Mitigation of Eutrophication in a Shallow Lake: The Influences of Submerged Macrophytes on Phosphorus and Bacterial Community Structure in Sediments
Abstract
:1. Introduction
2. Materials and Methods
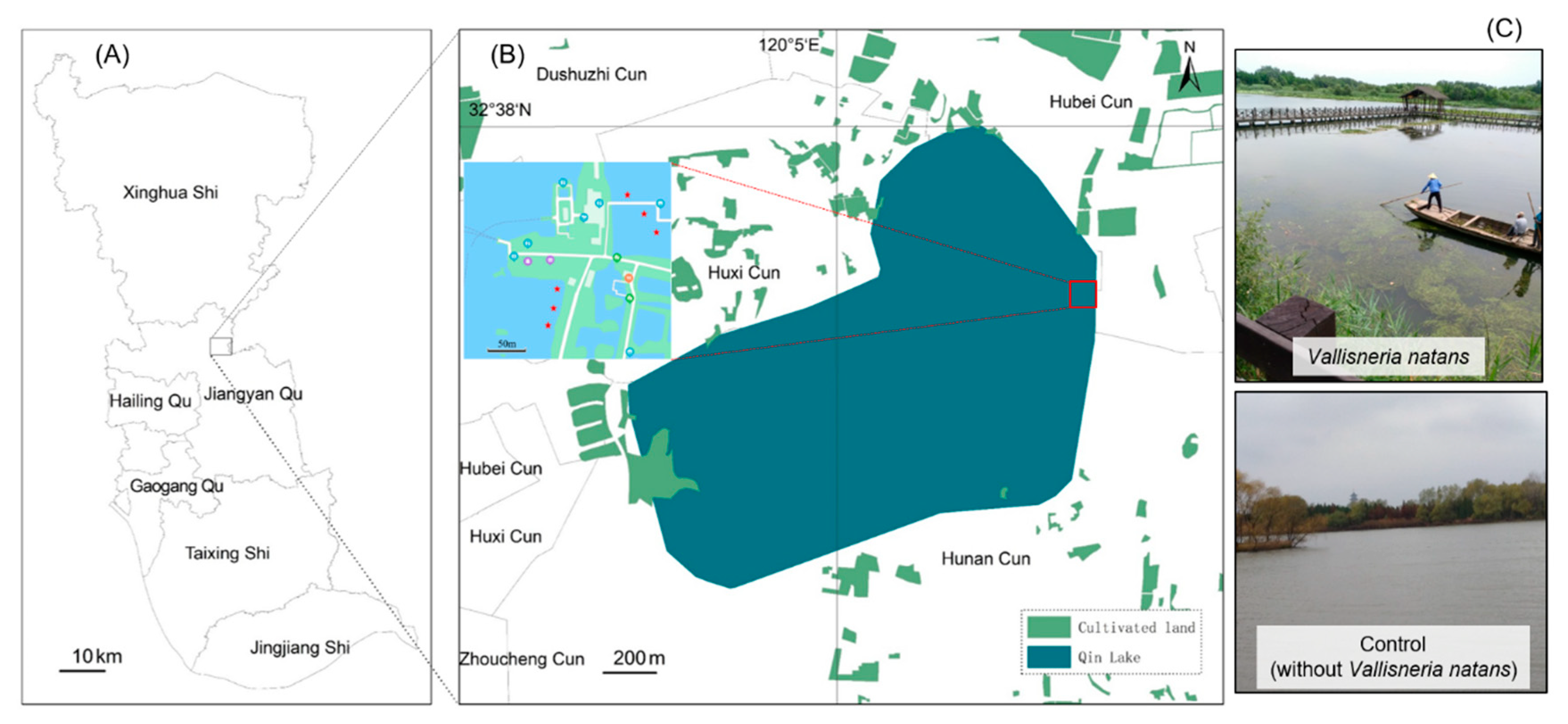
2.1. Sampling Site
2.2. Sample Collection
2.3. Physicochemical Analysis
2.4. DNA Extraction, PCR Amplification, and Sequencing
2.5. Data Analyses
3. Results
3.1. pH and Dissolved Oxygen Concentration in the Lake Water
3.2. Organic Matter, Fe, and P Contents in the Lake Sediment
3.3. Bacterial Community Composition in the Lake Sediment
3.3.1. Bacterial Community Diversity
3.3.2. Bacterial Community Composition
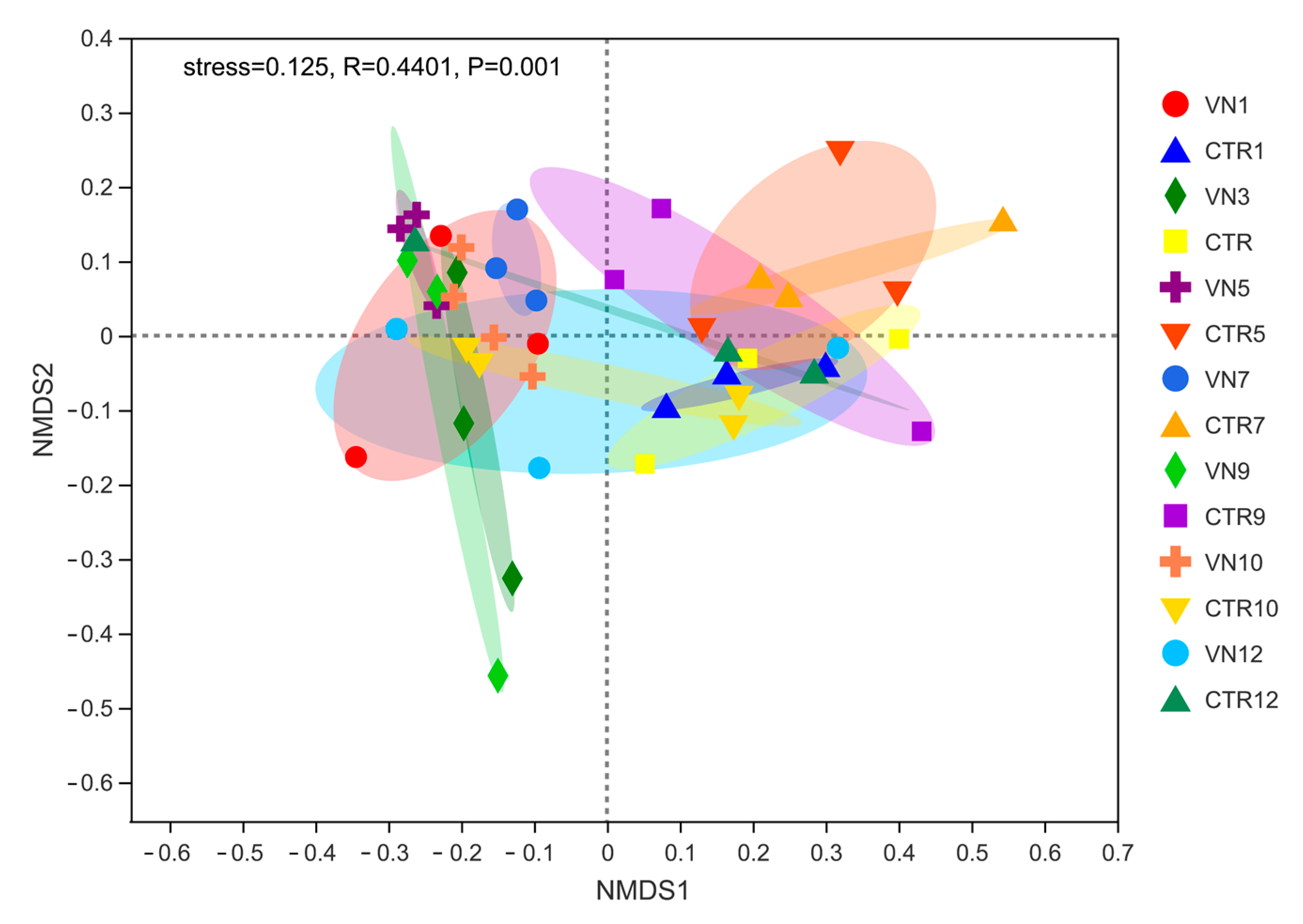
3.3.3. Differences in Bacterial Community Composition
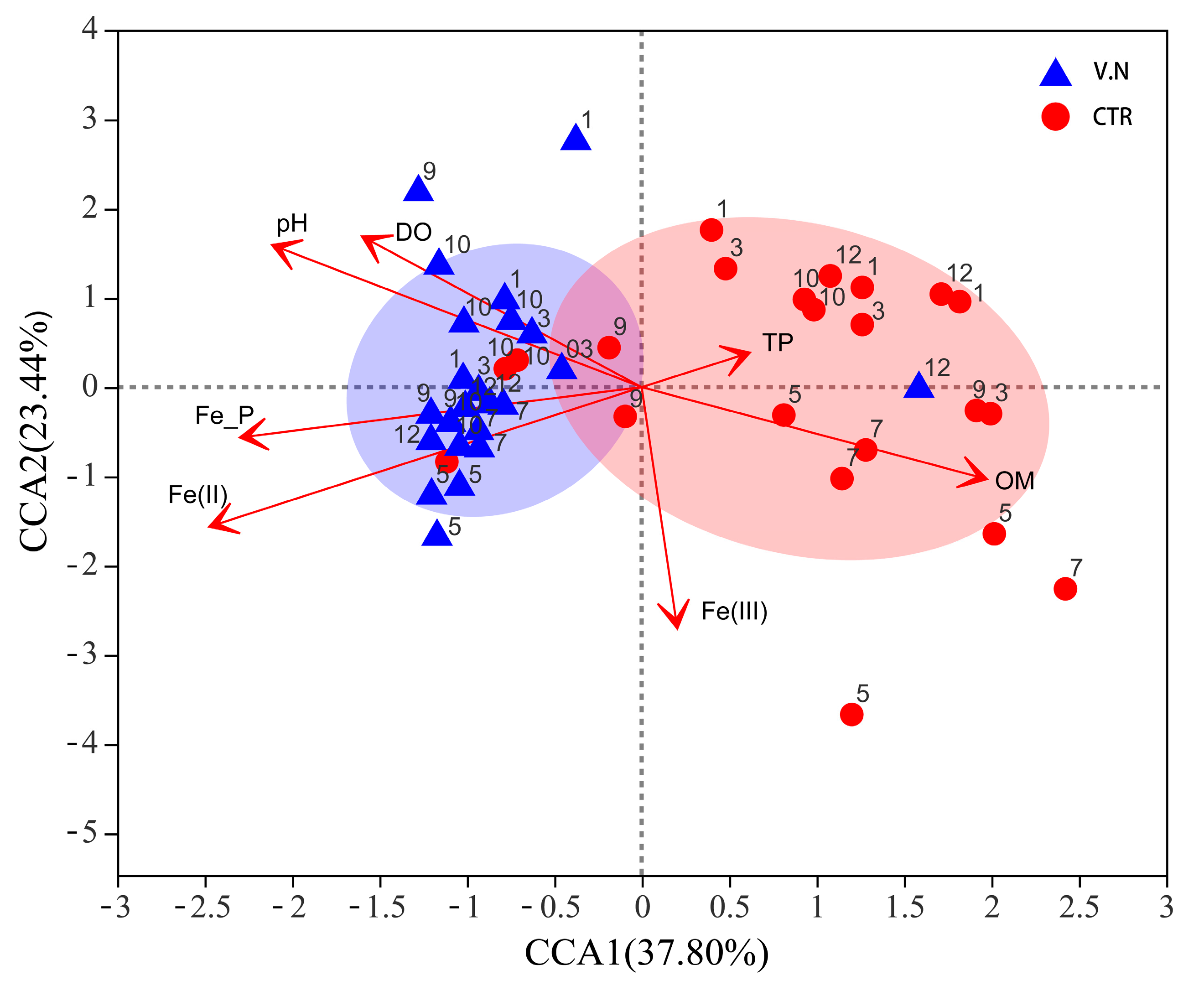
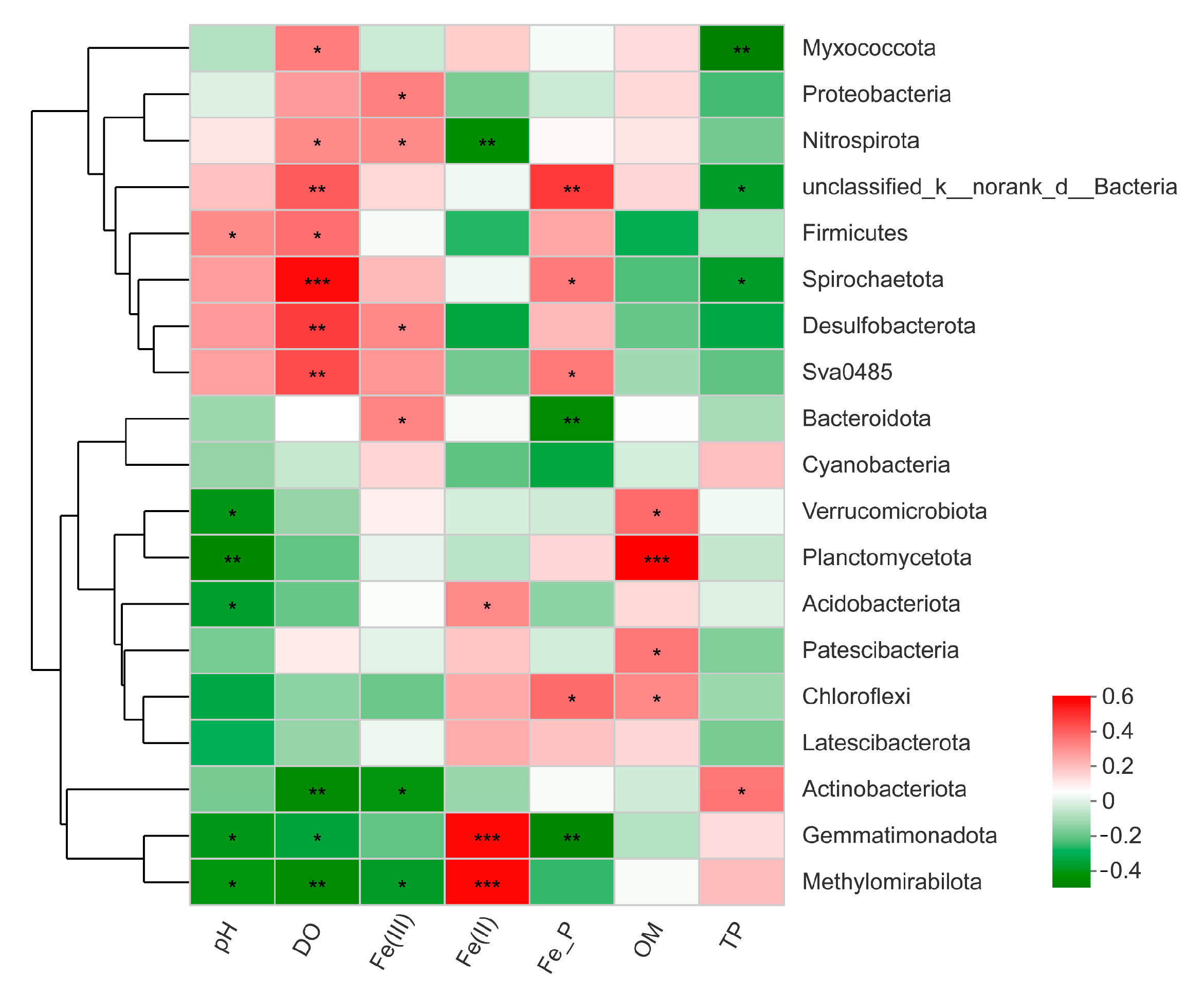
3.4. Relationship between Bacterial Communities and Environmental Factors
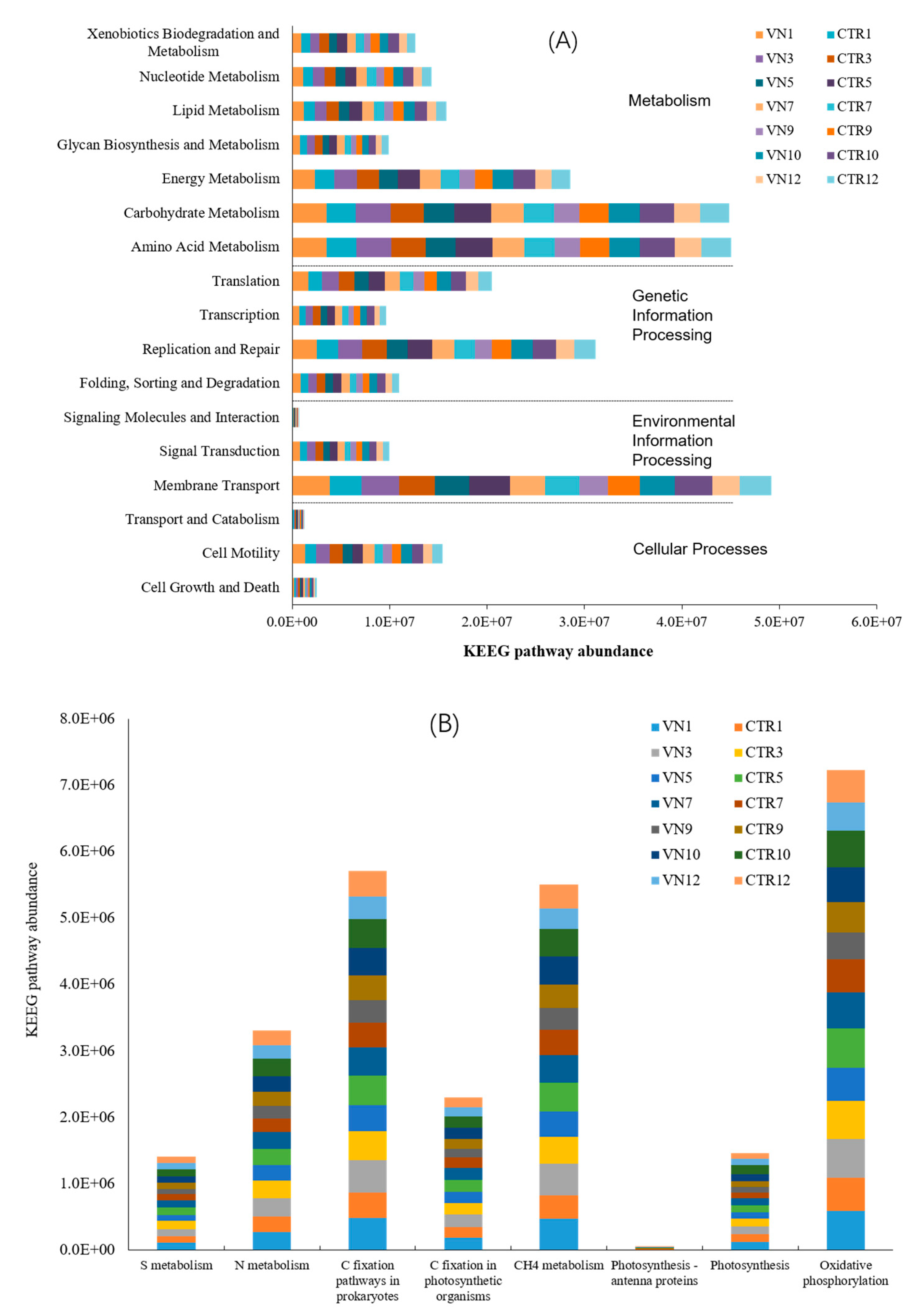
3.5. Prediction of Bacterial Functions
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Le, C.; Zha, Y.; Li, Y.; Sun, D.; Lu, H.; Yin, B. Eutrophication of lake waters in China: Cost, causes, and control. Environ. Manag. 2010, 45, 662–668. [Google Scholar] [CrossRef]
- Brett, M.T.; Benjamin, M.M. A review and reassessment of lake phosphorus retention and the nutrient loading concept. Freshw. Biol. 2007, 53, 194–211. [Google Scholar] [CrossRef]
- Li, L.L.; Tang, X.M.; Gao, G.; Shao, K.Q.; Gong, Z.J.; Chen, D.; Zhang, Y.H. Influence of submerged vegetation restoration on bacterial diversity and community composition in West Lake. J. Lake Sci. 2013, 25, 188–198. [Google Scholar]
- Søndergaard, M.; Jeppesen, E.; Lauridsen, T.; Skov, C.; van Nes, E.; Roijackers, R.; Lammens, E.; Portielje, R. Lake restoration: Successes, failures and long-term effects. J. Appl. Ecol. 2007, 44, 1095–1105. [Google Scholar] [CrossRef]
- Kleeberg, A.; Herzog, C.; Hupfer, M. Redox sensitivity of iron in phosphorus binding does not impede lake restoration. Water Res. 2013, 47, 1491–1502. [Google Scholar] [CrossRef]
