Abstract
The present study explored the plant growth promotion and bioremediation potential of bacteria inhabiting wastewater irrigated agricultural soils. Thirty out of 75 bacterial isolates (40%), 29/75 (39%) and 28/75 (37%) solubilized Zn, K and PO4 during plate essays respectively. Fifty-six percent of the isolates produced siderophores, while 30% released protease in vitro. Seventy-four percent of bacteria resisted Pb, Ni and Cd at various concentrations added to the culture media plates. Sixteen out of 75 (26%) isolates were able to fix N in Nbf medium. Among these 16 N fixers, N fixing nifH, nifD and nifK genes was detected through PCR in 8, 7 and 1 strain respectively using gene specific primers designed in the study with Enterobacter sp. having all three (nifHKD) genes. Isolated bacteria showed resemblance to diverse genera such as Bacillus, Pseudomonas, Enterobacter, Citrobacter, Acinetobacter, Serratia, Klebsiella and Enterococcus based on 16S rRNA gene sequence analysis. In addition to showing the best mineral solubilization and metal resistance potential, Citrobacter sp. and Enterobacter sp. also removed 87%, 79% and 43% and 86%, 78% and 51% of Ni, Cd and Pb, respectively, from aqueous solution. These potent bacteria may be exploited both for bioremediation and biofertilization of wastewater irrigated soils leading to sustainable agriculture.
1. Introduction
Agriculture plays a critical role in the economy of many countries. However, the agricultural sector is facing several challenges including scarcity of fresh water and fertilizers and increased cost of agricultural inputs [1]. Reuse of sewage and industrial wastewater is a potential solution to limited availability of freshwater [2]. In developing countries, due to the lack of treatment facilities, municipal and industrial wastewater is disposed of directly into streams and rivers or is used to fertilize and irrigate crops. As wastewater is a huge source of different heavy metals, pathogens as well as organic contaminants, its irrigation affects soil productivity and health of living organisms [3]. Even at a low concentration, heavy metals persist for a long period in soil from where they can enter the food chain [4]. In addition, application of chemical fertilizers may worsen the conditions by adding further chemicals into the soil.
Various chemical (bio-reduction and chelate extraction) [5] and physical methods (soil leaching and absorbent fixation method) [6] are used to remediate contaminated soils. Use of chelators is inevitable in all these methods, but excessive use of chelators pollutes soil and groundwater [7]. These conventional methods are also expensive, non- eco-friendly and need experts to operate machines involved [8]. Moreover, physicochemical methods do not completely eradicate pollution but only change the nature of the problem [9]. Thus, sustainable agriculture requires alternative means of bioremediation. In this scenario, biological methods for remediation (bioremediation) may offer a cheap alternative [10]. Bioremediation processes include bioventing, bioleaching, landfarming, bioaugmentation, biostimulation and phytoremediation [11]. Microbial remediation is a biological method for the removal of toxic metals using microorganisms such as bacteria, fungi and algae [12].
Soil microbes are proven to affect metal bioavailability and mobility through redox changes, acidification, producing iron chelators, transforming metals to less or non-toxic forms (biotransformation) [13], trapping these metals inside their cells (bioaccumulation) [14] and by binding metals within their cell wall (biosorption) [15]. Among microbes, metal resistant plant growth promoting (PGP) bacteria may serve as potential candidates for bioremediation and biofertilization thus mitigating the use of chemical fertilizers in addition to soil cleanup. These bacteria can carry out detoxification of toxic metals, N-fixation, siderophore and phytohormone production and transformation of nutrient elements [16]. Thus, metal resistant PGP bacteria, specifically indigenous PGP bacteria, can improve the efficacy of metal removal from soils and increase plant yield [17]. Therefore, to achieve the goals of agricultural sustainability, there is a need to isolate and screen potent PGP bacteria that can tolerate high concentrations of toxic metals, which after being inoculated may serve the dual purpose of bioremediation and biofertilization [18] of wastewater irrigated soils. These metal tolerant PGP bacteria prove useful in reducing the cost and labor of maintaining soil health and fertility.
As bacteria adapt to various toxins and heavy metals and possess PGP traits, we predicted that bacteria present in wastewater irrigated soils would be resistant to heavy metals and that they may help in plant growth promotion by detoxifying harmful metals and by solubilizing plant nutrients and producing various phytohormones and enzymes. To test this hypothesis, we characterized bacteria isolated from wastewater irrigated agricultural soils and studied their potential for nutrient solubilization, nitrogen fixation and enzyme production as well as their resistance and biosorption ability for selected heavy metals. Most earlier studies have either studied the biofertilization or bioremediation potential of bacteria. Dual potential of bacteria for biofertilization as well as bioremediation has been scarcely explored, especially in Pakistan. Thus, identification of potent strains in the current study could be a step towards the development of a bio-inoculum for bioremediation and biofertilization of wastewater irrigated soils.
2. Materials and Methods
2.1. Soil Sampling
To isolate potent bacteria, surface soil samples (0–15 cm) were collected from wastewater irrigated agricultural soils of major industrial cities of Punjab, Pakistan, i.e., Lahore (LHE), Gujranwala (GRN), Gujrat (GJT), Sialkot (SKT), Faisalabad (FSD) and Wazirabad (WZB) (Table 1). Five random sub-samples were collected with the help of a wooden core borer from each sampling site within a radius of 500 m. Each sample was taken at least 30 m away from the other. The soil samples were placed in sterile zip lock bags and shipped to Applied Microbiology and Biotechnology Lab, COMSATS University, Islamabad, Pakistan, on ice and were stored at 4 °C until further use. Gravel and stones were removed from the soils and samples from same representative location were combined to make a composite sample. The physicochemical analysis of soil samples was carried out according to the standard methods [19] (Supplementary Materials Table S1).

Table 1.
Location and major industrial units of sampling cities.
2.2. Isolation of Bacteria
Bacteria were isolated from soil using a serial dilution method. Briefly, 10 g of soil was added to 90 mL of 1% normal saline solution in a 250 mL flask. The flask was then shaken for 20 min on a rotary shaker. One mL of the suspension was taken out of the flask and added to 9 mL of normal saline solution, and serial dilutions were made up to 10−9 dilution. One mL of each dilution was spread on the nutrient agar and MacConkey agar media plates and were incubated for up to 3 days at 28 ± 2 °C to observe bacterial growth [20]. Bacterial colonies were studied for their colony morphology such as size, shape, margins, elevations, texture and opacity. Single colonies were re-streaked on to fresh LB agar plates and incubated under similar conditions. The process was repeated three times to purify the colonies. Bacterial colonies were preserved at −20 °C in 50% sterile glycerol solution for future use.
