Evaluation and Improvement of PCM Melting in Double Tube Heat Exchangers Using Different Combinations of Nanoparticles and PCM (The Case of Renewable Energy Systems)
Abstract
:1. Introduction
2. Numerical Approach
2.1. Governing Equations
- (1)
- Friction between the nanoparticles and the base fluid is negligible.
- (2)
- Viscosity dissipation is not considered.
- (3)
- Volumetric tolerance for melting PCM is assumed to be neglected.
- (4)
- The temperature difference in the base fluid can be ignored.
- (5)
- The thermal properties of PCM are assumed to be constant, except for density in the momentum term.
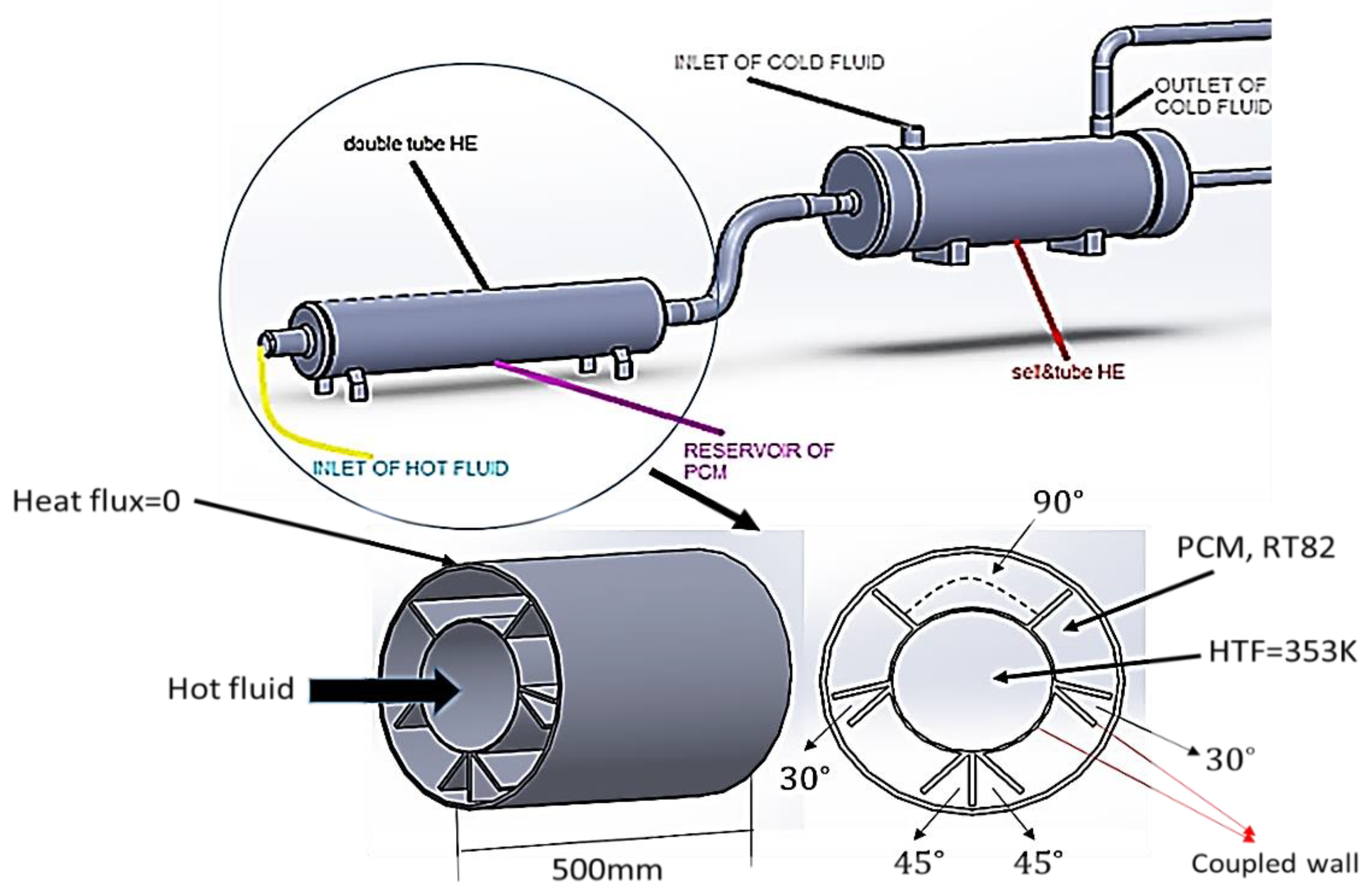
2.2. Initial and Boundary Conditions
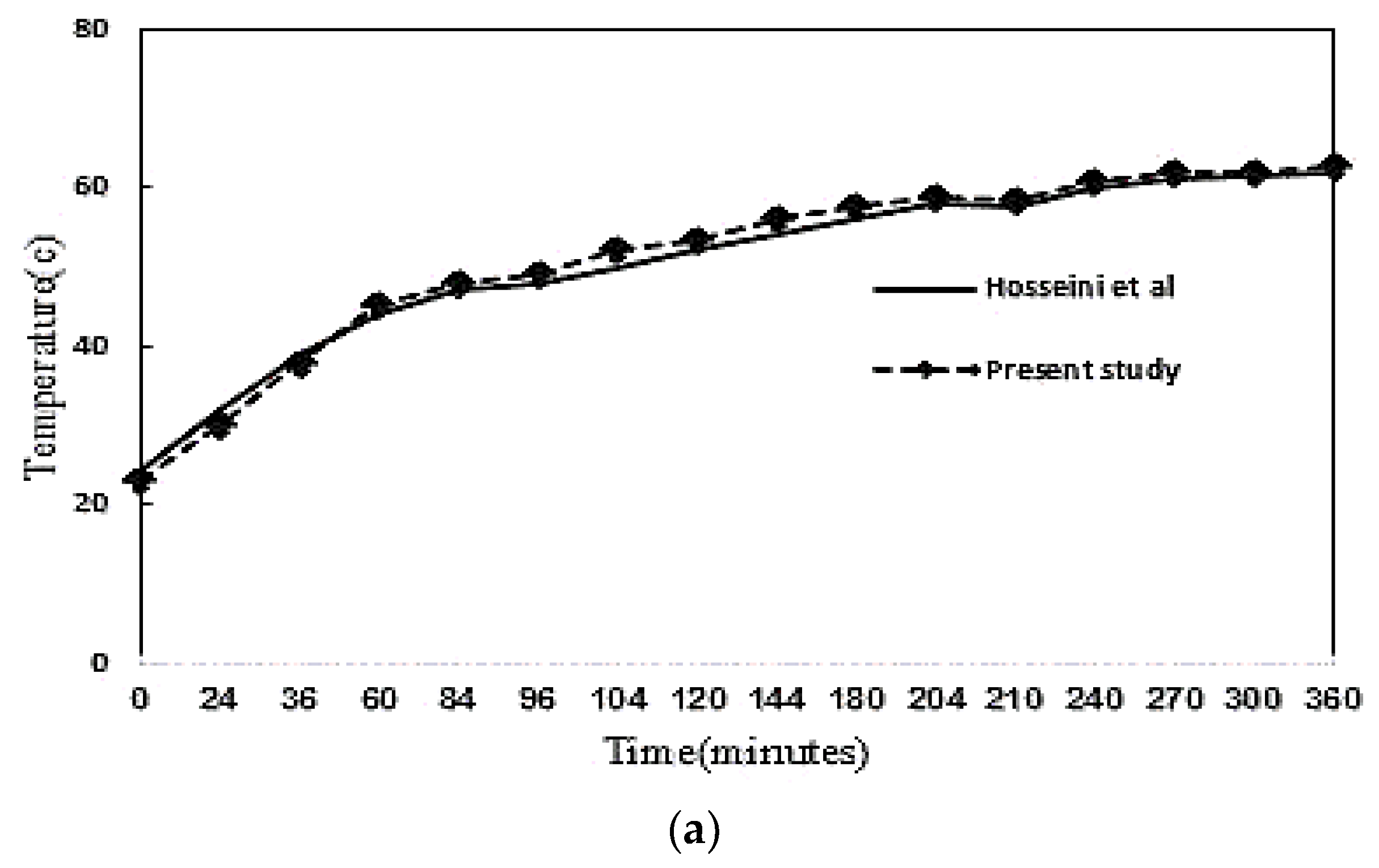
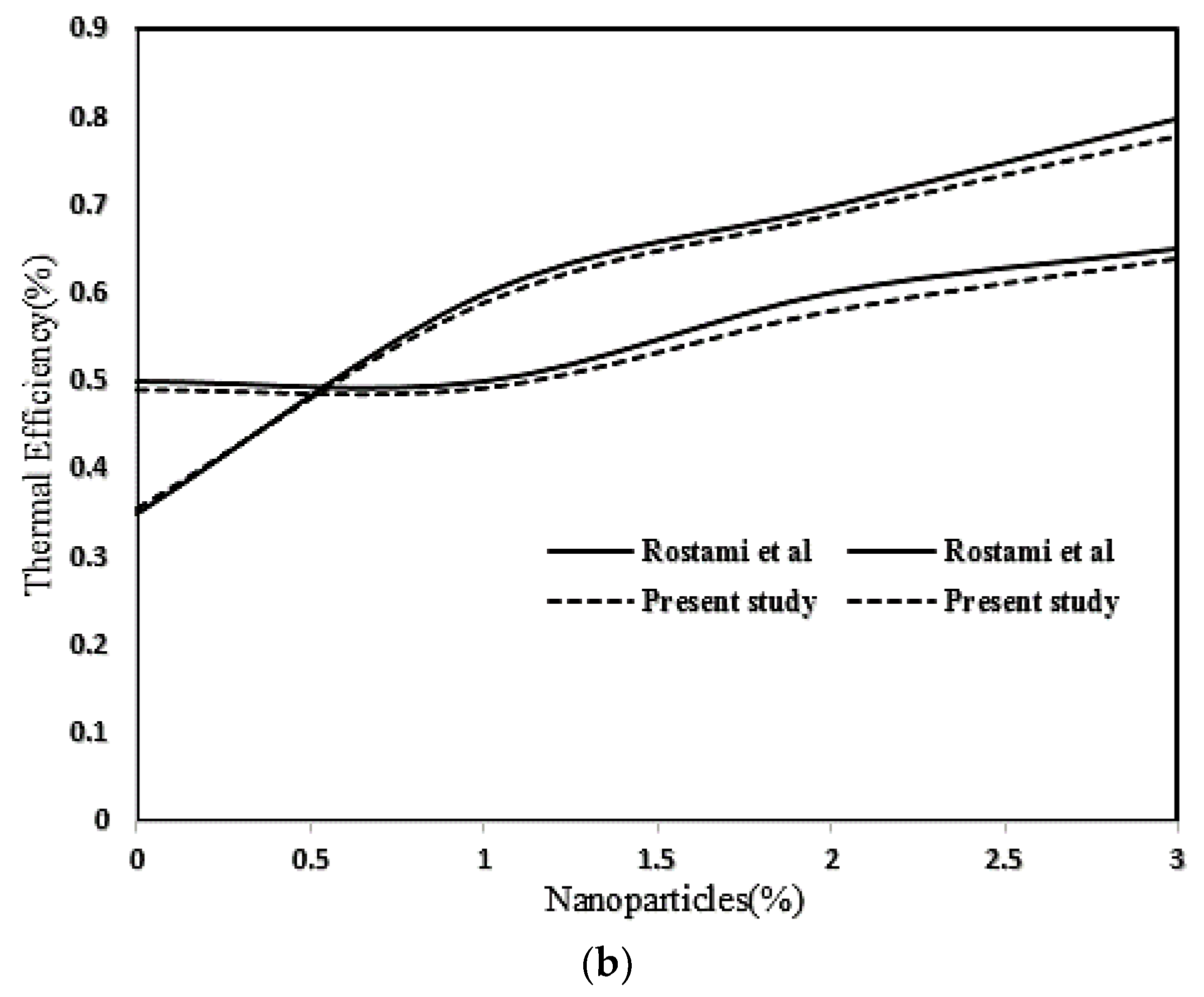
2.3. Numerical Procedure and Validation
3. Result and Discussion
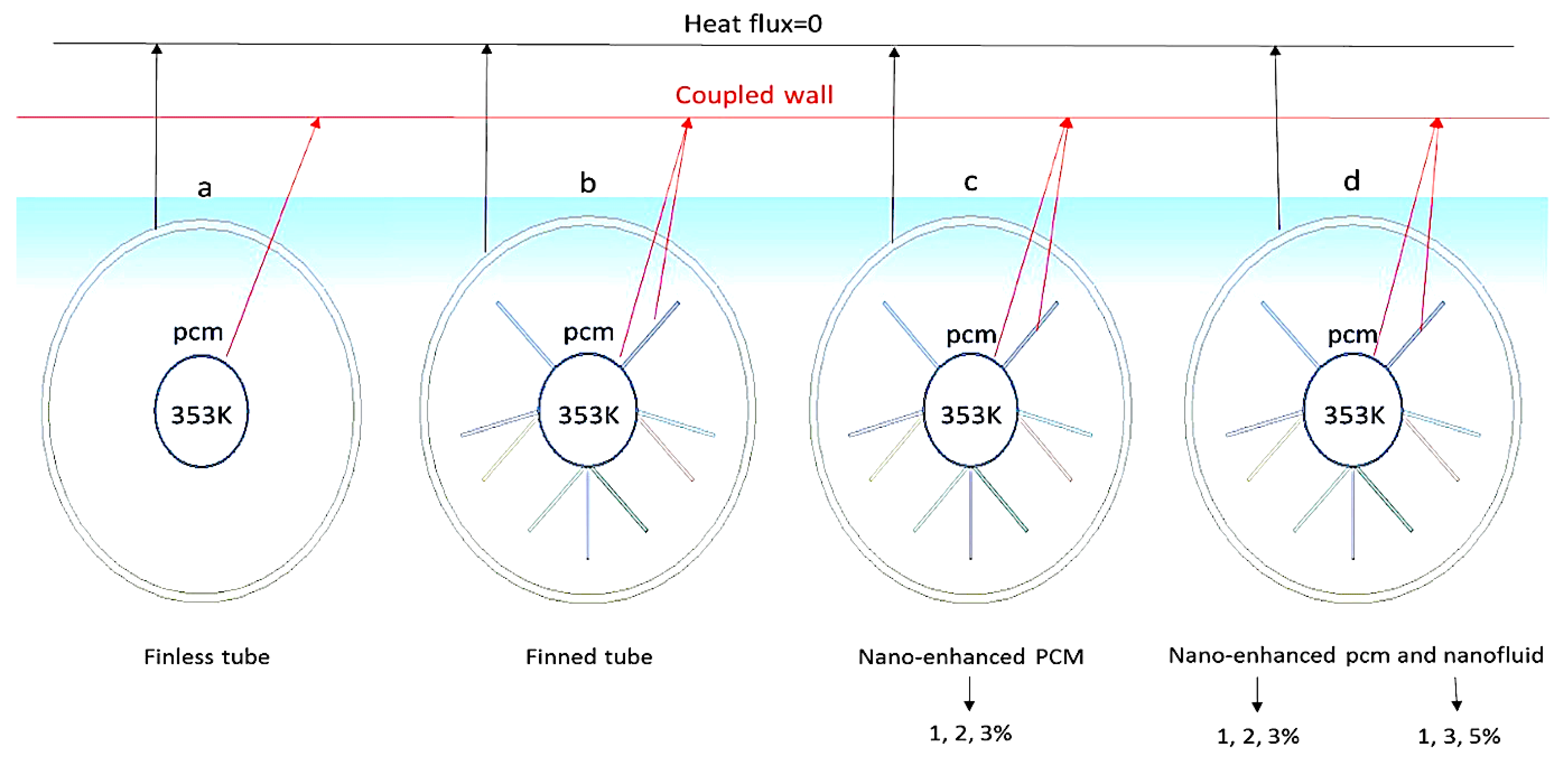
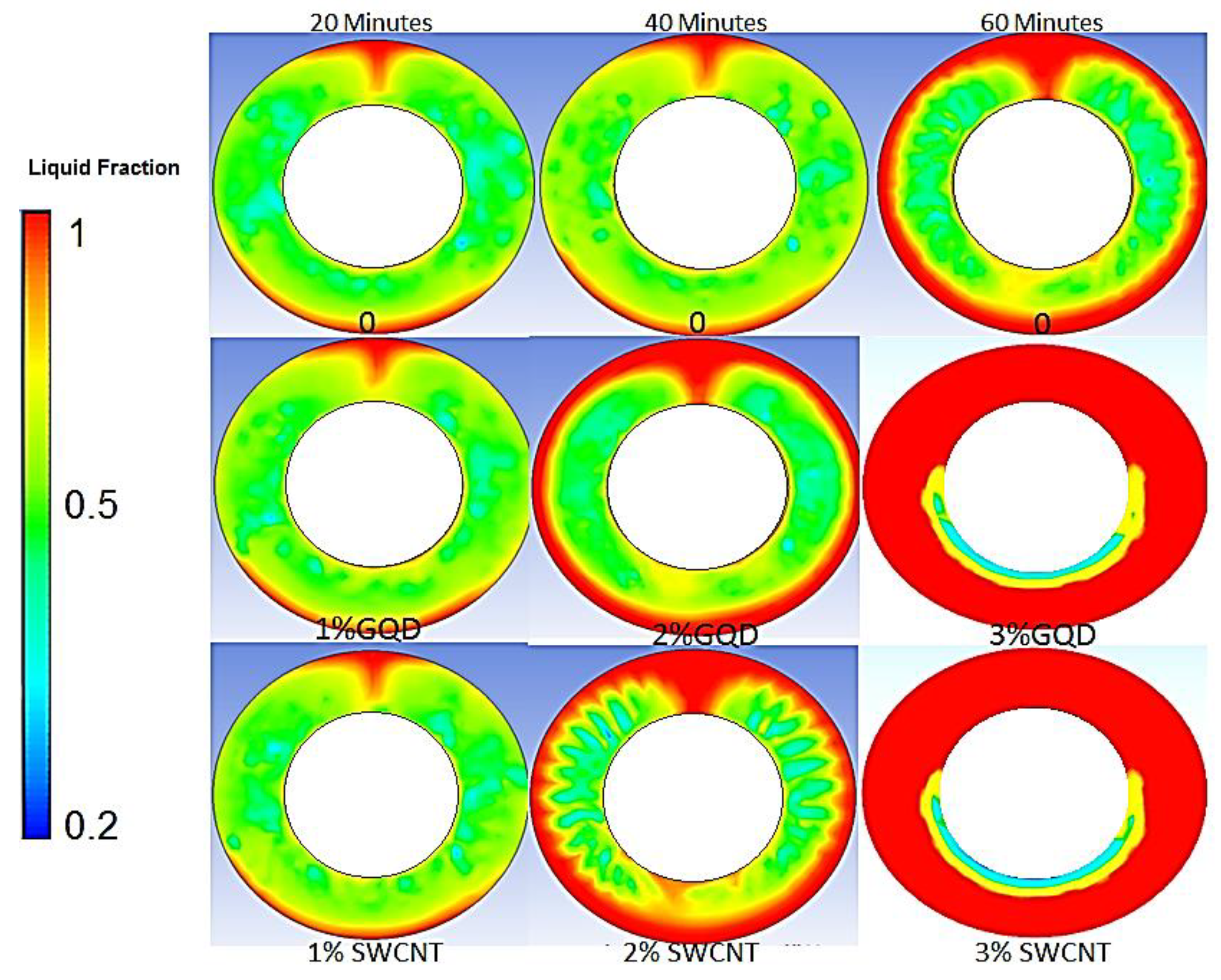
