Analysis of Non-Structural Carbohydrate in Relation with Shoot Elongation of Rice under Complete Submergence
Abstract
:1. Introduction
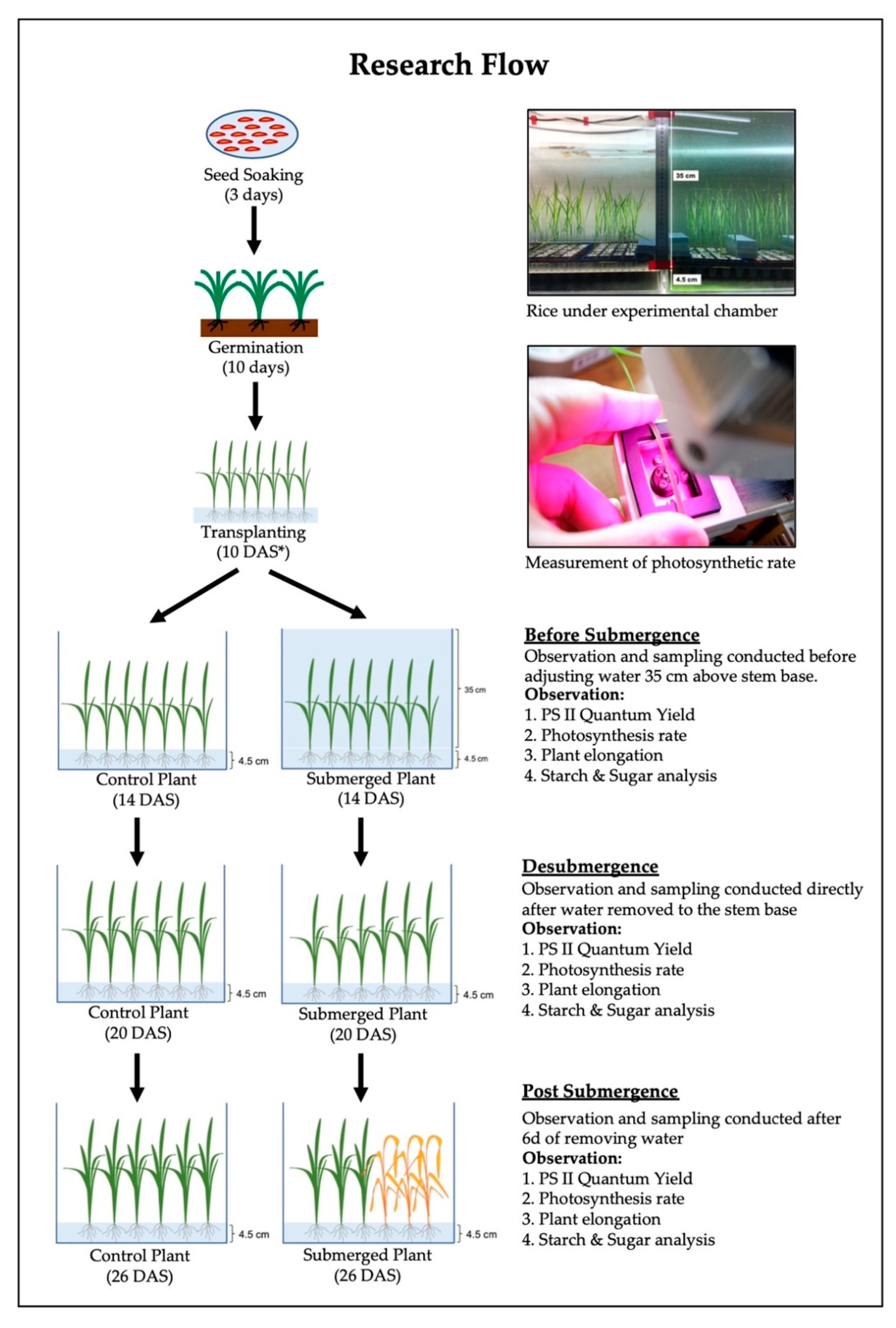
2. Materials and Methods
2.1. Photosystem II (PS II) Quantum Yield
2.2. Net Photosynthesis Rate
2.3. Shoot Elongation (cm)
2.4. Starch and Sugar Analysis
2.5. Statistical Analysis
3. Results
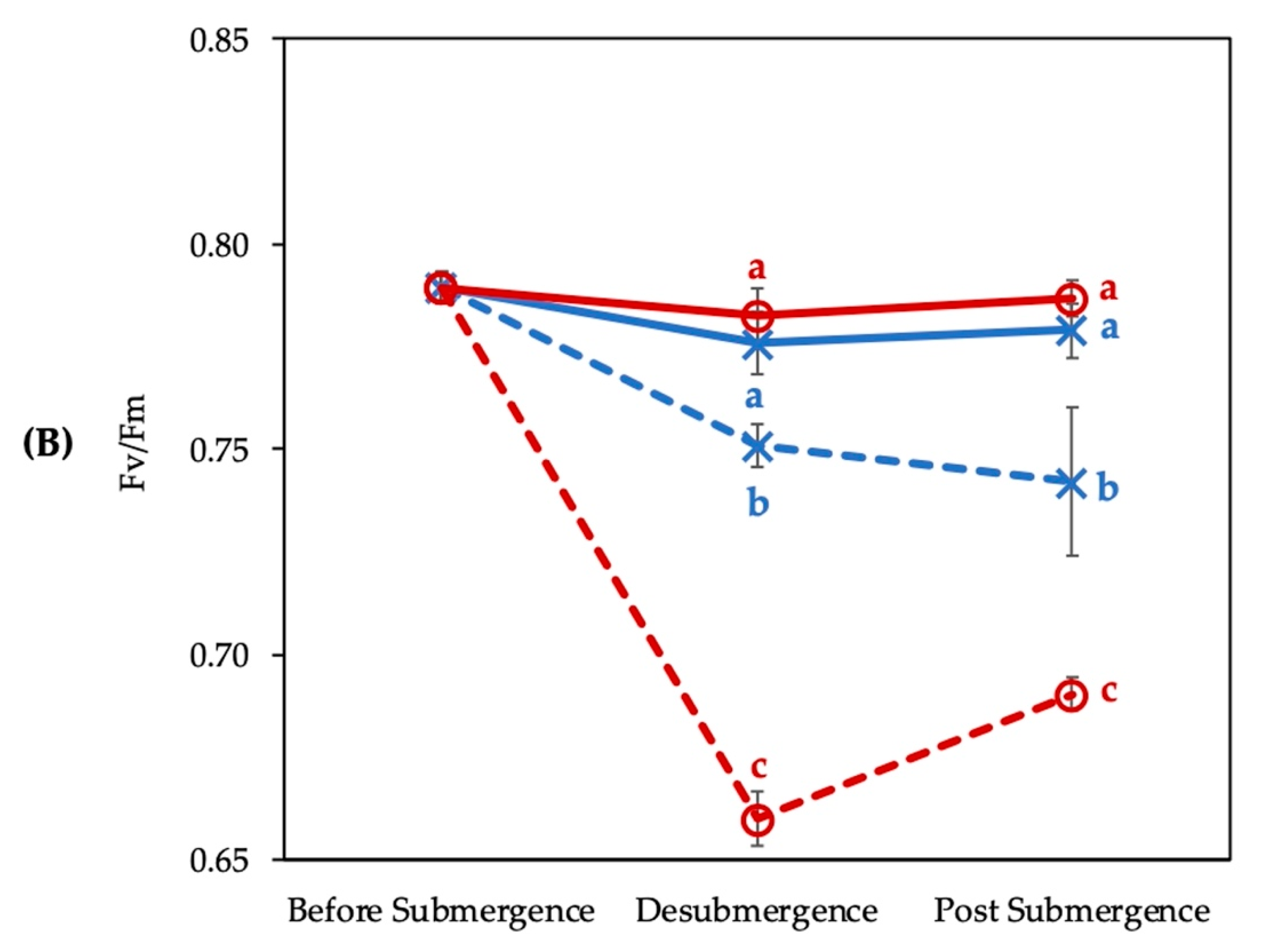
3.1. Photosynthesis Rate and Fv/Fm of Chlorophyll Fluorescence Affected by Submergence
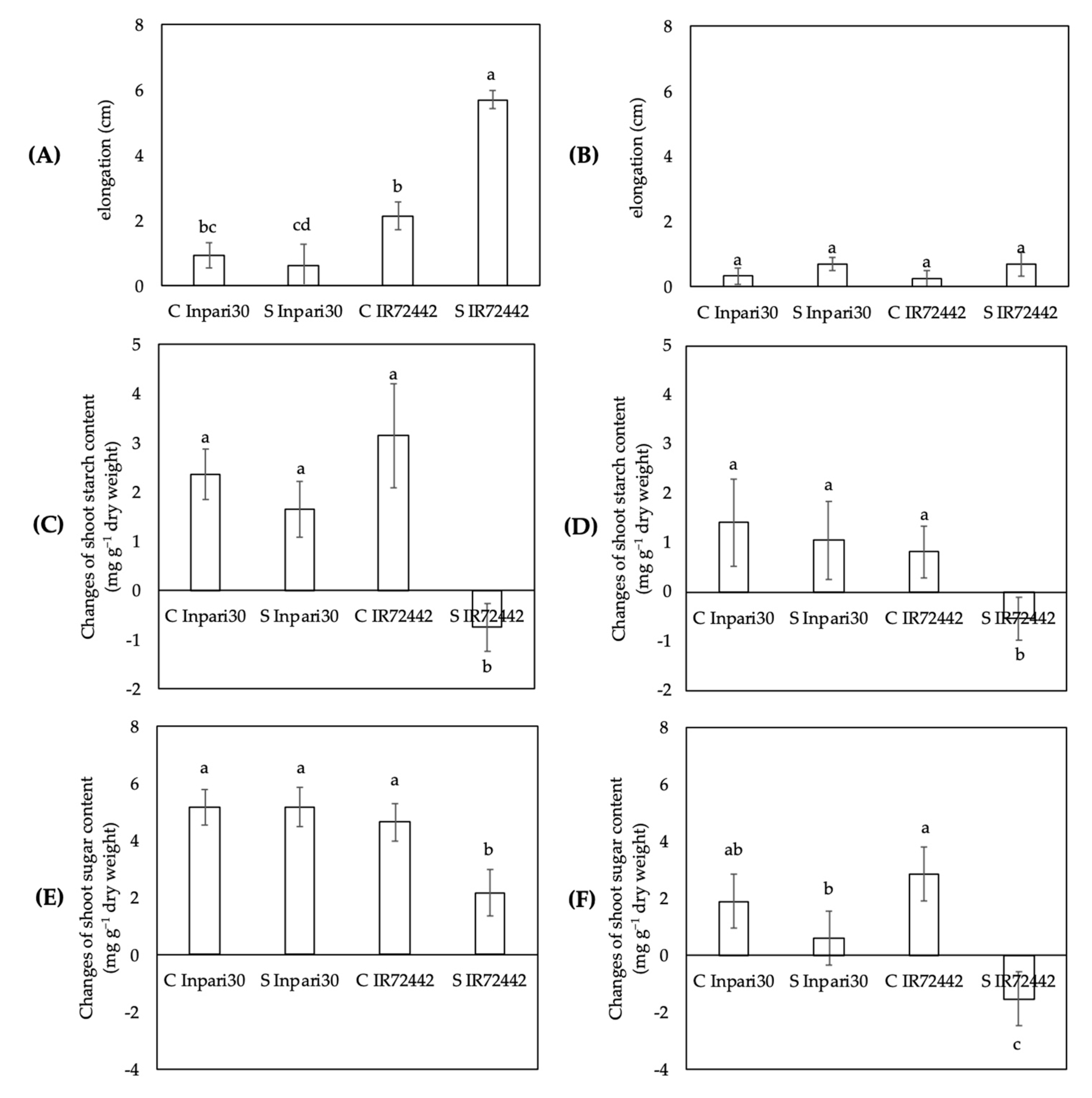
3.2. Changes in Starch and Soluble Sugar Content in Relation to Elongation
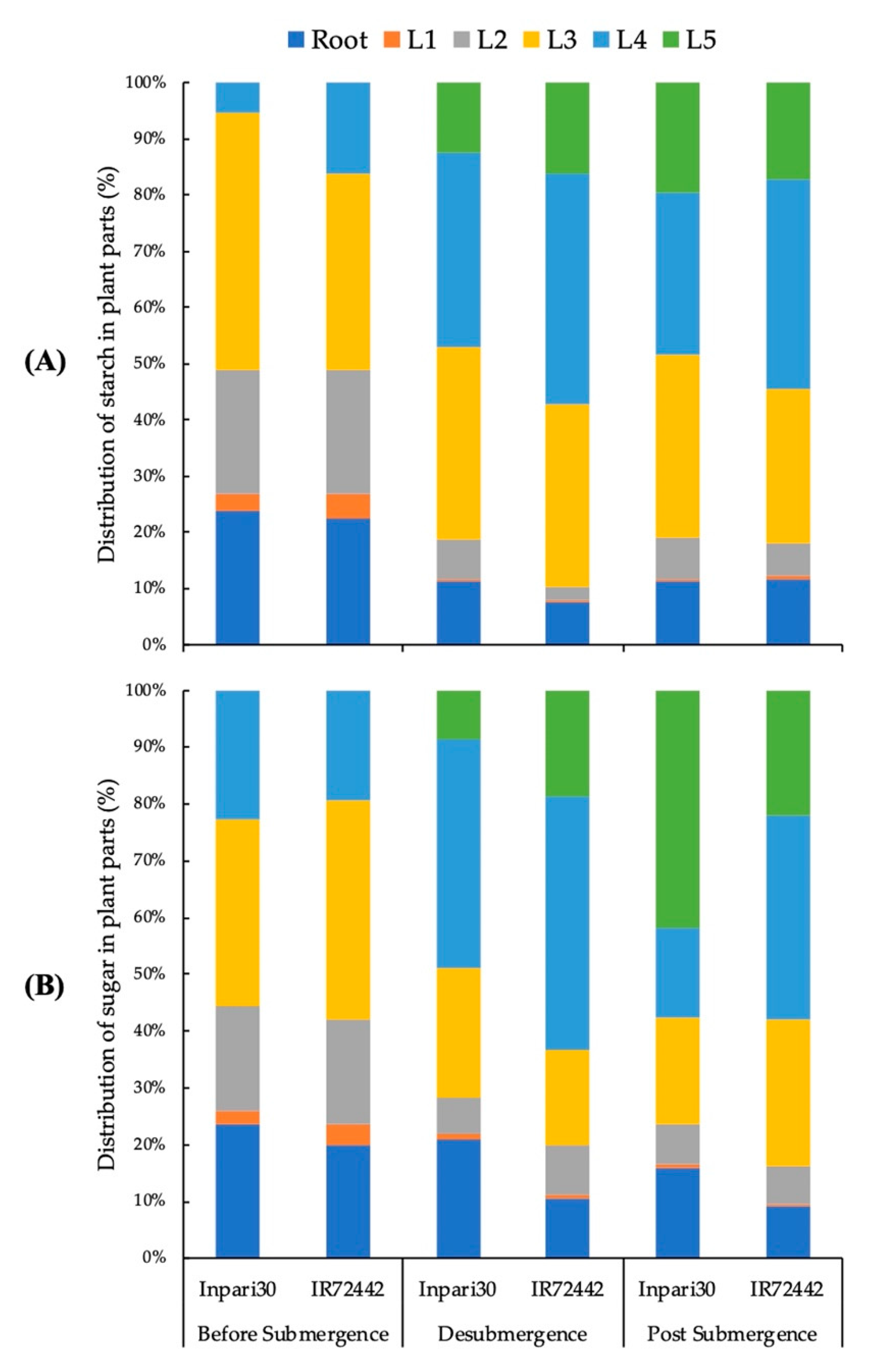
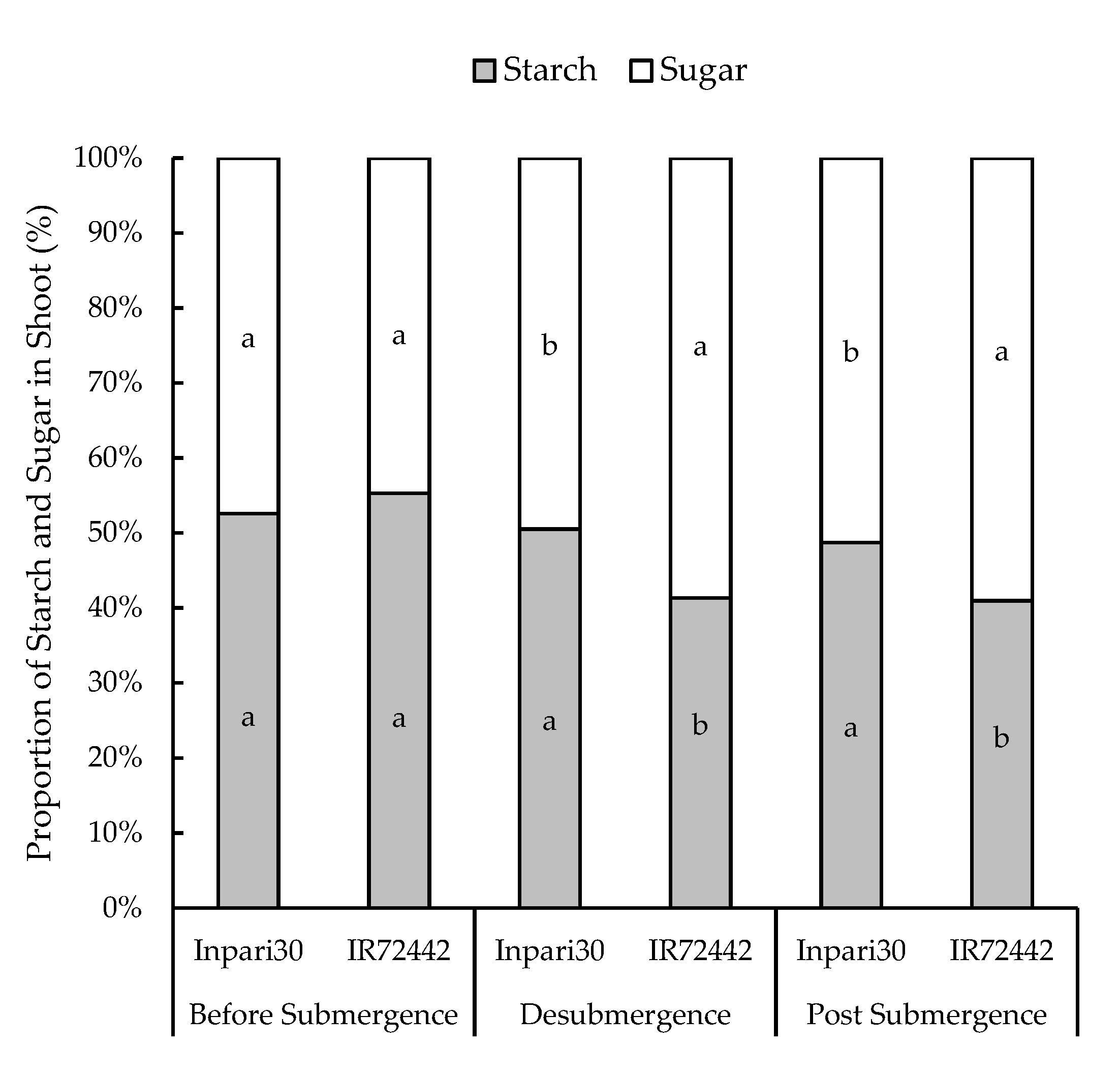
3.3. Distribution of Starch and Soluble Sugar to the Plant Organs
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Geiger, D.R.; Servaites, J.C.; Fuchs, M.A. Role of starch in carbon translocation and partitioning at the plant level. Funct. Plant Biol. 2000, 27, 571–582. [Google Scholar] [CrossRef]
- Chatterton, N.J.; Silvius, J.E. Photosynthate partitioning into starch in soybean leaves: I. Effects of photoperiod versus photosynthetic period duration. Plant Physiol. 1979, 64, 749–753. [Google Scholar] [CrossRef] [PubMed]
