Abstract
Potato is an economically important vegetable crop in Egypt. Weed infestation, especially broad-leafed, during the vegetative growth stage substantially affects both crop yield and tuber quality. In the current study, the impact of new ready-mix pre-emergent herbicides on broadleaf weeds, tuber yield, and quality was evaluated. The two-year field experiment comprised the following treatments: (1) Un-weeded control, (2) Hand hoeing, (3) Sencor, (4) Ecopart, (5) Zeus, (6) Kroki, and (7) Flomex. The results showed that weed control treatments significantly reduced the weed density compared to un-weeded control and the herbicides efficacy reached over 90%. The herbicidal treatments also significantly increased the activity of antioxidant enzymes peroxidases (POX) and catalase (CAT) and improved the non-enzymatic antioxidant (carotenoids) compared to un-weeded control. Conversely, the higher content of malondialdehyde (MDA) in potato leaves was obtained for un-weeded control. Moreover, weed control treatments caused significant enhancement in plant growth parameters, yield, and its components in addition to tuber quality of potato. Compared to the un-weeded control, maximum tuber yield was observed in Flomex followed by Ecopart, Kroki, Zeus, and Sencor, respectively. The higher number of tubers and total yield were recorded in plants treated with Flomex plus compared to all the other treatments. Higher content of total soluble sugar, total soluble protein, and total starch content was observed in weed control treatments compared with un-weeded control. Based on Pearson’s correlation and heatmap analysis, the changes in agro-physiological parameters data are linked to the herbicidal treatments. The results indicate that the applied herbicides could be alternative products for Sencor and an option for controlling broadleaved weeds. However, further studies are needed to ensure their efficacy and safety under other conditions.
1. Introduction
Potato (Solanum tuberosum L.) is the world’s leading vegetable crop and is grown in 79% of the world’s countries [1]. According to FAO statistics, the world production of potatoes reached 400 million tons, which were harvested from 19.25 million ha, in 2019 [2]. Potato is the fourth most important crop after rice, wheat, and maize in global production volume and contributing to the world food needs. In Egypt, potato is one of the main crops and the second most important vegetable crops after tomato in economic value [3,4]. However, potato is susceptible to weed infestation that emerges earlier than the crop and leads to heavy losses in yield. Thus, the occurrence of weeds in potato fields represents a major constraint for potato cultivation [5]. Weed competition in potatoes can reduce yield and potato tuber quality as well [5,6]. If annual weeds are left uncontrolled, the tuber yield losses can reach up to 86.0%, depending on the severity of infestation [5]. Weeds also interfere with harvest, causing more potatoes to be left in the field and increasing tuber injury. Additionally, weeds may serve as a harbor for insects, nematodes, and diseases of potatoes [7,8]. Thus, controlling weeds, mostly broadleaves, is an essential element and major component of successful potato production. Mechanical and cultural weed control means are showed to be less effective and costly in addition to the current scarcity of hand labor [9].
Furthermore, cultivation performed at three and five weeks after potato emergence injured roots and reduced yield [10]. Consequently, herbicides are presently most favored by farmers as they offer efficient, cost-effective, and reliable weed control methods compared to other measures [9]. Although the efficacy of herbicides against weeds has been primarily studied, there are not enough reports available on their effects on the potato growth and quality of tubers [11,12]. Potato quality and tuber chemical constituents content mainly total sugars, starch, and proteins, were shown to be altered after herbicide application [13,14].
Metribuzin is the most commonly used pre-emergence selective herbicide for numerous years, mainly for annual broadleaf weed control in potatoes, since there are no registered foliar post-emergence herbicides for broadleaf weeds. However, due to over-reliance on metribuzin as a single herbicide with the same mode of action, some farmers are complaining that metribuzin no longer provides satisfactory weed control in certain areas throughout the crop season. Moreover, negative effects may occur on new certain potato cultivars after the application of metribuzin [15]. Poor efficiency of metribuzin could be attributed to shifting of weed flora and placing selection pressure on weed populations; this could eventually select for resistant weeds, which pose a threat to the sustainability of weed control and potato farming. Failure to control weeds would result in escalating the cost and time for weed management in potato cultivation. For sustainable weed management to be achieved, a change to the current herbicide use patterns is needed through herbicide use diversification [16]. Besides, the most sustainable approaches in weed control are based on integrating herbicides with mechanical and cultural practices to maintain weed control efficiency, yield production, and conserve the environment [17,18].
