Abstract
Soil lead (Pb) contamination is a major environmental and public health risk. Switchgrass (Panicum virgatum), a second-generation biofuel crop, is potentially useful for the long-term phytoremediation and phytoextraction of Pb contaminated soils. We evaluated the efficacy of a coordinated foliar application of plant growth regulators and soil fungicide and a chelator in order to optimize phytoextraction. Plants were grown in soil culture under controlled conditions. First, three exogenous nitric oxide (NO) donors were evaluated at multiple concentrations: (1) S-nitroso-N-acetylpenicillamine (SNAP); (2) sodium nitroprusside (SNP); and (3) S-nitrosoglutathione (GSNO). Second, the effect of SNP (0.5 μM) was examined further with the model chelate EDTA and the soil fungicide propicanazole. Third, a combined foliar application of SNP and gibberellic acid (GA3) was examined with EDTA and propicanazole. The soil application of propiconazole (a broad-spectrum fungicides) reduced AMF colonization and allowed greater Pb phytoextraction. The foliar application of SNP resulted in similar concentrations of Pb (roots and foliage) to plants that were challenged with chelates and soil fungicides. The combined foliar application of SNP and GA3 resulted in significantly greater average Pb concentration (243 mg kg−1) in plant foliage in comparison to control plants (182 mg kg−1) and plants treated with GA3 alone (202 mg kg−1). The combined foliar application of SNP and GA3 resulted in the greatest phytoextraction efficiency and could therefore potentially improve phytoextraction by switchgrass grown in Pb contaminated soils.
1. Introduction
Anthropogenic lead (Pb) soil contamination is mainly derived from industrial sources such as mining, battery recycling, agricultural pesticides, and from tetraethyllead ((CH3CH2)4Pb), an additive formerly found in automotive fuels [1,2,3,4,5]. Airborne Pb from the smelting and burning of tetraethyllead containing fuel falls out of the atmosphere and deposits in topsoil [1]. Even residential areas have been found to be contaminated with high levels of soil Pb contamination [6,7,8]. It is estimated that 207,000 Pb contaminated sites, comprising millions of hectares, exist throughout the United States [9].
Environmental Pb contamination is a recognized global health problem; long-term low-level Pb exposure can result in neurological dysfunctions [10,11,12,13,14,15]. Even low (50 mg kg−1) concentrations of Pb in soil is correlated with increased risk of elevated blood lead level (BLL) in humans [16,17]. Children may additionally acquire prenatal neurological damage due to epigenetic effects from Pb accumulation in parents or grandparents [18]. Currently, there is no blood Pb level known that is not considered harmful to human health [19]. Measures have been put in place to reduce human exposure to environmental Pb contamination including site closing, ground covers, and in extreme cases, soil removal [20]. Although these measures may reduce interactions with contaminated soils, they still leave the harmful contaminant in the environment [20]. Levels of Pb contaminated soil have previously been correlated to Pb levels in tissues of domesticated animals and wildlife [21,22,23,24].
The removal of contaminants from soil can be employed through phytoextraction, an emerging heavy metal remediation technique [25]. In phytoextraction, the contaminant is absorbed and sequestered within plant shoots, resulting in a low degree of soil disruption [26]. The process of phytoextraction of Pb contaminated soil typically begins by soil acidification and chelation [27]. Lead ions are positively charged, causing them to bind to particles in the topsoil and resist leaching into deeper soil horizons [28,29]. Several studies have found that the synthetic chelator ethylenediaminetetraacetic acid (EDTA) is a highly effective Pb-chelation agent that forms soluble Pb complexes in the soil [26,30,31]. The chelator EDTA has been used extensively in phytoremediation studies [32].
Soil microorganisms such as arbuscular mycorrhizal fungi (AMF) form symbiotic relationship with plants, facilitate nutrient uptake, and prevent the uptake of harmful elements, such as Pb [33,34,35,36]. Switchgrass benefits from AMF symbiosis through macro-nutrient homeostasis [37]. The AMF act as a barrier against Pb uptake of plants and are commonly found associated with roots of plants growing on contaminated soils [35,38,39]. In most circumstances, plants that have symbiosis with AMF exhibit low Pb in the foliage. While AMF benefits the plant when growing on contaminated soil, it is disadvantageous to phytoextraction efforts [33,39,40]. To counteract the protecting effect of the AMF, contaminated soils must be treated with a broad-spectrum fungicide such as propiconazole to inhibit the function of the AMF [41]. Studies have shown that another soil fungicide, benomyl treatment, especially prior to EDTA application, improved Pb uptake and translocation [42,43].
An exogenous application of plant growth regulators has been shown to increase phytoextraction efficiency [44,45]. Nitric oxide (NO) is an important cellular signaling molecule in plants and appears to play a role in plant iron (Fe) maintenance and stress response signaling [44,46,47]. It has been suggested that NO contributes to iron homeostasis in two ways; first, as a reducing agent to change iron from Fe3+ to Fe2+, and secondly, by the formation of dinitrosyl-iron complexes (DNICs) which may facilitate iron transport through cellular membranes [48,49]. Nitric oxide can be exogenously applied to have a similar effect as endogenous NO [50]. Nitric oxide donor molecules have been shown to significantly reduce initial heavy metal toxicity in plants as well as increase metal uptake [47,51,52,53].
The plant growth regulator gibberellic acid (GA3) has several physiological effects on plants including growth stimulation [54]. The exogenous application of GA3 was found to enhance phytoextraction by ryegrass (Lolium perenne) [55]. The optimal concentration of GA3 was found to be 1 μM to increase growth of ryegrass and increase the proportion of Pb in the cell wall [55]. Higher doses (100 μM) of GA3 had adverse effects on plants and Pb phytoextraction [55]. Moreover, the foliar application of GA3 was found to counteract the negative effect of EDTA on the growth of maize (Zea mays) [45].
Switchgrass (Panicum virgatum L.) is a C4 perennial grass adapted to a broad range of climates, topography, and soil conditions throughout North America [56,57]. As a perennial grass, switchgrass may be harvested more than once in a growing period, and it will continue to grow for up to 10 years [58]. Another attribute contributing to the selection of switchgrass is its high tolerance for Pb in soils [59] and high biomass production. Previous studies have estimated that switchgrass var. “Alamo” is capable of generating 17,800 kg of harvestable tissue per hectare (ha) [60]. Switchgrass is regarded as a second-generation biofuel crop and its biomass could be used in advanced biofuel production [61,62]. The phytoremediation of contaminated soils using second-generation bioenergy crops such as switchgrass has great potential [63,64,65,66,67]. The cultivation of biofuel crops on marginal lands may improve energy security and aid in mitigating climate change [68]. In addition, the cultivation of bioenergy crops on marginal lands may reduce the need for using primary agricultural lands for biofuel production. The cost of this biomass production is estimated to be much lower than that of other high biomass yield crops [69]. The switchgrass cultivar “Alamo” (AP13) originating from Live Oak County, Texas, is particularly well acclimated for use in Georgia [70]. Derived from the “Alamo” cultivar, EG 1101, a high biomass yield isolate, was provide by the University of Georgia and used in this study.