- Bai, G.; Zhang, Y.; Yan, P.; Yan, W.; Kong, L.; Wang, L.; Wang, C.; Liu, Z.; Liu, B.; Ma, J.; et al. Spatial and seasonal variation of water parameters, sediment properties, and submerged macrophytes after ecological restoration in a long-term (6 year) study in Hangzhou west lake in China: Submerged macrophyte distribution influenced by environmental variables. Water Res. 2020, 186, 116379. [Google Scholar] [CrossRef]
- Lin, Q.; Fan, M.; Jin, P.; Riaz, L.; Wu, Z.; He, F.; Liu, B.; Ma, J. Sediment type and the clonal size greatly affect the asexual reproduction, productivity, and nutrient absorption of Vallisneria natans. Restor. Ecol. 2019, 28, 408–417. [Google Scholar] [CrossRef]
- Xing, X.; Ding, S.; Liu, L.; Chen, M.; Yan, W.; Zhao, L.; Zhang, C. Direct evidence for the enhanced acquisition of phosphorus in the rhizosphere of aquatic plants: A case study on Vallisneria natans. Sci. Total. Environ. 2018, 616–617, 386–396. [Google Scholar] [CrossRef]
- Yin, X.; Lu, J.; Wang, Y.; Liu, G.; Hua, Y.; Wan, X.; Zhao, J.; Zhu, D. The abundance of nirS-type denitrifiers and anammox bacteria in rhizospheres was affected by the organic acids secreted from roots of submerged macrophytes. Chemosphere 2020, 240, 124903. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Liu, S.; Jahan, T.E.; Liu, B.; He, F.; Zhou, Q.; Wu, Z. Short term succession of artificially restored submerged macrophytes and their impact on the sediment microbial community. Ecol. Eng. 2017, 103, 50–58. [Google Scholar] [CrossRef]
- Xu, P.; Xiao, E.; Xu, D.; Li, J.; Zhang, Y.; Dai, Z.; Zhou, Q.; Wu, Z. Enhanced phosphorus reduction in simulated eutrophic water: A comparative study of submerged macrophytes, sediment microbial fuel cells, and their combination. Environ. Technol. 2017, 39, 1144–1157. [Google Scholar] [CrossRef]
- Malecki-Brown, L.M.; White, J.; Brix, H. Alum application to improve water quality in a municipal wastewater treatment wetland: Effects on macrophyte growth and nutrient uptake. Chemosphere 2010, 79, 186–192. [Google Scholar] [CrossRef] [Green Version]