In this regard, herbicide premixes or ready-mixes broaden the spectrum of weed species controlled, as they often include more than one mode of action and, thereby, can be an efficient tool for controlling diverse weeds in a single growing season. Ready-mix herbicides in sugar cane were shown to be more effective against weeds than the single application [19]. The synergistic effect of these pre-packaged herbicides would also help to delay the imminent resistance risk and shift in weed population, which is a problem linked to the dependence on a single herbicide [19,20]. Herbicide combinations also as pre-packaged commercial formulations helped to tackle herbicide-resistant Phalaris minor in wheat fields [21]. Therefore, there is a need to prolong and sustain the efficacy of current herbicides. In Egypt, farmers mostly favor using pre-emergence herbicides for broadleaf weeds in potatoes, which may be because of their easiness in application and effectiveness starting at the early growth stages of the crop where competitive ability with weeds is lower. Therefore, the aim of this study was to (1) assess the efficacy of three new selective pre-emergence premix herbicides (under evaluation) under the commercial trade names Zeus, Kroki, and Flomex against broadleaf weeds, and these new herbicides contain two or more herbicides with different modes of action; (2) evaluate the effect of these herbicides on potato growth, yield, and quality attributes; and (3) determine the efficiency of pyraflufen ethyl as a newly registered herbicide in potatoes in addition to metribuzin.
2. Materials and Methods
2.1. Experimental Layout
Two field experiments were conducted at the Experimental Research Station, Giza, Egypt, during the winter seasons in 2019–2020 and 2020–2021. The soil of the experimental field was loamy clay in texture and slightly alkaline in reaction (pH 7.5). Potato seeds (cv. Bern) were cut (3–4 pieces) and left for 20 days until sprouting (about 1 cm) in a ventilated storage room. The potato seeds were planted at 25 cm between seeds and 0.5 cm between the rows. Seeds were irrigated after 28 days from planting and continuing through the experiment at 21 day-intervals. Potato plants were subjected to 660, 325.5, and 352.5 Kg·ha−1 for N, P2O5, and K2O, respectively. Flood irrigation was applied uniformly throughout the study. The experiment plots were arranged in a randomized complete block design with four replications. Seven treatments were included for annual broadleaved weed control as follows: untreated control (un-weeded served as a control), hand hoeing, metribuzin, pyraflufen ethyl, three ready premix herbicides of (MCPA 32% + flumetsulam 1%), (clomazone 11% + fomesafen 5.5% + quizalofop ethyl 1.5%), and (flufenacet 6% + pendimethalin 30%).
The description of the tested herbicides is summarized in Table 1. All herbicides were applied pre-emergence two weeks after potato planting and before emergence. The application of the herbicides was based on the recommended rate as specified by the manufacturer. Herbicides sprayed with operated knapsack sprayer fitted with flat fan nozzle using spray volume of 500 L ha−1. Herbicides were used, without any surfactant or additive. Water was applied for the untreated control in the same manner used for the other treatments. Potato protection against other diseases and insects was applied according to standard recommendations.

Table 1.
The tested herbicides used in the experiment.
The potato tubers were harvested four months after seed cultivation. Data on weed efficacy were collected after one month from herbicide application and plant growth parameters were taken 60 days after potato seed cultivation, while those for tuber yield and quality was recorded at harvesting time, after 120 days (Figure 1).
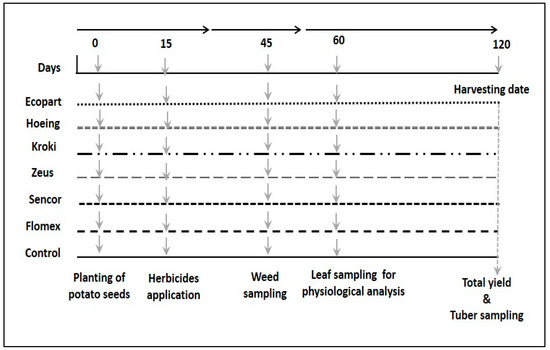
Figure 1.
Scheme of the experimental layout. Horizontal lines characterize different weed control treatments. Herbicidal treatments were applied before potato tuber transplanting at seven days. Samples of weed were collected after 30 days from tuber transplanting, and tuber samples were collected at the end of the season (after 120 days).
2.1.1. Weed Control Efficacy
The dominant broadleaf weed species associated with potato in the experimental field were identified, and the corresponding infestation ratio is shown in Table 2.

Table 2.
Dominant weed species found in the potato field in the experiment.
Data on aboveground weed fresh weight per 1 m2 was taken randomly four weeks after herbicide application. The herbicide efficacy was calculated based on fresh weight, using the following formula by Yadav et al. [6].
where WFWC = weed fresh weight in the un-weeded control; WFWT = weed fresh weight in treatment.
2.1.2. Growth Parameter and Yield Components
Plant samples from each treatment were selected randomly for recording the leaf greening index (SPAD, 502 Minolta Co, Osaka, Japan), plant height, fresh and dry weight of shoots, and the number of stems. The total yield of each treatment and the number of tubers per plant, the mean of tuber length, diameter, and weight were also determined.
2.1.3. LEAF Photosynthetic Pigments
Chlorophyll a, chlorophyll b, carotenoids, and total chlorophyll contents in fresh leaves were quantified following the method of Lichtenthaler et al. [22]. Leaf samples (500 mg) were ground in 80% acetone, then centrifuged at 1000× g for 10 min at 4 °C and the extracts were analyzed to record the absorbance of the supernatant at 647, 663, and 470 nm using a 96-well plate (Infinite 200PRO Nano Quant). The values of photosynthetic pigments were expressed in mg g−1 FW.
