Formulation of Organic Wastes as Growth Media for Cultivation of Earthworm Nutrient-Rich Eisenia foetida
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of the Cultivation Media
2.2. Cultivation Process
2.3. Experimental Design and Statistical Analysis
3. Results and Discussion
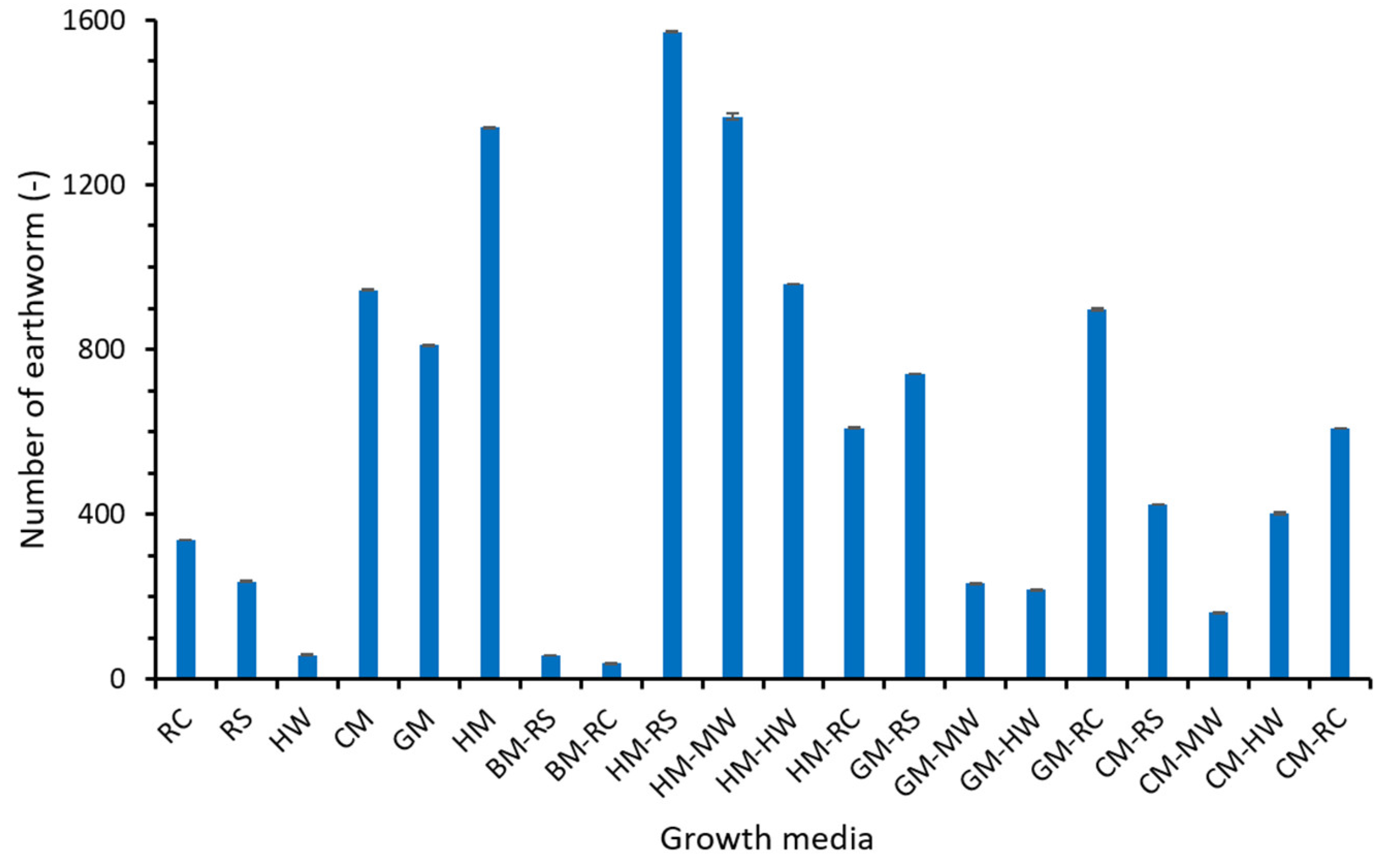
3.1. Mortality Rate
3.2. Cocoon Production
3.3. Cocoon Hatching
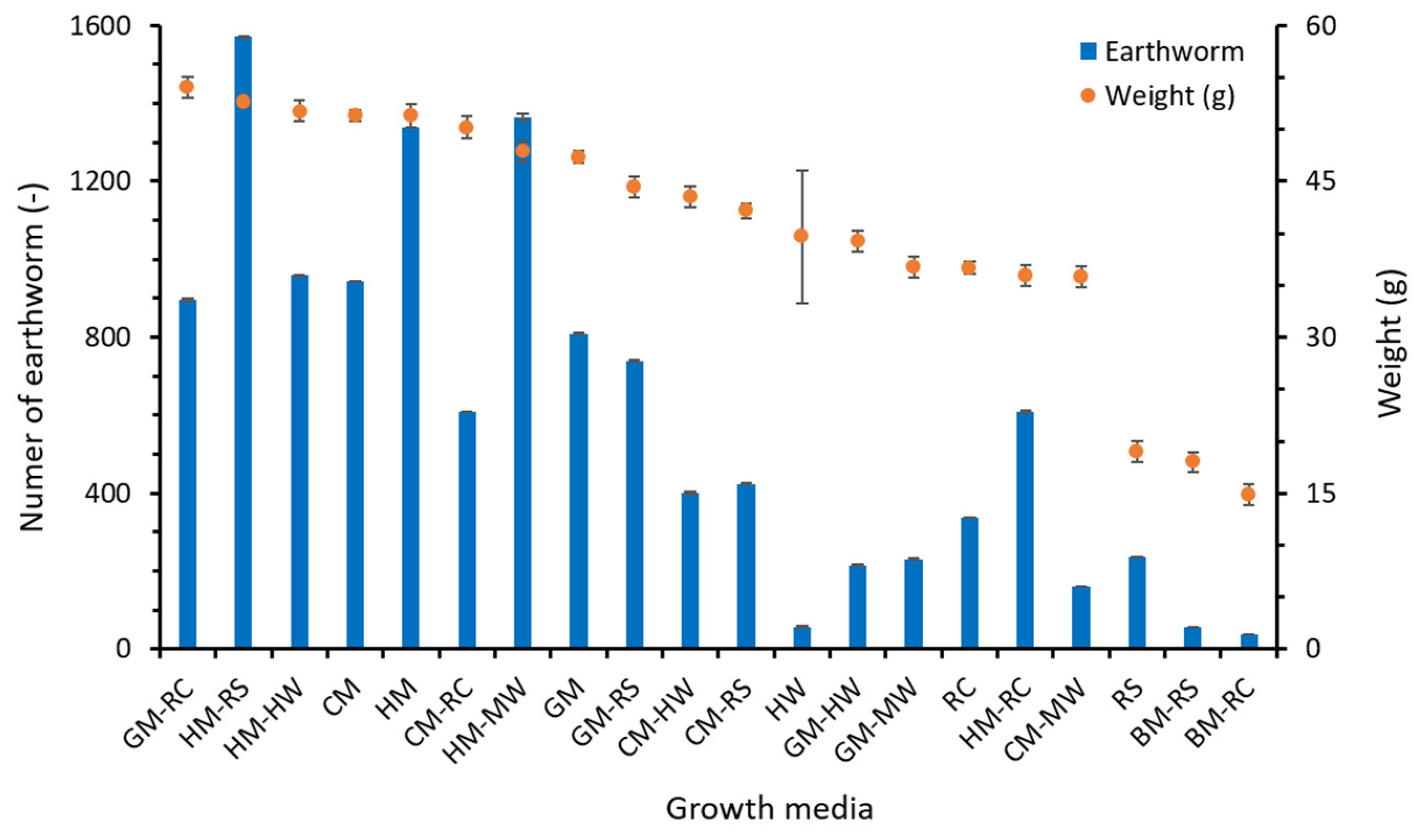
3.4. Biomass Production
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ghosh, C. Integrated Vermi-Pisciculture––An Alternative Option for Recycling of Solid Municipal Waste in Rural India. Bioresour. Technol. 2004, 93, 71–75. [Google Scholar] [CrossRef] [PubMed]
- Nair, J.; Sekiozoic, V.; Anda, M. Effect of Pre-Composting on Vermicomposting of Kitchen Waste. Bioresour. Technol. 2006, 97, 2091–2095. [Google Scholar] [CrossRef] [PubMed]
- Oyedele, D.J.; Schjønning, P.; Amusan, A.A. Physicochemical Properties of Earthworm Casts and Uningested Parent Soil from Selected Sites in Southwestern Nigeria. Ecol. Eng. 2006, 28, 106–113. [Google Scholar] [CrossRef]
- Adhikary, S. Vermicompost, the Story of Organic Gold: A Review. Agric. Sci. 2012, 3, 905–917. [Google Scholar] [CrossRef] [Green Version]
- Edwards, C.A. (Ed.) Earthworm Ecology, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2004; ISBN 978-0-8493-1819-1. [Google Scholar]
- Lim, P.N.; Wu, T.Y.; Shyang Sim, E.Y.; Lim, S.L. The Potential Reuse of Soybean Husk as Feedstock of Eudrilus Eugeniae in Vermicomposting. J. Sci. Food Agric. 2011, 91, 2637–2642. [Google Scholar] [CrossRef]
- Garg, P.; Gupta, A.; Satya, S. Vermicomposting of Different Types of Waste Using Eisenia foetida: A Comparative Study. Bioresour. Technol. 2006, 97, 391–395. [Google Scholar] [CrossRef]
- Gunya, B.; Masika, P.J.; Hugo, A.; Muchenje, V. Nutrient Composition and Fatty Acid Profiles of Oven-Dried and Freeze-Dried Earthworm Eisenia foetida. J. Food Nutr. Res. 2016, 4, 343–348. [Google Scholar] [CrossRef]