2.3. Screening of Heavy Metal Tolerant Plant Growth Promoting Bacteria
Plant growth promoting traits of isolated bacteria such as nutrients (Zn, K and PO4) solubilization, siderophore and protease production and N-fixation was carried out through plate assays. Bacteria with PGP traits were further explored for their resistance to Pb, Ni and Cd and removal of these metals from aqueous solution.
2.4. Detection of Plant Growth Promoting Traits
2.4.1. Nutrient Solubilization
Nutrient (Zn, K and PO4) solubilizing ability of bacteria isolated from wastewater irrigated agricultural soils was studied on modified Bunt and Rovira agar [21], Aleksandrov agar [22] and Pikovskaya agar [23], respectively. Bacterial strains were grown for 18 h in LB broth, and 3 µL of liquid culture was spotted on respective nutrient solubilizing agar media plates. The inoculated agar plates were incubated at 28 ± 2 °C for 72 h. A clear halo zone around bacterial colonies was indicative of their ability to solubilize the nutrient. All the experiments were carried out in triplicate. Nutrient solubilizing index for isolated bacteria was calculated by the following formula [24]:
2.4.2. Detection of Nitrogen Fixation Potential
Nitrogen-free malate semi solid medium (Nfb) was used to assess the N-fixation ability of the isolated bacteria. Test tubes containing Nfb media were inoculated with freshly grown bacterial culture using a stabbing needle. After inoculation, test tubes were incubated for 3 to 10 days at 28 ± 2 °C. Uninoculated Nfb medium was used as a control. Formation of a blue colored pellicle zone indicated N-fixation ability of bacterial isolates [25].
2.4.3. Siderophore and Protease Production
Production of protease and siderophores by bacterial isolates was detected by following the methods described earlier [26,27]. Briefly, siderophore production ability of bacterial isolates was determined by a Chrome Azurol S assay and the appearance of an orange zone around bacterial colonies indicated the secretion of siderophores. Protease production was studied by inoculating the bacteria on nutrient agar with added skimmed milk powder. Formation of a halo zone around bacterial colonies provided evidence of enzyme production. Solubilization and discoloration index were calculated as described in Section 2.4.1.
2.5. Heavy Metal Tolerance Assay
2.5.1. Preparation of Stock Solutions
Stock solutions of selected heavy metals (Pb, Ni and Cd) were prepared by dissolving their respective analytical grade salts, i.e., Pb(NO3)2, NiSO4.6H2O and CdCl2.2H2O, in MilliQ water. The solutions were sterilized by filtration using Millipore membrane (0.22 µm pore size) and stored at 4 °C. All the glassware used in the experiment was acid washed to avoid metal binding.
2.5.2. Determination of Minimum Inhibitory Concentration
Minimum inhibitory concentration (MIC) of above-mentioned heavy metals for bacterial isolates with at least one tested plant growth promoting trait was determined with the plate dilution method [28]. Various concentrations of heavy metals ranging from 0.1 to 8 mM were made by complementing respective heavy metal salt solutions in LB agar inoculated with 18 h old bacterial culture. Inoculated LB plates were incubated at 28 ± 2 °C for 48 h. In all the experiments, positive control plates were prepared without supplemented heavy metals. Minimum inhibitory concentration for each bacterial isolate was determined as the lowest concentration at which bacteria failed to grow completely.
2.6. In Vitro Heavy Metal Removal Assay
A scoring criterion was developed for bacterial strains (Supplementary Materials Table S2). Bacterial strains with highest overall score for plant growth promotion and minimum inhibitory concentration value of tested heavy metals were further assessed for their metal removal potential from aqueous solution. For this, bacterial cultures were incubated at 28 ± 2 °C for 24 h on a shaking incubator at 120 rpm until the logarithmic phase was attained (5 × 108 cells mL−1). Bacterial cells were centrifuged at 7000× g for 10 min, washed twice with phosphate buffer and were then suspended in the same buffer. Cells were inoculated into 100 mL of LB broth in a 250 mL conical flask supplemented with 10 mgL−1 of each of Pb, Ni and Cd. Flasks were then incubated at 28 ± 2 °C and agitated at 150 rpm. After 24 h of incubation, cells were harvested by centrifugation under the same experimental conditions. The amount of remaining metal present in the supernatant was measured with an atomic absorption spectrophotometer. Control sets, without added bacterial cells, were used to compare and obtain the net value of the metal uptake by bacteria. All the experiments were performed in triplicate.
where:
- Ci = initial concentration of heavy metal (control).
- C = concentration of heavy metal in the supernatant after incubation.
2.7. Molecular Identification of Bacterial Strains
Bacterial isolates showing both PGP traits and heavy metal tolerance were identified by 16S rRNA gene sequence analysis. DNA of bacterial isolates was extracted using the CTAB method with some modifications, and 16S rRNA gene amplification was carried out using P1 and P6 primers [29]. Polymerase chain reaction (PCR) included template DNA (40–50 ng), Taq polymerase (1.5 unit), dNTPs (150 uM), MgCl2 (1.5 mM) and 20 pmol of each of forward and reverse primer. The gene was amplified using a thermocycler (PeqLab, Erlangen, Germany) under conditions as described by Tan et al. [29]. Polymerase chain reaction products were purified using a GeneJET PCR purification kit (Thermo Scientific, Waltham, MA, USA) following the manufacturer’s instructions and sent to Macrogen Inc., Seoul, Korea, for sequencing. Bacterial taxonomy was established using the Basic Local Alignment Search Tool (BLAST) algorithm and submitted to NCBI GenBank. Accession numbers of the submissions were also obtained. The phylogenetic tree of bacterial 16S rRNA sequences was obtained using the neighbor-joining method in MEGA X.
2.8. Detection of Nitrogen Fixing (nif) Genes in Putative Nitrogen Fixing Bacteria
Based on 16S rRNA gene sequence identification, species specific primers for bacterial N-fixation genes nifH, nifD and nifK were designed in this study by retrieving the reported sequences from GenBank and aligning at clustal W. Amplification was carried out in a 25 µL reaction mixture containing 2 µL (3.6 ng µL−1) of template DNA; 200 µM each of dTTP, dCTP, dGTP and dATP; 1.5 U Taq polymerase; 1.5 mM MgCl2; 40 µg of both forward and reverse primers and PCR buffer. The amplification was performed using a thermocycler with conditions of initial denaturation at 96 °C for 5 min followed by 30 cycles (96 °C for 30 s, annealing temperature for 45 s and 72 °C for 90 s) and final extension at 72 °C for 10 min (Table 2).

Table 2.
Primer sequences, PCR conditions for the amplification and amplicon size of nitrogen fixation (nif) genes.
