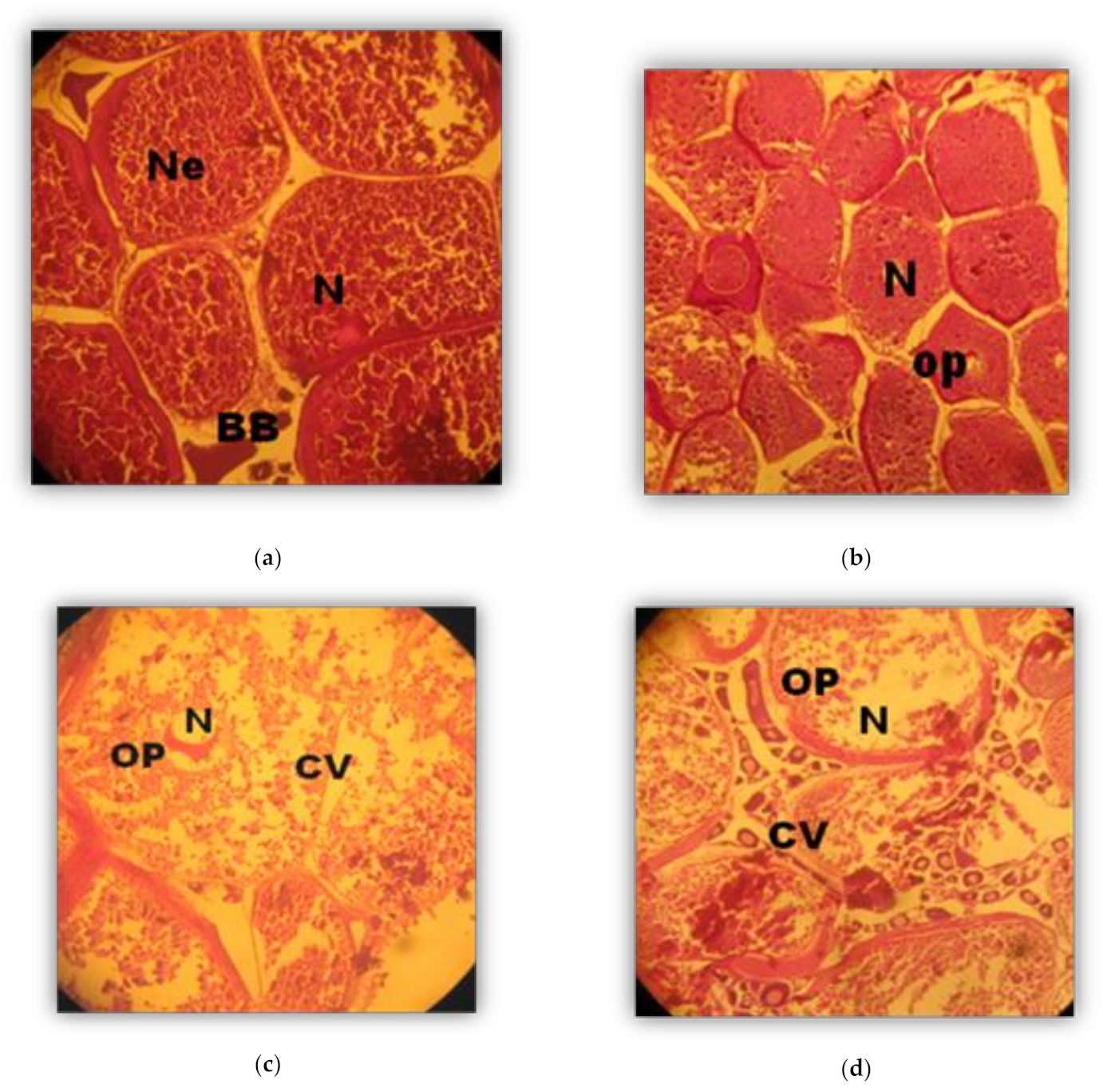
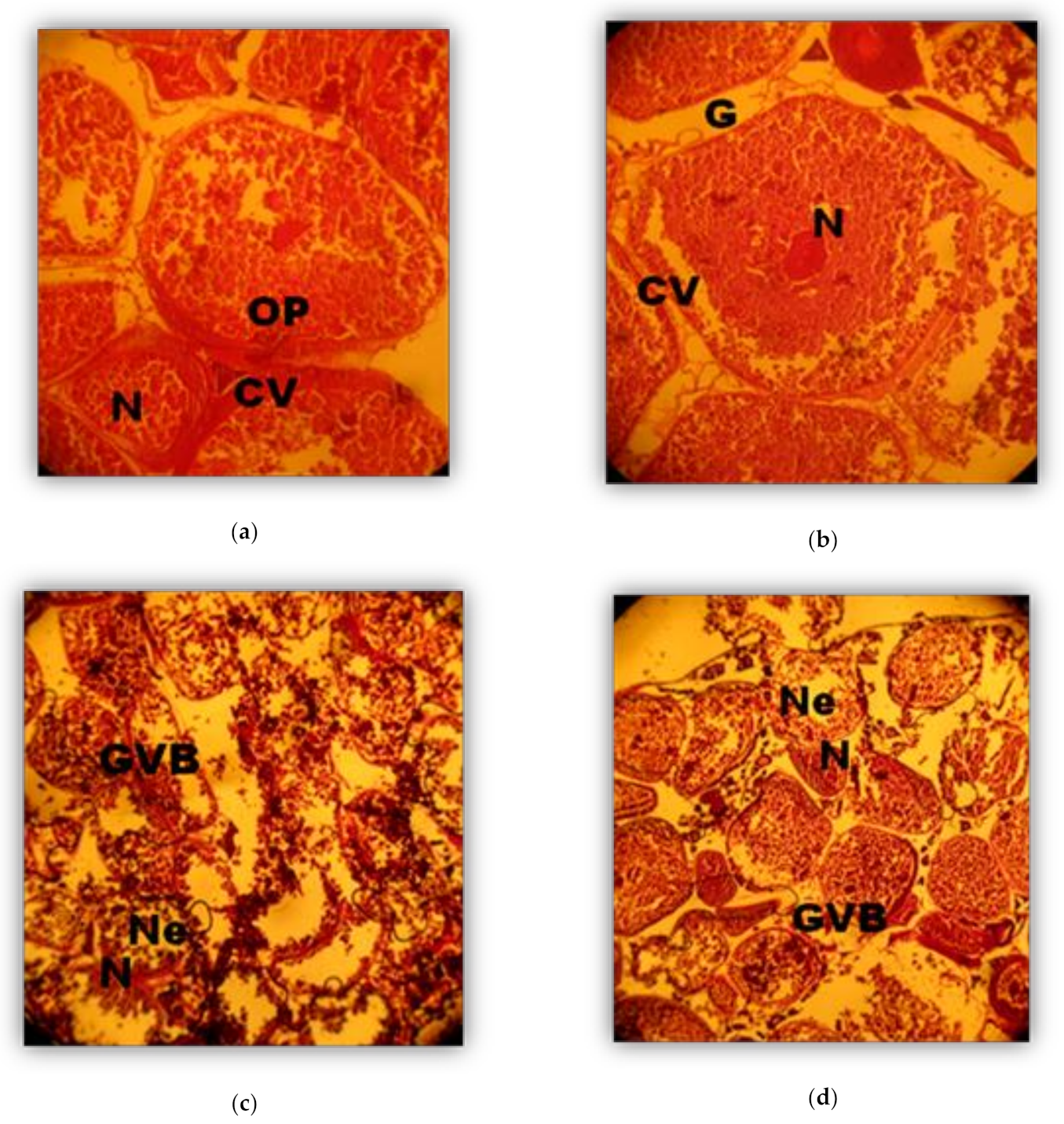
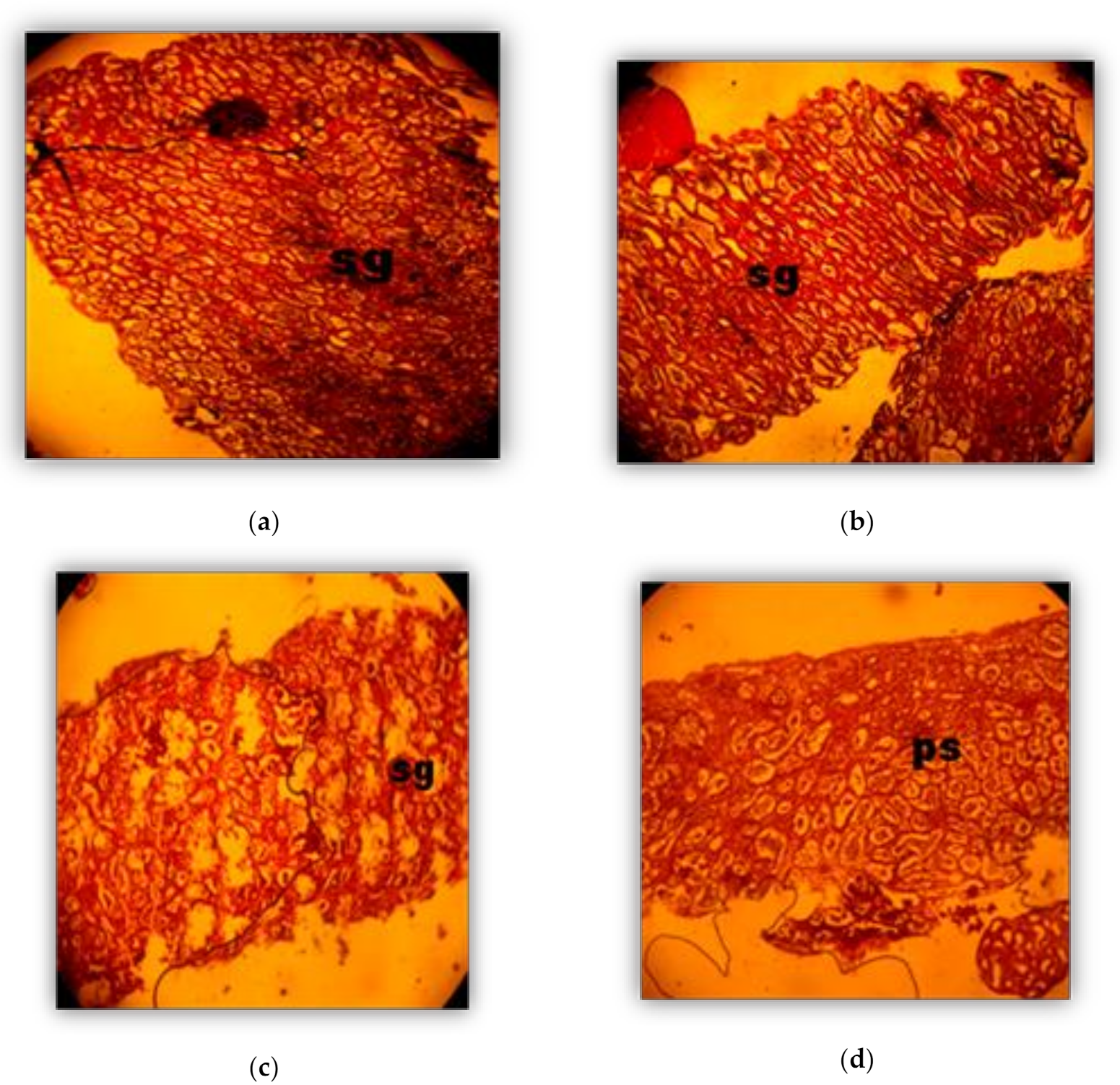
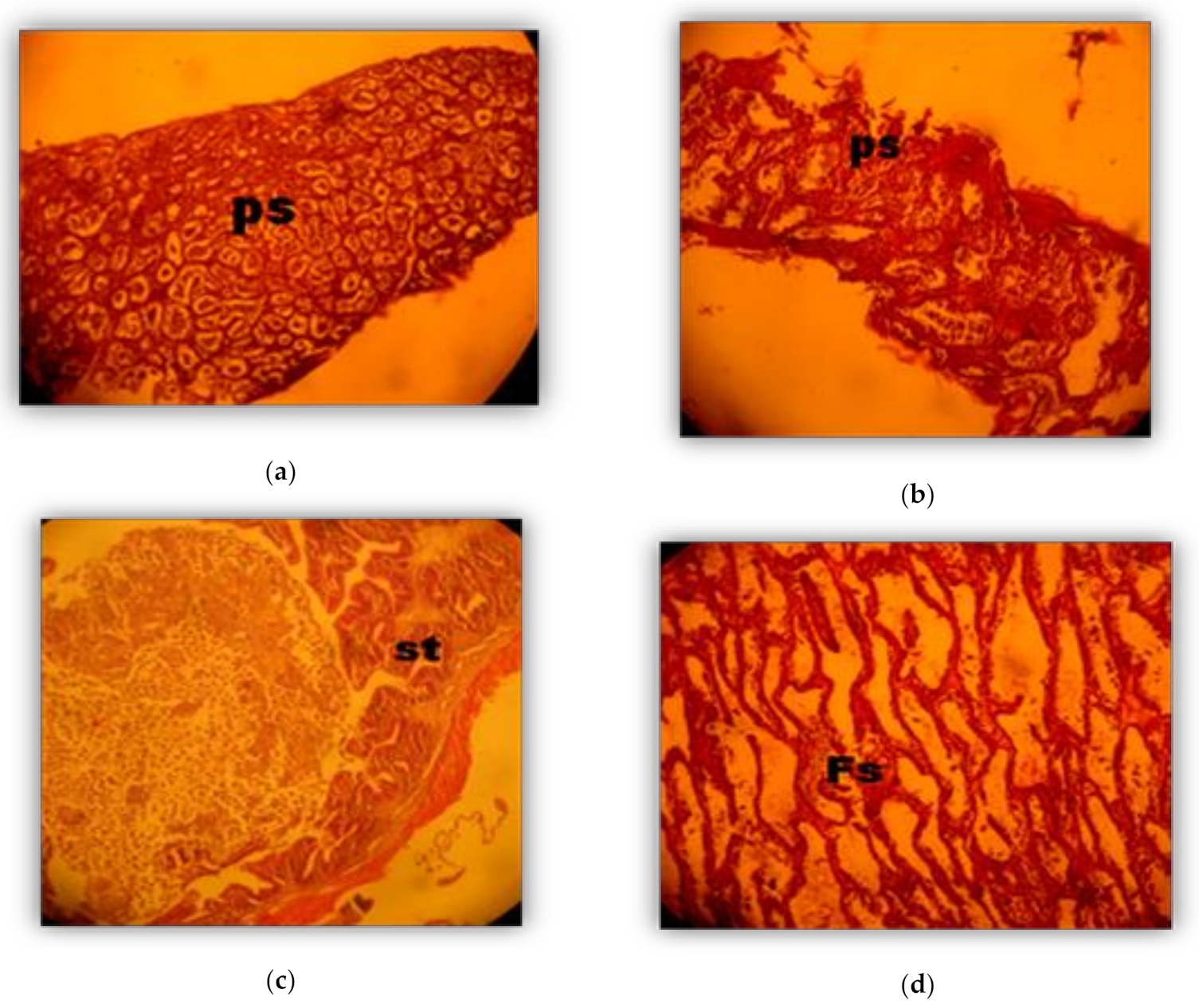
2.4. Histological Analysis
2.5. Proximate Analysis
2.6. Statistical and Growth Analysis
3. Results
3.1. Composition of Experimental Diet and Growth Performance
Phases (in weeks) of Oocyte development (Oogenesis)
3.2. Phases of Male Gonad Development (Spermatogenesis)
4. Discussion
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Ethical Approval
References
- Fiore, G. Farming and Food Security: An Assessment of Animal Production and Environmental Impact; EUR 28792 EN; Publications Office of the European Union: Luxembourg, 2017; ISBN 978-92-79-73832-6. [Google Scholar] [CrossRef]
- FAO—Food and Agriculture Organization. The Future of Food and Agriculture: Alternative Pathways to 2050. 2018. Available online: http://www.fao.org/3/CA1553EN/ca1553en.pdf (accessed on 22 November 2020).