- Zhao, D.-Y.; Liu, P.; Fang, C.; Sun, Y.-M.; Zeng, J.; Wang, J.-Q.; Ma, T.; Xiao, Y.-H.; Wu, Q.L. Submerged macrophytes modify bacterial community composition in sediments in a large, shallow, freshwater lake. Can. J. Microbiol. 2013, 59, 237–244. [Google Scholar] [CrossRef]
- Zhao, D.; Wang, S.; Huang, R.; Zeng, J.; Huang, F.; Yu, Z. Diversity and composition of bacterial community in the rhizosphere sediments of submerged macrophytes revealed by 454 pyrosequencing. Ann. Microbiol. 2017, 67, 313–319. [Google Scholar] [CrossRef]
- He, R.; Zeng, J.; Zhao, D.; Huang, R.; Yu, Z.; Wu, Q.L. Contrasting patterns in diversity and community assembly of phragmites australis root-associated bacterial communities from different seasons. Appl. Environ. Microbiol. 2020, 86. [Google Scholar] [CrossRef]
- Kalff, J.; Rooney, N. Submerged Macrophyte-bed effects on water-column phosphorus, chlorophyll a and bacterial production. Ecosystems 2003, 6, 797–807. [Google Scholar] [CrossRef]
- Hitchcock, J.N.; Mitrovic, S.M.; Kobayashi, T.; Westhorpe, D.P. Responses of estuarine bacterioplankton, phytoplankton and zooplankton to dissolved organic carbon (doc) and inorganic nutrient additions. Chesap. Sci. 2009, 33, 78–91. [Google Scholar] [CrossRef]
- Subbarao, G.V.; Ishikawa, T.; Ito, O.; Nakahara, K.; Wang, H.Y.; Berry, W.L. A bioluminescence assay to detect nitrification inhibitors released from plant roots: A case study with Brachiaria humidicola. Plant Soil 2006, 288, 101–112. [Google Scholar] [CrossRef]
- Davison, W. Iron and manganese in lakes. Earth Sci. Rev. 1993, 34, 119–163. [Google Scholar] [CrossRef]
- Gunnars, A.; Blomqvist, S.; Johansson, P.; Andersson, C. Formation of Fe(III) oxyhydroxide colloids in freshwater and brackish seawater, with incorporation of phosphate and calcium. Geochim. Cosmochim. Acta 2002, 66, 745–758. [Google Scholar] [CrossRef]
- Obi, C.C.; Adebusoye, S.A.; Ugoji, E.O.; Ilori, M.; Amund, O.O.; Hickey, W.J. Microbial communities in sediments of Lagos Lagoon, Nigeria: Elucidation of community structure and potential impacts of contamination by municipal and industrial wastes. Front. Microbiol. 2016, 7, 1213. [Google Scholar] [CrossRef] [Green Version]