2.9. Statistical Analysis
Data were statistically analyzed by applying one-way ANOVA, and comparison between mean values was conducted using Statistix 8.1. Significance of difference among treatments was tested by using the least significant difference (LSD) at significance level of p ≤ 0.05.
3. Results
Overall, a total of 75 bacterial isolates were obtained from sampling cities (Supplementary Materials Table S3), of which 14 each were isolated from of Lahore, Faisalabad and Sialkot. Thirteen isolates were obtained from Gujrat followed by 10 isolates each from Gujranwala and Wazirabad.
3.1. Nutrient Solubilization
Thirty out of 75 (40%) bacterial isolates demonstrated Zn solubilization activity by solubilizing insoluble ZnO and producing solubilization indices ranging from 2.2 to 4.8. Higher solubilization indices (SI) of 4.8 and 4.6 were observed for bacterial strains LWN1 and GWN3 respectively followed by GWM3 and WWN7 which showed an SI of 4.1. An intermediate SI of 3.7 was shown by SWN4, SWM6 and JWN5 followed by GWN2 and GWM1 showing SI of 3.5 and 3.4 respectively. Minimum SI of 2.2 was observed for WWN3 (Figure 1a).
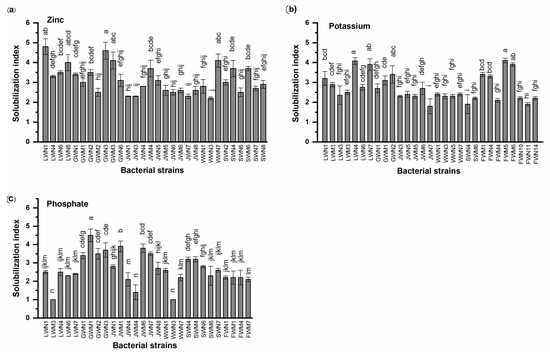
Figure 1.
Nutrient solubilization by bacterial strains isolated from wastewater irrigated agricultural soils of major industrial cities of Punjab, Pakistan. Values are mean of three replicates. Bars represent standard errors. Different letters above bars indicate significant differences between treatments at p < 0.05 level. (a) Zinc solubilization (b) Potassium solubilization (c) Phosphate solubilization.
Similarly, 29/75 (39%) bacterial isolates were able to solubilize K. Maximum SI was observed in the case of FWM5 and LWN4. Both strains showed an SI of 4.1 followed by LWN7 and FWM6 with an SI of 3.9. Intermediate K solubilization ability was demonstrated by FWM1 (SI 3.9), FWN4 (SI 3.4) and LWN1 (SI 3.2). Minimum SI of 1.8 was shown by bacterial strain JWN7 (Figure 1b).
In addition, 28/75 (37%) isolates were able to solubilize PO4 showing a solubilization index ranging from 1 to 4.5. Maximum SI of 4.5 was shown by bacterial isolate GWM1. Among others notable PO4 solubilizers, isolates JWM1 and JWM6 showed SI of 3.9 and 3.8, respectively. Minimum solubilization index of 1 was shown by bacterial isolate WWN3 (Figure 1c).
3.2. Nitrogen Fixation Potential by Plate Assay
Among all the isolates tested, only 16/75 (26%) showed nitrogenase activity by changing the color of Nfb medium from green to blue. Five strains showed color change after 72 h of incubation, six bacterial strains displayed nitrogenase activity on the 7th day while five bacterial isolates did the same after 10 days of incubation (Figure S1). Uninoculated NFb medium was kept as a negative control, which remained green indicating no nitrogenase activity.
3.3. Siderophore and Protease Production
Isolated bacteria also showed protease and siderophore production ability. Siderophore production was shown by 42/75 (56%) bacterial isolates. Higher siderophore production indices were observed for bacterial isolates FWM7 (SI 4.9), JWN3 (SI 4.8) and GWM2 (SI 4.7) followed by LWM3 and GWM1 showing siderophore production indices of 4.2 and 4.1 respectively. Minimum siderophore production index of 2.1 was shown by bacterial isolate SWN2 (Figure 2a).
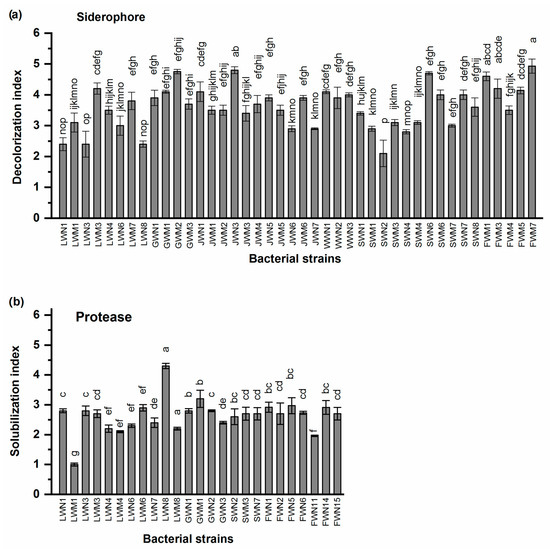
Figure 2.
(a) Siderophore production and (b) protease production ability of bacterial strains isolated from wastewater irrigated agricultural soils of major industrial cities of Punjab, Pakistan. Values are mean of three replicates. Bars represent standard errors. Different letters above bars indicate significant differences between treatments at p < 0.05 level.
Protease production was shown by 25/75 (33%) bacterial strains. Maximum protease production index of 4.3 was shown by bacterial strain LWN8. Bacterial strain GWM1 showed intermediate protease production index of 3.2, while LWM1 showed minimum protease production index of 1 (Figure 2b).
3.4. Minimum Inhibitory Concentration of Tested Heavy Metals
Bacterial isolates with PGP potential also showed resistance to various concentration of selected heavy metals (Figure 3a). Minimum inhibitory concentration of tested heavy metals for isolated bacteria ranged from 0.2 to 8 mM (Supplementary Materials Table S4). Bacterial strain FWN9 showed maximum resistance to all the three heavy metals used in the study. Five isolates were able to tolerate 8 mM of Ni, while 14 isolates were able to tolerate Pb at 8 mM. On the other hand, only one strain was able to tolerate 6 mM of Cd. Thus, Cd was the most toxic among tested metals, followed by Ni and Pb.
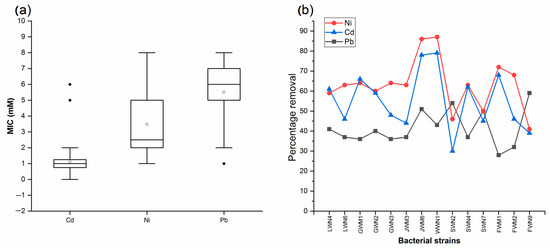
Figure 3.