- Das, A.; Nanda, B.B.; Sarkar, R.K.; Lodh, S.B. Effect of Complete Submergence on the Activity of Starch Phosphorylase Enzyme in Rice (Oryza sativa L.) Leaves. J. Plant Biochem. Biotechnol. 2000, 9, 41–43. [Google Scholar] [CrossRef]
- Catling, D. Rice in Deep Water; Palgrave Macmillan: London, UK, 1999. [Google Scholar]
- Mommer, L.; Pons, T.L.; Visser, E.J. Photosynthetic consequences of phenotypic plasticity in response to submergence: Rumex palustris as a case study. J. Exp. Bot. 2006, 57, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Nurrahma, A.; Yabuta, S.; Junaedi, A.; Sakagami, J.I. Characterizing the photosynthetic ability of the submergence-tolerant rice variety of Inpari30 via maximum quantum yield performance during transient flooding stress and recovery. Aust. J. Crop Sci. 2021, 15. [Google Scholar] [CrossRef]
- Sarkar, R. Saccharide content and growth parameters in relation with flooding tolerance in rice. Biol. Plant. 1997, 40, 597–603. [Google Scholar] [CrossRef]
- Das, K.K.; Sarkar, R.K.; Ismail, A.M. Elongation ability and non-structural carbohydrate levels in relation to submergence tolerance in rice. Plant Sci. 2005, 168, 131–136. [Google Scholar] [CrossRef]