2.1.4. Leaf Malondialdehyde Content (MDA)
Fifty mg of a fresh leaf was homogenized in 0.5 mL of 0.1% (w/v) trichloroacetic acid (TCA). The homogenate was filtered and incubated with 0.5% (v/v) thiobarbituric acid (TBA) in 20% TCA at 95 °C. The absorbance was read at 440, 532, and 600 nm using a microplate reader (Infinite 200 PRO, Tecan Group Ltd., Männedorf, Switzerland). MDA content was expressed as nmol·g−1 FW [23].
2.1.5. Leaf Proline Content
Free proline content was estimated according to Shabnam et al. [24]. Briefly, 50 mg of dry leaves were homogenized in 1.5 mL of 3% (v/v) sulfosalicylic acid solution. Then, the homogenate was filtrated and mixed with a 1.25% (w/v) ninhydrin reagent. Finally, leaf content (mg proline g−1 DW) was calculated using pure proline as a standard based on the absorbance at 508 nm using a microplate reader (Infinite 200 PRO, Tecan Group Ltd., Männedorf, Switzerland).
2.1.6. Leaf Membrane Stability Index (MSI)
Membrane stability indices (MSI) of leaves were estimated using the method stated by Rady [25]; briefly, a 200 mg sample of fresh leaf tissue was placed in a test tube containing 10 mL of double-distilled water heated at 40 °C in a water bath for 30 min. First, the electrical conductivity (C1) of the solution was recorded using a conductivity bridge. Then, the second sample was boiled at 100 °C for 10 min, and the conductivity was also measured (C2). The MSI of potato leaves was calculated by following formula.
2.1.7. Activities of leaf Antioxidant Enzymes
A 50 mg of fresh leaves was powdered in liquid nitrogen and homogenized in 0.5 mL potassium phosphate buffer (50 mM, pH 7.0) containing 2% (w/v) PVP and 0.1 mM EDTA. Homogenates were centrifuged at 15,000× g for 30 min at 4 °C and the supernatants were used to determine peroxidase activities (POX, EC 1.11.1.7). The catalase activity (CAT, EC: 1.11.1.6) was determined using the methods described by Aebi [26].
2.1.8. Chemical Composition of Tubers
Tuber Total Soluble Sugar Content
The total soluble sugar content of 50 mg of tuber was extracted in 1.5 mL of distilled water at 50 °C for 15 min, according to the method described by Giannoccaro et al. [27] and quantified calorimetrically using anthrone-sulfuric acid.
Tuber Soluble Protein Content and Dry Matter
Total soluble protein content was determined using the Lowry method [28]. The dry matter content of potato tuber was assessed using the oven-dry method, then using the following equation: Dry matter (%) = Dry weight/Fresh weight × 100, to calculate the percentage of dry matter content.
Tuber Total Soluble Solid and Total Starch Content
Total soluble solids content (TSS) in fresh potato tubers was determined using a Chinese-made digital refractometer TD-45. The starch content of potato tubers was determined using a formula described by Burton [29]:
where specific gravity = tuber fresh weight/tuber volume.
Starch (%) content = 17.546 + 199.07 (specific gravity − 1. 0988)
2.1.9. Statistical Analysis
Data were statistically analyzed using statistica 7 software (version 7, StatSoft Inc., Tulsa, OK, USA) and treatment average was compared using the Tukey test. The interactions between treatments and years for all studied parameters were insignificant, so data were combined over the two growing seasons. Pearson’s correlation analysis was statistically performed using the SPSS program (version 16.0 for Windows, Chicago, IL, USA). Finally, based on Pearson’s correlation results, hierarchical clustering was developed and transformed into a two-dimensional heatmap plot. The heatmap was drawn with the aid of ClustVis Tool (R package version 0.10.2.1) [30].
3. Results and Discussion
3.1. Efficacy of Herbicides
At 28 days after herbicide application to the germinating broadleaf weeds, excellent weed control efficiency (over 90%) was obtained compared to the un-weeded control, as illustrated in Figure 2. The lowest weed control efficacy was obtained in the case of hoeing (72.0%). None of the tested herbicides caused visible foliar injury symptoms except Kroki, which contains 11% clomazone, as slight foliar phytotoxicity was observed. Similar results were obtained by Klara and Zvonimir [31] as a mixture of flufenacet and metribuzin have proved to be a very good and selective herbicide for control of a wide array of annual weeds in potato.
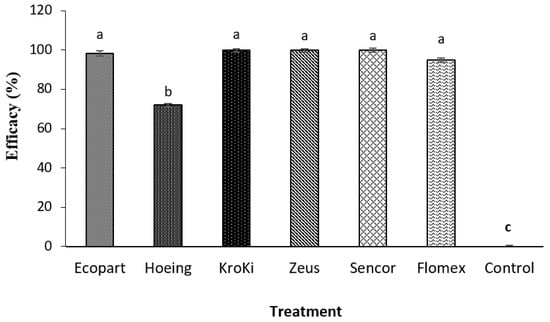
Figure 2.