This study examined phytoextraction efficiency of switchgrass in the following ways: (1) effect of different exogenous NO donors; (2) effect of foliar application of SNP along with chelate (EDTA) and soil fungicide (propicanazole); (3) effect of combined foliar application of SNP and the plant hormone gibberellic acid (GA3) along with chelate and soil fungicide.
2. Materials and Methods
2.1. Soil
Plants were grown in soil that was collected from a brownfield in downtown Atlanta, Georgia, USA. Atlanta soils are generally clay-rich acidic ultisols with low base cation saturation [71]. The mineral compositions reflect the generally granitic or gneissic parent materials of the Georgia Piedmont that have weathered to produce soils rich in quartz, feldspar, mica, Fe-oxyhydroxides, kaolinite, and illite [72]. This soil was tested using ICP-AES for elemental content at the University of Georgia, Stable Isotope Ecology lab, Athens, Georgia. Soil testing revealed that the soil contained 108 mg kg−1 of Pb, 7765 mg kg−1 of iron (Fe) and 21,850 mg kg−1 aluminum (Al). The soil pH was 5.5. The soil was spiked to 350 mg kg−1 Pb using a standard Pb spiking solution (Pb(NO3)2) at a concentration of 1000 mg kg−1 that had been diluted to the necessary concentration with DI H2O [62,73]. After the soil was spiked, it was mixed to homogenize Pb distribution.
2.2. Plant Growth Conditions
Plants were grown under controlled environmental conditions in the Science Greenhouse at Kennesaw State University (KSU), Kennesaw, GA, USA, at an average temperature of 22.9 °C (30.6 °C max and 15.6 °C min). The soil was left unsterilized in order to maintain the indigenous soil microbiota; however, debris larger than 0.5 cm were removed by hand. Pots were filled with 5000 g of contaminated soil and planted with approximately 30 seeds of switchgrass at a depth of 0.25 cm to allow for maximum germination [57]. Seedlings were thinned to three per pot. The pots were placed on wire-topped greenhouse benches with individual plastic saucers placed under each pot to prevent soil loss and cross contamination. Natural light varied over time but not across treatments with the sun availability as per the greenhouse conditions and supplemented with 14 h of artificial cool-white-fluorescent overhead light (10,000 Lux) each day.
2.3. Selection of Exogenous NO Donor
Three exogenous NO donors were tested: (1) sodium nitroprusside (SNP) (Na2[Fe(CN)5NO]), which has shown promises in phytoextraction applications in studies using Arabidopsis thaliana by providing a protection against Pb uptake [52]; (2) S-nitroso-N-acetylpenicillamine (SNAP) (C7H12N2O4S), which may be active in upregulating cell division in plants, potentially causing the uptake of excess soil contaminants [74]; and (3) S-nitrosoglutathione (GSNO) (C10H16N4O7S), which has shown to be active in Pb stressed plants, alleviating heavy metal oxidation and stress [75,76]. The effect of three exogenous NO donor molecules (SNP, SNAP, GSNO) were tested in three different concentrations (0.1 μM, 0.2 μM, 0.5 μM) on switchgrass.
2.4. Phytoextraction by Switchgrass Enhanced by Coordinated Application of SNP, Chelate and Soil Fungicide
Seeds of P. virgatum were sown into 12 pots that were filled with contaminated soil (5 kg). The pots were randomly divided into three treatments: (1) control, (2) EDTA + propicanazole (EP); (3) EDTA + propicanazole + SNP (EPS). Three plants were grown in each pot that were arranged in a complete randomized block design, with re-randomization every seven days. Plants were given DI water (100 mL) twice a week until soil chemical exposure began. Phytoextraction was induced by a coordinated foliar application of SNP and soil application of soil fungicide and chelate. The soil fungicide propiconazole (trade name Infuse®) is a short-lived fungal suppressant and is usually required in multiple applications [41]. It was prepared in a 2 mg L−1 solution with DI H2O and applied as a soil drench at 20, 40, 60, and 80 days after planting (dap) [41]. The model chelate EDTA was applied as 1.0 mmol kg−1 soil [43]. The EDTA application occurred at 91 dap. This application difference was determined from findings of a previous study, suggesting that applying EDTA chelator after AMF suppression by soil fungicide resulted in more efficient Pb uptake than simultaneous application [43]. The EDTA treatments were prepared using granular EDTA mixed with 90 mL DI H2O; the solution was then vortexed and applied to pots in appropriate treatments. Of the three exogenous NO donor being tested, SNP (0.5 μM) was selected for this experiment due to several factors, including efficacy and potential costs associated with its use in large-scale field applications. Crystallized SNP was dissolved in DI H2O to a concentration of 0.5 μM and applied as a 20.0 mL foliar leaf spray at 100, 110, and 120 dap. At 135 dap, the plants showed slight yellowing (chlorosis) of leaves and all plants were harvested. The plants were removed from the pots and rinsed with DI H2O to remove soil traces. Root samples were divided and three root samples from each pot were stored in 70% ethanol at 5 °C for later AMF staining. The remaining roots and shoots were dried for 48 h in an oven at 65 °C. Once the plant tissues were dried, dry mass (DM) was recorded for each sample prior to acid digestion.
2.5. Phytoextraction by Switchgrass Enhanced by Coordinated Application of SNP, GA3, Chelate and Soil Fungicide
Seeds of P. virgatum were sown into 40 pots that were filled with contaminated soil (5 kg). The pots were randomly divided into four treatments: (1) control, (2) SNP (0.5 μM), (3) GA3 (1.0 μM), (4) SNP and GA3 (0.5 μM and 1.0 μM, respectively). Three plants were grown in each pot that were arranged in a complete randomized block design, with re-randomization every seven days. The plants were given DI water (100 mL) twice a week until soil chemical exposure began. Phytoextraction was induced by a coordinated foliar application of SNP and soil application of soil fungicide and chelate. At 100, 110, 120 dap, SNP (0.5 μM) was exogenously applied on plant’s foliage in treatments 2 and 4 (5 mL of SNP was exogenously applied to each pot). At the same time, the plant hormone gibberellic acid GA3 (1.0 μM) was applied exogenously on plants in treatment 3 (10 mL was applied to each pot) and to plants in treatment 4 where combined application of SNP and GA3, was applied as well. The plants were given the soil fungicide propiconazole at 20, 40, 60 and 80 dap as described above. This was followed by EDTA application at 90 dap as described above. All plants were harvested at 140 dap and treated as described above.