- EUMOFA—European Market Observatory for Fisheries and Aquaculture Products Union. Blue Bioeconomy: Situation Report and Perspective. 2018, pp. 1–144. Available online: https://www.eumofa.eu/documents/20178/84590/Blue+bioeconomy_Final.pdf (accessed on 15 January 2021). [CrossRef]
- Onyeneke, R.U.; Igberi, C.O.; Aligbe, J.O.; Iruo, F.A.; Amadi, M.U.; Iheanacho, S.C.; Osuji, E.E.; Munonye, J.; Uwadoka, C. Climate change adaptation actions by fish farmers: Evidence from the Niger Delta Region of Nigeria. Aust. J. Agric. Resour. Econ. 2020, 59, 1–29. [Google Scholar] [CrossRef]
- Rana, K.J.; Siriwardena, S.; Hasan, M.R. Impact of Rising Feed Ingredient Prices on Aquafeeds and Aquaculture Production; Fisheries and Aquaculture Technical Paper. No. 541; FAO: Rome, Italy, 2009. [Google Scholar]
- FAO. The State of World Fisheries and Aquaculture 2018—Meeting the Sustainable Development Goals; FAO: Rome, Italy, 2018. Available online: http://www.fao.org/documents/card/en/c/I9540EN/ (accessed on 16 July 2020).
- Maiolo, S.; Parisi, G.; Biondi, N.; Lunelli, F.; Emilio, T.E.; Pastres, R. Fishmeal partial substitution within aquafeed formulations: Life cycle assessment of four alternative protein sources. Int. J. Life Cycle Assess. 2020. [Google Scholar] [CrossRef]
- Thorarinsdottir, R.I.; Jokumsen, A.; Bjornsson, B.T.; Torrissen, O. Local Raw Materials for Production of Fish Feed for Aquaculture; Nordic Innovation Centre Project No. 10102; Norden: Oslo, Norway, 2011. [Google Scholar]
- Gephart, J.A.; Golden, C.D.; Asche, F.; Belton, B.; Brugere, C.; Froehlich, H.E.; Fry, J.P.; Halpern, B.S.; Hicks, C.C.; Jones, R.C.; et al. Scenarios for global aquaculture and its role in human nutrition. Rev. Fish. Sci. Aquac. 2020. [Google Scholar] [CrossRef]
- Wilfart, A.; Espagnol, S.; Dauguet, S.; Tailleur, A.; Gac, A.; Garcia-Launay, F. ECOALIM: A Dataset of Environmental Impacts of Feed Ingredients Used in French Animal Production. PLoS ONE 2016, 11, e0167343. [Google Scholar] [CrossRef] [PubMed]
- The Danish National Bioeconomy Panel New Protein Value Chains—Recommendations to the Danish Government for the Proteins of the Future. Ministry of Environment and Food-Ministry of Environment and Food of Denmark. 2018. Available online: https://mfvm.dk/fileadmin/user_upload/MFVM/Miljoe/Biooekonomi/Recommendations_from_the_National_Bioeconomy_Panel_Proteins_for_the_future__PDF_.pdf (accessed on 20 October 2020).
- Iheanacho, S.C.; Ogueji, E.; Igberi, C.; Avwemoya, F.; Amadi-Eke, A.; Yaji, A.; Mbah, C. Suitability of discarded cashewnut meal (Anacardium occidentale L.) as replacement of soy bean meal (Glycine max) in the diet of juvenile African catfish Clarias gariepinus (Burchell, 1822). Indian J. Fish. 2019, 66, 78–86. [Google Scholar] [CrossRef]
- Ogunji, J.O.; Iheanacho, S.C.; Abe, G.A.; Ikeh, O.R. Assessing effects of substituting dietary fish mea with Boiled Donkey and Cow Blood Meal on Growth Performance and Digestive Enzyme Activities of Clarias gariepinus Juvenile. J. World Aquac. Soc. 2020, 51, 1066–1079. [Google Scholar] [CrossRef]
- Bio-Based Industries Consortium-BbIC. Biobased Industries Joint Undertaken: A ₤3.7 Billion Partnership between the European Union and the Bio-Based Industries Consotium. 2019. Available online: https://www.bbi-europe.eu/ (accessed on 20 October 2020).
- Ogunji, J.O.; Wirth, M. Alternative protein sources as substitutes for fish meal in the diet of young Tilapia Oreochromis niloticus (Linn.). Isr. J. Aquac. Bamidgeh 2001, 53, 34–43. [Google Scholar]
- Ogunji, J.O. Alternative Protein Sources in Diets for Farmed Tilapia; Animalscience.com Reviews No. 13; CAB International Publishing: Oxford, UK, 2004; Available online: https://www.cabi.org/cabreviews/review/20053092365 (accessed on 21 January 2019).
- Ogueji, E.; Iheanacho, S.C.; Mbah, C.; Yaji, A.J.; Ezemagu, U. Effect of partial and complete replacement of soybean with discarded cashew nut (Anacardium occidentale L.) on liver and stomach histology of Clarias gariepinus (Burchell, 1822). Aquac. Fish. 2020, 5, 86–91. [Google Scholar] [CrossRef]
- FAO. The Effect of COVID-19 on Fisheries and Aquaculture in Asia. 2020. Available online: https://reliefweb.int/sites/reliefweb.int/files/resources/CA9545EN.pdf (accessed on 21 October 2020).
