Potential Development of Sustainable 3D-Printed Meat Analogues: A Review
Abstract
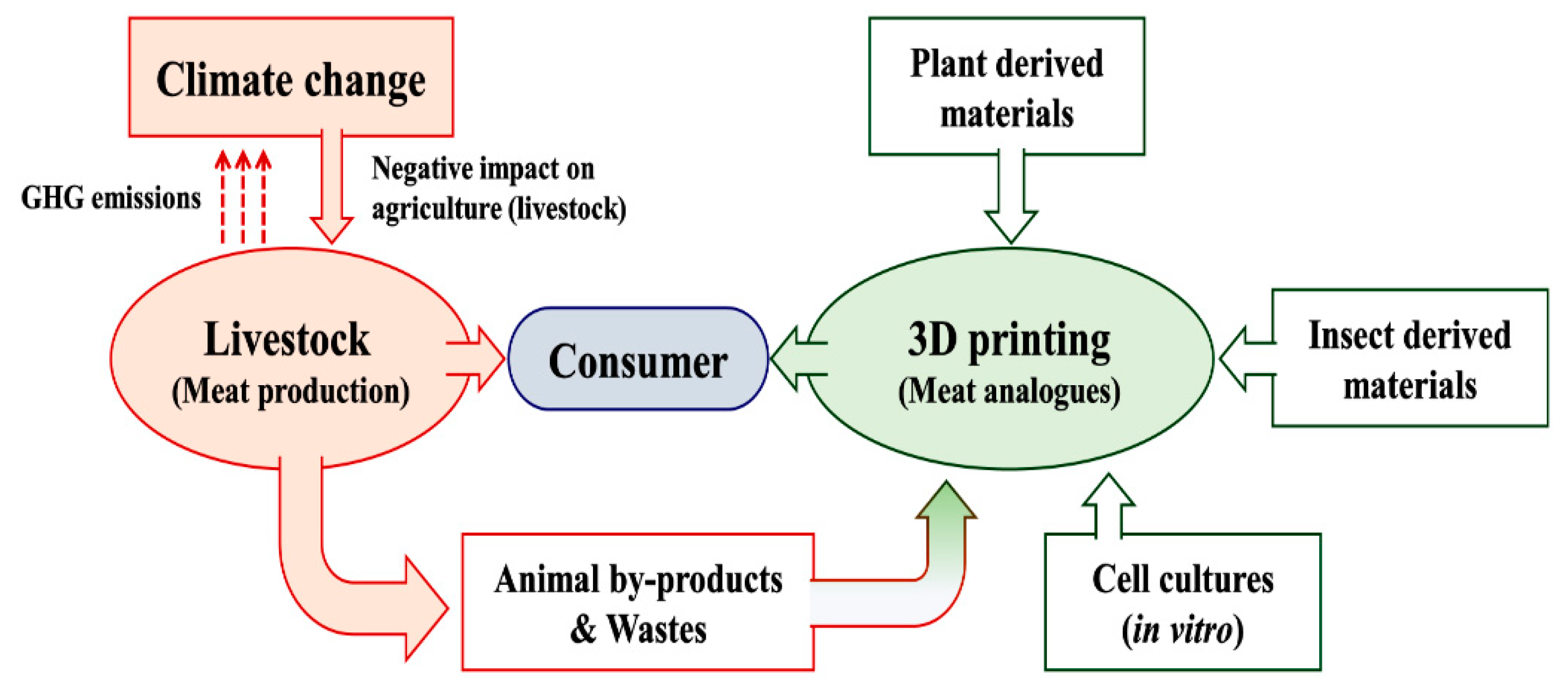
1. Introduction
2. Overview of 3D Printing Technology
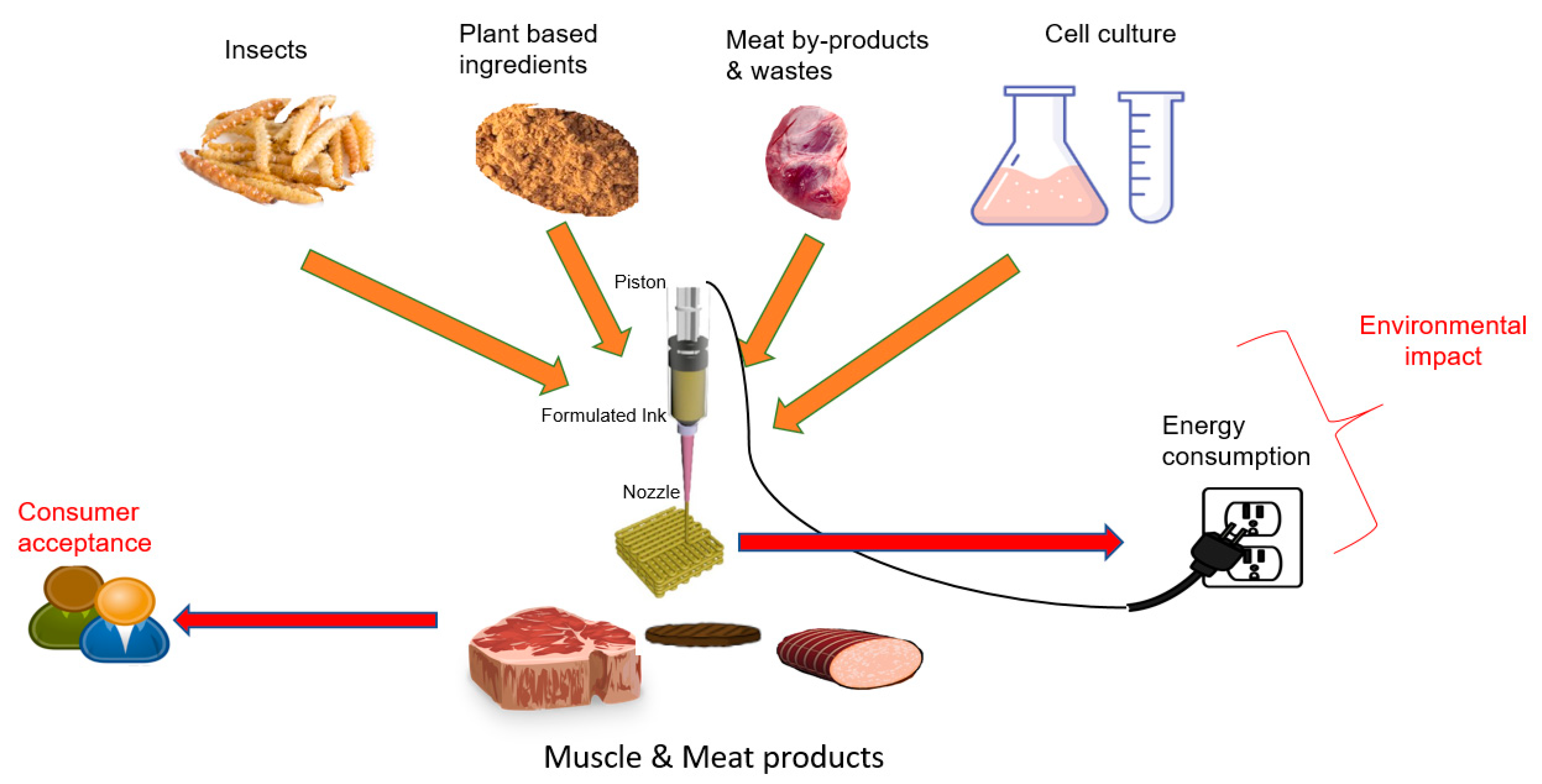
3. Technological Feasibility of Meat Analog Fabrication
4. Materials-Based 3DP Approaches
4.1. Biomaterials-Based 3DP: Bioprinting
4.2. Native Printable Materials
4.3. Non-Native Printable Materials
4.3.1. Meat-Byproducts-Based 3D Printing
4.3.2. Plant-Materials-Based 3D Printing
4.4. Alternative Materials: Insect-Derived 3D Structures
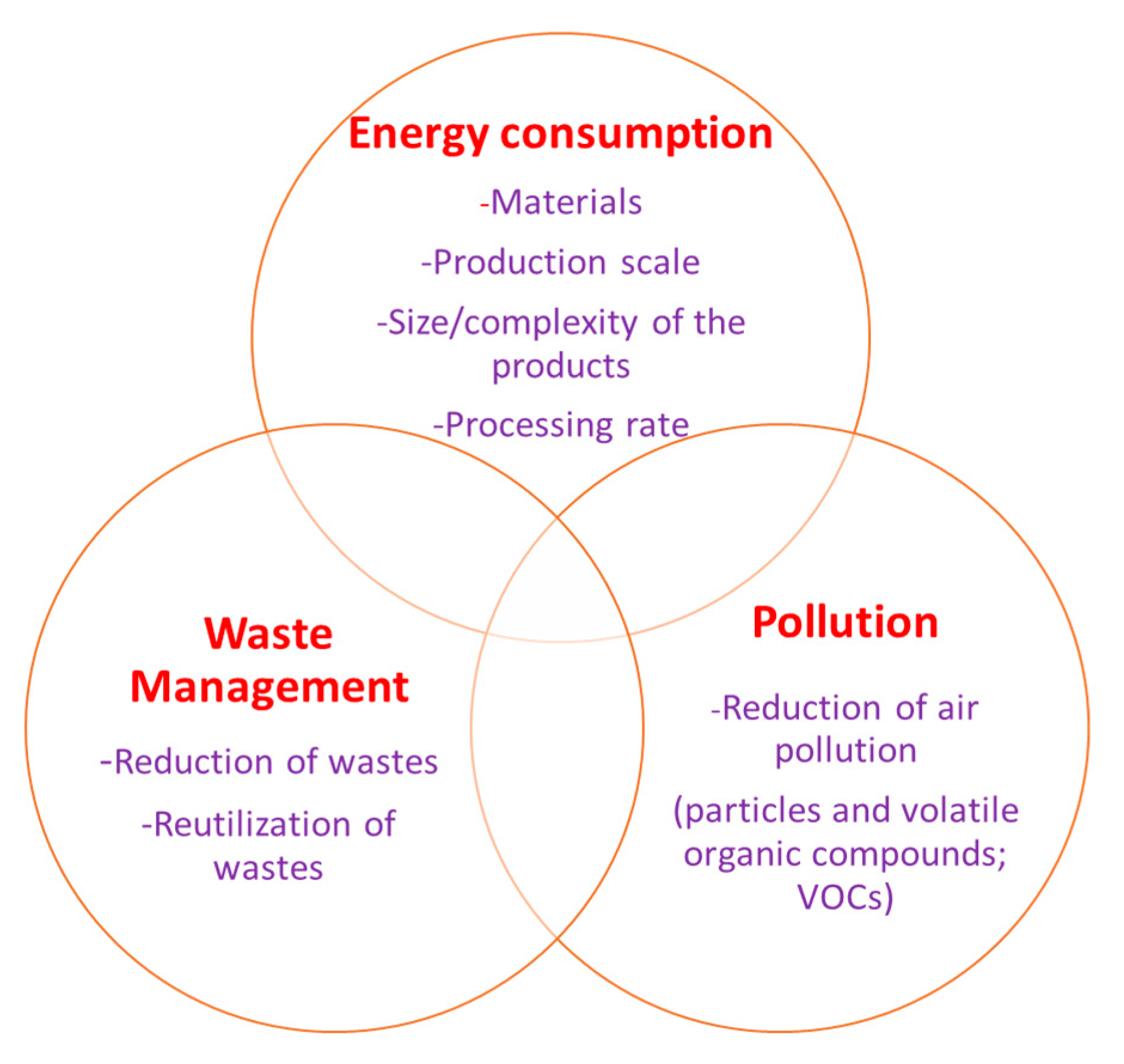
5. Environmental Sustainability
5.1. Energy Consumption
5.2. Air Pollution
5.3. Sustainable Supply Chain Management (SSCM)
6. Consumer Acceptance
7. Future Prospects
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- McMichael, A.J.; Powles, J.W.; Butler, C.D.; Uauy, R. Food, livestock production, energy, climate change, and health. Lancet 2007, 370, 1253–1263. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations (FAO). Climate Change and Food Security: A Framework Document; FAO: Rome, Italy, 2008; Available online: http://www.fao.org/3/k2595e/k2595e00.pdf (accessed on 11 July 2020).