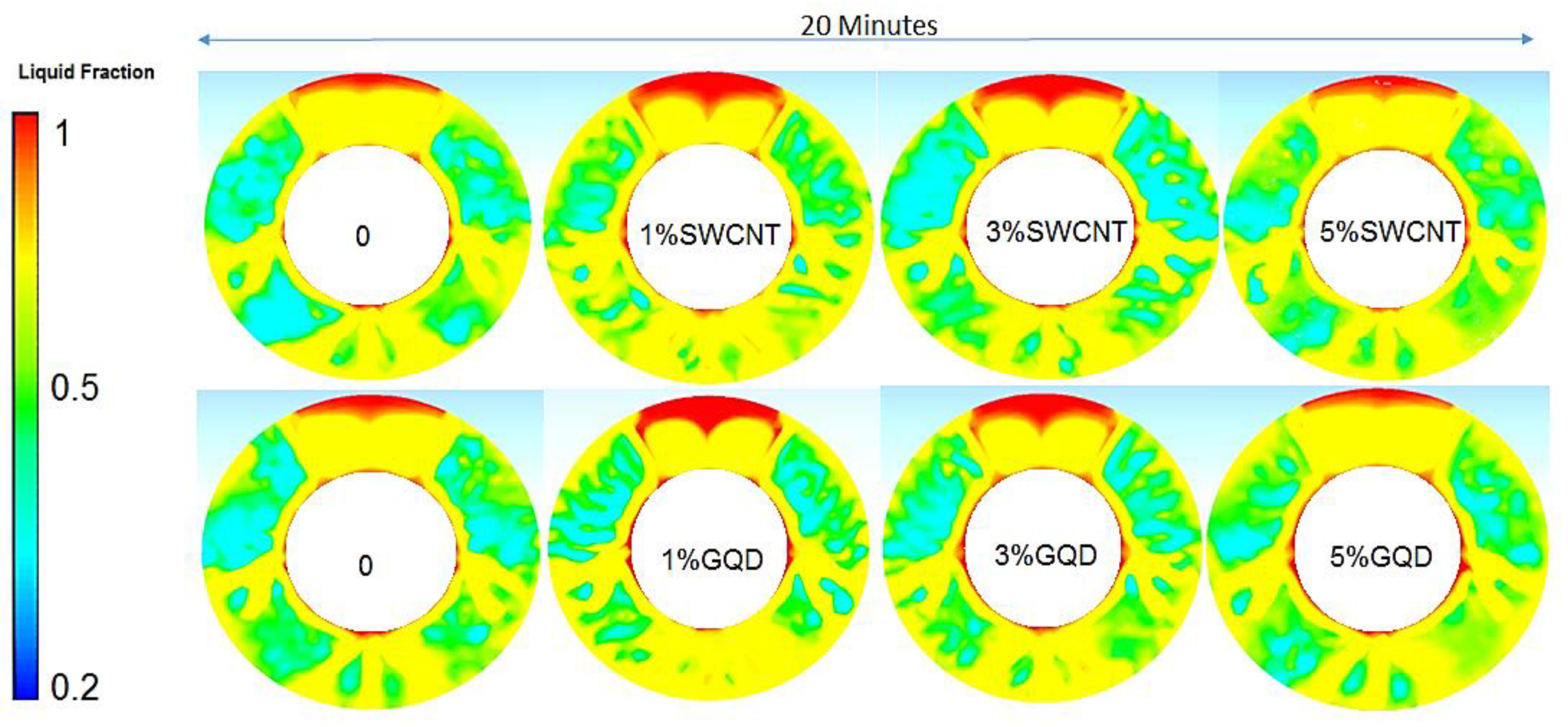
3.1. Effect of Nanoparticles (Nano-Enhanced PCM) on PCM Melting Process
3.2. Effect of Fin Tubes and Nanoparticles (Nano-HTF) on the PCM Melting Process
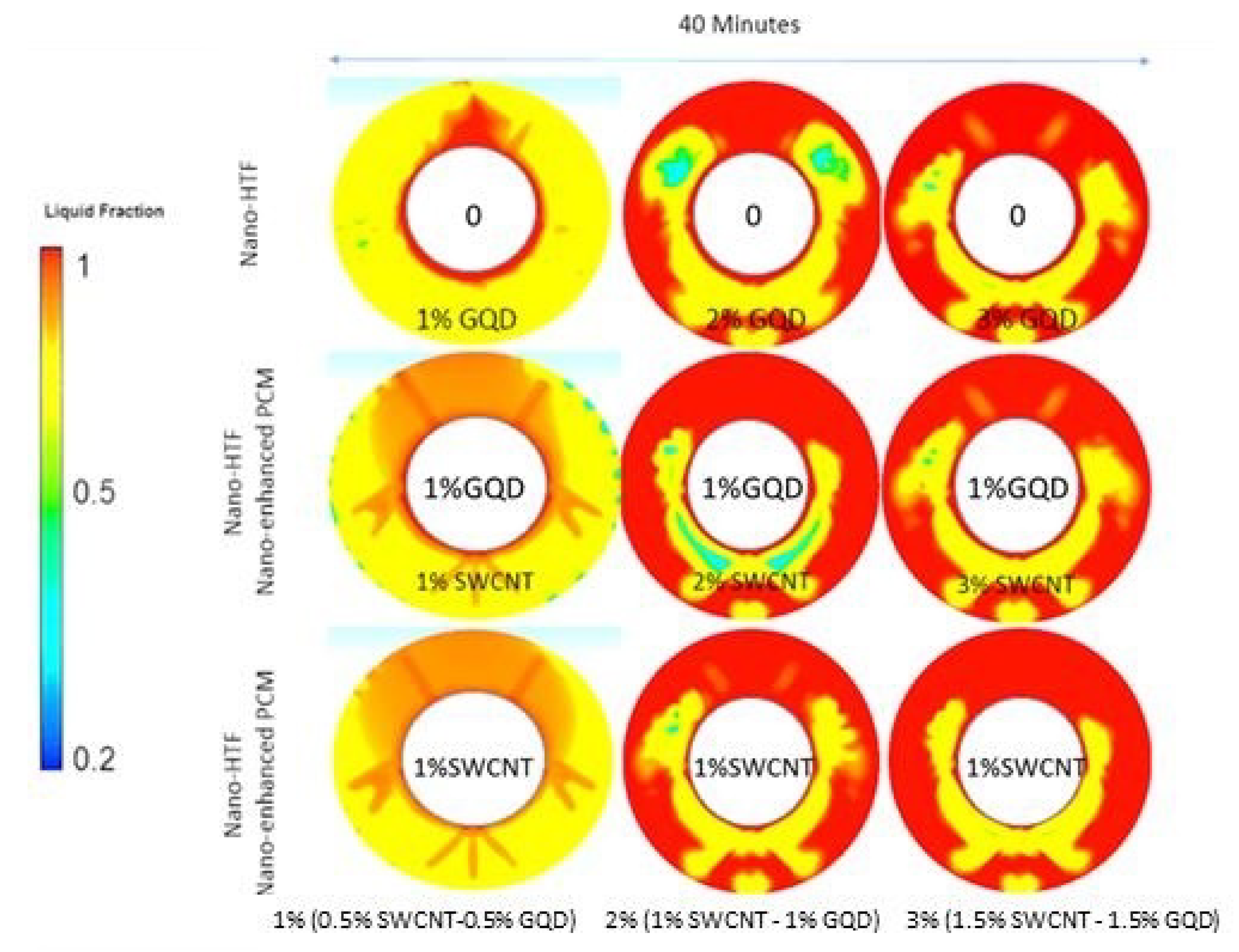
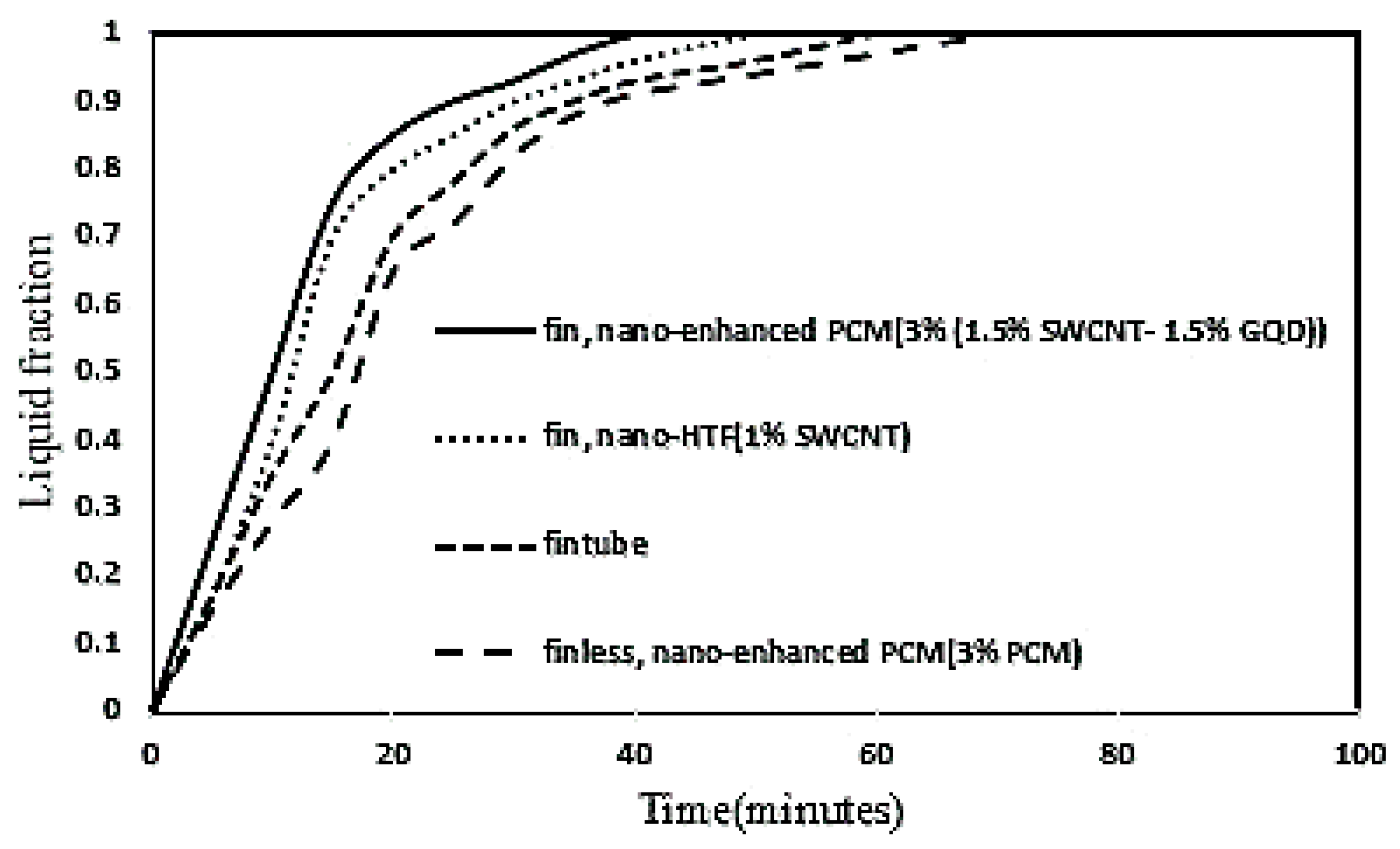
3.3. Effect of Fin Tubes and Nanoparticles (Nano-HTF and Nano-Enhanced PCM) on the PCM Melting Process
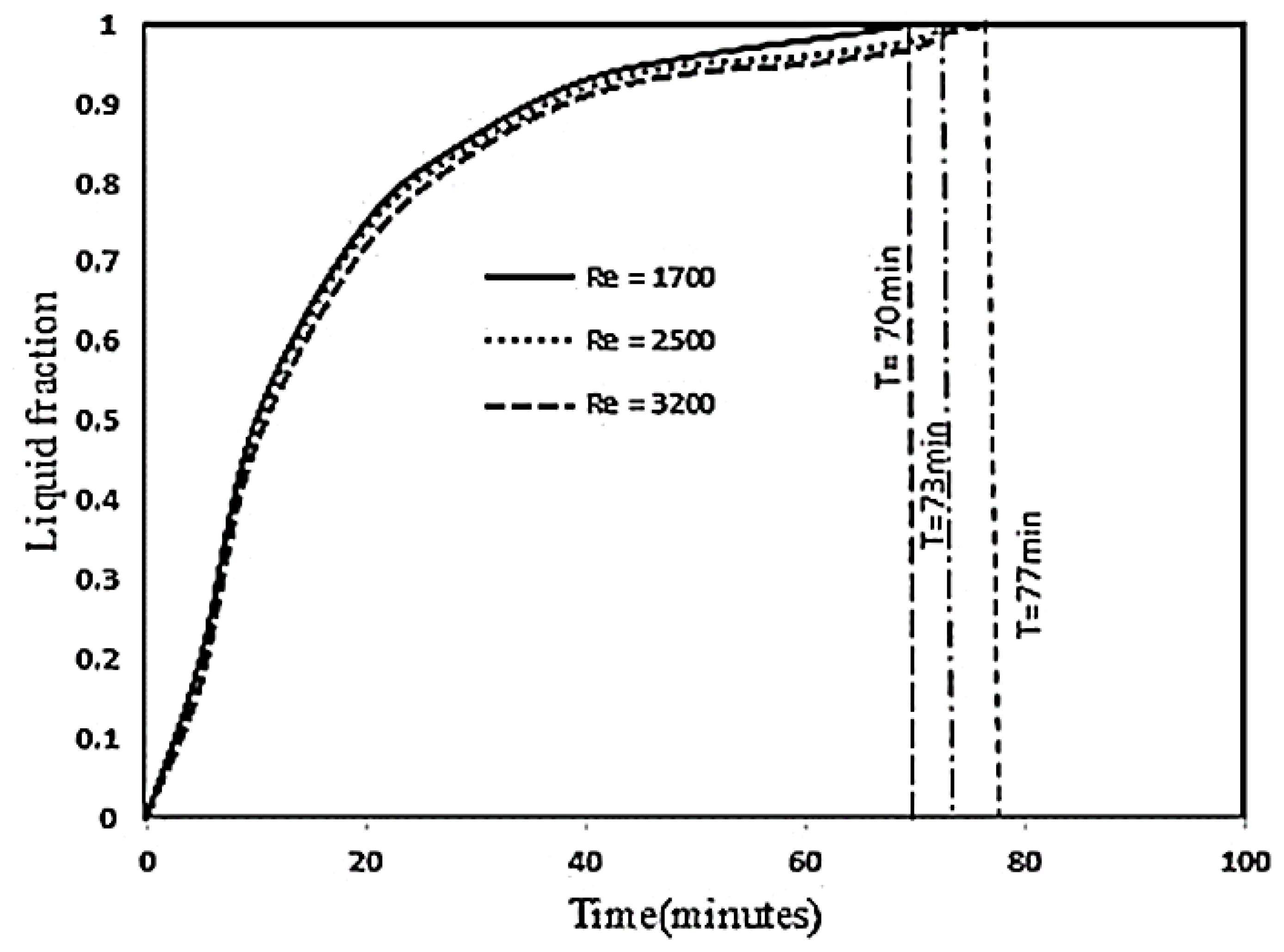
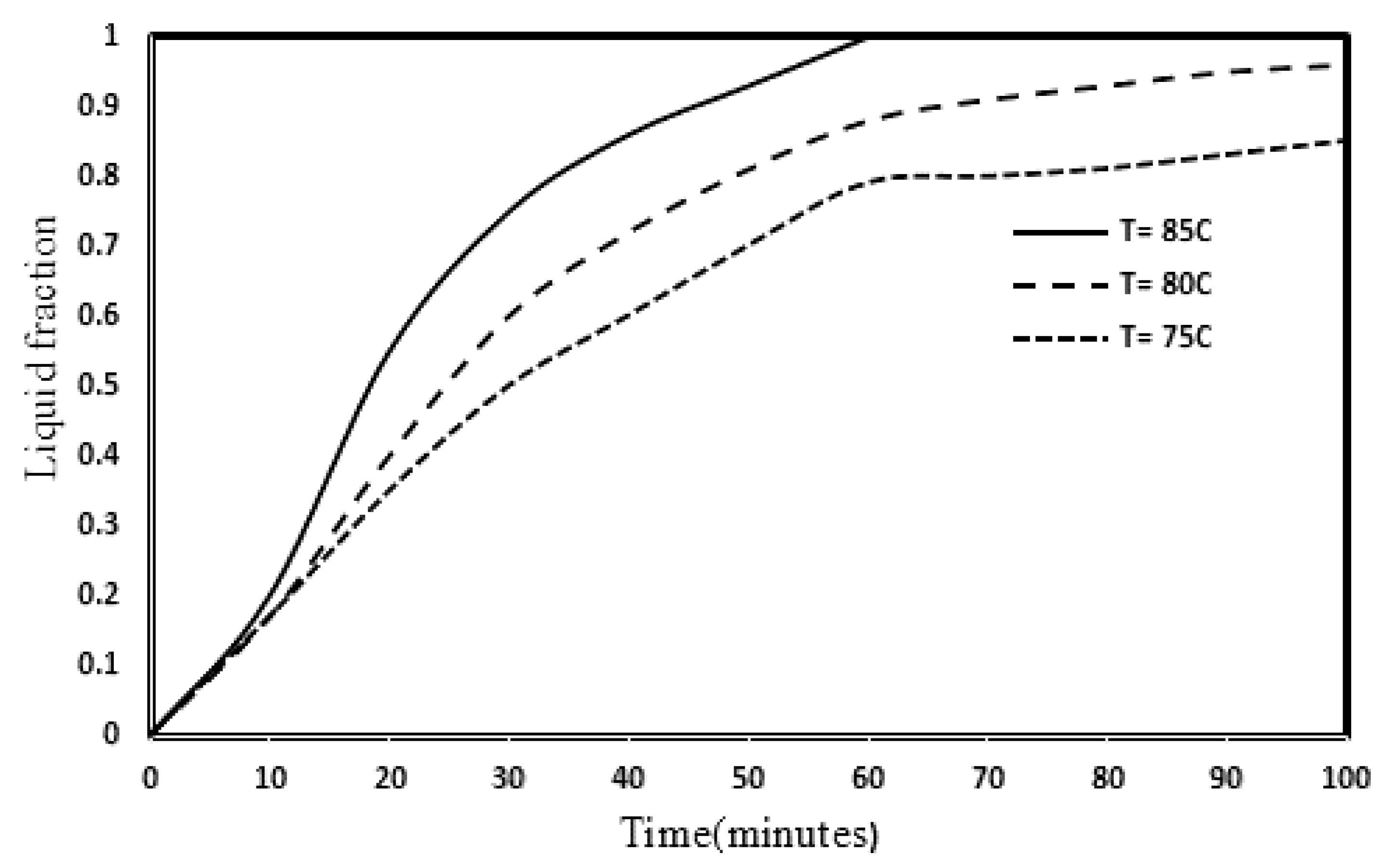