- Chaves, R.C.; de Paula, R.Q.; Gücker, B.; Marriel, I.E.; Teixeira, A.O.; Boëchat, I.G. An Alternative Fish Feed Based on Earthworm and Fruit Meals for Tilapia and Carp Postlarvae. Rev. Bras. Biociênc. 2015, 13, 15–24. [Google Scholar]
- Loh, T.C.; Fong, L.Y.; Foo, H.L.; Thanh, N.T.; Sheikh-Omar, A.R. Utilisation of Earthworm Meal in Partial Replacement of Soybean and Fish Meals in Diets of Broilers. J. Appl. Anim. Res. 2009, 36, 29–32. [Google Scholar] [CrossRef]
- Edwards, C.A.; Blaxter, K.L.; Fowden, L. Production of Feed Protein from Animal Waste by Earthworms. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1985, 310, 299–307. [Google Scholar] [CrossRef]
- Parolini, M.; Ganzaroli, A.; Bacenetti, J. Earthworm as an Alternative Protein Source in Poultry and Fish Farming: Current Applications and Future Perspectives. Sci. Total Environ. 2020, 734, 139460. [Google Scholar] [CrossRef]
- Stafford, E.A.; Tacon, A.G.J. The Nutritional Evaluation of Dried Earthworm Meal (Eisenia foetida, Savigny, 1826) Included at Low Levels in Production Diets for Rainbow Trout, Salmo Gairdneri Richardson. Aquac. Res. 1985, 16, 213–222. [Google Scholar] [CrossRef]
- Fosgate, O.T.; Babb, M.R. Biodegradation of Animal Waste by Lumbricus terrestris. J. Dairy Sci. 1972, 55, 870–872. [Google Scholar] [CrossRef]
- Hayati, S.N.; Herdian, H.; Damayanti, E.; Istiqomah, L.; Julendra, H. Profil Asam Amino Ekstrak Cacing Tanah (Lumbricus Rubellus) Terenkapsulasi Dengan Metode Spray Drying. J. Teknol. Indones. 2011, 34, 1–7. [Google Scholar]
- Musyoka, S.N.; Liti, D.M.; Ogello, E.; Waidbacher, H. Utilization of the Earthworm, Eisenia fetida (Savigny, 1826) as an Alternative Protein Source in Fish Feeds Processing: A Review. Aquac. Res. 2019, 50, 2301–2315. [Google Scholar] [CrossRef] [Green Version]
- Nahmani, J.; Hodson, M.E.; Black, S. Effects of Metals on Life Cycle Parameters of the Earthworm Eisenia fetida Exposed to Field-Contaminated, Metal-Polluted Soils. Environ. Pollut. 2007, 149, 44–58. [Google Scholar] [CrossRef]