- Intergovernmental Panel on Climate Change (IPCC). Climate Change and Land: Special Report on Climate Change, Desertification, Land Degradation, Sustainable Land Management, Food Security, and Greenhouse Gas Fluxes in Terrestrial Ecosystems. 2019. Available online: https://www.ipcc.ch/site/assets/uploads/2019/08/4.-SPM_Approved_Microsite_FINAL.pdf (accessed on 12 July 2020).
- Gerber, P.J.H.; Steinfeld, B.; Henderson, A.; Mottet, C.; Opio, J.; Dijkman, A.; Falcucci, A.; Tempio, G. Tackling Climate Change through Livestock—A Global Assessment of Emissions and Mitigation Opportunities; Food and Agriculture Organization of the United Nations (FAO): Rome, Italy, 2013. [Google Scholar]
- Eshel, G.; Shepon, A.; Makov, T.; Milo, R. Land, irrigation water, greenhouse gas, and reactive nitrogen burdens of meat, eggs, and dairy production in the United States. Proc. Natl. Acad. Sci. USA 2014, 111, 11996–12001. [Google Scholar] [CrossRef] [PubMed]
- Flachowsky, G.; Kamphues, J. Carbon footprints for food of animal origin: What are the most preferable criteria to measure animal yields? Animals 2012, 2, 108–126. [Google Scholar] [CrossRef] [PubMed]
- Jackson, B.; Lee-Woolf, C.; Higginson, F.; Wallace, J.; Agathou, N. Strategies for Reducing the Climate Impacts of Red Meat/Dairy Consumption in the UK; WWF and Imperial College: London, UK, 2009; pp. 1–84. [Google Scholar]
- de Bakker, E.; Dagevos, H. Reducing meat consumption in today’s consumer society: Questioning the citizen-consumer gap. J. Agric. Environ. Ethics 2012, 25, 877–894. [Google Scholar] [CrossRef]
- Springmann, M.; Clark, M.; Mason-D’Croz, D.; Wiebe, K.; Bodirsky, B.L.; Lassaletta, L.; Willett, W. Options for keeping the food system within environmental limits. Nature 2018, 562, 519–525. [Google Scholar] [CrossRef] [PubMed]
- Smetana, S.; Mathys, A.; Knoch, A.; Heinz, V. Meat alternatives: Life cycle assessment of most known meat substitutes. Int. J. Life Cycle Assess. 2015, 20, 1254–1267. [Google Scholar] [CrossRef]
- Lynch, J.; Pierrehumbert, R. Climate impacts of cultured meat and beef cattle. Front. Sustain. Food Syst. 2019, 3, 5. [Google Scholar] [CrossRef] [PubMed]
- Allied, M.R. Meat Substitute Market by Product Type, Source and Category: Global Opportunity Analysis and Industry Forecast, 2018–2025. 2018. Available online: https://www.alliedmarketresearch.com/press-release/global-meat-substitute-market.html (accessed on 13 July 2020).