(a) Boxplot of MIC values for bacterial strains grown on heavy metal supplemented LB agar. (b) Net heavy metal removal ability of bacterial strains isolated from wastewater irrigated agricultural soils of major industrial cities of Punjab, Pakistan. Control removal: Cd = 0.9%, Ni = 0.8% and Pb = 0.5%.
3.5. In Vitro Heavy Metal Removal by Bacterial Strains
Bacterial strains with good PGP score and higher minimum inhibitory concentrations for tested heavy metals were selected for their heavy metal removal ability. Removal by control samples was 0.5% for Cd, 0.7% for Ni and 0.9% for Pb. Bacterial strain WWN1 showed maximum net removal of 87% for Ni and 79% for Cd from the aqueous solution followed by JWM6 which showed 86% and 78% net removal of Ni and Cd, respectively. On the other hand, maximum net removal of Pb (59%) was observed for bacterial strain FWN9. Hence, among all three heavy metals, Pb showed the least biosorption (Figure 3b).
3.6. Molecular Identification of Bacterial Isolates
Bacterial isolates belonged to various genera, i.e., Citrobacter, Serratia (16%), Acinetobacter, Enterobacter, Escherichia (12%), Bacillus, Pseudomonas, Klebsiella (8%) and Enterococcus (4%) (Table 3). Eighty-eight percent of the bacteria were Gram-negative. Figure 4 and Figure 5 illustrate the phylogenetic tree and relative plant growth promoting score and heavy metals minimum inhibitory concentration of bacterial strains respectively.

Table 3.
Basic Local Alignment Search Tool (BLAST) homology of 16S rRNA genes of bacterial strains with PGP traits and heavy metal resistance isolated from wastewater irrigated agricultural soils of major industrial cities of Punjab, Pakistan.
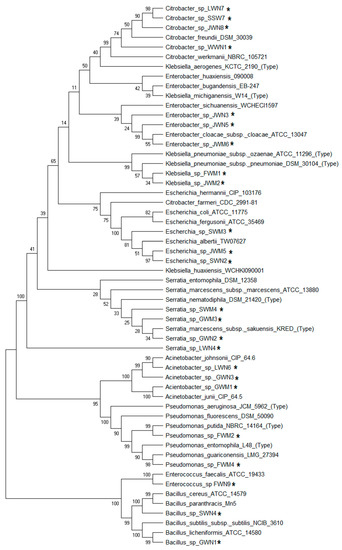
Figure 4.
Phylogenetic tree of bacterial strains isolated from wastewater irrigated agricultural soils of major industrial cities of Punjab, Pakistan, showing plant growth promotion and heavy metal resistance based on the 16S rRNA gene constructed using MEGA6. All the sequences were aligned through MUSCLE, edited manually and trimmed from start to finish to equalize the sequence lengths. Phylogenetic trees were constructed using the neighbor-joining method with a bootstrap value of 1000. * shows strains isolated in the current study.
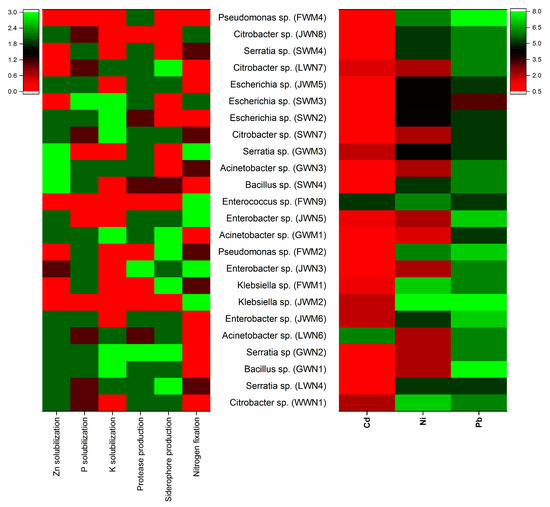
Figure 5.
Heatmaps indicating the relative plant growth promoting score and heavy metal minimum inhibitory concentration of bacterial strains isolated from wastewater irrigated agricultural soils of major industrial cities of Punjab, Pakistan. Green corresponds to the maximum value, and red corresponds to the minimum value within each trait.
3.7. Amplification of Nitrogen Fixing (nif) Gene
Bacterial strains showing nitrogen fixation ability on semi solid medium were further studied for nif gene amplification. Approximately 818 bp of the nifH gene amplicon was observed in eight bacterial strains, i.e., JWM2 (Klebsiella sp.), JWN3 (Enterobacter sp.), JWN8 (Citrobacter sp.), JWN5 (Enterobacter sp.), JWN3 (Enterobacter sp.), LWN4 (Serratia sp.), LWN6 (Klebsiella sp.) and SWN7 (Citrobacter sp.). nifD with amplicon size of 1340 bp was amplified in seven bacterial strains, i.e., JWN3 (Enterobacter sp.), SWM3 (Escherichia sp.), JWM2 (Klebsiella sp.), LWN4 (Serratia sp.), SWN7 (Citrobacter sp.), LWN6 (Klebsiella sp.) and JWN5 (Enterobacter sp.) while nifK gene with amplicon size of 1065 bp was detected only in JWN3 (Enterobacter sp.) (Figure 6).
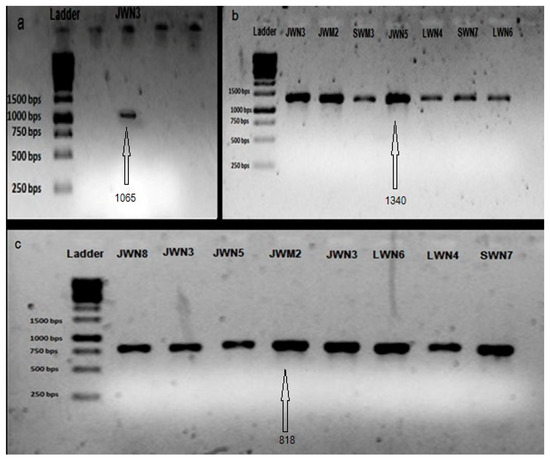
Figure 6.
Amplification of (a) nifK, (b) nifH and (c) nifD of bacterial strains isolated from wastewater irrigated agricultural soils of major industrial cities of Punjab, Pakistan.
The number of bacterial isolates with Zn, K and P solubilization potential, N-fixation, siderophore production and protease production is shown in Supplementary Materials Figure S2.