- Cui, N.; Zhang, X.; Cai, M.; Zhou, L.; Chen, G.; Zou, G. Roles of vegetation in nutrient removal and structuring microbial communities in different types of agricultural drainage ditches for treating farmland runoff. Ecol. Eng. 2020, 155, 105941. [Google Scholar] [CrossRef]
- Wang, L.Z.; Wang, G.X.; Yu, Z.F.; Zhou, B.B.; Ge, X.G.; Chen, Q.M.; Li, Z.G. Effects of Vallisneria natans on sediment phosphorus fractions and transfer during the growth period. J. Environ. Sci. 2011, 23, 753–760. [Google Scholar]
- Chen, Z.F.; Chen, K.; Yang, S.J. Analysis of remediation effect of aquatic plants on freshwater ecosystem. Mol. Plant 2019, 17, 4501–4506. [Google Scholar]
- Feng, J.M.; Zhang, H.R. Conservation and measures of Qin Lake wetland in Jiangyan. Pollut. Control. Technol. 2011, 24, 23–25. [Google Scholar]
- Kostka, J.E.; Iii, G. Partitioning and speciation of solid phase iron in saltmarsh sediments. Geochim. Cosmochim. Acta 1994, 58, 1701–1710. [Google Scholar] [CrossRef]
- Springer, U.; Klee, J. Prüfung der leistungsfähigkeit von einigen wichtigen verfahren zur bestimmung des kohlenstoffs mittels chromschwefelsaure sowie vorschlag einer neuen schnellmethode. J. Plant Nutr. Soil Sci. 1954, 64, 1–26. [Google Scholar]
- Bao, S.D. Soil Agro-Chemistrical Analysis, 3rd ed.; China Agriculture Press: Beijing, China, 2000; pp. 76–78. [Google Scholar]
- Ruban, V.; López-Sánchez, J.F.; Pardo, P.; Rauret, G.; Muntau, H.; Quevauviller, P. Harmonized protocol and certified reference material for the determination of extractable contents of phosphorus in freshwater sediments—A synthesis of recent works. Anal. Bioanal. Chem. 2001, 370, 224–228. [Google Scholar] [CrossRef]
- Dennis, K.L.; Wang, Y.; Blatner, N.R.; Wang, S.; Saadalla, A.; Trudeau, E.; Roers, A.; Weaver, C.; Lee, J.J.; Gilbert, J.A.; et al. Adenomatous polyps are driven by microbe-instigated focal inflammation and are controlled by IL-10–producing T cells. Cancer Res. 2013, 73, 5905–5913. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stackebrandt, E.; Goebel, B.M. Taxonomic note: A place for DNA-DNA reassociation and 16S rRNA sequence analysis in the present species definition in bacteriology. Int. J. Syst. Evol. Microbiol. 1994, 44, 846–849. [Google Scholar] [CrossRef] [Green Version]