- Stephens, N.; Di Silvio, L.; Dunsford, I.; Ellis, M.; Glencross, A.; Sexton, A. Bringing cultured meat to market: Technical, socio-political, and regulatory challenges in cellular agriculture. Trends Food Sci. Technol. 2018, 78, 155–166. [Google Scholar] [CrossRef] [PubMed]
- Dick, A.; Bhandari, B.; Prakash, S. 3D printing of meat. Meat Sci. 2019, 153, 35–44. [Google Scholar] [CrossRef]
- Godoi, F.C.; Prakash, S.; Bhandari, B.R. 3D printing technologies applied for food design: Status and prospects. J. Food Eng. 2016, 179, 44–54. [Google Scholar] [CrossRef]
- Sun, J.; Zhou, W.; Huang, D.; Fuh, J.Y.H.; Hong, G.S. An overview of 3D printing technologies for food fabrication. Food Bioprocess Technol. 2015, 8, 1605–1615. [Google Scholar] [CrossRef]
- Severini, C.; Derossi, A.; Ricci, I.; Caporizzi, R.; Fiore, A. Printing a blend of fruit and vegetables. New advances on critical variables and shelf life of 3D edible objects. J. Food Eng. 2018, 220, 89–100. [Google Scholar] [CrossRef]
- Keerthana, K.; Anukiruthika, T.; Moses, J.A.; Anandharamakrishnan, C. Development of fiber-enriched 3D printed snacks from alternative foods: A study on button mushroom. J. Food Eng. 2020, 287, 110116. [Google Scholar] [CrossRef]
- Gebler, M.; Schoot Uiterkamp, A.J.M.; Visser, C. A global sustainability perspective on 3D printing technologies. Energy Policy 2014, 74, 158–167. [Google Scholar] [CrossRef]
- Munaz, A.; Vadivelu, R.K.; St. John, J.; Barton, M.; Kamble, H.; Nguyen, N.-T. Three-dimensional printing of biological matters. J. Sci. Adv. Mater. Devices 2016, 1, 1–17. [Google Scholar] [CrossRef]
- Noorani, R. 3D Printing: Technology, Applications, and Selection; CRC Press: Milton, UK, 2017. [Google Scholar]
- Garrett, B. 3D Printing: New Economic Paradigms and Strategic Shifts. Glob. Policy 2014, 5, 70–75. [Google Scholar] [CrossRef]
- Min, S.; Ko, I.K.; Yoo, J.J. State-of-the-Art Strategies for the Vascularization of Three-Dimensional Engineered Organs. Vasc. Spec. Int. 2019, 35, 77–89. [Google Scholar] [CrossRef]
- Liu, C.; Ho, C.; Wang, J. The development of 3D food printer for printing fibrous meat materials. In Proceedings of the IOP Conference Series: Materials Science and Engineering, 2nd International Conference on Innovative Engineering Materials (ICIEM 2017), Philadelphia, PA, USA, 21–23 October 2017. [Google Scholar]
- Dekkers, B.L.; Boom, R.M.; van der Goot, A.J. Structuring processes for meat analogues. Trends Food Sci. Technol. 2018, 81, 25–36. [Google Scholar] [CrossRef]
- Lipton, J.; Arnold, D.; Nigl, F.; Lopez, N.; Cohen, D.; Norén, N.; Lipson, H. Multi-material food printing with complex internal structure suitable for conventional post-processing. In Proceedings of the Solid Freeform Fabrication Symposium, Austin, TX, USA, 17–19 August 2010; pp. 809–815. [Google Scholar]
- Chen, T.; Lin, Y.-C. Feasibility Evaluation and Optimization of a Smart Manufacturing System Based on 3D Printing: A Review. Int. J. Intell. Syst. 2017, 32, 394–413. [Google Scholar] [CrossRef]
- Azzollini, D.; Fogliano, F. Potential and Challenges of Edible Insects in 3D Food Printing. Available online: https://3dfoodprintingconference.com/wp-content/uploads/2017/07/Domenico-Azzollini-wur.pdf (accessed on 17 July 2020).
- Caporizzi, R.; Derossi, A.; Severini, C. Cereal-Based and Insect-Enriched Printable Food. In Fundamentals of 3D Food Printing and Applications; Academic Press: Cambridge, MA, USA, 2019; pp. 93–116. [Google Scholar]
- Zhang, B.; Gao, L.; Ma, L.; Luo, Y.; Yang, H.; Cui, Z. 3D Bioprinting: A Novel Avenue for Manufacturing Tissues and Organs. Engineering 2019, 5, 777–794. [Google Scholar] [CrossRef]
- Hsu, L.; Jiang, X. ‘Living’ Inks for 3D Bioprinting. Trends Biotechnol. 2019, 37, 795–796. [Google Scholar] [CrossRef] [PubMed]
- Food and Agriculture Organization of the United Nations (FAO). Meat Processing Products; FAO: Rome, Italy, 2014; Available online: http://www.fao.org/ag/againfo/themes/en/meat/Processing_product.html (accessed on 18 July 2020).
- Shahrubudin, N.; Koshy, P.; Alipal, J.; Kadir, M.; Lee, T.C. Challenges of 3D printing technology for manufacturing biomedical products: A case study of Malaysian manufacturing firms. Heliyon 2020, 6, e03734. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, P.; An, J.; Chua, C.K.; Tan, L.P. Bioprinting of 3D in vitro skeletal muscle models: A review. Mater. Des. 2020, 193, 108794. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, I.; Seol, Y.; Ko, I.K.; Yoo, J.J.; Atala, A.; Lee, S.J. Neural cell integration into 3D bioprinted skeletal muscle constructs accelerates restoration of muscle function. Nat. Commun. 2020, 11, 1025. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.M.; Yeong, W.Y. Design and Printing Strategies in 3D Bioprinting of Cell-Hydrogels: A Review. Adv. Healthc. Mater. 2016, 5, 2856–2865. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Huang, Y.; Du, M. Farm animals for studying muscle development and metabolism: Dual purposes for animal production and human health. Anim. Front. 2019, 9, 21–27. [Google Scholar] [CrossRef]