2.6. Acid Digestion & Chemical Analysis of Plant Samples
Dried plant material was digested using the HotBlock digestion system (Environmental Express®, Inc., Charleston, SC, USA). Dried plant tissues (1.0 g) were digested in 38% HCl (10.0 mL) and 70% HNO3 (10.0 mL) in Environmental Express® 100.0 mL plastic digestion tubes following a modified EPA Method 3050B. The tubes were capped and rested at room temperature for 24 h, then refluxed at 95 °C in an Environmental Express® HotBlock system for 55 min. Samples were capped, allowed to cool for another 24 h period, and had their volume brought to 50 mL using trace-metal grade DI H2O before being vacuum filtered. The filtered samples were chemically analyzed using the Varian SpectraAA 220 FAAS in the Department of Chemistry and Biochemistry at KSU.
2.7. Trypan Blue Staining of Roots for AMF Assessment
Preserved root samples from both experiments were cleared and stained for AMF evaluation [43,77]. The samples were placed in 10% KOH solution and heated in a water bath at 90 °C for 30 min to clear roots of non-chitinous cellular structures. Cleared roots were rinsed in DI H2O five times and placed in 2.5% HCl for 30 min at room temperature for acidification. The roots were stained in 0.05% trypan blue for 15 min at 90 °C, and then de-stained in glycerol acidified with 2.5% HCl for 2 h to remove excess trypan blue and stored in acidic glycerol in a 5 °C refrigerator prior to AMF assessment.
Cleared and stained root samples from both experiments were evaluated for AMF colonization using the root-segment method [78]. The root specimen (n = 150) pieces (1 cm) per treatment were mounted on microscope slides and observed under a bright field microscope at 200× and 400× magnifications. AMF root colonization was calculated as the number of root segments colonized by AMF divided by the total number of root segments examined [78]. Colonization percentages were established by counting the occurrence of different fungal structures: hyphae, arbuscles, and vesicles for each treatment.
2.8. Statistical Analysis and Remediation Calculation
Data were statistically analyzed using one-way analysis of variance (ANOVA) followed by post hoc Fisher’s Least Significant Difference (LSD) using IBM SPSS Statistics 27. Additionally, a relationship between categorical treatments and foliage Pb concentrations was calculated by regression analysis. Statistical significance was accepted at the level of p < 0.05.
Bioaccumulation factor (BCF) measures the ability of the plant to accumulate Pb from the soil and is defined as the direct ratio of Pb in the total harvestable plant tissues to Pb in the soil [79,80]. A BCF of ≥ 1.0 is considered to be a successful phytoextraction [80].
3. Results
Coordinated chemical treatments showed dramatic increases in Pb concentrations in plants. Plants treated with propiconazole and EDTA had a significantly higher average Pb concentration (172 mg kg−1) in their foliage compared to control plants (12.4 mg kg−1) (Figure 1). Plants treated with propiconazole and EDTA and SNP showed a high value in the foliage (176 mg kg−1), a 1330% increase in shoot Pb concentration compared to control plants (Figure 1). Similarly, Pb concentrations in roots of plants treated with propiconazole and EDTA (1070 mg kg−1) and with the addition of SNP (822 mg kg−1) were significantly higher compared to control plants (97.4 mg kg−1) (Figure 2). The foliar application of SNP was not found to increase the Pb concentration in the foliage or roots (Figure 1 and Figure 2). The effectiveness of the combined chemical application was further demonstrated by the use of the bioaccumulation factor. Plants treated with propiconazole and EDTA and with the addition of SNP had significantly higher bioaccumulation factor compared to control plants (Figure 3). The foliar application of SNP did not increase the bioaccumulation factor (Figure 3). The soil application of propiconazole had a dramatic impact on total AMF colonization in roots (Figure 4). The soil application of propiconazole significantly reduced AMF hyphae colonization to 48% compared to 92.5% in the roots of control plants (Figure 4). It was also observed that the soil application of propiconazole negatively affected AMF fungal structures such as arbuscules. The suppression of AMF activity through the application of propiconazole resulted in greater Pb accumulation in the foliage. Combined foliar application of GA3 and SNP increased Pb concentrations in the foliage. Plants treated with a foliar application of GA3 and SNP (243 mg kg−1) had significantly higher Pb concentrations in the foliage compared to control plants (182 mg kg−1) and plants that received GA3 alone (202 mg kg−1) (Figure 5). Plants that received a foliar application of SNP alone and plants that received GA3 and SNP did not differ significantly in Pb concentration in the foliage (Figure 5). The Pb concentration in leaves increased stepwise between treatments (Figure 5). A linear regression of treatment categories (see Figure 5) vs. foliage Pb concentrations showed a strong relationship (R2 = 0.97) between the average Pb concentration in leaves and treatments.
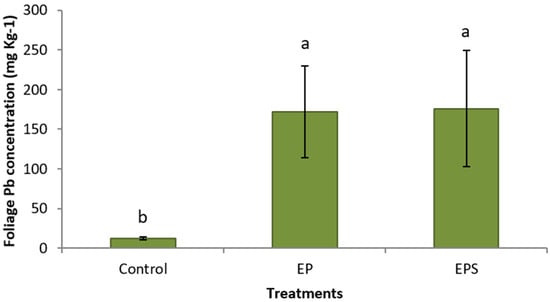
Figure 1.
Mean (+/− SD) foliage Pb concentration (mg kg−1) of Panicum virgatum at time of harvest. Means for columns designated with the same letter are not statistically significantly different (α = 0.05). (Treatments: E—EDTA, P—propiconazole, S—SNP).
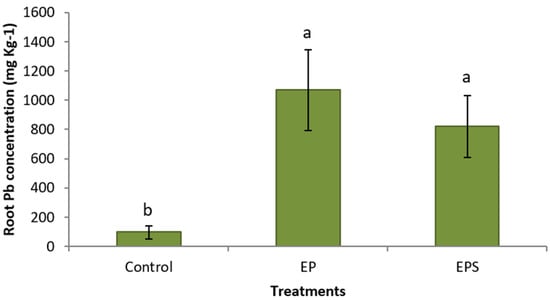
Figure 2.
Mean (+/− SD) root Pb concentration (mg kg−1) of Panicum virgatum at time of harvest. Means for columns designated with the same letter are not statistically significantly different (α = 0.05). (Treatments: E—EDTA, P—propiconazole, S—SNP).