Effect of weed control treatments on weed population density Means within each column followed by the same letter are not statistically different at p ≤ 0.05 according to Tukey test; vertical bars indicate to standard error (±SE).
The efficacy of metribuzin was almost equal to other herbicides. Pre-emergence application of the tested herbicides facilitated weed control, which was not achieved efficiently with hoeing [32]. Herbicides not only reduced weed biomass but also increased tubers yield compared with hoeing. This could be explained as a decline in weed fresh weight and then removal of competition with weeds and improving plant growth requirements and yield formation [33]. Results exhibited that manual weeding alone is not sufficient for weed control, which is in line with Barbaś et al. [34] who found that chemical control of weeds (as weed biomass) in potato was more effective than a mechanical method. Moreover, in case of the current higher labor costs, scarcity, and the risk of spreading plant diseases that penetrate plants through physical damage, hoeing is an unfavorable method for farmers [35].
3.2. Potato Growth Parameters
With the exception of Sencor, very limited information is available regarding the effects of the newly tested herbicides on vegetative growth. As shown in Table 3, all tested herbicides and hoeing twice significantly enhanced all tested growth parameters. Flomex recorded taller plants, Flomex and Zeus showed higher shoot fresh weight and dry weight, and Ecopart showed a higher number of stems compared with other treatments and the control. Additionally, the greatest content of photosynthetic pigments (greening index, chlorophyll a, chlorophyll b, and total chlorophyll) were observed in all tested herbicides compared with the control. The current results indicated that the treatment with herbicides had a positive effect on reducing weed density and improved plant growth. This improvement could be due to the little competition with associated weeds for light, water, and nutrient absorption [36,37].

Table 3.
Effect of herbicides on vegetative growth of potato plants. Means within each column followed by the same letter are not statistically different at p ≤ 0.05 according to Tukey test, ±value indicates to standard error (±SE).
3.3. Leaf Membrane Stability Index (MSI) and Lipid Peroxidation (MDA)
Data in Figure 3A,B show that weed control treatments revealed apparent differences in MSI index and MDA. The leaf membrane stability index value decreased markedly with herbicidal treatments application compared to un-weeded control and hand hoeing (Figure 3A). The maximum values of MSI were obtained for un-weeded control and hoeing, with no significant difference among them, compared to the other weed control treatments. In contrast, the minimum value recorded by Flomex followed by Ecopart, Kroki, Zeus, and Sencor compared to un-weed treatment (Control). Our results agree with Mahmoud et al. [38] and Arvin et al. [39] who found that MSI decreased with increasing stress conditions, such as the toxicity of herbicides.
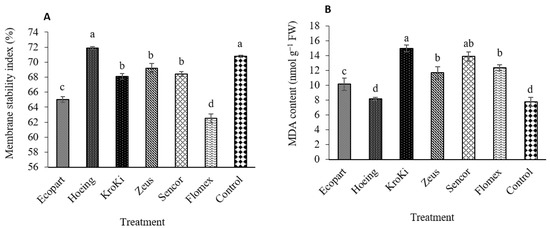
Figure 3.
Effect of weed control treatments on membrane stability index (A) and lipid peroxidation (MDA, B) of potato leaves. Columns followed by the same letter are not statistically different at p ≤ 0.05 according to the Tukey test. Vertical bars indicate standard error (±SE).
Regarding to lipid peroxidation, lipid peroxidation produces malondialdehyde (MDA), a product of the breakdown of fatty acids of membranes, which causes a collapse to plant cells [40]. Weed control treatments revealed significant differences in MDA. As shown in Figure 3B, the maximum values of leaf MDA content were recorded by herbicidal treatments while minimum values were obtained for hand hoeing and un-weeded control. Compared to un-weeded control, the value of MDA content was increased by 52.7%, 45.4%, 21.8%, 18.1%, and 17.08% for Kroki, Ecopart, Zeus, Sencor, and Flomex, respectively. These results are contradictory to those by Alves et al. [41], who observed that lipid peroxidation (MDA formed) in shoot tissues of Vicia sativa clearly increased after fomesafen application. Additionally, after bentazone application in two peanut cultivars, MDA contents increased, indicating occurrence of lipid peroxidation and oxidative stress [42]. In a recent study, it was reported that the fenoxaprop showed negative effects on levels of lipid peroxidation (MDA) in wheat [43]. Abiotic stress, as herbicides toxicity, results in an increase in the amount of MDA in buckwheat compared with the control [44].