4. Discussion
We predicted that bacteria isolated from wastewater irrigated soils would be resistant to heavy metals and would possess PGP traits and metal removal potential. This hypothesis was supported by our findings and we isolated diverse groups of metal resistant bacteria with PGP and metal removal traits. Although it is known that presence of heavy metals in soil might decrease the overall microbial biodiversity, interaction among different organisms may play a key role in species survival in abiotic stress conditions [30]. Therefore, we were also able to retrieve various groups of bacteria from contaminated soils.
Of the total 75 bacterial isolates, 40%, 39% and 37%, solubilized Zn, K and PO4, respectively in vitro. Zinc solubilizers acidify soil, sequester Zn cations and decrease pH of the nearby soil [31]. Other mechanisms possibly involved in Zn solubilization include production of siderophores [32] and proton oxidoreductive systems in cell membranes and chelated ligands [33].
Potassium is another nutrient that is required by various developmental processes of plants. Organic acids produced by bacteria that are involved in the conversion of insoluble K into available K are tartaric acid, citric acid, succinic acid and α-ketogluconic acid [34].
Phosphorous is also crucial for normal plant growth. However, a high level of heavy metals and other contaminants in soil reduces plant P uptake [35]. This deficiency can be compensated by using PO4 solubilizing bacteria [36]. It has been well described that PGP bacteria solubilize P by releasing organic acids. These organic acids chelate PO4 bound cations with their carboxyl and hydroxyl groups and convert them into soluble forms for plants [37]. Two enzymes, phytase and acid phosphatase are mainly responsible for solubilization of organic P in soil. These enzymes catalyze the hydrolysis of phosphomonoesters at lower pH [38]. Thus, PGP bacteria possess several mechanisms to increase the bioavailability of important plant nutrients.
Besides mineral solubilization, N is also mainly obtained through bacterial N-fixation. In our study, 26% of isolates displayed N-fixation potential in vitro. It is well known that nif genes are responsible for bacterial nitrogenase activity. The structural subunit of dinitrogenase reductase and two subunits of dinitrogenase are encoded by nifD, nifH and nifK genes respectively. Among all these nif genes, nifH is the most important. Therefore, nifH gene provides evidence for potential N-fixation and is identified in both unculturable and cultured microorganisms from different environments [39]. The nifH gene identified in our isolates has also been reported in 70% of N-fixation bacterial strains isolated from Zn contaminated soil [40]. Similarly, nifD detected in our isolates has also been reported as proof of N-fixation ability of bacteria [41]. Moreover, in this study, JWN3 (Enterbacter sp.) possessed all three (nifHKD) N-fixation genes, similar to an earlier study where these three genes were also reported in Enterobacter sp. and Klebsiella sp. [42].
In addition to nutrient solubilization and N-fixation, 56% of isolates were able to produce siderophores, and 30% were able to produce protease. Bacterial siderophores improve growth and chlorophyll content of plants growing in heavy-metal-contaminated soils [43]. Bacterial siderophores also chelate different heavy metals and high levels of heavy metals in soil stimulate the biosynthesis of bacterial siderophores [44]. Therefore, siderophores play an important role in heavy metal bioremediation and in plant growth promotion. It is also recognized that PGP bacteria have certain mechanisms to counter the deleterious effect of plant pathogens by the production of catalytic enzymes such as protease [45].
Isolated bacteria possessing innate PGP traits also exhibited resistance to heavy metals. Among tested heavy metals, Cd was more toxic than Pb and Ni. Previous studies have also reported that Cd poses more toxicity [4]. In our study, higher MIC of metals can be linked with continuous exposure of bacteria to heavy metals [46]. Co-existence of PGP traits and metal resistance in bacteria during the current study agrees with previous reports [47].
In the present study, isolates with dual properties of plant growth promotion and heavy metal tolerance belonged to diverse genera. These genera have been previously identified as important PGP bacterial genera. For instance, bacteria belonging to Klebsiella, Escherichia, Serratia, Bacillus, Enterobacter and Pseudomonas not only show PGP traits but also possess metal resistance mechanisms which makes them suitable for their use in both plant growth promotion and bioremediation [48]. Similarly, Acinetobacter sp. and Bacillus sp. isolated from heavy-metal-contaminated soils have the ability to produce indole acetic acid (IAA) and siderophores even under high levels of heavy metal stress [49]. Thus, the higher levels of heavy metals in soil may not disable the PGP ability of bacteria.
Based on collective scores of both PGP traits and heavy metal MIC, higher scoring strains were used for heavy metal biosorption studies. Two bacterial strains, Citrobacter sp. and Enterobacter sp. showed the best metal removal. In our study, bacterial isolates showed a biosorption trend of heavy metals as Ni > Cd > Pb. A reason for this specific behavior could be the ionic radius of these three heavy metals. Smaller ionic radius of Ni (0.69 Å) than Cd (0.96 Å) and Pb (1.19 Å) might be the reason for higher biosorption of Ni as Tobin et al. [50] suggested that molecules with smaller ionic radius tend to sorb more quickly. Based on ionic radius, biosorption of Ni followed by Cd has been well supported by earlier findings [51,52]. Bacteria can accumulate metal in their cell walls, protein polyphosphate complexes, carbohydrates and complex formations with carboxyl groups of peptidoglycans in the bacterial cell wall [42]. Therefore, the above-mentioned strains can be used as potential immobilizing agents for heavy metals in polluted soils.
Thus, overall, the two best performing in vitro strains based on plant growth promoting traits, metal resistance and metal removal belonged to Enterobacter sp. and Citrobacter sp. A previous study has also shown that Enterobacter sp. and Citrobacter sp. isolated from heavy-metal-contaminated soils were able to increase the plant biomass as well as significantly reduce the heavy metal uptake [53]. Therefore, these species have the potential to be used as biofertilizer and biopesticides with multiple plant growth promoting and stress tolerant activities both in stress and non-stress environments [54,55,56,57,58]. Our results also showed the abundance of Gram-negative species (88%), which may be due to their lower sensitivity to metal ions compared to Gram-positive bacteria [59].