3.4. Influence of Inlet Temperature and Reynolds Numbers on the PCM Melting Process
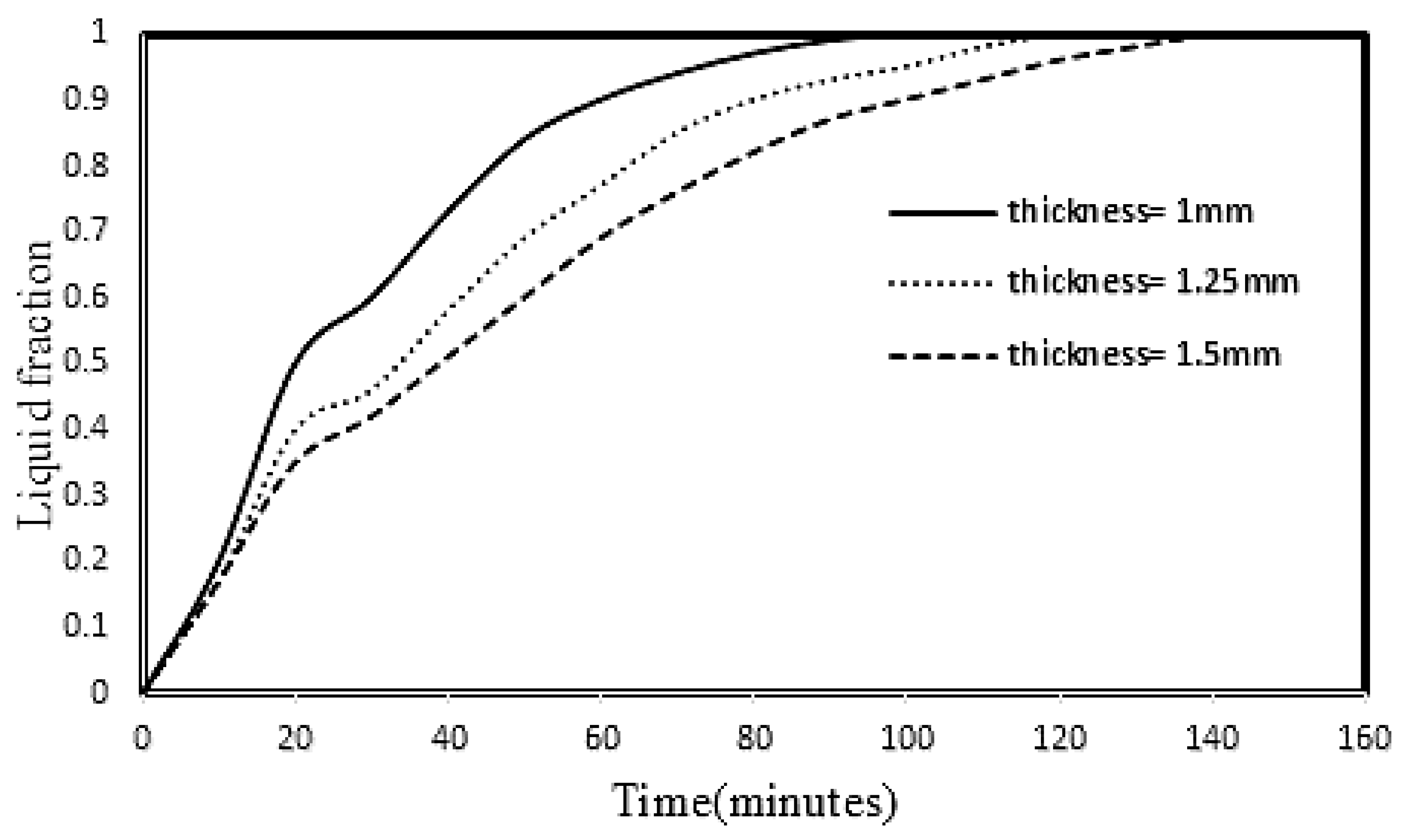
3.5. Effect of HTF Pipe and Fin Thickness on PCM Melting
3.6. Influence of Fin Tube, Nanoparticle Parameters on Melting Process of PCM
4. Conclusions
- -
- At first, the melting process of PCM heat conduction mechanism was recognized as the dominant phenomenon, but in the continuation of the melting process and with the appearance of a mushy state in PCM, the heat convection mechanism was identified as the superior mechanism.
- -
- With the increase of HTF inlet temperature, the melting rate of PCM increased, so that with increasing temperature from 75 to 80 and from 80 to 85, the melting rate of PCM increased by 21% and 23%, respectively.
- -
- Increasing the Reynolds number in the laminar and transient flow range has not had a significant effect on the melting rate of PCM, because with increasing the Reynolds number from 1700 to 2500 and from 2500 to 3200 due to reducing the fluid retention time, PCM melting rate decreased by 2 and 4%, respectively.
- -
- The PCM melting process was improved by reducing the thickness of the HTF tube and the thickness of the fin, because the thermal resistance caused by the wall of the HTF tube was reduced, so that by increasing the thickness of the tube and the fan from 1 mm to 1.25 and 1.5 mm, the rate PCM melting decreased by 18% and 31%, respectively.