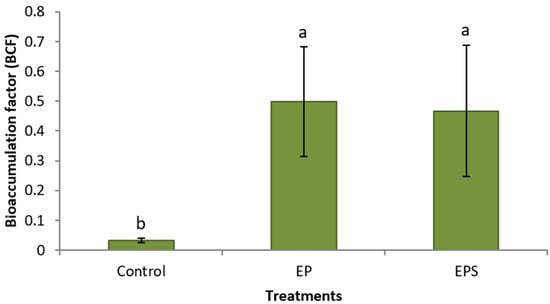
Figure 3.
Mean (+/− SD) bioaccumulation factor (BCF) of Panicum virgatum. Means for columns with same letter not statistically significant (α = 0.05). (Treatments: E—EDTA, P—propiconazole, S—SNP).
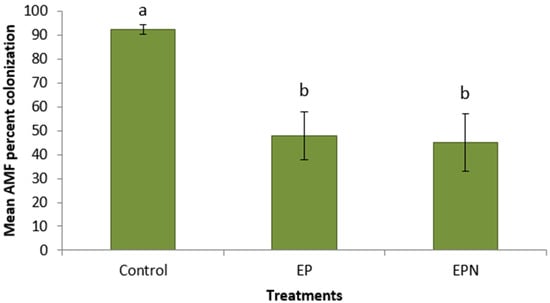
Figure 4.
Average (+/− SD) percentage (%) of total arbuscular mycorrhizal fungi colonization in P. virgatum roots at time of harvest. Means for columns with same letter not statistically significantly different (α = 0.05). (Treatments: E—EDTA, P—propiconazole, N—SNP).
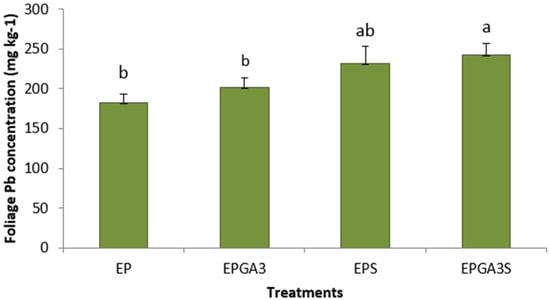
Figure 5.
Mean (+/− SD) foliage Pb concentration (mg kg−1) of Panicum virgatum at time of harvest. Means for columns designated with the same letter are not statistically significantly different (α = 0.05). (Treatments: E—EDTA, P—propiconazole, gibberellic acid—GA3, S—SNP).
4. Discussion
The objective of this study was to examine the impact of exogenous NO donor on plants used in phytoextraction. A foliar application of the NO donor resulted in a significantly higher concentration of Pb in foliage compared to control plants. However, the foliar application of the NO donor SNP in addition to the model chelate agent EDTA and propiconazole fungicide treatments did not affect Pb uptake of plants. Similarly, it was found that SNP application did not have an effect on heavy metal accumulation in bean plants [11]. It was previously suggested that NO plays an important role in maintaining Fe homeostasis and may ameliorate the negative effects of Fe stress in plants [48,81]. This study found no statistically significant differences in shoot and root Fe concentration between SNP treated plants or the control plants. These results are consistent with other study findings showing that although exogenous NO donors can be used to simulate the function of endogenous NO, the application of exogenous NO donors may not change the amount of Fe absorbed into roots or translocated into shoots [48,49]. Overall, the foliar NO donor SNP (0.5 μM) application resulted in a dry mass effect in plants that was not statistically significant compared to the control plants and other treatments that did not receive SNP application. Previous studies have shown that the application of SNP may accelerate switchgrass seed germination, but not plant growth [52,82,83]. Further research into the appropriate timing and application method of exogenous NO donors may suggest more beneficial methods to optimize switchgrass growth in a phytoextraction context, but the results of this study suggest that exogenous NO donors, specifically SNP, applied as foliar spray did not stimulate switchgrass growth or Pb uptake, a finding consistent with [83].
The foliar application of SNP and the plant growth regulator GA3 resulted in significantly higher Pb concentration in the foliage compared to control plants and plants treated with GA3 alone. Although GA3 foliar application did not result in an improved biomass of plants, phytoextraction was improved. Similarly, the foliar application of GA3 was found to enhance phytoextraction by ryegrass (Lolium perenne) [55]. An optimal concentration of GA3 was found to be critical for improving phytoextraction by ryegrass [55]. The foliar application of GA3 was not found to improve any toxic effects on switchgrass although GA3 was previously found to counteract the negative effect of EDTA on the growth of maize (Zea mays) [45].
The phytoextraction of Pb by switchgrass enhanced with chemical applications has many implications in future research for both the phytoremediation and bioenergy industries. This study showed that EDTA treatments increased Pb accumulation by plants and this finding agrees with previous studies [43,84,85]. However, EDTA is persistent in soil and may mobilize Pb and other metals through the soil column and into ground water, thus increasing the risk of human exposure [86,87,88,89]. In addition to its soil effect, EDTA has been observed to negatively impact plant health and reduce biomass [90,91]. Due to these issues, natural acids and other chelates with shorter soil persistence are being studied as alternatives to the synthetic chelator EDTA. A previous study suggests that combined citric acid and soil fungicide application could achieve similar results to EDTA application [85]. Furthermore, the soil application of nitrilotriacetic acid (NTA) and alkyl polyglucoside (APG) has shown improved Pb uptake of plants compared to EDTA [92].
The application of propiconazole demonstrated the ability of this soil fungicides to reduce AMF root colonization. The symbiotic association of AMF and switchgrass provides a barrier against phytoextraction, so fungal suppressants must be applied during phytoextraction. This study showed that plants treated with the fungicide propiconazole exhibited significant decreases in mean AMF percent colonization compared to the control plants. These trends were conserved when observing AMF colonization by fungal structure as well; all propiconazole treatments resulted in significantly reduced vesicle and arbuscle percentages, a finding consistent with [41].
Plants treated with exogenous NO donor showed no growth effects. Optimizing plant biomass is important for maximizing Pb soil remediations and also has applications in the bioenergy industry. While the results of this study showed no significant difference between the control plants and plants treated with exogenous NO donors, no clear consensus on the appropriate timing of exogenous NO donor application exists. This lack of consensus suggests further study into the possible timing of exogenous NO donor applications may be necessary to determine if timing is critical for the production of greater harvestable biomass.
5. Conclusions
This study demonstrated that the phytoextraction of Pb by switchgrass was enhanced by a combined foliar application of the exogenous nitric oxide donor SNP and GA3. These results could aid in the phytoextraction of Pb contaminated soils and bioenergy production. The merits of Pb phytoextraction extend beyond the removal of harmful heavy metals. Switchgrass biomass is currently harvested as a lingo-cellulosic biofuel feedstock. Optimizing biomass production and the Pb uptake of plants has significant implications for phytoextraction and bioenergy production. Cultivating biofuel crops such as switchgrass on contaminated sites for phytoextraction could potentially remediate Pb soil contamination, with the long-term goal of reclaiming the land for future uses.