5. Conclusions
This study showed that diverse groups of bacteria thrive in contaminated environments which may retain PGP ability even under heavy metal and organic and inorganic pollutant stress. These bacteria are adapted to polluted environments due to their metal resistance capabilities. Two bacterial strains Citrobacter sp. and Enterobacter sp. showed the best overall score for PGP traits and heavy metal resistance and removal among isolated bacteria. These potent strains can be further used to bioremediate and biofertilize the heavy-metal-contaminated agricultural soils and to achieve the goals of sustainable agriculture.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/su13147792/s1, Figure S1: Nitrogen fixation ability of bacterial strains isolated from wastewater irrigated agricultural soils of major industrial cities of Punjab, Pakistan. Figure S2: Venn diagram showing number of bacterial isolates with plant growth promoting traits including nutrient (Zn, K, P) solubilization, nitrogen fixation, siderophore production and protease production from wastewater irrigated agricultural soils of major industrial cities of Punjab, Pakistan. Table S1: Physicochemical characteristics of wastewater irrigated agricultural soils of major industrial cities of Punjab, Pakistan. Table S2: Scoring criteria for plant growth promotion traits of bacterial strains isolated from wastewater irrigated agricultural soils of major industrial cities of Punjab, Pakistan. Table S3: Morphological characteristics of bacterial strains isolated from wastewater irrigated agricultural soils of major industrial cities of Punjab, Pakistan. Table S4: Plant growth promotion score and minimum inhibitory concentration (MIC) of bacterial strains isolated from wastewater irrigated agricultural soils of major industrial cities of Punjab, Pakistan. Values for PGP correspond to score index mentioned in supplementary materials Table S2.
Author Contributions
Conceptualization, M.N.H.; Methodology, A.W.A., S.S., Z.J., H.Y., A.N., R.N. and T.M.Q.; Software, A.W.A., S.M. (Shah Mulk) and S.M.U.S.; Formal Analysis, A.W.A., S.M. (Shah Mulk), H.Y., Z.J. and T.M.Q.; Investigation, A.W.A., S.S. and S.M. (Saqib Mumtaz); Resources, S.M., M.N.H. and S.M.U.S.; Data Curation, A.W.A., S.M. (Shah Mulk), Z.H.; Writing—Original Draft Preparation, A.W.A. and S.S.; Writing—Review and Editing, S.M. (Saqib Mumtaz), M.N.H., H.Y., Z.J., Z.H., A.N., R.N. and A.W.; Visualization, A.W.A. and A.W.; Supervision, S.M. (Saqib Mumtaz) and M.N.H.; Project Administration, S.M. (Saqib Mumtaz); Funding Acquisition, S.M. (Saqib Mumtaz). All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by Higher Education Commission of Pakistan (5381/Federal/NRPU/R&D/HEC/2016).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Acknowledgments
The authors would like to thank Tamoor Ahmad, Department of Biosciences, COMSATS University Islamabad for his help in soil sampling.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Vallino, E.; Ridolfi, L.; Laio, F. Measuring economic water scarcity in agriculture: A cross-country empirical investigation. Environ. Sci. Policy 2020, 114, 73–85. [Google Scholar] [CrossRef]
- Valipour, M.; Singh, V.P. Global experiences on wastewater irrigation: Challenges and prospects. In Balanced Urban Development: Options and Strategies for Liveable Cities; Springer: Berlin/Heidelberg, Germany, 2016; pp. 289–327. [Google Scholar]
- Anwar, S.; Nawaz, M.F.; Gul, S.; Rizwan, M.; Ali, S.; Kareem, A. Uptake and distribution of minerals and heavy metals in commonly grown leafy vegetable species irrigated with sewage water. Environ. Monit. Assess. 2016, 188, 541. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Pan, C.; Xiao, A.; Yang, X.; Zhang, G. Isolation, identification, and environmental adaptability of heavy-metal-resistant bacteria from ramie rhizosphere soil around mine refinery. 3 Biotech 2017, 7, 5. [Google Scholar] [CrossRef]
- Ayangbenro, A.S.; Babalola, O.O. A new strategy for heavy metal polluted environments: A review of microbial biosorbents. Int. J. Environ. Res. Public Health 2017, 14, 94. [Google Scholar] [CrossRef] [PubMed]
- Khalid, S.; Shahid, M.; Bibi, I.; Sarwar, T.; Shah, A.H.; Niazi, N.K. A review of environmental contamination and health risk assessment of wastewater use for crop irrigation with a focus on low and high-income countries. Int. J. Environ. Res. Public Health 2018, 15, 895. [Google Scholar] [CrossRef]
- Yadav, B.K.; Sidhu, A.S. Dynamics of potassium and their bioavailability for plant nutrition. In Potassium Solubilizing Microorganisms for Sustainable Agriculture; Springer: Berlin/Heidelberg, Germany, 2016; pp. 187–201. [Google Scholar]
- Krishnamoorthy, R.; Venkateswaran, V.; Senthilkumar, M.; Anandham, R.; Selvakumar, G.; Kim, K.; Kang, Y.; Sa, T. Potential Microbiological Approaches for the Remediation of Heavy Metal-Contaminated Soils. In Plant-Microbe Interactions in Agro-Ecological Perspectives; Springer: Berlin/Heidelberg, Germany, 2017; pp. 341–366. [Google Scholar]
- Sharma, S.; Tiwari, S.; Hasan, A.; Saxena, V.; Pandey, L.M. Recent advances in conventional and contemporary methods for remediation of heavy metal-contaminated soils. 3 Biotech 2018, 8, 216. [Google Scholar] [CrossRef]
- Wang, T.; Sun, H.; Ren, X.; Li, B.; Mao, H. Evaluation of biochars from different stock materials as carriers of bacterial strain for remediation of heavy metal-contaminated soil. Sci. Rep. 2017, 7, 12114. [Google Scholar] [CrossRef]
- Beškoski, V.P.; Gojgić-Cvijović, G.; Milić, J.; Ilić, M.; Miletić, S.; Šolević, T.; Vrvić, M.M. Ex situ bioremediation of a soil contaminated by mazut (heavy residual fuel oil)–A field experiment. Chemosphere 2011, 83, 34–40. [Google Scholar] [CrossRef]
- Ajao, A.; Adebayo, G.; Yakubu, S. Bioremediation of Textile Industrial Effluent using mixed culture of Pseudomonas aeruginosa and Bacillus subtilis immobilized on agaragar in a Bioreactor. J. Microbiol. Biotechnol. 2017, 1, 50–56. [Google Scholar]
- Reitz, T.; Merroun, M.L.; Selenska-Pobell, S. Interactions of Paenibacillus sp. and Sulfolobus acidocaldarius strains with U (VI). In Uranium, Mining and Hydrogeology; Springer: Berlin/Heidelberg, Germany, 2008; pp. 703–710. [Google Scholar]
- Ahemad, M.; Malik, A. Bioaccumulation of heavy metals by zinc resistant bacteria isolated from agricultural soils irrigated with wastewater. Bacteriol. J. 2011, 2, 12–21. [Google Scholar] [CrossRef]
- Llorens, I.; Untereiner, G.; Jaillard, D.; Gouget, B.; Chapon, V.; Carriere, M. Uranium interaction with two multi-resistant environmental bacteria: Cupriavidus metallidurans CH34 and Rhodopseudomonas palustris. PLoS ONE 2012, 7, e51783. [Google Scholar] [CrossRef] [PubMed]
- Etesami, H. Bacterial mediated alleviation of heavy metal stress and decreased accumulation of metals in plant tissues: Mechanisms and future prospects. Ecotoxicol. Environ. Saf. 2018, 147, 175–191. [Google Scholar] [CrossRef] [PubMed]
- Kidd, P.S.; Alvarez-Lopez, V.; Becerra-Castro, C.; Cabello-Conejo, M.; Prieto-Fernandez, A. Potential role of plant-associated bacteria in plant metal uptake and implications in phytotechnologies. Adv. Bot. Res. 2017, 83, 87–126. [Google Scholar] [CrossRef]
- Ullah, A.; Heng, S.; Munis, M.F.H.; Fahad, S.; Yang, X. Phytoremediation of heavy metals assisted by plant growth promoting (PGP) bacteria: A review. Environ. Exp. Bot. 2015, 117, 28–40. [Google Scholar] [CrossRef]
- Okalebo, J.R.; Gathua, K.W.; Woomer, P.L. Laboratory Methods of Soil and Plant Analysis: A Working Manual Second Edition. Available online: https://www.google.com.hk/url?sa=t&rct=j&q=&esrc=s&source=web&cd=&ved=2ahUKEwintMessNLxAhUQvpQKHfqKD18QFjABegQIAxAD&url=https%3A%2F%2Fwww.researchgate.net%2Ffile.PostFileLoader.html%3Fid%3D5882f0c8ed99e15dce797d03%26assetKey%3DAS%253A452837697167361%25401484976328229&usg=AOvVaw34bHXsDqOXDYvlj2ICtI0M (accessed on 1 May 2021).