- Žaltauskaitė, J.; Sodienė, I. Effects of Cadmium and Lead on the Life-Cycle Parameters of Juvenile Earthworm Eisenia fetida. Ecotoxicol. Environ. Saf. 2014, 103, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Hatanaka, K.; Ishioka, Y.; Furuichi, E. Cultivation of Eisenia fetida using dairy waste sludge cake. In Earthworm Ecology; Satchell, J.E., Ed.; Springer: Dordrecht, The Netherlands, 1983; pp. 323–329. ISBN 978-94-009-5967-5. [Google Scholar]
- Minnich, J. The Earthworm Book: How to Raise and Use Earthworms for Your Farm and Garden; Rodale Press: Emmaus, PA, USA, 1977; ISBN 978-0-87857-193-2. [Google Scholar]
- Huang, K.; Li, F.; Wei, Y.; Chen, X.; Fu, X. Changes of Bacterial and Fungal Community Compositions during Vermicomposting of Vegetable Wastes by Eisenia foetida. Bioresour. Technol. 2013, 150, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Vodounnou, D.S.J.V.; Kpogue, D.N.S.; Tossavi, C.E.; Mennsah, G.A.; Fiogbe, E.D. Effect of Animal Waste and Vegetable Compost on Production and Growth of Earthworm (Eisenia fetida) during Vermiculture. Int. J. Recycl. Org. Waste Agric. 2016, 5, 87–92. [Google Scholar] [CrossRef] [Green Version]
- Loh, T. Vermicomposting of Cattle and Goat Manures by Eisenia foetida and Their Growth and Reproduction Performance. Bioresour. Technol. 2005, 96, 111–114. [Google Scholar] [CrossRef]
- Domínguez, J.; Velando, A.; Ferreiro, A. Are Eisenia fetida (Savigny, 1826) and Eisenia andrei Bouché (1972) (Oligochaeta, Lumbricidae) Different Biological Species? Pedobiologia 2005, 49, 81–87. [Google Scholar] [CrossRef]
- Suthar, S. Vermicomposting of Vegetable-Market Solid Waste Using Eisenia fetida: Impact of Bulking Material on Earthworm Growth and Decomposition Rate. Ecol. Eng. 2009, 35, 914–920. [Google Scholar] [CrossRef]