- Kim, J.H.; Seol, Y.; Ko, I.K.; Kang, H.W.; Lee, Y.K.; Yoo, J.J.; Atala, A.; Lee, S.J. 3D Bioprinted Human Skeletal Muscle Constructs for Muscle Function Restoration. Sci. Rep. 2018, 8, 12307. [Google Scholar] [CrossRef]
- Kang, H.W.; Lee, S.J.; Ko, I.K.; Kengla, C.; Yoo, J.J.; Atala, A. A 3D bioprinting system to produce human-scale tissue constructs with structural integrity. Nat. Biotechnol. 2016, 34, 312–319. [Google Scholar] [CrossRef]
- Post, M.J. 11-Proteins in cultured beef. In Proteins in Food Processing, 2nd ed.; Yada, R.Y., Ed.; Woodhead Publishing: Sawston, UK, 2018; pp. 289–298. [Google Scholar]
- Choi, Y.-J.; Jun, Y.-J.; Kim, D.Y.; Yi, H.-G.; Chae, S.-H.; Kang, J.; Cho, D.-W. A 3D cell printed muscle construct with tissue-derived bioink for the treatment of volumetric muscle loss. Biomaterials 2019, 206, 160–169. [Google Scholar] [CrossRef]
- García-Lizarribar, A.; Fernández-Garibay, X.; Velasco-Mallorquí, F.; Castaño, A.G.; Samitier, J.; Ramon-Azcon, J. Composite Biomaterials as Long-Lasting Scaffolds for 3D Bioprinting of Highly Aligned Muscle Tissue. Macromol. Biosci. 2018, 18, 1800167. [Google Scholar] [CrossRef]
- MacQueen, L.A.; Alver, C.G.; Chantre, C.O.; Ahn, S.; Cera, L.; Gonzalez, G.M.; O’Connor, B.B.; Drennan, D.J.; Peters, M.M.; Motta, S.E.; et al. Muscle tissue engineering in fibrous gelatin: Implications for meat analogs. NPJ Sci. Food 2019, 3, 20. [Google Scholar] [CrossRef] [PubMed]
- Duan, B.; Hockaday, L.A.; Kang, K.H.; Butcher, J.T. 3D Bio-printing of heterogeneous aortic valve conduits with alginate/gelatin hydrogels. J. Biomed. Mater. Res. Part A 2013, 101, 1255–1264. [Google Scholar] [CrossRef] [PubMed]
- Capel, A.J.; Rimington, R.P.; Fleming, J.W.; Player, D.J.; Baker, L.A.; Turner, M.C.; Lewis, M.P. Scalable 3D Printed Molds for Human Tissue Engineered Skeletal Muscle. Front. Bioeng. Biotechnol. 2019, 7, 20. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; McCarthy, A.; Zhang, Y.S.; Xie, J. Decorating 3D Printed Scaffolds with Electrospun Nanofiber Segments for Tissue Engineering. Adv. Biosyst. 2019, 3, 1900137. [Google Scholar] [CrossRef]
- VanDusen, K.W.; Syverud, B.C.; Williams, M.L.; Lee, J.D.; Larkin, L.M. Engineered skeletal muscle units for repair of volumetric muscle loss in the tibialis anterior muscle of a rat. Tissue Eng Part A 2014, 20, 2920–2930. [Google Scholar] [CrossRef]
- Modern Meadow. 2020. Available online: http://www.modernmeadow.com/about-us/ (accessed on 15 June 2020).
- Kouzani, A.Z.; Adams, S.; Whyte, D.J.; Oliver, R.; Hemsley, B.; Palmer, S.; Balandin, S. 3D printing of food for people with swallowing difficulties. KnE Eng. 2017, 2, 23–29. [Google Scholar] [CrossRef]
- Lupton, D.; Turner, B. Food of the future? Consumer responses to the idea of 3D-printed meat and insect-based foods. Food Foodways 2018, 26, 269–289. [Google Scholar] [CrossRef]
- Portanguen, S.; Tournayre, P.; Sicard, J.; Astruc, T.; Mirade, P.-S. Toward the design of functional foods and biobased products by 3D printing: A review. Trends Food Sci. Technol. 2019, 86, 188–198. [Google Scholar] [CrossRef]
- United Nations (UN). Unece Standard Edible Meat Co-Products; UN: Geneva, Switzerland, 2015. [Google Scholar]
- Lynch, S.A.; Mullen, A.M.; O’Neill, E.; Drummond, L.; Álvarez, C. Opportunities and perspectives for utilisation of co-products in the meat industry. Meat Sci. 2018, 144, 62–73. [Google Scholar] [CrossRef]
- Jayathilakan, K.; Sultana, K.; Radhakrishna, K.; Bawa, A.S. Utilization of byproducts and waste materials from meat, poultry and fish processing industries: A review. J. Food Sci. Technol. 2012, 49, 278–293. [Google Scholar] [CrossRef]
- Hsieh, Y.H.; Ofori, J.A. Blood-derived products for human consumption. Revel. Sci. 2011, 1, 14–21. [Google Scholar]
- Kubberød, E.; Ueland, Ø.; Tronstad, A.; Risvik, E. Attitudes towards meat and meat-eating among adolescents in Norway: A qualitative study. Appetite 2002, 38, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Stangierski, J.; Baranowska, H.M. The Influence of Heating and Cooling Process on the Water Binding in Transglutaminase-Modified Chicken Protein Preparation, Assessed Using Low-Field NMR. Food Bioprocess Technol. 2015, 8, 2359–2367. [Google Scholar] [CrossRef]
- Rivera, J.A.; Sebranek, J.G.; Rust, R.E.; Tabatabai, L.B. Composition and protein fractions of different meat by-products used for petfood compared with mechanically separated chicken (MSC). Meat Sci. 2000, 55, 53–59. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, M.; Bhandari, B.; Yang, C. Investigation on fish surimi gel as promising food material for 3D printing. J. Food Eng. 2018, 220, 101–108. [Google Scholar] [CrossRef]
- Ramachandraiah, K.; Choi, M.-J.; Hong, G.-P. Micro- and nano-scaled materials for strategy-based applications in innovative livestock products: A review. Trends Food Sci. Technol. 2018, 71, 25–35. [Google Scholar] [CrossRef]