- Hassan, M.N.; Afghan, S.; Hafeez, F.Y. Suppression of red rot caused by Colletotrichum falcatum on sugarcane plants using plant growth-promoting rhizobacteria. BioControl 2010, 55, 531–542. [Google Scholar] [CrossRef]
- Bunt, J.; Rovira, A. Microbiological studies of some subantarctic soils. J. Soil Sci. 1955, 6, 119–128. [Google Scholar] [CrossRef]
- Aleksandrov, V.; Blagodyr, R.; Ilev, I. Liberation of phosphoric acid from apatite by silicate bacteria. Mikrobiol. Z. 1967, 29, 1. [Google Scholar] [CrossRef]
- Pikovskaya, R. Mobilization of phosphorus in soil in connection with vital activity of some microbial species. Mikrobiologiya 1948, 17, 362–370. [Google Scholar]
- Rashid, M.; Khalil, S.; Ayub, N.; Alam, S.; Latif, F. Organic acids production and phosphate solubilization by phosphate solubilizing microorganisms (PSM) under in vitro conditions. Pak. J. Biol. Sci. 2004, 7, 187–196. [Google Scholar] [CrossRef]
- Putrie, R.F.W.; Widowati, T.; Lekatompessy, S.J.; Sukiman, H. Nitrogen Fixing Potential of Endophytic Bacteria Isolated from Aloe barbadensis Miller and Aloe sp. Microbiol. Indones. 2016, 10, 2. [Google Scholar] [CrossRef][Green Version]
- Denizci, A.; Kazan, D.; Abeln, E.; Erarslan, A. Newly isolated Bacillus clausii GMBAE 42: An alkaline protease producer capable to grow under higly alkaline conditions. J. Appl. Microbiol. 2004, 96, 320–327. [Google Scholar] [CrossRef] [PubMed]
- Schwyn, B.; Neilands, J. Universal chemical assay for the detection and determination of siderophores. Anal. Biochem. 1987, 160, 47–56. [Google Scholar] [CrossRef]
- Ezzouhri, L.; Castro, E.; Moya, M.; Espinola, F.; Lairini, K. Heavy metal tolerance of filamentous fungi isolated from polluted sites in Tangier, Morocco. Afr. J. Microbiol. Res. 2009, 3, 35–48. [Google Scholar]
- Tan, Z.-Y.; Xu, X.-D.; Wang, E.-T.; Gao, J.-L.; Martinez-Romero, E.; Chen, W.-X. Phylogenetic and genetic relationships of Mesorhizobium tianshanense and related rhizobia. Int. J. Syst. Evol. Microbiol. 1997, 47, 874–879. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Ramakrishna, W. Effect of multiple metal resistant bacteria from contaminated lake sediments on metal accumulation and plant growth. J. Hazard. Mater. 2011, 189, 531–539. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Prasad, S.; Yadav, K.K.; Shrivastava, M.; Gupta, N.; Nagar, S.; Bach, Q.-V.; Kamyab, H.; Khan, S.A.; Yadav, S. Hazardous heavy metals contamination of vegetables and food chain: Role of sustainable remediation approaches—A review. Environ. Res. 2019, 179, 108792. [Google Scholar] [CrossRef] [PubMed]
- Saravanan, V.; Madhaiyan, M.; Thangaraju, M. Solubilization of zinc compounds by the diazotrophic, plant growth promoting bacterium Gluconacetobacter diazotrophicus. Chemosphere 2007, 66, 1794–1798. [Google Scholar] [CrossRef]
- Chang, H.-B.; Lin, C.-W.; Huang, H.-J. Zinc-induced cell death in rice (Oryza sativa L.) roots. Plant Growth Regul. 2005, 46, 261–266. [Google Scholar] [CrossRef]
- Meena, V.S.; Maurya, B.R.; Verma, J.P.; Aeron, A.; Kumar, A.; Kim, K.; Bajpai, V.K. Potassium solubilizing rhizobacteria (KSR): Isolation, identification, and K-release dynamics from waste mica. Ecol. Eng. 2015, 81, 340–347. [Google Scholar] [CrossRef]
- Halstead, R.; Finn, B.; MacLean, A. Extractability of nickel added to soils and its concentration in plants. Can. J. Soil Sci. 1969, 49, 335–342. [Google Scholar] [CrossRef]
- Zaidi, S.; Usmani, S.; Singh, B.R.; Musarrat, J. Significance of Bacillus subtilis strain SJ-101 as a bioinoculant for concurrent plant growth promotion and nickel accumulation in Brassica juncea. Chemosphere 2006, 64, 991–997. [Google Scholar] [CrossRef]
- Khan, M.S.; Zaidi, A.; Wani, P.A. Role of phosphate-solubilizing microorganisms in sustainable agriculture—A review. Agron. Sustain. Dev. Agron. Sustain. Dev. 2007, 27, 29–43. [Google Scholar] [CrossRef]
- Golovan, S.; Wang, G.; Zhang, J.; Forsberg, C.W. Characterization and overproduction of the Escherichia coli appA encoded bifunctional enzyme that exhibits both phytase and acid phosphatase activities. Can. J. Microbiol. 1999, 46, 59–71. [Google Scholar] [CrossRef]
- Dai, Z.; Guo, X.; Yin, H.; Liang, Y.; Cong, J.; Liu, X. Identification of nitrogen-fixing genes and gene clusters from metagenomic library of acid mine drainage. PLoS ONE 2014, 9, e87976. [Google Scholar] [CrossRef]
- Dadook, M.; Mehrabian, S.; Irian, S. Identification of ten N2-fixing bacteria using 16S rRNA and their response to various zinc concentrations. Int. J. Cell. Mol. Biol. 2013, 2013, 1–8. [Google Scholar] [CrossRef]