- Singh, S.; Mackill, D.J.; Ismail, A.M. Physiological basis of tolerance to complete submergence in rice involves genetic factors in addition to the SUB1 gene. AoB Plants 2014, 6, plu060. [Google Scholar] [CrossRef] [Green Version]
- Gautam, P.; Nayak, A.; Lal, B.; Bhattacharyya, P.; Tripathi, R.; Shahid, M.; Mohanty, S.; Raja, R.; Panda, B. Submergence tolerance in relation to application time of nitrogen and phosphorus in rice (Oryza sativa L.). Environ. Exp. Bot. 2014, 99, 159–166. [Google Scholar] [CrossRef]
- Xu, K.; Mackill, D.J. A major locus for submergence tolerance mapped on rice chromosome 9. Mol. Breed. 1996, 2, 219–224. [Google Scholar] [CrossRef]
- Xu, K.; Xu, X.; Fukao, T.; Canlas, P.; Maghirang-Rodriguez, R.; Heuer, S.; Ismail, A.M.; Bailey-Serres, J.; Ronald, P.C.; Mackill, D.J. Sub1A is an ethylene-response-factor-like gene that confers submergence tolerance to rice. Nature 2006, 442, 705–708. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panda, D.; Sarkar, R.K. Role of non-structural carbohydrate and its catabolism associated with Sub 1 QTL in rice subjected to complete submergence. Exp. Agric. 2012, 48, 502. [Google Scholar] [CrossRef]