- Shannon, C.E. A mathematical theory of communication. Bell Syst. Tech. J. 1948, 27, 379–423. [Google Scholar] [CrossRef] [Green Version]
- Chao, A.; Yang, M.C.K. Stopping rules and estimation for recapture debugging with unequal failure rates. Biometrika 1993, 80, 193. [Google Scholar] [CrossRef]
- Fan, X.; Ding, S.; Gong, M.; Chen, M.; Gao, S.; Jin, Z.; Tsang, D. Different influences of bacterial communities on Fe (III) reduction and phosphorus availability in sediments of the cyanobacteria- and macrophyte-dominated zones. Front. Microbiol. 2018, 9, 2636. [Google Scholar] [CrossRef]
- Liu, M.; Ran, Y.; Peng, X.; Zhu, Z.; Liang, J.; Ai, H.; Li, H.; He, Q. Sustainable modulation of anaerobic malodorous black water: The interactive effect of oxygen-loaded porous material and submerged macrophyte. Water Res. 2019, 160, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.Y.; Xie, Y.F.; Liu, Z.W. The response to external phosphorus loadings of experimental aquatic systems with submerged plants of different root to shoot ratios. Chin. J. Appl. Environ. Biol. 2016, 22, 747–751. [Google Scholar]
- Qian, Y.; Cheng, C.L.; Drouillard, K.; Zhu, Q.Z.; Feng, H.; He, S.Z.; Fang, Y.H.; Qiao, S.N.; Kolenčíka, M.; Chang, X.X. Bioaccumulation and growth characteristics of Vallisneria natans (Lour.) Hara after chronic exposure to metal-contaminated sediments. Environ. Sci. Pollut. Res. 2019, 26, 20510–20519. [Google Scholar] [CrossRef]
- Klatt, C.G.; Liu, Z.; Ludwig, M.; Kühl, M.; Jensen, S.I.; Bryant, D.A.; Ward, D.M. Temporal metatranscriptomic patterning in phototrophic Chloroflexi inhabiting a microbial mat in a geothermal spring. ISME J. 2013, 7, 1775–1789. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Liu, S.; Zhang, Y.; Liu, B.; Zeng, L.; He, F.; Zhou, Q.; Wu, Z. Effects of planted versus naturally growing Vallisneria natans on the sediment microbial community in West Lake, China. Microb. Ecol. 2017, 74, 278–288. [Google Scholar] [CrossRef]