- Henneberg, W.; Stohmann, F. Über Das Erhaltungsfutter Volljährigen Rindviehs. J. Landwirtsch 1859, 3, 485–551. [Google Scholar]
- Greenfield, H.; Southgate, D.A.T. Food Composition Data: Production, Management, and Use; FAO: Rome, Italy, 2003; ISBN 978-92-5-104949-5. [Google Scholar]
- Langan, A.M.; Shaw, E.M. Responses of the Earthworm Lumbricus terrestris (L.) to Iron Phosphate and Metaldehyde Slug Pellet Formulations. Appl. Soil Ecol. 2006, 34, 184–189. [Google Scholar] [CrossRef]
- Esmaeili, A.; Khoram, M.R.; Gholami, M.; Eslami, H. Pistachio Waste Management Using Combined Composting-Vermicomposting Technique: Physico-Chemical Changes and Worm Growth Analysis. J. Clean. Prod. 2020, 242, 118523. [Google Scholar] [CrossRef]
- Abbott, I.; Parker, C.A. Interactions between Earthworms and Their Soil Environment. Soil Biol. Biochem. 1981, 13, 191–197. [Google Scholar] [CrossRef]
- Edwards, C.A.; Fletcher, K.E. Interactions between Earthworms and Microorganisms in Organic-Matter Breakdown. Agric. Ecosyst. Environ. 1988, 24, 235–247. [Google Scholar] [CrossRef]
- Monroy, F.; Aira, M.; Domínguez, J.; Velando, A. Seasonal Population Dynamics of Eisenia fetida (Savigny, 1826) (Oligochaeta, Lumbricidae) in the Field. Comptes Rendus Biol. 2006, 329, 912–915. [Google Scholar] [CrossRef]
- Luo, Y.; Zang, Y.; Zhong, Y.; Kong, Z. Toxicological Study of Two Novel Pesticides on Earthworm Eisenia foetida. Chemosphere 1999, 39, 2347–2356. [Google Scholar] [CrossRef]
- Rao, J.V.; Kavitha, P. Toxicity of Azodrin on the Morphology and Acetylcholinesterase Activity of the Earthworm Eisenia foetida. Environ. Res. 2004, 96, 323–327. [Google Scholar] [CrossRef]
- Zhu, X.; Lian, B.; Yang, X.; Liu, C.; Zhu, L. Biotransformation of Earthworm Activity on Potassium-Bearing Mineral Powder. J. Earth Sci. 2013, 24, 65–74. [Google Scholar] [CrossRef]
- Singh, S.; Singh, J.; Vig, A.P. Earthworm as Ecological Engineers to Change the Physico-Chemical Properties of Soil: Soil vs Vermicast. Ecol. Eng. 2016, 90, 1–5. [Google Scholar] [CrossRef]