3.4. Enzymatic Antioxidants
Data in Figure 4A,B show the activity of POX and CAT in untreated and treated plots. All herbicides significantly increased the activity of the POX and CAT compared to un-weeded control. The maximum activity of POX and CAT was observed with Ecopart followed by Flomex, Sencor, Zeus, and Kroki. In contrast, the minimum POX and CAT activity was observed with hoeing treatment. Data in Figure 4C show that the carotenoid concentration increased by 43.22%, 35.20%, 32.28%, 31.06%, 30.78%, and 30% in treatments of Sencor, Flomex, Hoeing, Zeus, Kroki, and Ecopart, respectively, relative to untreated control. Herbicides are known to cause oxidative stress in which antioxidant alteration is generated in response to herbicide action. This change is responsible for an important part of cellular and tissue damage via formation of reactive oxygen species (ROS). The ROS scavenging antioxidant-defense system includes proline and enzymatic components, which serve to balance the production and detoxification of ROS [45,46,47]. As a cascade effect [48], the ROS formed resulting in the degradation of lipids (lipid peroxidation) leading to loss of chlorophyll, carotenoids, and disruption of cell membranes. In general, the antioxidant enzyme activities, ROS levels, and lipid peroxidation are elevated in plants treated with herbicides. However, in some cases, high herbicide concentrations decrease the antioxidant enzyme activities [49]. Both POX and CAT represent the major enzymes of preventing accumulation of H2O2 [50]. Malondialdehyde (MDA) content, as a non-enzymatic indicator for oxidative stress and membrane damage, is determined to analyze the extent of lipid peroxidation [50]. The herbicides metribuzin as photosystem II inhibitor and pyraflufen-ethyl, as inhibition of protoporphyrinogen oxidase (PPO), were found to cause oxidative stress and produce ROS in plants [49]. Metribuzin, also showed an increase in CAT activity in Rhapanus sativus. Likewise, three other premix herbicides apparently exhibited similar responses in potato plants. Exposure of preemergent herbicide oxyfluorfen resulted in increased activity of superoxide dismutase and CAT in rice compared to control [49]. In the research of Alves et al. [41], the effect of herbicide exposure on the promotion of oxidative stress and response of antioxidant defense mechanism depended on the species used. In their study, fomesafen causes a significant decrease in CAT activity in Vicia sativa, but activity increased in Avena sativa. Similarly, in our study, ready-mix herbicide Kroki, which contains fomesafen, promoted elevated levels of CAT, MDA. Stimulation of CAT has been also observed in wheat plants under herbicide stress [42]. In contrast, CAT activity was able to be inhibited in soybean by other herbicides, and, thus, the decrease in CAT activity by atrazine was able to explain the accumulated levels of H2O2. In contrast to our findings, treatment with bentazone did not significantly change the content of the leaf carotenoids content in soybean [51,52]. Additionally, higher concentration of ascorbic acid (vitamin C), as another antioxidant factor, was determined after application of metribuzin and its mixtures in potato in comparison to the control [53]. Furthermore, proline is an osmoprotectant that has been considered to be a powerful antioxidant [45]. Proline can act as an inhibitor of lipid peroxidation (MDA) and ROS scavenger [45]. These results were in accordance with Fayez and Kristen [53] who found that proline content significantly increased in Pisum and Vicia roots after chlorsulfuron application.
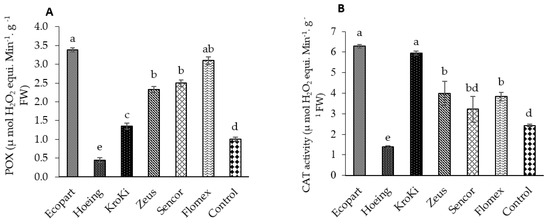
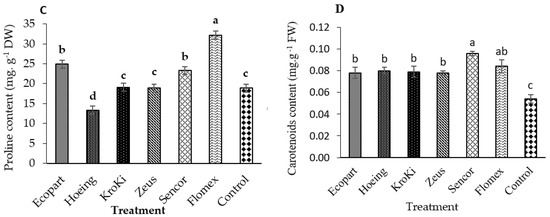
Figure 4.
Effect of weed control treatments on CAT activity (A), POX activity (B), Proline content (C), and Carotenoid content (D) of potato leaves. Columns followed by the same letter are not statistically different at p ≤ 0.05 according to the Tukey test. Vertical bars indicate to standard error (±SE).
3.5. Tuber Yield and Quality
It can be noted from Table 4 that the tuber length, diameter, weight, numbers per plant, and total tubers yield were considerably affected by weed density and the herbicide treatments. All the treatments significantly reduced the weed density and improved tubers yield and its components. The plants treated with Ecopart showed the highest values of tuber length, diameter, and weight of all remaining treatments. The highest number of tubers was observed in plots treated with Flomex compared to all treatments. The maximum tubers yield was recorded also in case of Flomex and Ecopart, while the lowest was recorded in the untreated control. The total tuber number increased by 84%, 80.9%, 77.2%, 76.6%, 74.8%, and 73.1% with Flomex, Ecopart, Sencor, Zeus, Hoeing, and Kroki, respectively, compared to the untreated control. No significant differences in dry matter content were noted among the weeded and un-weeded treatments (Table 4).