- Velmurugan, N.; Kalpana, D.; Cho, J.-Y.; Lee, G.-H.; Park, S.-H.; Lee, Y.-S. Phylogenetic analysis of culturable marine bacteria in sediments from South Korean Yellow Sea. Microbiology 2011, 80, 261–272. [Google Scholar] [CrossRef]
- Shen, L.-D.; Zheng, P.-H.; Ma, S.-J. Nitrogen loss through anaerobic ammonium oxidation in agricultural drainage ditches. Biol. Fertil. Soils 2015, 52, 127–136. [Google Scholar] [CrossRef]
- Coates, J.D.; Ellis, D.J.; Gaw, C.V.; Lovley, D.R. Geothrix fermentans gen. nov., sp. nov., a novel Fe(III)-reducing bacterium from a hydrocarbon-contaminated aquifer. Int. J. Syst. Evol. Microbiol. 1999, 49, 1615–1622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Terashima, M.; Aama, A.; Sato, M.; Yumoto, I.; Kamagata, Y.; Kato, S. Culture-dependent and -independent identification of polyphosphate-accumulating Dechloromonas spp. predominating in a full-scale oxidation ditch wastewater treatment plant. Microbes Environ. 2016, 31, 449–455. [Google Scholar] [CrossRef] [Green Version]
- Pang, S.; Zhang, S.; Lv, X.; Han, B.; Liu, K.; Qiu, C.; Wang, C.; Wang, P.; Toland, H.; He, Z. Characterization of bacterial community in biofilm and sediments of wetlands dominated by aquatic macrophytes. Ecol. Eng. 2016, 97, 242–250. [Google Scholar] [CrossRef]
- Takeshi, Y.; Yuji, S.; Satoshi, H.; Hiroyuki, I.; Akiyoshi, O.; Hideki, H.; Yoichi, K. Anaerolinea thermolimosa sp. nov., Levilinea saccharolytica gen. nov., sp. nov. and Leptolinea tardivitalis gen. nov., sp. nov., novel filamentous anaerobes, and description of the new classes Anaerolineae classis nov. and Caldilineae classis nov. in the bacterial phylum Chloroflexi. Int. J. Syst. Evol. Microbiol. 2006, 56, 1331–1340. [Google Scholar]
- Basso, O.; Caumette, P.; Magot, M. Desulfovibrio putealis sp. nov., a novel sulfate-reducing bacterium isolated from a deep subsurface aquifer. Int. J. Syst. Evol. Microbiol. 2005, 55, 101–104. [Google Scholar] [CrossRef] [Green Version]