- Nordic Council of Ministrers. Nordic Alternative Protein Potentials: Mapping of Regional Bioeconomy Opportunities. 2016. Available online: http://www.diva-portal.org/smash/get/diva2:1033296/FULLTEXT02.pdf (accessed on 17 November 2020). [CrossRef]
- Nordic Council of Ministers. Ten Trends for the Sustainable Bioeconomy in Nordic Arctic and Baltic Sea Region. 2020. Nordpub Copenhegan. Available online: https://www.norden.org/en/publication/ten-trends-sustainable-bioeconomy-nordic-arctic-and-baltic-sea-region-responsible (accessed on 15 January 2021). [CrossRef]
- Bioeconomy Council. Bioeconomy for Sustainable Nutrition New Approaches to the Protein Supply of the Future. 2017, pp. 1–9. Available online: https://biooekonomierat.de/fileadmin/Publikationen/Englisch/BOERMEMO_final_english_1.pdf (accessed on 15 January 2021).
- FAO. Species Fact Sheet–Clarias Gariepinus (Burchell, 1822) Clarias gariepinus. 2020. Available online: http://www.fao.org/fishery/species/2982/en (accessed on 22 November 2020).
- William, B.B.; Olaosebikan, B.D.; Adeleke, A.; Fagbenro, O.A. Status of African catfish farming in Nigeria. In Proceedings of the Workshop on the Development of Genetic Improvement Program for African Catfish Clarias gariepinus, Accra, Ghana, 5–9 November 2007; pp. 49–56. [Google Scholar]
- Fagbenro, O.A.; Adeparusi, E.O.; Jimoh, W.A. Haematological profile of blood of African Catfish (Clarias gariepinus Burchell, 1822) fed sunflower and sesame meal based diet. J. Fish. Aquat. Sci. 2013, 8, 80–86. [Google Scholar] [CrossRef][Green Version]
- Khan, F.; Khan, M.A. Growth, feed conversion, and nutrient retention efficiency of African Catfish, Clarias gariepinus, (Burchell, 1822) fingerling fed diets with varying levels of protein. J. Appl. Aquac. 2011, 23, 304–316. [Google Scholar]
- Iheanacho, S.C.; Odo, G.E.; Ezewudo, B.I. Adulteration of Aquafeed with melamine and melamine-formaldehyde chemicals. Ex situ study of impact on haematology and antioxidant systems in Clarias gariepinus. Aquac. Res. 2020. [Google Scholar] [CrossRef]
- Çek, S.; Yilmaz, E. The effect of varying dietary energy on gonad development at first sexual maturity of the sharptooth catfish (Clarias gariepinus Burchell, 1822). Aquac. Int. 2009, 17, 553–563. [Google Scholar] [CrossRef]
- Balogun, A.M.; Fashakin, E.A. Replacement of groundnut cake with processed Soybean meals in diets for the African mud catfish, Clarias gariepinus. J. Appl. Trop. Agric. 1996, 1, 5–11. [Google Scholar]
- Iheanacho, S.; Ikwo, T.; Igweze, N.; Chukwuidha, C.; Ogueji, E.; Onyeneke, R. Effect of different dietary inclusion levels of melon seed (Citrullus lanatus) peel on growth, Haematology and Histology of Oroechromis niloticus Juvenile. Turk. J. Fish. Aquat. Sci. 2018, 18, 377–384. [Google Scholar] [CrossRef]
- Milton, J.; Bhat, A.A.; Haniffa, M.A.; Hussain, S.A.; Rather, I.A.; Al-Anazi, K.M.; Waleed Hailan, W.A.Q.; Farah, M.A. Ovarian development and histological observations of threatened dwarf snakehead fish, Channagachua (Hamilton, 1822). Saudi J. Biol. Sci. 2018, 25, 149–153. [Google Scholar] [CrossRef]
- Idowu, E.O. Cyclical changes in the histology of the gonads (ovary and testes) of African pike. Hepsetusodoe. Afr. J. Biotechnol. 2017, 16, 1032–1041. [Google Scholar] [CrossRef][Green Version]
- Mandić, M.; Regner, S. Variation in fish egg size in several pelagic fish species. Study Mar. 2014, 27, 31–46. [Google Scholar]
- Ogunji, J.O.; Kolas, W.; Wirth, M.; Schulz, C.; Rennert, B. Housefly maggot meal (magmeal) as a protein source for Oreochromis niloticus (Linn). Asian Fish. Sci. 2008, 21, 319–331. [Google Scholar]
- Ogunji, J.O.; Nimpptsch, J.; Wiegand, C.; Schulz, C. Evaluation of the influence of housefly maggot meal (magmeal) diets on catalase, glutathione, s-transferase and glycogen concentration of the liver of Oreochromis niloticus fingerling. Comp. Biochem, Physiol. A 2007, 20, 942–947. [Google Scholar] [CrossRef]