Author Contributions
Conceptualization, S.G., T.M. and M.K.; methodology, S.G., T.M., M.K.; software, S.G., T.M., M.K.; validation, S.G., T.M. and M.K.; formal analysis, A.B., S.G., M.K.; investigation, A.B., S.G., M.K., T.M.; resources, S.G., M.K.; data curation, S.G.; writing—original draft preparation, A.B.; writing—review and editing, A.B., S.G., T.M. and M.K.; visualization, A.B., S.G.; supervision, S.G., T.M. and M.K.; project administration, S.G., T.M.; funding acquisition, S.G., T.M. and M.K. All authors have read and agreed to the published version of the manuscript.
Funding
U.S. National Science Foundation, Environmental Engineering Program. Award number 1705924.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
This study was supported by a grant from the U.S. National Science Foundation, Environmental Engineering Program, Award number 1705924. We would like to thank Myesha Choudhury for her help.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lagerwerff, J.V.; Specht, A.W. Contamination of roadside soil and vegetation with cadmium, nickel, lead, and zinc. Environ. Sci. Technol. 1970, 4, 583–586. [Google Scholar] [CrossRef]
- Gulson, B.L.; Davis, J.J.; Mizon, K.J.; Korsch, M.J.; Bawden-Smith, J. Sources of lead in soil and dust and the use of dust fallout as a sampling medium. Sci. Total. Environ. 1995, 166, 245–262. [Google Scholar] [CrossRef]
- Mielke, H.W.; Reagan, P.L. Soil is an important pathway of human lead exposure. Environ. Health Perspect. 1998, 106 (Suppl. S1), 217. [Google Scholar] [PubMed] [Green Version]
- Johnson, A.W.; Gutierrez, M.; Gouzie, D.; McAliley, M.L. State of remediation and metal toxicity in the tri-state mining district, USA. Chemosphere 2016, 144, 1132–1141. [Google Scholar] [CrossRef] [PubMed]
- Nacke, H.; Gonçalves, A.; Schwantes, D.; Nava, I.; Strey, L.; Coelho, G. Availability of heavy metals (Cd, Pb, and Cr) in agriculture from commercial fertilizers. Arch. Environ. Contam. Toxicol. 2013, 64, 537–544. [Google Scholar] [CrossRef] [PubMed]
- Deocampo, D.M.; Reed, P.J.; Kalenuik, A.P. Road dust lead (Pb) in two neighborhoods of urban Atlanta, (GA, USA). Int. J. Environ. Res. Public Health 2012, 9, 2020–2030. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Solt, M.J.; Deocampo, D.M.; Norris, M. Spatial distribution of lead in Sacramento, California, USA. Int. J. Environ. Res. Public Health 2015, 12, 3174–3187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mielke, H.W.; Conzales, C.R.; Powell, E.T.; Mielke, P.W. Spatiotemporal dynamic transformations of soil lead and children’s blood lead ten years after Hurricane Katrina: New grounds for primary prevention. Environ. Int. 2016, 94, 567–575. [Google Scholar] [CrossRef]
- EPA (Environmental Protection Agency). Cleaning Up the Nation’s Waste Sites: Markets and Technology Trends; EPA/542/R-96/005; Office of Solid Waste and Emergency Response: Washington, DC, USA, 1997.
- Nevin, R. How lead exposure relates to temporal changes in IQ, violent crime, and unwed pregnancy. Environ. Res. 2000, 83, 1–22. [Google Scholar] [CrossRef] [Green Version]
- Canfield, R.L.; Henderson, R.; Cory-Slechta, D.A.; Cox, C.; Jusko, T.A.; Lanphear, B.P. Intellectual impairment in children with blood lead concentrations below 10 μg per deciliter. N. Engl. J. Med. 2003, 348, 1517–1526. [Google Scholar] [CrossRef] [Green Version]
- Skerfving, S.; Löfmark, L.; Lundh, T.; Mikoczy, Z.; Strömberg, U. Late effects of low blood lead concentrations in children on school performance and cognitive functions. Neurotoxicology 2015, 49, 114–120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bakulski, K.M.; Rozek, L.S.; Dolinoy, D.C.; Paulson, H.L.; Hu, H. Alzheimer’s disease and environmental exposure to lead: The epidemiologic evidence and potential role of epigenetics. Curr. Alzheimer Res. 2012, 9, 563–573. [Google Scholar] [CrossRef]
- Chen, H.; Kwong, J.C.; Copes, R.; Tu, K.; Villeneuve, P.J.; van Donkelaar, A.; Hystad, P.; Martin, R.V.; Murray, B.J.; Jessiman, B.; et al. Living near major roads and the incidence of dementia, Parkinson’s disease, and multiple sclerosis: A population-based cohort study. Lancet 2017, 389, 718–726. [Google Scholar] [CrossRef]
- Fathabadi, B.; Dehghanifiroozabadi, M.; Aaseth, J.; Sharifzadeh, G.; Nakhaee, S.; Rajabpour-Sanati, A.; Amirabadizadeh, A.R.; Mehrpour, O. Comparison of blood lead levels in patients with Alzheimer’s Disease and healthy people. Am. J. Alzheimer’s Dis. Other Dement. 2018, 33, 541–547. [Google Scholar] [CrossRef]
- Malcoe, L.H.; Lynch, R.A.; Keger, M.C.; Skaggs, V.J. Lead sources, behaviors, and socioeconomic factors in relation to blood lead of Native American and white children: A community-based assessment of a former mining area. Environ. Health Perspect. 2002, 110, 221–231. [Google Scholar] [CrossRef]
- Levin, R.; Brown, M.J.; Kashtock, M.E.; Jacobs, D.E.; Whelan, E.A.; Rodman, J.; Schock, M.R.; Padilla, A.; Sinks, T. Lead exposures in US children, 2008: Implications for prevention. Environ. Health Perspect. 2008, 116, 1285–1293. [Google Scholar] [CrossRef] [Green Version]
- Sen, A.; Heredia, N.; Senut, M.C.; Land, S.; Hollocher, K.; Lu, X.; Dereski, M.O.; Ruden, D.M. Multigenerational epigenetic inheritance in humans: DNA methylation changes associated with maternal exposure to lead can be transmitted to the grandchildren. Sci. Rep. 2015, 5, 14466. [Google Scholar] [CrossRef] [Green Version]
- Paulson, J.A.; Brown, M.J. The CDC blood lead reference value for children: Time for a change. Environ. Health 2019, 18, 16. [Google Scholar] [CrossRef] [Green Version]