- Rozan, T.F.; Taillefert, M.; Trouwborst, R.E.; Glazer, B.T.; Ma, S.F.; Herszage, J.L.; Valdes, L.M.; Price, K.S.; Luther, G.W., III. Iron-sulfur-phosphorus cycling in the sediments of a shallow coastal bay: Implications for sediment nutrient release and benthic macroalgal blooms. Limnol. Oceanogr. 2002, 47, 1346–1354. [Google Scholar] [CrossRef] [Green Version]
- Sun, X.; Zhou, Y.; Tan, Y.; Wu, Z.; Lu, P.; Zhang, G.; Yu, F. Restoration with pioneer plants changes soil properties and remodels the diversity and structure of bacterial communities in rhizosphere and bulk soil of copper mine tailings in Jiangxi Province, China. Environ. Sci. Pollut. Res. 2018, 25, 22106–22119. [Google Scholar] [CrossRef]
- Weber, K.; Achenbach, L.A.; Coates, J.D. Microorganisms pumping iron: Anaerobic microbial iron oxidation and reduction. Nat. Rev. Genet. 2006, 4, 752–764. [Google Scholar] [CrossRef] [Green Version]
- Lovley, D.R.; Holmes, D.E.; Nevin, K.P. Dissimilatory Fe(III) and Mn(IV) Reduction. Adv. Microb. Physiol. 2004, 49, 219–286. [Google Scholar] [CrossRef] [Green Version]
- Kellermann, C.; Griebler, C. Thiobacillus thiophilus sp. nov., a chemolithoautotrophic, thiosulfate-oxidizing bacterium isolated from contaminated aquifer sediments. Int. J. Syst. Evol. Microbiol. 2009, 59, 583–588. [Google Scholar] [CrossRef] [Green Version]
- Bednaík, A.; Blaser, M.; Matou, A.; Tuer, M.; Rulík, M. Sediment methane dynamics along the Elbe River. Limnologica 2019, 79, 125716. [Google Scholar] [CrossRef]
- Stanley, E.H.; Casson, N.J.; Christel, S.T.; Crawford, J.T.; Loken, L.C.; Oliver, S.K. The ecology of methane in streams and rivers: Patterns, controls, and global significance. Ecol. Monogr. 2016, 86, 146–171. [Google Scholar] [CrossRef]
- Chen, F.; Zheng, Y.; Hou, L.; Niu, Y.; Gao, D.; An, Z.; Zhou, J.; Yin, G.; Dong, H.; Han, P.; et al. Microbial abundance and activity of nitrite/nitrate-dependent anaerobic methane oxidizers in estuarine and intertidal wetlands: Heterogeneity and driving factors. Water Res. 2021, 190, 116737. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Liu, C.; He, K.; Shen, Q.; Zhong, J. Dramatic temporal variations in methane levels in black bloom prone areas of a shallow eutrophic lake. Sci. Total Environ. 2021, 767, 144868. [Google Scholar] [CrossRef] [PubMed]