- -
- The best melting rate of PCM has been obtained by using finned tubes and a simultaneous combination of nano-enhanced PCM (3% (1.5% SWCNT-1.5% GQD)) and 1% SWCNT (nano-HTF). This is because both the heat transfer surfaces and the thermal conductivity of the PCM and HTF were increased, so that it improved the melting rate of the PCM by 39% compared to the conventional model in double tube heat exchangers.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Nomenclatures
| CP | Specific heat capacity (J/kg K) |
| g | gravity (m/s2) |
| h | sensible enthalpy (J/kg) |
| L | latent heat (J/kg) |
| K | thermal conductivity (W/m K) |
| H | total enthalpy (J) |
| T | temperature (K) |
| S | source term |
| mass flow rate (kg/s) | |
| V | velocity (m/s) |
| ri | inner radius (m) |
| ro | outer radius (m) |
| Ts | solidus temperature (K) |
| Tl | liquidus temperature (K) |
| HTF | heat transfer fluid |
| PCM | phase change material |
| Greek symbols | |
| μ | dynamic viscosity (Pa s) |
| ρ | density (kg/m3) |
| φ | mass fraction |
| ε | small parameter for avoiding division by zero |
| β | thermal expansion coefficient (1/K) |
| λ | liquid fraction |
| Γ | latent heat of fusion (kJ/kg K) |
| Subscripts | |
| ref | reference |
| w | wall |
| bf | base fluid |
| nf | nanofluid |
| np | nanoparticle |
References
- Rostami, S.; Afrand, M.; Shahsavar, A.; Sheikholeslami, M.; Kalbasi, R.; Aghakhani, S.; Shadloo, M.S.; Oztop, H.F. A review of melting and freezing processes of PCM/nano-PCM and their application in energy storage. Energy 2020, 211, 118698. [Google Scholar] [CrossRef]
- Huang, X.; Zhu, C.; Lin, Y.; Fang, G. Thermal properties and applications of microencapsulated PCM for thermal energy storage: A review. Appl. Therm. Eng. 2019, 147, 841–855. [Google Scholar] [CrossRef]
- Kalapala, L.; Devanuri, J.K. Influence of operational and design parameters on the performance of a PCM based heat exchanger for thermal energy storage—A review. J. Energy Storage 2018, 20, 497–519. [Google Scholar] [CrossRef]
- Medrano, M.; Yilmaz, M.O.; Nogués, M.; Martorell, I.; Roca, J.; Cabeza, L.F. Experimental evaluation of commercial heat exchangers for use as PCM thermal storage systems. Appl. Energy 2009, 86, 2047–2055. [Google Scholar] [CrossRef]
- Anish, R.; Mariappan, V.; Joybari, M.M. Experimental investigation on the melting and solidification behavior of erythritol in a horizontal shell and multi-finned tube latent heat storage unit. Appl. Therm. Eng. 2019, 161, 161,114194. [Google Scholar] [CrossRef]
- Trp, A. An experimental and numerical investigation of heat transfer during technical grade paraffin melting and solidification in a shell-and-tube latent thermal energy storage unit. Sol. Energy 2005, 79, 648–660. [Google Scholar] [CrossRef]
- Alizadeh, M.; Hosseinzadeh, K.; Shahavi, M.H.; Ganji, D.D. Solidification acceleration in a triplex-tube latent heat thermal energy storage system using V-shaped fin and nano-enhanced phase change material. Appl. Therm. Eng. 2019, 163, 114436. [Google Scholar] [CrossRef]
- Ettouney, H.M.; Alatiqi, I.; Al-Sahali, A.; Al-Ali, S.A. Heat transfer enhancement by metal screens and metal spheres in phase change energy storage systems. Renew. Energy 2004, 29, 841–860. [Google Scholar] [CrossRef]
- Rahimi, M.; Ranjbar, A.A.; Ganji, D.D.G.-D.; Sedighi, K.; Hosseini, S.M.J.; Bahrampoury, R. Analysis of geometrical and operational parameters of PCM in a fin and tube heat exchanger. Int. Commun. Heat Mass Transf. 2014, 53, 109–115. [Google Scholar] [CrossRef]
- Lin, W.; Ling, Z.; Fang, X.; Zhang, Z. Experimental and numerical research on thermal performance of a novel thermal energy storage unit with phase change material. Appl. Therm. Eng. 2021, 186, 116493. [Google Scholar] [CrossRef]
- Regin, A.F.; Solanki, S.C.; Saini, J.S. Latent heat thermal energy storage using cylindrical capsule: Numerical and experimental investigations. Renew. Energy 2006, 31, 2025–2041. [Google Scholar] [CrossRef]
- Kibria, M.A.; Anisur, M.R.; Mahfuz, M.H.; Saidur, R.; Metselaar, I.H.S.C. Numerical and experimental investigation of heat transfer in a shell and tube thermal energy storage system. Int. Commun. Heat Mass Transf. 2014, 53, 71–78. [Google Scholar] [CrossRef]
- Ismail, K.A.R.; Lino, F.A.M.; da Silva, R.C.R.; de Jesus, A.B.; Paixão, L.C. Experimentally validated two dimensional numerical model for the solidification of PCM along a horizontal long tube. Int. J. Therm. Sci. 2014, 75, 184–193. [Google Scholar] [CrossRef]
- Başal, B.; Ünal, A. Numerical evaluation of a triple concentric-tube latent heat thermal energy storage. Sol. Energy 2013, 92, 196–205. [Google Scholar] [CrossRef]
- Hosseini, S.M.J.; Rahimi, M.; Bahrampoury, R. Experimental and computational evolution of a shell and tube heat exchanger as a PCM thermal storage system. Int. Commun. Heat Mass Transf. 2014, 50, 128–136. [Google Scholar] [CrossRef]
- Hosseini, S.M.J.; Ranjbar, A.A.; Sedighi, K.; Rahimi, M. A combined experimental and computational study on the melting behavior of a medium temperature phase change storage material inside shell and tube heat exchanger. Int. Commun. Heat Mass Transf. 2012, 39, 1416–1424. [Google Scholar] [CrossRef]
- Al-Abidi, A.A.; Mat, S.; Sopian, K.; Sulaiman, M.Y.; Mohammad, A.T. Experimental study of melting and solidification of PCM in a triplex tube heat exchanger with fins. Energy Build. 2014, 68, 33–41. [Google Scholar] [CrossRef]
- Esapour, M.; Hosseini, S.M.J.; Ranjbar, A.A.; Pahamli, Y.; Bahrampoury, R. Phase change in multi-tube heat exchangers. Renew. Energy 2016, 85, 1017–1025. [Google Scholar] [CrossRef]
- Esapour, M.; Hosseini, M.J.; Ranjbar, A.A.; Bahrampoury, R. Numerical study on geometrical specifications and operational parameters of multi-tube heat storage systems. Appl. Therm. Eng. 2016, 109, 351–363. [Google Scholar] [CrossRef]
- Kousha, N.; Hosseini, S.M.J.; Aligoodarz, M.R.; Pakrouh, R.; Bahrampoury, R. Effect of inclination angle on the performance of a shell and tube heat storage unit—An experimental study. Appl. Therm. Eng. 2017, 112, 1497–1509. [Google Scholar] [CrossRef]
- Mat, S.; Al-Abidi, A.A.; Sopian, K.; Sulaiman, M.Y.; Mohammad, A.T. Enhance heat transfer for PCM melting in triplex tube with internal–external fins. Energy Convers. Manag. 2013, 74, 223–236. [Google Scholar] [CrossRef]
- Adine, H.A.; Qarnia, H.E. Numerical analysis of the thermal behavior of a shell and tube heat storage unit using phase change materials. Appl. Math. Model. 2009, 33, 2132–2144. [Google Scholar] [CrossRef]
- Pakrouh, R.; Hosseini, S.M.J.; Ranjbar, A.A.; Bahrampoury, R. A numerical method for PCM-based pin fin heat sinks optimization. Energy Convers. Manag. 2015, 103, 542–552. [Google Scholar] [CrossRef]