Table 4.
Effect of weed control treatments on tubers yield and its components.
The presented results in Figure 5A–D show that the chemical composition of potato tuber including TSS, starch content, total soluble sugar, and total soluble protein content, significantly influenced by weed density and promoted by herbicide application (p ≤ 0.05). The highest content of TSS, total soluble sugar, and starch content in potato tubers was obtained in Hoeing, Kroki, and Zeus treatments, and the lowest content was recorded in the un-weeded control. On the other side, the maximum content of tuber total soluble protein was recorded in plants treated with Ecopart followed by Hoeing, Kroki, Zeus, Sencor, and Flomex; however, the minimum content was observed in the control plots. Insignificant differences in tuber total soluble protein content were observed in Hoeing, Kroki, Zeus, Sencor, and Flomex treatments. Based on our results, hand hoeing and herbicides significantly increased the tuber yield compared to the un-weeded control. This improved tuber yield could be due to the low competition of weeds with potato plants, especially at the early growth stages, reflecting on the number, weight, diameter, and length of the tubers. The new premix herbicide Flomex recorded a higher and a significant tuber yield than metribuzin. These results agree with the previous work of Jovović et al. [54], who pointed out that acetochlor recorded a higher yield than metribuzin. Moreover, Deshi et al. [55] observed that combinations of herbicides recorded significantly higher tuber yield than the herbicide alone, and also higher than the control plots. In contrast, Zarzecka et al. [13] and Hussain et al. [56] found that the highest yields were harvested in plots sprayed with metribuzin. Flomex, which contains flufenacet and belongs to a new group, oxyacetamide, is acting by inhibiting the biosynthesis of very-long-chain fatty acids (VLCFAs), which is essential for cell division and the synthesis of cuticular waxes and suberin in the plants [50]. This new mode of herbicidal action is novel regarding potato production in Egypt, which could make Flomex more effective for controlling weeds and, thus, increase the tuber quantity and quality. Numerous studies reported that the pre-emergence herbicides influenced sugar, dry matter, protein, polyphenols, and starch content in the potato tuber [30,31,49]. Our results also indicated a major significant increase in the content of total sugars in potato tubers after applying the herbicide metribuzin (Sencor). This data agrees with Zarzecka et al. [13,33]. In contrast, Gugała et al. [49] concluded that the applied herbicides they tested did not significantly impact the content of sugars, but only a slight increase in total sugar content was found. The elevated levels of sugars measured may be due to the triggering of the activity of the enzyme, which catalyzes sucrose to glucose and fructose. The herbicides tested in the study also contributed to increasing total protein content except for metribuzin. Our results are in harmony with Barba’s and Sawicka [57] and Zarzecka et al. [33] who reported an increase in the protein content in tubers after herbicide spraying compared to the control plots. High protein content measured after application of pyraflufen ethyl (Ecopart) in our study is similar to those obtained by El-Quesni [58] who indicated that total protein was increased in soybean when applied with oxyfluorfen (herbicide with a similar mode of action). Conversely, reduced amounts of protein after using the herbicide metribuzin may be attributed to differences in the potato cultivar type, methods of application, the type of the herbicides, biostimulants used, and hydrothermal conditions during their experiment [53].
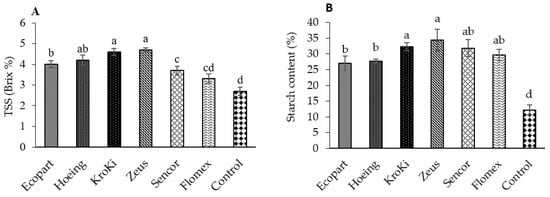
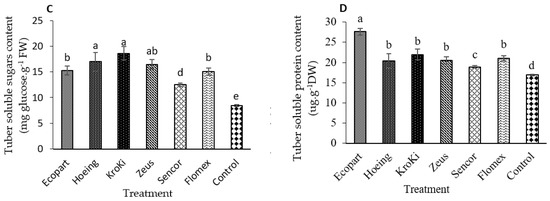
Figure 5.
Effect of weed control treatments on chemical composition (A—TSS, B—Starch content, C—total soluble sugar and D—total soluble protein) of potato tubers. Columns followed by the same letter are not statistically different at p ≤ 0.05 according to the Tukey test. Vertical bars indicate to standard error (±SE).
Concerning starch content, higher starch levels in tubers were measured after applying the herbicide metribuzin related to the control plots as supported by Zarzecka et al. [33] and Baranowska [59]. Our findings also agree with Arora et al. [60] who confirmed that the presence of weeds during the entire growing season reduced tuber dry matter, protein, and starch content. Likewise, in the research of Zarzecka et al. [13], dry matter was not affected by weed control methods including herbicide metribuzin, rather by weather conditions over the study years. Zarzecka et al. [13,33] concluded that the tuber dry matter content did not depend on the type of herbicides; but the influencing factor in the years of their study was the atmospheric conditions.