| Sampling Time | Treatment | Sobs | Shannon | ACE | Coverage |
|---|---|---|---|---|---|
| January | V.N | 3582.3 b | 6.67 | 4680.2 | 0.972 |
| CTR | 4509.3 a* | 7.15 | 6665.2 | 0.94 | |
| March | V.N | 3698.3 | 7.01 | 4375.3 | 0.98 |
| CTR | 4074.3 | 6.95 | 6216.0 | 0.95 | |
| May | V.N | 3849.3 | 6.73 | 5602.9 | 0.96 |
| CTR | 3162.5 | 6.37 | 4054.8 | 0.98 | |
| July | V.N | 5077.3 | 7.32 | 7054.0 | 0.95 |
| CTR | 4948.0 | 7.24 | 7172.5 | 0.95 | |
| September | V.N | 4309.8 | 7.01 | 7053.4 a | 0.95 |
| CTR | 4163.8 | 7.01 | 6076.5 b | 0.95 | |
| October | V.N | 3096.3 | 6.49 b | 5440.9 | 0.95 |
| CTR | 3940.5 | 7.18 a | 5138.7 | 0.96 | |
| December | V.N | 3648.3 | 6.80 | 6562.4 | 0.94 |
| CTR | 3835.3 | 6.92 | 6468.6 | 0.95 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Zhang, S.; Que, T.; Kaksonen, A.H.; Qian, X.; Zhuang, X.; Bohu, T. Mitigation of Eutrophication in a Shallow Lake: The Influences of Submerged Macrophytes on Phosphorus and Bacterial Community Structure in Sediments. Sustainability 2021, 13, 9833. https://doi.org/10.3390/su13179833
Wang J, Zhang S, Que T, Kaksonen AH, Qian X, Zhuang X, Bohu T. Mitigation of Eutrophication in a Shallow Lake: The Influences of Submerged Macrophytes on Phosphorus and Bacterial Community Structure in Sediments. Sustainability. 2021; 13(17):9833. https://doi.org/10.3390/su13179833
Chicago/Turabian StyleWang, Juanjuan, Siwen Zhang, Tianyang Que, Anna H. Kaksonen, Xiaoqing Qian, Xuliang Zhuang, and Tsing Bohu. 2021. "Mitigation of Eutrophication in a Shallow Lake: The Influences of Submerged Macrophytes on Phosphorus and Bacterial Community Structure in Sediments" Sustainability 13, no. 17: 9833. https://doi.org/10.3390/su13179833
APA StyleWang, J., Zhang, S., Que, T., Kaksonen, A. H., Qian, X., Zhuang, X., & Bohu, T. (2021). Mitigation of Eutrophication in a Shallow Lake: The Influences of Submerged Macrophytes on Phosphorus and Bacterial Community Structure in Sediments. Sustainability, 13(17), 9833. https://doi.org/10.3390/su13179833