- Septiningsih, E.M.; Hidayatun, N.; Sanchez, D.L.; Nugraha, Y.; Carandang, J.; Pamplona, A.M.; Collard, B.C.; Ismail, A.M.; Mackill, D.J. Accelerating the development of new submergence tolerant rice varieties: The case of Ciherang-Sub1 and PSB Rc18-Sub1. Euphytica 2015, 202, 259–268. [Google Scholar] [CrossRef]
- Nugraha, Y.; Hidayatun, N.; Trisnaningsih, T.; Yuliani, D. Phenotypic performance of Ciherang SUB1 near isogenic line as an adaptive variety for flooding conditions. Indones. J. Agric. Sci. 2017, 18, 7–16. [Google Scholar] [CrossRef] [Green Version]
- Nurrahma, A.H.I.; Junaedi, A.; Purnamawati, H.; Sakagami, J.I. Rice Root Distribution of Four Rice Varieties to Different Depth of Submergence. Agrivita J. Agric. Sci. 2017, 39, 9. [Google Scholar] [CrossRef] [Green Version]
- Sone, C.; Ito, O.; Sakagami, J.I. Characterizing submergence survival strategy in rice via chlorophyll fluorescence. J. Agron. Crop Sci. 2012, 198, 152–160. [Google Scholar] [CrossRef]
- Das, B.; Dash, P.; Roy, A. Role of total sugar and starch content of rice seedlings at different ages in variable submergence tolerance. Oryza-Int. J. Rice 2009, 46, 304–309. [Google Scholar]