- Singh, S.; Sharma, A.; Khajuria, K.; Singh, J.; Vig, A.P. Soil Properties Changes Earthworm Diversity Indices in Different Agro-Ecosystem. BMC Ecol. 2020, 20, 27. [Google Scholar] [CrossRef] [PubMed]
- Gates, G.E. Burmese Earthworms: An Introduction to the Systematics and Biology of Megadrile Oligochaetes with Special Reference to Southeast Asia. Trans. Am. Philos. Soc. 1972, 62, 1–326. [Google Scholar] [CrossRef]
- Yadav, K.D.; Tare, V.; Ahammed, M.M. Vermicomposting of Source-Separated Human Faeces for Nutrient Recycling. Waste Manag. 2010, 30, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Haukka, J.K. Growth and Survival of Eisenia fetida (Sav.) (Oligochaeta: Lumbricidae) in Relation to Temperature, Moisture and Presence of Enchytraeus Albidus (Henle) (Enchytraeidae). Biol. Fertil. Soils 1987, 3, 99–102. [Google Scholar] [CrossRef]
- Mashur, M. Produksi kokon dan biomassa cacing tanah Eisenia foetida pada berbagai media budidaya limbah peternakan. Biosci. J. Ilmiah. Biol. 2020, 8, 48. [Google Scholar] [CrossRef]
- Garg, V.K.; Yadav, Y.K.; Sheoran, A.; Chand, S.; Kaushik, P. Livestock Excreta Management through Vermicomposting Using an Epigeic Earthworm Eisenia foetida. Environmentalist 2006, 26, 269–276. [Google Scholar] [CrossRef]


| No. | Code | Media | Source of Collection |
|---|---|---|---|
| 1 | CM | Cow manure | Local farmer |
| 2 | HM | Horse manure | Local farmer |
| 3 | GM | Goat manure | Local farmer |
| 4 | BM | Broiler chicken manure | Local farmer |
| 5 | MW | Market organic waste | Wet market |
| 6 | HW | Household organic waste | Household waste |
| 7 | RS | Rice straw | Local rice farm |
| 8 | RC | Beef rumen content | Local slaughterhouse |
| 9 | CM-MW | CM + MW | The media were from from equal wt.% |
| 10 | CM-HW | CM + HW | |
| 11 | CM-RS | CM + RS | |
| 12 | CM-RC | CM + RC | |
| 13 | HM-MW | HM + MW | |
| 14 | HM-HW | HM + HW | |
| 15 | HM-RS | HM + RS | |