- Ajani, E.K.; Nwanna, I.C.; Musa, B.O. Replacement of fishmeal with maggot meal in the diets of Nile Tilapia Oreochronus niloticus. World Aquacult. 2004, 35, 52–54. [Google Scholar]
- Idowu, A.B.; Amusan, A.A.S.; Oyediran, A.G. The response of fingerlings Clarias gariepinus (Burchell, 1822) to the diet containing Housefly maggot (Musca domestica). Niger. J. Anim. Prod. 2003, 30, 139–144. [Google Scholar] [CrossRef]
- Fashina-Bombata, H.A.; Balogu, O. The effect of partial or total replacement of fish meal in the diet of tilapia (Oreochromisniloticus) fry. J. Prospect. Sci. 1997, 1, 178–181. [Google Scholar]
- Kroeckel, S.; Harjes, A.G.E.; Roth, I.; Katz, H.; Wuertz, S.; Susenbeth, A.; Schulz, C. When a turbot catches a fly: Evaluation of a pre-pupae meal of the Black Soldier Fly (Hermetiaillucens) as fish meal substitute—Growth performance and chitin degradation in juvenile turbot (Psetta maxima). Aquaculture 2012, 364–365, 345–352. [Google Scholar] [CrossRef]
- Bromage, N.R. Broodstockmanagement and seed quality. In Broodstock Management and Egg and Leaveae Quality; Bromage, N.R., Roberts, J., Eds.; Cambridge University Press: Cambridge, UK, 1995; pp. 1–24. [Google Scholar]
- Adeyemi, J.A.; Klerrks, P.L. Occurrence of copper acclimation in the least killifish (Heterardria formosa) and associated biochemical and physiological mechanism. Aquat. Toxicol. 2013, 130, 1–57. [Google Scholar] [CrossRef]
- Fasakin, E.A.; Balogun, A.M.; Ajayi, O.O. Evaluation of full-fat and defatted maggot meals in the feeding of Clariid catfish Clarias gariepinus fingerlings. Aquac. Res. 2003, 34, 733–738. [Google Scholar] [CrossRef]
- Ogunji, J.O.; Nimptsch, J.; Wiegand, C.; Schulz, C.; Rennert, B. Effect of housefly maggot meal (magmeal) diets on catalase, and glutathione S-transferase in the liver and gills of carp Cyprinus carpio fingerling. Int. Aquat. Res. 2011, 2, 39–44. [Google Scholar]
- Schulz, C.; Knaus, U.; Wirth, M.; Rennert, B. Effects of varying dietary fatty acid profile on growth performance, fatty acid, body and tissue composition of juvenile pike perch (Sander lucioperca). Aquac. Nutr. 2005, 11, 1–11. [Google Scholar] [CrossRef]
- Iheanacho, S.C.; Odo, G.E. Dietary exposure to polyvinyl chloride microparticles induced oxidative stress and hepatic damage in Clarias gariepinus (Burchell, 1822). Environ. Sci. Pollut. Res. 2020, 27, 21159–21173. [Google Scholar] [CrossRef]
- Copper, J.E.; Budgeon, C.R.; Foutz, C.A.; van Rossum, D.B.; Vansclow, D.J.; Hubley, M.J.; Clark, D.P.; Mandrell, D.T.; Cheng, K.C. Comparative analysis of fixation and embedding techniques for optimized histological preparation of zebrafish. Comp. Biochem. Physiol. 2018, 208, 38–46. [Google Scholar] [CrossRef]
- Baker, F.J.; Silverton, C.J.; Palliste, C. Introduction to Medical Laboratory Technology; Hodder Arnold Publication: Los Angeles, CA, USA, 1989; p. 156. [Google Scholar]
- FAO. North African Catfish-Clarias gariepinus (Burchell, 1822); FAO: Rome, Italy, 2014; Available online: http://www.fao.org/fishery/affris/species-profiles/north-african-catfish/north-african-catfish-home/en/ (accessed on 12 September 2014).
- Ali, M.Z.; Jauncey, K. Effects of feeding regime and dietary protein on growth and body composition in Clarias gariepinus (Burchell, 1822). Indian J. Fish 2004, 51, 407–416. [Google Scholar]
- Ogunji, J.O.; Rahat-Ul-Ain, S.T.; Schulz, C.; Kloas, W. Growth performance, nutrient utilization of Nile tilapia, Oreochromis niloticus fed housefly maggot meal (magmeal) diets. Turk. J. Fish. Aquat. Sci. 2008, 8, 141–147. [Google Scholar]
- Ekanem, A.P.; Eteng, S.U.; Nwosu, F.M.; Eyo, V.O. Comparative study of the growth and gonad development of Clarias gariepinus (Burchell, 1822) fed diets with plant and animal-based ingredients in concrete tanks. J. Agric. Sci. Technol. 2012, 2, 1203–1210. [Google Scholar]
- Alegbeleye, W.O.; Adetayo, J.A.; Ogunmoroti, T.A. Simple methods for off-season breeding. Aqabyte 1991, 4, 3–4. [Google Scholar]