- Jabeen, R.; Ahmad, A.; Iqbal, M. Phytoremediation of heavy metals: Physiological and molecular mechanisms. Bot. Rev. 2009, 75, 339–364. [Google Scholar] [CrossRef]
- Morgano, M.A.; Teixeira Martins, M.C.; Rabonato, L.C.; Milani, R.F.; Yotsuyanagi, K.; Rodriguez-Amaya, D.B. Inorganic contaminants in bee pollen from Southeastern Brazil. J. Agric. Food Chem. 2010, 58, 6876–6883. [Google Scholar] [CrossRef] [PubMed]
- Lambert, O.; Piroux, M.; Puyo, S.; Thorin, C.; Larhantec, M.; Delbac, F.; Pouliquen, H. Bees, honey and pollen as sentinels for lead environmental contamination. Environ. Pollut. 2012, 170, 254–259. [Google Scholar] [CrossRef]
- Van der Merwe, D.; Carpenter, J.W.; Nietfeld, J.C.; Miesner, J.F. Adverse health effects in Canada geese (Branta canadensis) associated with waste from zinc and lead mines in the Tri-State Mining District (Kansas, Oklahoma, and Missouri, USA). J. Wildl. Dis. 2011, 47, 650–660. [Google Scholar] [CrossRef] [PubMed]
- Beyer, W.N.; Franson, J.C.; French, J.B.; May, T.; Rattner, B.A.; Shearn-Bochsler, V.I.; Warner, S.E.; Weber, J.; Mosby, D. Toxic exposure of songbirds to lead in the southeast Missouri lead mining district. Arch. Environ. Contam. Toxicol. 2013, 65, 598–610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greipsson, S. Phytoremediation. Nat. Educ. Knowl. 2011, 13, 7. [Google Scholar]
- Peer, W.; Baxter, I.; Richards, E.; Freeman, J.; Murphy, A. Phytoremediation and hyperaccumulator plants. In Molecular Biology of Metal Homeostasis and Detoxification; Topics in Current Genetics; Tamas, M.J., Martinoia, E., Klomp, L.W.J., Eds.; Springer: Berlin/Heidelberg, Germany, 2006; Volume 14, pp. 299–340. [Google Scholar]
- Evangelou, M.W.; Ebel, M.; Schaeffer, A. Chelate assisted phytoextraction of heavy metals from soil, effect, mechanism, toxicity, and fate of chelating agents. Chemosphere 2007, 68, 989–1003. [Google Scholar] [CrossRef]
- Zimdahl, R.L.; Skogerboe, R.K. Behavior of lead in soil. Environ. Sci. Technol. 1977, 11, 1202–1207. [Google Scholar] [CrossRef]
- Schooley, T.; Weaver, M.; Mullins, D.; Eick, M. The history of lead arsenate use in apple production: Comparison of its impact in Virginia with other states. J. Pestic. Saf. Educ. 2009, 10, 22–53. [Google Scholar]
- Hovsepyan, A.; Greipsson, S. EDTA-enhanced phytoremediation of lead contaminated soil by corn. J. Plant. Nutr. 2005, 28, 2037–2048. [Google Scholar] [CrossRef]
- López, M.L.; Peralta-Videa, J.R.; Benitez, T.; Gardea-Torresdey, J.L. Enhancement of lead uptake by alfalfa (Medicago sativa) using EDTA and a plant growth promoter. Chemosphere 2005, 61, 595–598. [Google Scholar] [CrossRef]
- Bolan, N.; Kunhikrishnan, A.; Thangarajan, R.; Kumpiene, J.; Park, J.; Makino, T.; Kirkham, M.B.; Scheckel, K. Remediation of heavy metal(oid)s contaminated soil—To mobilize or to immobilize? J. Hazard. Mater. 2014, 266, 141–166. [Google Scholar] [CrossRef]
- Jeffries, P.; Gianinazzi, S.; Perotto, S.; Turnau, K.; Barea, J.M. The contribution of arbuscular mycorrhizal fungi in sustainable maintenance of plant health and soil fertility. Biol. Fertil. Soils 2003, 37, 1–16. [Google Scholar] [CrossRef]
- Reynolds, H.L.; Vogelsang, K.M.; Hartley, A.E.; Bever, J.D.; Schultz, P.A. Variable responses of old-field perennials to arbuscular mycorrhizal fungi and phosphorus source. Oecologia 2006, 147, 348–358. [Google Scholar] [CrossRef] [PubMed]
- Leung, H.-M.; Wang, Z.-W.; Ye, Z.-H.; Yung, K.-L.; Peng, X.-L.; Cheung, K.-C. Interactions between arbuscular mycorrhizae and plants in phytoremediation of metal-contaminated soils: A review. Pedosphere 2013, 23, 549–563. [Google Scholar] [CrossRef]
- Van der Heijden, M.G.A.; Martin, F.M.; Selosse, M.; Sanders, I.R. Mycorrhizal ecology and evolution: The past, the present, and the future. New Phytol. 2015, 205, 1406–1423. [Google Scholar] [CrossRef] [PubMed]
- Clark, R.B. Differences among mycorrhizal fungi for mineral uptake per root length of switchgrass grown in acidic soil. J. Plant. Nutr. 2002, 25, 1753–1772. [Google Scholar] [CrossRef]
- Cabral, L.; Soares, C.R.F.S.; Giachini, A.J.; Siqueira, J.O. Arbuscular mycorrhizal fungi in phytoremediation of contaminated areas by trace elements: Mechanisms and major benefits of their applications. World J. Microbiol. Biotechnol. 2015, 31, 1655–1664. [Google Scholar] [CrossRef] [PubMed]
- Wang, F. Occurrence of arbuscular mycorrhizal fungi in mining impacted sites and their contribution to ecological restoration: Mechanisms and application. Crit. Rev. Environ. Sci. Technol. 2017, 47, 1901–1957. [Google Scholar] [CrossRef]
- Xu, Z.; Ban, Y.; Li, Z.; Chen, H.; Yang, R.; Tang, M. Arbuscular mycorrhizal fungi play a role in protecting roots from Pb damage associated with increased phytochelatin synthase gene expression. Environ. Sci. Pollut. Res. 2014, 22, 12671–12683. [Google Scholar] [CrossRef] [PubMed]
- Calonne, M.; Fontaine, J.; Debiane, D.; Laruelle, F.; Grandmougin, A.; Lounes-Hadj, S.A. Side effects of the sterol biosynthesis inhibitor fungicide, propiconazole, on a beneficial arbuscular mycorrhizal fungus. Commun. Agric. Appl. Biol. Sci. 2010, 76, 891–902. [Google Scholar]
- Hovsepyan, A.; Greipsson, S. Effect of arbuscular mycorrhizal fungi on phytoextraction by corn (Zea mays) of lead-contaminated soil. Int. J. Phytoremediat. 2004, 6, 305–321. [Google Scholar] [CrossRef]
- Perry, V.R.; Krogstad, E.J.; El-Mayas, H.; Greipsson, S. Chemically enhanced phytoextraction of lead-contaminated soils. Int. J. Phytoremediat. 2012, 14, 703–713. [Google Scholar] [CrossRef]