- Adak, M.K.; Ghosh, N.; Dasgupta, D.K.; Gupta, S. Impeded Carbohydrate Metabolism in Rice Plants under Submergence Stress. Rice Sci. 2011, 18, 116–126. [Google Scholar] [CrossRef]
- Panda, D.; Sarkar, R.K. Mechanism associated with nonstructural carbohydrate accumulation in submergence tolerant rice (Oryza sativa L.) cultivars. J. Plant Interact. 2014, 9, 62–68. [Google Scholar] [CrossRef]
- Horwitz, W. Official Methods of Analysis of AOAC International. Volume I, Agricultural Chemicals, Contaminants, Drugs; Horwitz, W., Ed.; AOAC International: Gaithersburg, MI, USA, 1997. [Google Scholar]
- AACC International. Approved Methods of Analysis; AACC International: St. Paul, MN, USA, 2010. [Google Scholar]
- Winkel, A.; Pedersen, O.; Ella, E.; Ismail, A.M.; Colmer, T.D. Gas film retention and underwater photosynthesis during field submergence of four contrasting rice genotypes. J. Exp. Bot. 2014, 65, 3225–3233. [Google Scholar] [CrossRef] [Green Version]
- Panda, D.; Sharma, S.G.; Sarkar, R.K. Chlorophyll fluorescence parameters, CO2 photosynthetic rate and regeneration capacity as a result of complete submergence and subsequent re-emergence in rice (Oryza sativa L.). Aquat. Bot. 2008, 88, 127–133. [Google Scholar] [CrossRef]