- Ogunji, J.O.; Kloas, W.; Wirth, M.; Neumann, N.; Pietsch, C. Effect of housefly maggot meal (magmeal) diets on the concentration plasma gluocose, cortisol and blood characteristics of Oreochromis niloticus fingerlings. J. Anim. Physiol. Anim. Nutr. 2008, 92, 511–518. [Google Scholar] [CrossRef] [PubMed]
- Renna, M.A.; Schiavone, A.; Gai, F.; Dabbou, F.; Lussiana, C.; Malfatto, V.; Prearo, M.T.; Capucchio, M.T.; Biasato, I.; Biasibetti, E.; et al. Evaluation of the suitability of a partially defatted black soldier fly (Hermetiaillucens L.) larvae meal as ingredient for rainbow trout (IWalbaum) diets. J. Anim. Sci. Biotechnol. 2017, 8, 57. [Google Scholar] [CrossRef] [PubMed]
- Hashemi, S.; Kashi, M.; Safikhani, H. Growth parameter, Length-Weight relationship and quality coefficient of klunzingeri Mullet (Liza klunzingeri (Day, 1888)) in the Coastal of Khuzestan (Northwest of Persian Gulf). J. Nov. Appl. Sci. 2013, 2, 60–64. [Google Scholar]
- Al-Deghayem, W.A.; Al-Balawi, H.F.; Kandeal, S.A.; Suliman, E.A. Gonadosomatic index and some hematological parameters in African catfish Clarias gariepinus (Burchell, 1822) as affected by feed type and temperature level. Braz. Arch. Biol. Technol. 2017, 60, 1–10. [Google Scholar] [CrossRef]
- Cek, S.; Bromage, N.R.; Randall, C.; Rana, K. Ogenesis hepatosomatic indexes, and sex ration in Rosy in rosy barb (Pontius conchonius). Turk. J. Fish Aquat. Sci. 2001, 1, 133–141. [Google Scholar]
- Anibueze, C.I.P.; Inyang, N.M. Oocytes Structure, fecundity and sex Ratio of Heterobranchuslongifilis (Valenciennes 1840) in Idodo River Basin (Nigeria) with Comments on the Breding Biology. J. Aquat. Sci. 2003, 15, 59–62. [Google Scholar]
- Arockiaraji, A.J.; Haniffa, M.A.; Seetharaman, S.; Singh, S. Cyclic Changes in gonadal maturation and histological observations of threatened freshwater catfish. “Narikeliru” MystusMonthanus (Jerdon, 1849). Acta. Ichthyol. Piscat. 2004, 34, 253–266. [Google Scholar] [CrossRef]
- Olaleye, V.F. A review of reproduction and gamete management in the African catfish Clarias gariepinus. Life J. Sci. 2005, 7, 63–70. [Google Scholar] [CrossRef]
- Jalabert, B. Particularities of reproduction and oogenesis in teleost fish compared to mammals. Reprod. Nutr. Dev. 2005, 45, 261–279. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S. The development of carp gonads in warm water aquaria. J. Fish Biol. 1975, 7, 775–782. [Google Scholar] [CrossRef]
- Bromage, N.R.; Cumaranatunga, P.R.T. Oocytes development in the rainbow trout with special reference to vitellogenesis and artresia. In Proceedings of the 3rd International Symposium on the Reproductive Physiology of Fish, St. John’s, NL, Canada, 2–7 August 1987; Idler, D.R., Crin, L.W., Walsh, J.M., Eds.; Marine Sciences Research Laboratory, Memorial University of Newfoundland: St John’s, NL, Canada, 1987; p. 194. [Google Scholar]
- Çek, S.; Yilmaz, E. Gonadal development and sex ratio of shaptooth catfish (Clarias gariepinus Burchel, 1822) cultured under laboratory conditions. Turk. J. Zool. 2007, 31, 35–46. [Google Scholar]
- Ogunji, J.O.; Rahe, R.E. Larval development of the African catfish Heterobranchus longifilis val., 1840 (Teleostei; Clariidae) and its larval behaviour. J. Aquac. Trop. 1999, 14, 11–25. [Google Scholar]
- Guraya, S.S. The biology of gonadal development, sex differentiation and maturation and sex reversal in fish: Cellular, molecular and endocrinological aspects. Proc. Indian Nat. Sci. Acad. 2000, 66, 167–194. [Google Scholar]
- Schulz, R.W.; Vander Corput, L.; Janssen-Dommerholt, J.; Goos, H.J. Sexual steroids during puberty in male African Catfish (Clarias gariepinus): Serum levels and gonadototropin stimulated tesicular secretion in vitro. J. Comp. Physiol. Biol. 1994, 164, 195–205. [Google Scholar] [CrossRef]
- Kurbanov, A.; Kamilov, B. Maturation of African catfish, Clarias gariepinus, in condition of seasonal climate of Uzbekistan. Int. J. Fish. Aquat. Stud. 2017, 5, 236–239. [Google Scholar]