- Pahamli, Y.; Hosseini, M.J.; Ranjbar, A.A.; Bahrampoury, R. Analysis of the effect of eccentricity and operational parameters in PCM-filled single-pass shell and tube heat exchangers. Renew. Energy 2016, 97, 344–357. [Google Scholar] [CrossRef]
- Kok, B. Examining effects of special heat transfer fins designed for the melting process of PCM and Nano-PCM. Appl. Therm. Eng. 2020, 170, 114989. [Google Scholar] [CrossRef]
- Sun, X.; Liu, L.; Mo, Y.; Li, J.; Li, C. Enhanced thermal energy storage of a paraffin-based phase change material (PCM) using nano carbons. Appl. Therm. Eng. 2020, 181, 115992. [Google Scholar] [CrossRef]
- Ebadi, S.; Tasnim, S.H.; Aliabadi, A.A.; Mahmud, S. Geometry and nanoparticle loading effects on the bio-based nano-PCM filled cylindrical thermal energy storage system. Appl. Therm. Eng. 2018, 141, 724–740. [Google Scholar] [CrossRef]
- Li, Z.; Shahsavar, A.; Al-Rashed, A.A.; Talebizadehsardari, P. Effect of porous medium and nanoparticles presences in a counter-current triple-tube composite porous/nano-PCM system. Appl. Therm. Eng. 2020, 167, 114777. [Google Scholar] [CrossRef]
- Kristiawan, B.; Santoso, B.; Wijayanta, A.T.; Aziz, M.; Miyazaki, T. Heat Transfer Enhancement of TiO2/Water Nanofluid at Laminar and Turbulent Flows: A Numerical Approach for Evaluating the Effect of Nanoparticle Loadings. Energies 2018, 11, 1584. [Google Scholar] [CrossRef] [Green Version]
- Kristiawan, B.; Wijayanta, A.T.; Enoki, K.; Miyazaki, T.; Aziz, M. Heat transfer enhancement of TiO2/water nanofluids flowing inside a square minichannel with a microfin structure: A numerical investigation. Energies 2019, 12, 3041. [Google Scholar] [CrossRef] [Green Version]
- Kristiawan, B.; Rifa’I, A.I.; Enoki, K.; Wijayanta, A.T.; Miyazaki, T. Enhancing the thermal performance of TiO2/water nanofluids flowing in a helical microfin tube. Powder Technol. 2020, 376, 254–262. [Google Scholar] [CrossRef]
- Nitsas, M.T.; Koronaki, I.P. Thermal analysis of pure and nanoparticle-enhanced PCM—Application in concentric tube heat exchanger. Energies 2020, 13, 3841. [Google Scholar] [CrossRef]
- Fong, M.; Kurnia, J.; Sasmito, A.P. Application of phase change material-based thermal capacitor in double tube heat exchanger—A numerical investigation. Energies 2020, 13, 4327. [Google Scholar] [CrossRef]
- ANSYS. Fluent Theory Guide; Ansys. Inc.: Canonsburg, PA, USA, 2012. [Google Scholar]
- Al-Abidi, A.A.; Bin Mat, S.; Sopian, K.; Sulaiman, M.Y.; Mohammad, A.T. CFD applications for latent heat thermal energy storage: A review. Renew. Sustain. Energy Rev. 2013, 20, 353–363. [Google Scholar] [CrossRef]
- Mahdi, J.M.; Lohrasbi, S.; Ganji, D.D.; Nsofor, E. Accelerated melting of PCM in energy storage systems via novel configuration of fins in the triplex-tube heat exchanger. Int. J. Heat Mass Transf. 2018, 124, 663–676. [Google Scholar] [CrossRef]
- Mahdi, J.M.; Nsofor, N.C. Solidification enhancement of PCM in a triplex-tube thermal energy storage system with nanoparticles and fins. Appl. Energy 2018, 211, 975–986. [Google Scholar] [CrossRef]
- Vajjha, R.S.; Das, D.K. Experimental determination of thermal conductivity of three nanofluids and development of new correlations. Int. J. Heat Mass Transf. 2009, 52, 4675–4682. [Google Scholar] [CrossRef]
- Vajjha, R.S.; Das, D.K.; Namburu, P.K. Numerical study of fluid dynamic and heat transfer performance of Al2O3 and CuO nanofluids in the flat tubes of a radiator. Int. J. Heat Fluid Flow 2010, 31, 613–621. [Google Scholar] [CrossRef]
- Patankar, S.V. Numerical Heat Transfer and Fluid Flow; CRC Press: Boca Raton, FL, USA, 1980; ISBN 9781315275130. [Google Scholar]
- Leonard, B. A stable and accurate convective modelling procedure based on quadratic upstream interpolation. Comput. Methods Appl. Mech. Eng. 1979, 19, 59–98. [Google Scholar] [CrossRef]
- Rostami, M.H.; Najafi, G.; Motevalli, A.; Sidik, N.A.C.; Harun, M.A. Evaluation and improvement of thermal energy of heat exchangers with SWCNT, GQD nanoparticles and PCM (RT82). J. Adv. Res. Fluid Mech. Therm. Sci. 2020, 79, 153–168. [Google Scholar] [CrossRef]
- Mahdi, J.M.; Lohrasbi, S.; Ganji, D.D.; Nsofor, E.C. Simultaneous energy storage and recovery in the triplex-tube heat exchanger with PCM, copper fins and Al2O3 nanoparticles. Energy Convers. Manag. 2019, 180, 949–961. [Google Scholar] [CrossRef]
- Rostami, M.H.; Ghobadian, B.; Najafi, G.; Motevali, A.; Sidik, N.A.C. CFD study based on effect of employing the single-walled carbon nanotube (SWCNT) and graphene quantum dots (GQD) nanoparticles and a particular fin configuration on the thermal performance in the shell and tube heat exchanger. CFD Lett. 2020, 12, 37–60. [Google Scholar] [CrossRef]
- Rostami, M.H.; Najafi, G.; Ghobadin, B.; Motevali, A. Thermal performance investigation of SWCNT and graphene quantum dots nanofluids in a shell and tube heat exchanger by using fin blade tubes. Heat Transf. 2020, 49, 4783–4800. [Google Scholar] [CrossRef]


| Properties | RT82 | Copper Fin | GQD | SWCNT |
|---|---|---|---|---|
| ρ (kg/m3) | 770 | 8920 | 400 | 2600 |
| CP (J/kgK) | 2000 | 380 | 643 | 425 |
| k (W/mK) | 0.2 | 400 | 3000 | 6600 |
| (Ns/m2) | 0.03499 | - | - | - |
| (kJ/kg) | 176 | - | - | - |
| (K) | 350 | - | - | - |
| (K) | 358 | - | - | - |
| (1/K) | 0.001 | - | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Motevali, A.; Hasandust Rostami, M.; Najafi, G.; Yan, W.-M. Evaluation and Improvement of PCM Melting in Double Tube Heat Exchangers Using Different Combinations of Nanoparticles and PCM (The Case of Renewable Energy Systems). Sustainability 2021, 13, 10675. https://doi.org/10.3390/su131910675
Motevali A, Hasandust Rostami M, Najafi G, Yan W-M. Evaluation and Improvement of PCM Melting in Double Tube Heat Exchangers Using Different Combinations of Nanoparticles and PCM (The Case of Renewable Energy Systems). Sustainability. 2021; 13(19):10675. https://doi.org/10.3390/su131910675
Chicago/Turabian StyleMotevali, Ali, Mohammadreza Hasandust Rostami, Gholamhassan Najafi, and Wei-Mon Yan. 2021. "Evaluation and Improvement of PCM Melting in Double Tube Heat Exchangers Using Different Combinations of Nanoparticles and PCM (The Case of Renewable Energy Systems)" Sustainability 13, no. 19: 10675. https://doi.org/10.3390/su131910675
APA StyleMotevali, A., Hasandust Rostami, M., Najafi, G., & Yan, W.-M. (2021). Evaluation and Improvement of PCM Melting in Double Tube Heat Exchangers Using Different Combinations of Nanoparticles and PCM (The Case of Renewable Energy Systems). Sustainability, 13(19), 10675. https://doi.org/10.3390/su131910675