| 16 | HM-RC | HM + RC | |
| 17 | GM-MW | GM + MW | |
| 18 | GM-HW | GM + HW | |
| 19 | GM-RS | GM + RS | |
| 20 | GM-RC | GM + RC | |
| 21 | BM-MW | BM + MW | |
| 22 | BM-HW | BM + HW | |
| 23 | BM-RS | BM + RS | |
| 24 | BM-RC | BM + RC |
| Cultivation Media | Water (%) | Ash (%) | Protein (%) | Fat (%) | Fiber (%) |
|---|---|---|---|---|---|
| RC | 16.96 | 14.99 | 17.6 | 0.04 | 33.76 |
| MW | 21.41 | 17.16 | 18.66 | 2.69 | 33.86 |
| RS | 17.61 | 26.88 | 8.33 | 0.07 | 20.83 |
| HW | 28.38 | 16.71 | 21.12 | 2.73 | 23.72 |
| CM | 15.37 | 51.97 | 10.62 | 0.54 | 16.21 |
| GM | 19.69 | 21.15 | 17.84 | 0.92 | 32.9 |
| HM | 17.74 | 31.54 | 13.2 | 0.14 | 25.73 |
| BM | 23.87 | 24.35 | 24.93 | 1.25 | 16.53 |
| BM-RS | 18.76 | 33 | 15.49 | 0.15 | 17.27 |
| BM-MW | 21.82 | 26.3 | 22.29 | 2.42 | 9.47 |
| BM-HW | 25.94 | 23.15 | 24.29 | 1.56 | 21.17 |
| BM-RC | 18.49 | 21.33 | 14.79 | 0.52 | 25.95 |
| HM-RS | 16.07 | 30.28 | 9.39 | 0.62 | 26.24 |
| HM-MW | 15.73 | 30.21 | 9.86 | 0.34 | 27.44 |
| HM-HW | 16.89 | 29.06 | 9.27 | 0.55 | 27.4 |
| HM-RC | 15.62 | 26.24 | 10.44 | 0.13 | 31.73 |
| GM-RS | 17.16 | 24.34 | 15.9 | 0.46 | 31.58 |
| GM-MW | 18.78 | 19.37 | 19.71 | 0.89 | 35.24 |
| GM-HW | 20.51 | 18.45 | 18.71 | 2.05 | 35.65 |
| GM-RC | 18.66 | 20.44 | 17.89 | 0.63 | 36.99 |
| CM-RS | 14.85 | 38.1 | 10.86 | 0.35 | 15.9 |
| CW-MW | 15.52 | 41.74 | 11.73 | 0.05 | 21.54 |
| CM-HW | 18.67 | 40.3 | 13.73 | 0.17 | 16.2 |
| CM-RC | 16.08 | 33.74 | 11.5 | 0.18 | 18.44 |
| Cultivation Media | N (%) | P (%) | K (%) | C (%) | C/N (-) | Organic (%) |
|---|---|---|---|---|---|---|
| RC | 2.82 | 0.78 | 0.7 | 42.23 | 14.98 | 70.63 |
| MW | 2.99 | 0.4 | 1.66 | 46.02 | 15.39 | 79.16 |
| RS | 1.33 | 0.09 | 0.95 | 40.62 | 30.54 | 69.87 |
| HW | 3.38 | 0.48 | 1.97 | 46.27 | 13.69 | 79.59 |
| CM | 1.7 | 0.49 | 1.11 | 26.68 | 15.69 | 45.89 |
| GM | 2.85 | 0.41 | 1.39 | 43.81 | 15.37 | 75.35 |
| HM | 2.11 | 0.74 | 1.03 | 38.11 | 18.06 | 65.55 |