- Inamullah; Isoda, A. Adaptive Responses of Soybean and Cotton to Water Stress II. Changes in CO2 Assimilation Rate, Chlorophyll Fluorescence and Photochemical Reflectance Index in Relation to Leaf Temperature. Plant Prod. Sci. 2005, 8, 131–138. [Google Scholar] [CrossRef]
- Maxwell, K.; Johnson, G.N. Chlorophyll fluorescence—A practical guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef]
- Lemoine, R.; La Camera, S.; Atanassova, R.; Dédaldéchamp, F.; Allario, T.; Pourtau, N.; Bonnemain, J.-L.; Laloi, M.; Coutos-Thévenot, P.; Maurousset, L. Source-to-sink transport of sugar and regulation by environmental factors. Front. Plant Sci. 2013, 4, 272. [Google Scholar] [CrossRef] [Green Version]
- Pradhan, C.; Mohanty, M. Submergence Stress: Responses and adaptations in crop plants. In Molecular Stress Physiology of Plants; Rout, G.R., Das, A.B., Eds.; Springer: Chennai, India, 2013; pp. 331–357. [Google Scholar] [CrossRef]
- Hattori, Y.; Nagai, K.; Ashikari, M. Rice growth adapting to deepwater. Curr. Opin. Plant Biol. 2011, 14, 100–105. [Google Scholar] [CrossRef]
- Krishnan, P.; Ravi, I.; Krishnayya, G.R. Leaf senescence in submerged rice plants. Exp. Agric. 1999, 35, 345–355. [Google Scholar] [CrossRef]