- Darine, S.; Christophe, V.; Gholamreza, D. Emulsification properties of proteins extracted from beef lungs in the presence of xanthan gum using a continuous rotor/stator system. LWT Food Sci. Technol. 2011, 44, 1179–1188. [Google Scholar] [CrossRef]
- Alvarez, C.; Drummond, L.; Mullen, A.M. Protein recovered from meat co-products and processing streams as pork meat replacers in Irish breakfast sausages formulations. LWT Food Sci. Technol. 2018, 96, 679–685. [Google Scholar] [CrossRef]
- Toldrá, F.; Reig, M.; Aristoy, M.C.; Mora, L. Generation of bioactive peptides during food processing. Food Chem. 2018, 267, 395–404. [Google Scholar] [CrossRef]
- Alao, B.O.; Falowo, A.B.; Chulayo, A.; Muchenje, V. The Potential of Animal By-Products in Food Systems: Production, Prospects and Challenges. Sustainability 2017, 9, 1089. [Google Scholar] [CrossRef]
- Nawaz, A.; Li, E.; Irshad, S.; Xiong, Z.; Xiong, H.; Shahbaz, H.M.; Siddique, F. Valorization of fisheries by-products: Challenges and technical concerns to food industry. Trends Food Sci. Technol. 2020, 99, 34–43. [Google Scholar] [CrossRef]
- Govindharaj, M.; Roopavath, U.K.; Rath, S.N. Valorization of discarded Marine Eel fish skin for collagen extraction as a 3D printable blue biomaterial for tissue engineering. J. Clean. Prod. 2019, 230, 412–419. [Google Scholar] [CrossRef]
- Yang, X.; Lu, Z.; Wu, H.; Li, W.; Zheng, L.; Zhao, J. Collagen-alginate as bioink for three-dimensional (3D) cell printing based cartilage tissue engineering. Mater. Sci. Eng. 2018, 83, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.; Ahn, S.; Yoon, H.; Kim, Y.; Chun, W. A cryogenic direct-plotting system for fabrication of 3D collagen scaffolds for tissue engineering. J. Mater. Chem. 2009, 19, 8817–8823. [Google Scholar] [CrossRef]
- Asgar, M.A.; Fazilah, A.; Huda, N.; Bhat, R.; Karim, A.A. Nonmeat protein alternatives as meat extenders and meat analogs. Compr. Rev. Food Sci. Food Saf. 2010, 9, 513–529. [Google Scholar] [CrossRef]
- Vinnari, M. The future of meat consumption—Expert views from Finland. Technol. Forecast. Soc. Chang. 2008, 75, 893–904. [Google Scholar] [CrossRef]
- Bohrer, B.M. An investigation of the formulation and nutritional composition of modern meat analogue products. Food Sci. Hum. Wellness 2019, 8, 320–329. [Google Scholar] [CrossRef]
- Kyriakopoulou, K.; Dekkers, B.; van der Goot, A.J. Chapter 6—Plant-based meat analogues. In Sustainable Meat Production and Processing; Galanakis, C.M., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 103–126. [Google Scholar]
- Lille, M.; Nurmela, A.; Nordlund, E.; Metsä-Kortelainen, S.; Sozer, N. Applicability of protein and fiber-rich food materials in extrusion-based 3D printing. J. Food Eng. 2018, 220, 20–27. [Google Scholar] [CrossRef]
- Bloomberg. 2019. Available online: https://www.bloomberg.com/news/articles/2019-11-22/fake-meat-companies-are-racing-to-3d-print-steaks (accessed on 10 June 2020).
- NOVAMEAT. Plant based meat. 2020. Available online: https://www.novameat.com/ (accessed on 11 June 2020).
- Ismail, I.; Hwang, Y.H.; Joo, S.T. Meat analog as future food: A review. J. Anim. Sci. Technol. 2020, 62, 111–120. [Google Scholar] [CrossRef]
- Meat, Livestock Australia. 3D Printed Food Conference- MLA Introducing Potential High Valued Red Meat Opportunities/Trends. 2017. Available online: https://www.mla.com.au/research-and-development/search-rd-reports/final-report-details/3D-printed-Food-conference-MLA-introducing-potential-high-valued-red-meat-opportunities-trends/3622# (accessed on 17 July 2020).
- Sun-Waterhouse, D.; Waterhouse, G.I.N.; You, L.; Zhang, J.; Liu, Y.; Ma, L.; Dong, Y. Transforming insect biomass into consumer wellness foods: A review. Food Res. Int. 2016, 89, 129–151. [Google Scholar] [CrossRef]
- Alexander, P.; Brown, C.; Arneth, A.; Dias, C.; Finnigan, J.; Moran, D.; Rounsevell, M.D.A. Could consumption of insects, cultured meat or imitation meat reduce global agricultural land use? Glob. Food Secur. 2017, 15, 22–32. [Google Scholar] [CrossRef]
- Jongema, Y. List of Edible Insects of the World. 2017. Available online: https://www.wur.nl/en/Research-Results/Chair-groups/Plant-Sciences/Laboratory-of-Entomology/Edible-insects/Worldwide-species-list.htm. (accessed on 30 June 2020).
- Melgar-Lalanne, G.; Hernández-Álvarez, A.J.; Salinas-Castro, A. Edible insects processing. Traditional and innovative technologies. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1166–1191. [Google Scholar] [CrossRef] [PubMed]
- Azzollini, D.; Wibisaphira, T.; Lakemond, C.M.M.; Fogliano, V. Toward the design of insect-based meat analogue: The role of calcium and temperature in coagulation behavior of Alphitobius diaperinus proteins. LWT 2019, 100, 75–82. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations (FAO). Composition Database for Biodiversity Version 2; BioFoodComp2. (Latest Update: 06 August 2014); FAO: Rome, Italy, 2012; Available online: http://www.fao.org/docrep/017/ap814e/ap814e.pdf (accessed on 15 September 2020).