| BM | 3.99 | 1.13 | 1.5 | 42.03 | 10.53 | 72.29 |
| BM-RS | 2.48 | 0.91 | 1.46 | 37.22 | 15.01 | 64.02 |
| BM-MW | 3.57 | 0.99 | 1.6 | 40.94 | 11.48 | 70.42 |
| BM-HW | 3.89 | 1.01 | 1.54 | 42.69 | 10.97 | 73.43 |
| BM-RC | 2.37 | 1.09 | 1.2 | 43.71 | 18.44 | 75.17 |
| HM-RS | 1.5 | 0.43 | 0.86 | 38.76 | 25.84 | 66.67 |
| HM-MW | 1.58 | 0.68 | 1.2 | 38.77 | 24.54 | 66.69 |
| HM-HW | 1.48 | 0.74 | 1.2 | 39.41 | 26.63 | 67.79 |
| HM-RC | 1.67 | 0.79 | 0.93 | 40.93 | 24.54 | 70.48 |
| GM-RS | 2.54 | 0.26 | 1.26 | 42.03 | 16.55 | 72.3 |
| GM-MW | 3.15 | 0.37 | 1.62 | 44.79 | 14.22 | 77.05 |
| GM-HW | 2.99 | 0.37 | 1.68 | 45.31 | 15.15 | 77.93 |
| GM-RC | 2.86 | 0.52 | 1.32 | 44.2 | 15.45 | 76.02 |
| CM-RS | 1.74 | 0.3 | 1.1 | 34.59 | 19.88 | 59.15 |
| CM-MW | 1.88 | 0.33 | 1.26 | 32.37 | 17.22 | 55.67 |
| CM-HW | 2.2 | 0.53 | 1.32 | 33.13 | 15.06 | 57.05 |
| CM-RC | 1.84 | 0.67 | 1 | 36.81 | 20.21 | 63.31 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mashur, M.; Bilad, M.R.; Hunaepi, H.; Huda, N.; Roslan, J. Formulation of Organic Wastes as Growth Media for Cultivation of Earthworm Nutrient-Rich Eisenia foetida. Sustainability 2021, 13, 10322. https://doi.org/10.3390/su131810322
Mashur M, Bilad MR, Hunaepi H, Huda N, Roslan J. Formulation of Organic Wastes as Growth Media for Cultivation of Earthworm Nutrient-Rich Eisenia foetida. Sustainability. 2021; 13(18):10322. https://doi.org/10.3390/su131810322
Chicago/Turabian StyleMashur, Mashur, Muhammad Roil Bilad, Hunaepi Hunaepi, Nurul Huda, and Jumardi Roslan. 2021. "Formulation of Organic Wastes as Growth Media for Cultivation of Earthworm Nutrient-Rich Eisenia foetida" Sustainability 13, no. 18: 10322. https://doi.org/10.3390/su131810322
APA StyleMashur, M., Bilad, M. R., Hunaepi, H., Huda, N., & Roslan, J. (2021). Formulation of Organic Wastes as Growth Media for Cultivation of Earthworm Nutrient-Rich Eisenia foetida. Sustainability, 13(18), 10322. https://doi.org/10.3390/su131810322