- Muthukumarasamy, R.; Kang, U.; Park, K.; Jeon, W.T.; Park, C.; Cho, Y.; Kwon, S.W.; Song, J.; Roh, D.H.; Revathi, G. Enumeration, isolation and identification of diazotrophs from Korean wetland rice varieties grown with long-term application of N and compost and their short-term inoculation effect on rice plants. J. Appl. Microbiol. 2007, 102, 981–991. [Google Scholar] [CrossRef]
- Väsänen, O.; Haahtela, K.; Bask, L.; Kari, K.; Salkinoja-Salonen, M.; Sundman, V. Diversity of nif gene location and nitrogen fixation among root-associated Enterobacter and Klebsiella strains. Arch. Microbiol. 1985, 141, 123–127. [Google Scholar] [CrossRef]
- Herrera-Quiterio, A.; Toledo-Hernández, E.; Aguirre-Noyola, J.L.; Romero, Y.; Ramos, J.; Palemón-Alberto, F.; Toribio-Jiménez, J. Antagonic and plant growth-promoting effects of bacteria isolated from mine tailings at El Fraile, Mexico. Rev. Argent. Microbiol. 2020. [Google Scholar] [CrossRef]
- Sinha, S.; Mukherjee, S.K. Cadmium–induced siderophore production by a high Cd-resistant bacterial strain relieved Cd toxicity in plants through root colonization. Curr. Microbiol. 2008, 56, 55–60. [Google Scholar] [CrossRef]
- Zheng, Y.; Xue, Q.-Y.; Xu, L.-L.; Xu, Q.; Lu, S.; Gu, C.; Guo, J.-H. A screening strategy of fungal biocontrol agents towards Verticillium wilt of cotton. Biol. Control. 2011, 56, 209–216. [Google Scholar] [CrossRef]
- Yu, Z.; Gunn, L.; Wall, P.; Fanning, S. Antimicrobial resistance and its association with tolerance to heavy metals in agriculture production. Food Microbiol. 2017, 64, 23–32. [Google Scholar] [CrossRef]
- Aleem, A.; Isar, J.; Malik, A. Impact of long-term application of industrial wastewater on the emergence of resistance traits in Azotobacter chroococcum isolated from rhizospheric soil. Bioresour. Technol. 2003, 86, 7–13. [Google Scholar] [CrossRef]
- Nadeem, S.M.; Zahir, Z.A.; Naveed, M.; Asghar, H.N.; Arshad, M. Rhizobacteria capable of producing ACC-deaminase may mitigate salt stress in wheat. Soil Sci. Soc. Am. J. 2010, 74, 533–542. [Google Scholar] [CrossRef]
- Zhang, Y.-F.; He, L.-Y.; Chen, Z.-J.; Zhang, W.-H.; Wang, Q.-Y.; Qian, M.; Sheng, X.-F. Characterization of lead-resistant and ACC deaminase-producing endophytic bacteria and their potential in promoting lead accumulation of rape. J. Hazard. Mater. 2011, 186, 1720–1725. [Google Scholar] [CrossRef] [PubMed]
- Tobin, J.M.; Cooper, D.; Neufeld, R. Uptake of metal ions by Rhizopus arrhizus biomass. Appl. Environ. Microbiol. 1984, 47, 821–824. [Google Scholar] [CrossRef] [PubMed]
- Ansari, M.I.; Malik, A. Biosorption of nickel and cadmium by metal resistant bacterial isolates from agricultural soil irrigated with industrial wastewater. Bioresour. Technol. 2007, 98, 3149–3153. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, S.; Rai, L. Biotechnological potential of Microcystis sp. in Cu, Zn and Cd biosorption from single and multimetallic systems. BioMetals 2001, 14, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Shan, S.; Guo, Z.; Lei, P.; Li, Y.; Wang, Y.; Zhang, M.; Cheng, W.; Wu, S.; Wu, M.; Du, D. Increased biomass and reduced tissue cadmium accumulation in rice via indigenous Citrobacter sp. XT1-2-2 and its mechanisms. Sci. Total Environ. 2020, 708, 135224. [Google Scholar] [CrossRef]
- Habib, S.; Kausar, H.; Saud, H.; Ismail, M.; Othman, R. Molecular characterization of stress tolerant plant growth promoting rhizobacteria (PGPR) for growth enhancement of rice. Int. J. Agric. Biol. 2016, 18, 184–191. [Google Scholar] [CrossRef]
- Pramanik, K.; Mitra, S.; Sarkar, A.; Maiti, T.K. Alleviation of phytotoxic effects of cadmium on rice seedlings by cadmium resistant PGPR strain Enterobacter aerogenes MCC 3092. J. Hazard. Mater. 2018, 351, 317–329. [Google Scholar] [CrossRef]
- Pramanik, K.; Mitra, S.; Sarkar, A.; Soren, T.; Maiti, T.K. Characterization of a Cd2+-resistant plant growth promoting rhizobacterium (Enterobacter sp.) and its effects on rice seedling growth promotion under Cd2+-stress in vitro. Agric. Nat. Resour. 2018, 52, 215–221. [Google Scholar] [CrossRef]
- Qamar, N.; Rehman, Y.; Hasnain, S. Arsenic-resistant and plant growth-promoting Firmicutes and γ-Proteobacteria species from industrially polluted irrigation water and corresponding cropland. J. Appl. Microbiol. 2017, 123, 748–758. [Google Scholar] [CrossRef] [PubMed]
- Gontia-Mishra, I.; Sapre, S.; Kachare, S.; Tiwari, S. Molecular diversity of 1-aminocyclopropane-1-carboxylate (ACC) deaminase producing PGPR from wheat (Triticum aestivum L.) rhizosphere. Plant Soil 2017, 414, 213–227. [Google Scholar] [CrossRef]
- Morozzi, G.; Cenci, G.; Scardazza, F.; Pitzurra, M. Cadmium uptake by growing cells of gram-positive and gram-negative bacteria. Microbios 1986, 48, 27–35. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).