- Bukkens, S.G.F. Insects in Human Diet: Nutritional Aspects. In Ecological Implications of Minilivestock, Role of Rodents, Frogs, Snails, and Insects for Sustainable Development; Paoletti, M.G., Ed.; Science Publishers: Enfield, NH, USA, 2005; pp. 545–577. [Google Scholar]
- Womeni, H.M.; Linder, M.; Tiencheu, B.; Mbiapo, F.T.; Villeneuve, P.; Fanni, J.; Parmentier, M. Oils of insects and larvae consumed in Africa: Potential sources of polyunsaturated fatty acids. OCL 2009, 16, 230–235. [Google Scholar] [CrossRef]
- Arango Gutiérrez, G.P.; Vergara Ruiz, R.A.; Mejía Vélez, H. Analisis composicional, microbiológico y digestibilidad de la proteína de la harina de larvas de hermetia illuscens l (diptera:stratiomyiidae) en angelópolis-antioquia, colombia. Rev. Fac. Nac. Agron. Medellín 2004, 57, 2491–2500. [Google Scholar]
- Newton, L.; Sheppard, C.; Watson, D.W.; Burtle, G.; Dove, R. Using the Black Soldier Fly, Hermetia illucens, As a Value-Added Tool for the Managementof Swine Manure; Report for Mike Williams; Director of the Animal and Poultry Waste Management Center, North Carolina State University: Raleigh, NC, USA, 2005. [Google Scholar]
- Soares, S.; Forkes, A. Insects Au Gratin—An Investigation into the Experiences of Developing a 3D Printer that uses Insect Protein Based Flour as a Building Medium for the Production of Sustainable Food. In Proceedings of the 16th International Conference on Engineering and Product Design Education, London South Bank University, Enschede, The Netherlands, 4–5 September 2014; Bohemia, E., Eger, A., Eggink, W., Kovacevic, A., Parkinson, B., Wits, W., Eds.; The Design Society: Glasgow, UK, 2014; pp. 426–431. [Google Scholar]
- Fombong, F.T.; Van Der Borght, M.; Vanden Broeck, J. Influence of freeze-drying and oven-drying post blanching on the nutrient composition of the edible insect Ruspolia differens. Insects 2017, 8, 102. [Google Scholar] [CrossRef]
- Khosravani, M.R.; Reinicke, T. On the environmental impacts of 3D printing technology. Appl. Mater. Today 2020, 20, 100689. [Google Scholar] [CrossRef]
- Reis, G.G.; Heidemann, M.S.; Matos, K.H.O.D.; Molento, C.F.M. Cell-Based Meat and Firms’ Environmental Strategies: New Rationales as per Available Literature. Sustainability 2020, 12, 9418. [Google Scholar] [CrossRef]
- Mattick, C.S.; Landis, A.E.; Allenby, B.R.; Genovese, N.J. Anticipatory life cycle analysis of in vitro biomass cultivation for cultured meat production in the United States. Environ. Sci. Technol. 2015, 49, 11941–11949. [Google Scholar] [CrossRef]
- Tuomisto, H.L.; Teixeira de Mattos, M.J. Environmental Impacts of Cultured Meat Production. Environ. Sci. Technol. 2011, 45, 6117–6123. [Google Scholar] [CrossRef]
- Oonincx, D.G.A.B.; de Boer, I.J.M. Environmental impact of the production of mealworms as a protein source for humans—A life cycle assessment. PLoS ONE 2012, 7, e51145. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Gillis, A.; Sheviryov, J.; Levkov, K.; Golberg, A. Towards waste meat biorefinery: Extraction of proteins from waste chicken meat with non-thermal pulsed electric fields and mechanical pressing. J. Clean. Prod. 2019, 208, 220–231. [Google Scholar] [CrossRef]
- Parodi, A.; De Boer, I.J.M.; Gerrits, W.J.J.; Van Loon, J.J.A.; Heetkamp, M.J.W.; Van Schelt, J.; Van Zanten, H.H.E. Bioconversion efficiencies, greenhouse gas and ammonia emissions during black soldier fly rearing—A mass balance approach. J. Clean. Prod. 2020, 271, 122488. [Google Scholar] [CrossRef]
- Ritchie, H.; Reay, D.S.; Higgins, P. Potential of meat substitutes for climate change mitigation and improved human health in high-income markets. Front. Sustain. Food Syst. 2018, 2, 16. [Google Scholar] [CrossRef]
- Kaipia, R.; Dukovska-Popovska, I.; Loikkanen, L. Creating sustainable fresh food supply chains through waste reduction. Int. J. Phys. Distrib. Logist. Manag. 2013, 43, 262–276. [Google Scholar] [CrossRef]
- Qiu, F.; Hu, Q.; Xu, B. Fresh Agricultural Products Supply Chain Coordination and Volume Loss Reduction Based on Strategic Consumer. Int. J. Environ. Res. Public Health 2020, 17, 7915. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, D.; Tseng, T.W.; Swartz, E. The Business of Cultured Meat. Trends Biotechnol. 2020, 38, 573–577. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, L.; Jiang, Y.; Faisal, S.; Wei, L.; Cao, C.; Wang, Q. Converting Peanut Protein Biomass Waste into “Double Green” Meat Substitutes Using a High-Moisture Extrusion Process: A Multiscale Method to Explore a Process for Forming a Meat-Like Fibrous Structure. J. Agric. Food Chem. 2019, 67, 10713–10725. [Google Scholar] [CrossRef]