| Proximate Components | Maggot Meal | Fish Meal | Maize |
|---|---|---|---|
| Crude protein (%) | 44.87 ± 0.01 | 61.55 ± 0.03 | 10.91 ± 0.03 |
| Crude fat (%) | 7.38 ± 0.01 | 6.76 ± 0.01 | 3.93 ± 0.01 |
| Crude fiber (%) | 6.88 ± 0.02 | 0.56 ± 0.01 | 1.95 ± 0.01 |
| Crude ash (%) | 7.95 ± 0.02 | 15.84 ± 0.01 | 1.08 ± 0.01 |
| Moisture content (%) | 7.35 ± 0.01 | 5.18 ± 0.01 | 8.28 ± 0.02 |
| NFE (%) | 25.57 ± 0.01 | 10.10 ± 0.02 | 84.03 ± 0.02 |
| Feed Ingredients (g) | Diets (Inclusion Levels) | |||
|---|---|---|---|---|
| D1 Control | D2 | D3 | D4 | |
| Fish | 520 | 380 | 360 | 280 |
| Maggot meal | 00 | 250 | 450 | 650 |
| Maize | 430 | 320 | 140 | 20 |
| Fish oil | 30 | 30 | 30 | 30 |
| Vitamin/mineral premix | 20 | 20 | 20 | 20 |
| Proximate Analysis 1 | ||||
| Crude protein | 40.99 ± 0.04 | 40.83 ± 0.02 | 40.78 ± 0.05 | 40.46 ± 0.08 |
| Fat | 4.42 ± 0.01 | 4.36 ± 0.01 | 3.96 ± 0.01 | 3.85 ± 0.01 |
| Fiber | 3.56 ± 0. 01 | 3.68 ± 0. 01 | 4.74 ± 0.01 | 4.87 ± 0.01 |
| Ash | 12.67 ± 0.01 | 13.03 ± 0.01 | 12.94 ± 0.01 | 12.86 ± 0.01 |
| NFE 2 | 38.36 ± 0.04 | 38.10 ± 0.03 | 37.59 ± 0.05 | 37.96 ± 0.08 |
| Dry Matter | 91.57 ± 0.01 | 91.63 ± 0.01 | 90.86 ± 0.01 | 90.78 ± 0.01 |
| Energy (kJ g−1) 3 | 18.31 ± 0.00 | 18.20 ± 0.00 | 17.94 ± 0.00 | 17.88 ± 0.00 |
| Diets | D1 (g) | D2 (g) | D3 (g) | D4 (g) |
|---|---|---|---|---|
| Initial weight | 1.66 ± 0.03 | 1.69 ± 0.00 | 1.62 ± 0.07 | 1.61 ± 0.00 |
| Final weight(g) * | 71.23 ± 4.42 | 90.65 ± 14.81 | 87.38 ± 1.05 | 90.94 ± 13.58 |
| Mean weight gain (g) 1 | 69.58 ± 4.44 | 88.97 ± 14.6 | 85.76 ± 1.11 | 89.33 ± 13.58 |
| SGR %2 | 2.09 ± 0.05 | 2.20 ± 0.09 | 2.22 ± 0.03 | 2.23 ± 0.09 |
| Male | Female | |||||
|---|---|---|---|---|---|---|
| Diets | Week 16 | Week 20 | Week 24 | Week 16 | Week 20 | Week 24 |
| D1 | 0.86 ± 0.06 | 1.25 ± 0.01 | 1.84 ± 0.54 | 3.51 ± 1.38 | 8.01 ± 1.35 | 15.25 ± 2.26 |
| D2 | 0.28 ± 0.02 | 0.92 ± 0.26 | 1.32 ± 0.18 | 3.47 ± 0.90 | 11.50 ± 1.84 | 18.05 ± 2.40 |
| D3 | 0.31 ± 0.10 | 0.93 ± 0.21 | 1.68 ± 0.44 | 2.26 ± 0.35 | 7.84 ± 2.01 | 18.48 ± 1.57 |
| D4 | 0.42 ± 0.10 | 0.60 ± 0.20 | 1.38 ± 0.02 | 3.11 ± 0.10 | 8.08 ± 1.64 | 18.10 ± 0.70 |
| Diet | Time (Weeks) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 2 | 4 | 6 | 8 | 10 | 12 | 16 | 20 | 24 | |
| D1 | R | R | R | R | R | R | A | B | D |
| D2 | R | R | R | R | R | R | B | C | E |
| D3 | R | R | R | R | R | R | B | D | F |
| D4 | R | R | R | R | R | R | C | D | F |
| Diet | Time (Weeks) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 2 | 4 | 6 | 8 | 10 | 12 | 16 | 20 | 24 | |
| D1 | R | R | R | R | R | R | A | B | C |
| D2 | R | R | R | R | R | R | A | B | C |
| D3 | R | R | R | R | R | R | A | C | D |
| D4 | R | R | R | R | R | R | B | D | E |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ogunji, J.O.; Iheanacho, S.C.; Mgbabu, C.N.; Amaechi, N.C.; Evulobi, O.O.C. Housefly Maggot Meal as a Potent Bioresource for Fish Feed to Facilitate Early Gonadal Development in Clarias gariepinus (Burchell,1822). Sustainability 2021, 13, 921. https://doi.org/10.3390/su13020921
Ogunji JO, Iheanacho SC, Mgbabu CN, Amaechi NC, Evulobi OOC. Housefly Maggot Meal as a Potent Bioresource for Fish Feed to Facilitate Early Gonadal Development in Clarias gariepinus (Burchell,1822). Sustainability. 2021; 13(2):921. https://doi.org/10.3390/su13020921
Chicago/Turabian StyleOgunji, Johnny O., Stanley C. Iheanacho, Christopher Nwokwa Mgbabu, Nuria C. Amaechi, and Onyedikachi O. C. Evulobi. 2021. "Housefly Maggot Meal as a Potent Bioresource for Fish Feed to Facilitate Early Gonadal Development in Clarias gariepinus (Burchell,1822)" Sustainability 13, no. 2: 921. https://doi.org/10.3390/su13020921
APA StyleOgunji, J. O., Iheanacho, S. C., Mgbabu, C. N., Amaechi, N. C., & Evulobi, O. O. C. (2021). Housefly Maggot Meal as a Potent Bioresource for Fish Feed to Facilitate Early Gonadal Development in Clarias gariepinus (Burchell,1822). Sustainability, 13(2), 921. https://doi.org/10.3390/su13020921