- Namdjoyan, S.; Kermanian, H. Exogenous nitric oxide (as sodium nitroprusside) ameliorates arsenic-induced oxidative stress in watercress (Nasturtium officinale R. Br.) plants. Sci. Hortic. 2013, 161, 350–356. [Google Scholar] [CrossRef]
- Hadi, F.; Bano, A.; Fuller, M.P. The improved phytoextraction of lead (Pb) and the growth of maize (Zea mays L.): The role of plant growth regulators (GA3 and IAA) and EDTA alone and in combinations. Chemosphere 2010, 80, 457–462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neill, S.J.; Desikan, R.; Clarke, A.; Hurst, R.D.; Hancock, J.T. Hydrogen peroxide and nitric oxide as signaling molecules in plants. J. Exp. Bot. 2002, 53, 1237–1247. [Google Scholar] [CrossRef] [PubMed]
- Khan, J.; Malangisha, G.K.; Ali, A.; Mahmoud, A.; Yang, J.; Zhang, M.; Hu, Z. Nitric oxide alleviates lead toxicity by inhibiting lead translocation and regulating root growth in watermelon seedlings. Hortic. Environ. Biotechnol. 2021. [Google Scholar] [CrossRef]
- Graziano, M.; Beligni, M.V.; Lamattina, L. Nitric oxide improves internal iron availability in plants. Plant. Physiol. 2002, 130, 1852–1859. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Graziano, M.; Lamattina, L. Nitric oxide and iron in plants: An emerging and converging story. Trends Plant. Sci. 2005, 10, 4–8. [Google Scholar] [CrossRef] [PubMed]
- Besson-Bard, A.; Gravot, A.; Richaud, P.; Auroy, P.; Duc, C.; Gaymard, F.; Taconnat, L.; Renou, J.P.; Pugin, A.; Wendehenne, D. Nitric oxide contributes to cadmium toxicity in Arabidopsis by promoting cadmium accumulation in roots and by up-regulating genes related to iron uptake. Plant. Physiol. 2009, 149, 1302–1315. [Google Scholar] [CrossRef] [Green Version]
- Lehotai, N.; Petô, A.; Weisz, M.; Erdei, L.; Kolbert, Z. Generation of reactive oxygen and nitrogen species in pea cultivars under copper exposure. Acta Bioligica. Szeged. 2011, 55, 273–278. [Google Scholar]
- Phang, C.; Leung, D.W.; Taylor, H.H.; Burritt, D.J. The protective effect of sodium nitroprusside (SNP) treatment on Arabidopsis thaliana seedlings exposed to toxic levels of Pb is not linked to avoidance of Pb uptake. Ecotoxicol. Environ. Saf. 2011, 74, 1310–1315. [Google Scholar] [CrossRef]
- Mihailovic, N.; Drazic, G. Incomplete alleviation of nickel toxicity in bean by nitric oxide supplementation. Plant. Soil Environ. 2011, 57, 396–401. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, C.; Vandenberghe, L.P.S.; de Oliveira, J.; Soccol, C.R. New perspectives of gibberellic acid production: A review. Crit. Rev. Biotechnol. 2012, 32, 263–273. [Google Scholar] [CrossRef] [PubMed]
- He, S.; He, Z.; Wu, Q.; Lei, W.; Zhang, X. Effects of GA3 on plant physiological properties, extraction, subcellular distribution and chemical forms of Pb in Lolium perenne. Int. J. Phytoremediat. 2015, 17, 1153–1159. [Google Scholar] [CrossRef] [PubMed]
- Gleeson, A.M. Phytoextraction of Lead from Contaminated Soil by Panicum virgatum L. and Associated Growth Responses. Master’s Thesis, Department of Biology, Queen’s University, Kingston, ON, Canada, 2007. [Google Scholar]
- Parrish, D.J.; Fike, J.H. The biology and agronomy of switchgrass for biofuels. Crit. Rev. Plant. Sci. 2005, 24, 423–459. [Google Scholar] [CrossRef]
- Briske, D.D. Developmental morphology and physiology of grasses. In Grazing Management: An Ecological Perspective; Timber Press: Portland, OR, USA, 1991; pp. 85–108. [Google Scholar]
- Levy, D.B.; Redente, E.F.; Uphoff, G.D. Evaluating the phytotoxicity of Pb-Zn tailings to big bluestem (Andropogon gerardii Vitman) and switchgrass (Panicum virgatum L.). Soil Sci. 1999, 164, 363–375. [Google Scholar] [CrossRef]
- Smith, L.L.; Allen, D.J.; Barney, J.N. Yield potential and stand establishment for 20 candidate bioenergy feedstocks. Biomass Bioenergy 2015, 73, 145–154. [Google Scholar] [CrossRef]
- Lewandowski, I.; Scurlock, J.M.; Lindvall, E.; Christou, M. The development and current status of perennial rhizomatous grasses as energy crops in the US and Europe. Biomass Bioenergy 2003, 25, 335–361. [Google Scholar] [CrossRef]
- Balsamo, R.A.; Kelly, W.J.; Satrio, J.A.; Ruiz-Felix, M.N.; Fetterman, M.; Wynn, R.; Hagel, K. Utilization of grasses for potential biofuel production and phytoremediation of heavy metal contaminated soils. Int. J. Phytoremediat. 2014, 17, 448–455. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.G.; Li, X.D.; Wang, C.C.; Chen, H.M.; Chua, H. Lead phytoextraction from contaminated soil with high-biomass plant species. J. Environ. Qual. 2002, 31, 1893–1900. [Google Scholar] [CrossRef] [PubMed]
- Van Ginneken, L.; Meers, E.; Guisson, R.; Ruttens, A.; Elst, K.; Tack, F.M.G.; Vangronsveld, J.; Diels, L.; Dejonghe, W. Phytoremediation for heavy metal-contaminated soils combined with bioenergy production. J. Environ. Eng. Landsc. Manag. 2007, 15, 227–236. [Google Scholar] [CrossRef] [Green Version]
- Hernández-Allica, J.; Becerril, J.M.; Garbisu, C. Assessment of the phytoextraction potential of high biomass crop plants. Environ. Pollut. 2008, 152, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.C.; Lai, H.Y.; Juang, K.W. Model evaluation of plant metal content and biomass yield for the phytoextraction of heavy metals by switchgrass. Ecotoxicol. Environ. Saf. 2012, 80, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Oh, K.; Li, T.; Cheng, H.; Hu, X.; He, C.; Yan, L.; Shinichi, Y. Development of profitable phytoremediation of contaminated soils with biofuel crops. J. Environ. Prot. 2013, 4, 58–64. [Google Scholar] [CrossRef]