- Jiang, G.; Ameer, K.; Kim, H.; Lee, E.-J.; Ramachandraiah, K.; Hong, G.-P. Strategies for Sustainable Substitution of Livestock Meat. Foods 2020, 9, 1227. [Google Scholar] [CrossRef]
- Zimon, D.; Madzik, P.; Domingues, P. Development of Key Processes along the Supply Chain by Implementing the ISO 22000 Standard. Sustainability 2020, 12, 61. [Google Scholar] [CrossRef]
- Murphy, S.V.; De Coppi, P.; Atala, A. Opportunities and challenges of translational 3D bioprinting. Nat. Biomed. Eng. 2020, 4, 370–380. [Google Scholar] [CrossRef] [PubMed]
- Lupton, D.; Bethaney, T. Both Fascinating and Disturbing: Consumer Responses to 3D Food Printing and Implications for Food Activism. In Digital Food Activism; Schneider, T., Ed.; Routledge: London, UK, 2018; pp. 151–167. [Google Scholar]
- Manstan, T.; McSweeney, M.B. Consumers’ attitudes towards and acceptance of 3D printed foods in comparison with conventional food products. Int. J. Food Sci. Technol. 2020, 55, 323–331. [Google Scholar] [CrossRef]
- Weinrich, R. Opportunities for the Adoption of Health-Based Sustainable Dietary Patterns: A Review on Consumer Research of Meat Substitutes. Sustainability 2019, 11, 4028. [Google Scholar] [CrossRef]

| Extrusion | Inkjet Printing | Binder Jetting | Bioprinting | |
|---|---|---|---|---|
| Merits | Availability of several materials, simple device | Availability of several materials, relatively improved printing and fabrication rate | Possibility of printing complex 3D structures | Printing of tissue analogs utilizing living cells |
| Demerits | Lack of superior intricate 3DP products | Preferable for simple designs | Lack of availability of a wide variety of materials | Lack of food-safe materials |
| Processing Factors | Height of fabricated products, size of nozzle diameter, printing rate, rate of nozzle motion | Speed of printing, size of nozzle diameter, Height of fabricated products, temperature | Speed of printing, nozzle diameter, thickness of fabricated layers, Head types | layer-by-layer assembly of multiple layers, requirement of multi-materials |
| Printing Technique | Materials | Cell Types | Printing Parameter | Features | References |
|---|---|---|---|---|---|
| Extrusion | dECM PCL contraints Gelatin | Human skeletal muscle cell | Temp: 18 °C | Mimicking native muscle Vascularization | [41] |
| Extrusion | CMCMA, Alginate-MA | C2C12 | Nozzle size: 200 micron | Mechanical property optimization | [42] |
| ITOP | Fibrinogen gelatin HA, glycerol, PCL pillar | hMPCs | 300 µ width | Increased cell viability | [35] |
| ITOP | Gelatin hydrogel poly (ε-caprolactone) (PCL) polymer | hMPCs | 300–400 µ | highly viable, organized cellular structure | [38] |
| Meat By-Products | Protein (%) | Moisture (%) | Fat (%) |
|---|---|---|---|
| Pork lungs | 16.6 | 79.1 | 2.1 |
| Pork kidneys | 16.2 | 77.7 | 4.0 |
| Chicken viscera | 11.2 | 69.6 | 16.9 |
| Mechanically separated meat (MSM): Chicken | 13.9 | 69.1 | 15.1 |
| Composition (Nutrient/Biomolecule) | Head | Flesh | Viscera |
|---|---|---|---|
| Crude protein | 9–21% | 14–22% | 18–23% |
| Crude fat | 1–6% | 2–5% | 2–7% |
| Moisture | 59–68% | 70–77% | 64–72% |
| Ash | 7–18% | 2–5% | 2–5% |
| Insect Type | Protein (%) | Lipid (%) | Other Nutrients | References |
|---|---|---|---|---|
| Ground cricket | 48–67% (dry wt) | 5–20% (species dependent) | Fiber 8.7–18%, Vitamin B12, Fe, Zn, Cu | [83,84] |
| Grasshopper | 13–28% (fresh wt) 57–69% (dry wt); | 3–67% (dry wt) | Crude fiber 8.5–12.5% Fe, Mn, Cu, Ca, P, Zn | [83,85] |
| Back soldier fly | 40–44% (dry wt) | 15–49% (dry wt) | Crude fiber 7% (dry wt) Mn, Zn, Ca, P | [86,87] |
| Weaver ant | 26–48.5% (dry wt) | 10–25% (fresh wt) | Vitamin B1, B2, B3, P, Mg, Na, Zn, Ca, Fe | [78] |
| Palm Weevil beetle | 7–36% (fresh wt) | 54% (dry wt) | Vitamin E | [83,84,85] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramachandraiah, K. Potential Development of Sustainable 3D-Printed Meat Analogues: A Review. Sustainability 2021, 13, 938. https://doi.org/10.3390/su13020938
Ramachandraiah K. Potential Development of Sustainable 3D-Printed Meat Analogues: A Review. Sustainability. 2021; 13(2):938. https://doi.org/10.3390/su13020938
Chicago/Turabian StyleRamachandraiah, Karna. 2021. "Potential Development of Sustainable 3D-Printed Meat Analogues: A Review" Sustainability 13, no. 2: 938. https://doi.org/10.3390/su13020938
APA StyleRamachandraiah, K. (2021). Potential Development of Sustainable 3D-Printed Meat Analogues: A Review. Sustainability, 13(2), 938. https://doi.org/10.3390/su13020938