Tubers total soluble solids were not significantly influenced after application of Sencor and Flomex compared to weed competition in the untreated weedy plots. A similar finding was reported by Luz et al. [35] who found that there was no significant difference between metribuzin and the un-weeded control. This may be because metribuzin inhibits photosynthesis by blocking the transport of electrons in photosystem II, thus, reducing carbon dioxide fixation and carbohydrate formation. However, other herbicides (having different modes of action) and hoeing significantly elevated this parameter’s value compared to the un-weeded control. The un-weeded control offered the least plant growth parameters, tuber quantity, and quality compared to the remaining other treatments. However, the potato yield was significantly increased by all the herbicides and the hoeing treatments [61,62,63,64,65,66,67]. This could be due to the effective weed control in herbicides treated plots and decreased weed infestation, providing a healthy environment for potato growth.
3.6. Correlation Study
The Pearson’s correlation analysis in Table 5 shows that the weed efficacy significantly and positively correlated to SPAD, chlorophyll (a&b), carotenoids, plant length, fresh and dry weight of the plant, total yield, and starch content (%). A similar relationship was recorded between weed efficacy and tuber soluble sugar content, tuber soluble protein content, TSS, and CAT. On the contrary, negative and significant relation between weed efficacy and MDA was noted. These results indicate the reduction of weed density using herbicidal treatments and hand hoeing had a significant impact on plant growth and productivity of potato plants as well as tuber quality. Furthermore, the total yield is related significantly and positively with greening index (SPAD), chlorophyll content (a&b), total soluble sugar, carotenoids, and POX activity. A similar correlation was also recorded between total yield and plant length, number of stems, fresh and dry weight number of tuber, and the tuber weight. Moreover, a positive link was observed between tuber starch content and SPAD. There is a significantly positive relationship between tuber soluble sugar content and MSI, TSS, SPAD, chlorophyll content (a&b), and CAT and correlated negatively with MDA content of potato leaves. Leaf MDA content correlated negatively and significantly with antioxidant compounds (POX, CAT, and carotenoids) and chlorophyll content (a&b). This correlation study clarifies that the weed control treatments significantly affected plant morphology and plant physiology, particularly chlorophyll content, antioxidant compounds content, leaf MSI, and leaf MDA that influence tuber yield and physiochemical quality of tuber. Meanwhile, the two-dimensional heatmap plot based on the 24 parameters clearly separated the control (un-weeded) in a separate cluster, while the Kroki, hoeing, and Ecopart treatments were grouped together in the same sub-cluster under the second cluster, indicating that these herbicides/treatments have the closest efficiency to the control group. Meanwhile, the second cluster comprised the Zeus, Sencor, and Flomex herbicidal treatments (Figure 6).

Table 5.
Pearson’s correlation analysis between morpho-physiological characteristics of the potato plant and physicochemical properties of potato tuber.
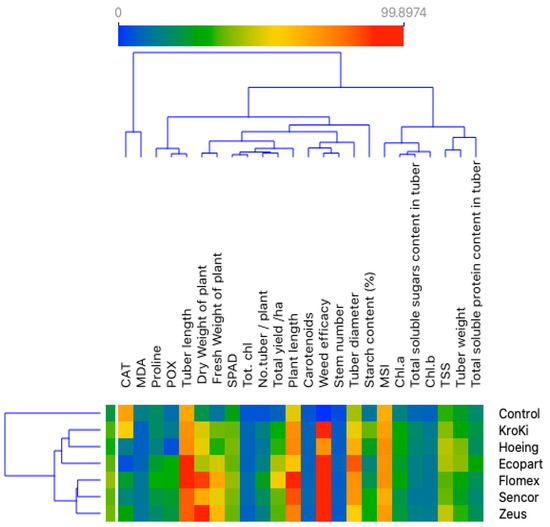
Figure 6.
A two-dimensional heatmap plot shows the correlation between the different herbicide treatments and the 24 morpho-physiological measured parameters included in the study.
4. Conclusions
The results of this study confirmed the significant influence of weeds on potato yields and the importance of controlling weeds with pre-emergence herbicides. Development of the new pre-emergence pre-mixes herbicides enabled a larger spectrum of control leading to greater weed control efficiency and increasing tuber yields compared to hand hoeing. Phytotoxicity and foliar injury symptoms were visible on plants treated with Kroki (mixture contains 11% clomazone) at the beginning of crop development, but symptoms disappeared at about 30 days after application. All weed control treatments caused an improvement in plant growth including greening index, leaf photosynthetic pigments, plant height, shoot fresh and dry weight, and the number of stems. Furthermore, the herbicidal treatments enhanced the activity of antioxidant enzymes (CAT and POX), proline and carotenoid content of potato leaves more than hand hoeing and un-weeded control. The potato tuber yield was almost equally increased by all the tested herbicides and the hoeing treatment in relation to the un-weeded control. Starch, protein, TSS, and protein, as major chemical constituents, seemed not to be affected by the herbicides as comparable values were found between hoeing and herbicides treatments. Hoeing treatment increased starch and total sugar content more than herbicides, while the total soluble protein was higher in case of Ecopart related to hoeing and un-weeded control. Although not all the tested herbicides have been adequately tested on potatoes, the current study suggests that they might be helpful options for weed management and may provide a possible alternative to metribuzin (Sencor).