- Setter, T.L.; Laureles, E.V. The beneficial effect of reduced elongation growth on submergence tolerance of rice. J. Exp. Bot. 1996, 47, 1551–1559. [Google Scholar] [CrossRef] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nurrahma, A.H.I.; Yabuta, S.; Junaedi, A.; Sakagami, J.-I. Analysis of Non-Structural Carbohydrate in Relation with Shoot Elongation of Rice under Complete Submergence. Sustainability 2021, 13, 670. https://doi.org/10.3390/su13020670
Nurrahma AHI, Yabuta S, Junaedi A, Sakagami J-I. Analysis of Non-Structural Carbohydrate in Relation with Shoot Elongation of Rice under Complete Submergence. Sustainability. 2021; 13(2):670. https://doi.org/10.3390/su13020670
Chicago/Turabian StyleNurrahma, Arinal Haq Izzawati, Shin Yabuta, Ahmad Junaedi, and Jun-Ichi Sakagami. 2021. "Analysis of Non-Structural Carbohydrate in Relation with Shoot Elongation of Rice under Complete Submergence" Sustainability 13, no. 2: 670. https://doi.org/10.3390/su13020670
APA StyleNurrahma, A. H. I., Yabuta, S., Junaedi, A., & Sakagami, J.-I. (2021). Analysis of Non-Structural Carbohydrate in Relation with Shoot Elongation of Rice under Complete Submergence. Sustainability, 13(2), 670. https://doi.org/10.3390/su13020670