- Qin, Z.; Zhaung, Q.; Cai, X. Bioenergy crop productivity and potential climate change mitigation from marginal lands in the United States: An ecosystem modeling perspective. GCB Bioenergy 2015, 7, 1211–1221. [Google Scholar] [CrossRef] [Green Version]
- McLaughlin, S.B.; Kszos, L.A. Development of switchgrass (Panicum virgatum) as a bioenergy feedstock in the United States. Biomass Bioenergy 2005, 28, 515–535. [Google Scholar] [CrossRef]
- Hancock, D.W. The Management and Use of Switch-Grass in Georgia; Extension Service Bulletin 1358; University of Georgia, College of Agricultural and Environmental Sciences: Athens, Georgia, 2009. [Google Scholar]
- Rose, S. Anion adsorption and desorption characteristics of a piedmont ultisol: Some implications for the fate of sulfate deposition. Water Air Soil Pollut. 1998, 101, 333–347. [Google Scholar] [CrossRef]
- Franklin, D.H.; West, L.T.; Radcliffe, D.E.; Hendrix, P.F. Characteristics and genesis of preferential flow paths in a piedmont ultisol. Soil Sci. Soc. Am. J. 2007, 71, 752–758. [Google Scholar] [CrossRef]
- Northcott, G.L.; Jones, K.C. Experimental approaches and analytical techniques for determining organic compound bound residues in soil and sediment. Environ. Pollut. 2000, 108, 19–43. [Google Scholar] [CrossRef]
- Ötvös, K.; Pasternak, T.P.; Miskolczi, P.; Domoki, M.; Dorjgotov, D.; Bottka, S.; Dudits, D.; Fehér, A. Nitric oxide is required for, and promotes auxin-mediated activation of, cell division and embryogenic cell formation but does not influence cell cycle progression in alfalfa cell cultures. Plant. J. 2005, 43, 849–860. [Google Scholar] [CrossRef]
- Gupta, D.K.; Huang, H.G.; Corpas, F.J. Lead tolerance in plants: Strategies for phytoremediation. Environ. Sci. Pollut. Res. 2013, 20, 2150–2161. [Google Scholar] [CrossRef]
- Saxena, I.; Shekhawat, G.S. Nitric oxide (NO) in alleviation of heavy metal induced phytotoxicity and its role in protein nitration. Nitric Oxide 2013, 32, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Koske, R.E.; Gemma, J.N. A modified procedure for staining roots to detect VA mycorrhizas. Mycol. Res. 1989, 92, 486–488. [Google Scholar] [CrossRef]
- Sun, X.G.; Tang, M. Comparison of four routinely used methods for assessing root colonization by arbuscular mycorrhizal fungi. Botany 2012, 90, 1073–1083. [Google Scholar] [CrossRef]
- McGrath, S.P.; Zhao, F.J. Phytoextraction of metals and metalloids from contaminated soils. Curr. Opin. Biotechnol. 2003, 14, 277–282. [Google Scholar] [CrossRef]
- Sun, Y.; Zhou, Q.; An, J.; Liu, W.; Liu, R. Chelator-enhanced phytoextraction of heavy metals from contaminated soil irrigated by industrial wastewater with the hyperaccumulator plant (Sedum alfredii Hance). Geoderma 2009, 150, 106–112. [Google Scholar] [CrossRef]
- Ramirez, L.; Simontacchi, M.; Murgia, I.; Zabaleta, E.; Lamattina, L. Nitric oxide, nitrosyl iron complexes, ferritin and frataxin: A well-equipped team to preserve plant iron homeostasis. Plant. Sci. 2011, 181, 582–592. [Google Scholar] [CrossRef]
- Kopyra, M.; Gwóźdź, E.A. Nitric oxide stimulates seed germination and counter active inhibitory effect of heavy metals and salinity on root growth of Lupinus luteus. Plant. Physiol. Biochem. 2003, 41, 1011–1017. [Google Scholar] [CrossRef]
- Sarath, G.; Bethke, P.C.; Jones, R.; Baird, L.M.; Hou, G.; Mitchell, R.B. Nitric oxide accelerates seed germination in warm-season grasses. Planta 2006, 223, 1154–1164. [Google Scholar] [CrossRef] [Green Version]
- Johnson, D.; Deocampo, D.; El-Mayas, H.; Greipsson, S. Induced phytoextraction of lead through chemical manipulation of switchgrass and corn; role of iron supplement. Int. J. Phytoremediat. 2016, 17, 1192–1203. [Google Scholar] [CrossRef]
- Aderholt, M.; Vogelien, D.L.; Koether, M.; Greipsson, S. Phytoextraction of contaminated urban soil by Panicum virgatum L. enhanced with application of a plant growth regulator (BAP) and citric acid. Chemosphere 2017, 175, 85–96. [Google Scholar] [CrossRef]
- Bucheli-Witschel, M.; Egli, T. Environmental fate and microbial degradation of aminopolycarboxylic acids. FEMS Microbiol. Rev. 2001, 25, 69–106. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Zhao, F.J.; Lombi, E.; McGrath, S.P. Leaching of heavy metals from contaminated soils using EDTA. Environ. Pollut. 2001, 113, 111–120. [Google Scholar] [CrossRef]
- Oviedo, C.; Rodríguez, J. EDTA: The chelating agent under environmental scrutiny. Quim. Nova 2003, 26, 901–905. [Google Scholar] [CrossRef] [Green Version]
- de Araújo, J.D.C.T.; do Nascimento, C.W.A. Phytoextraction of lead from soil from a battery recycling site: The use of citric acid and NTA. Water Air Soil Pollut. 2010, 211, 113–120. [Google Scholar] [CrossRef]
- Geebelen, W.; Vangronsveld, J.; Adriano, D.C.; Van Poucke, L.C.; Clijsters, H. Effects of Pb-EDTA and EDTA on oxidative stress reactions and mineral uptake in Phaseolus vulgaris. Physiol. Plant. 2002, 115, 377–384. [Google Scholar] [CrossRef]
- Hasegawa, H.; Rahman, M.A.; Saitou, K.; Kobayashi, M.; Okumura, C. Influence of chelating ligands on bioavailability and mobility of iron in plant growth media and their effect on radish growth. Environ. Exp. Bot. 2011, 71, 345–351. [Google Scholar] [CrossRef] [Green Version]
- Hart, G.; Koether, M.; McElroy, T.; Greipsson, S. Evaluation of chelating agents used in phytoextraction by switchgrass of lead (Pb) contaminated soils. J. Environ. Chem. Eng. 2021, submitted. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).