The availability of newer herbicides with different modes of action would also provide potato farmers with efficient options to manage difficult-to-control weeds. However, further investigation is needed on these herbicides in other situations such as soil types, planting time, different potato varieties, and geographical location in order to confirm the results of the present work for wider adaptability. Besides, the dissipation and residues of the tested herbicides in tubers and soil should also be tested for the safety of consumers. Based on the present data, integration of all possible approaches could offer sustainable weed control efficiency and positively affects potato yield and quality attributes.
Author Contributions
Conceptualization, I.S.A. and E.A.A.; methodology, I.S.A., E.A.A., A.K.N., H.S.E.-B., F.F.K. and M.M.E.-M.; software, M.A.M.A., I.S.A., E.A.A., H.S.E.-B. and F.F.K.; validation, M.M.E.-M. and E.A.A.; formal analysis, E.A.A. and M.A.M.A.; investigation, I.S.A., E.A.A. and A.K.N.; resources, I.S.A., M.A.M.A. and E.A.A.; data curation, E.A.A.; writing—original draft preparation, I.S.A., M.M.E.-M., M.A.M.A., H.S.E.-B. and E.A.A.; writing—review and editing, I.S.A., M.A.M.A. and E.A.A.; visualization, I.S.A. and M.A.M.A.; supervision, E.A.A. and M.A.M.A.; project administration, I.S.A.; funding acquisition, software I.S.A. All authors have read and agreed to the published version of the manuscript.
Funding
The authors received no specific funding for this work.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors are thankful for the Egyptian Ministry of Agriculture and Land Reclamation-Agriculture Research Center, for providing the commercial formulations of the herbicides under the study and for partial funding. Thanks are also extended to the Department of Vegetable Crops at Faculty of agriculture, Cairo University and Agricultural Genetic Engineering Research Institute (AGERI) for providing the scientific materials, tools and equipment used in this research work.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Janaki, P.; Archana, M.; Priya, S.R.; Nithya, C.; Chinnusamy, N.; Prabbahakaran, N.K. Effect of Herbicides on Potato and their Persistence in Acid Soil under Semiarid Tropical Condition. Adv. Plants Agric. Res. 2017, 7, 1–7. [Google Scholar] [CrossRef][Green Version]
- Ali, M.; Parmar, A.; Niedbała, G.; Wojciechowski, T.; El-Yazied, A.A.; El-Gawad, H.; Nahhas, N.; Ibrahim, M.; El-Mogy, M. Improved Shelf-Life and Consumer Acceptance of Fresh-Cut and Fried Potato Strips by an Edible Coating of Garden Cress Seed Mucilage. Foods 2021, 10, 1536. [Google Scholar] [CrossRef]
- Abdeldaym, E.A.; El-Sawy, M.B.I.; El-Helaly, M.A. Combined application of different sources of nitrogen fertilizers for improvement of potato yield and quality. Plant Arch. 2019, 19, 2513–2521. [Google Scholar]
- Abdeldaym, E.A.; Traversa, A.; Cocozza, C.; Brunetti, G. Effects of a 2-Year Application of Different Residual Biomasses on Soil Properties and Potato Yield. Clean Soil Air Water 2018, 46. [Google Scholar] [CrossRef]
- Mondani, F.; Golzardi, F.; Ahmadvand, G.; Ghorbani, R.; Moradi, R. Influence of weed competition on potato growth, production and radiation use efficiency. Notulae Sci. Biol. 2011, 3, 42–52. [Google Scholar] [CrossRef]
- Yadav, S.K.; Lal, S.S.; Srivastava, A.K.; Bag, T.K.; Singh, B.P. Efficacy of chemical and non-chemical meth-ods of weed management in rainfed potato (Solanum tuberosum). Indian J. Agric. Sci. 2015, 85, 382–386. [Google Scholar]
- Alvarez, J.M.; Hutchinson, P.J. Managing Hairy Nightshade to Reduce Potato Viruses and Insect Vectors. Outlooks Pest. Manag. 2005, 16, 249–252. [Google Scholar] [CrossRef]
- Cocozza, C.; Abdeldaym, E.A.; Brunetti, G.; Nigro, F.; Traversa, A. Synergistic effect of organic and inorganic fertilization on the soil inoculum density of the soilborne pathogens Verticillium dahliae and Phytophthora spp. under open-field conditions. Chem. Biol. Technol. Agric. 2021, 8, 1–11. [Google Scholar] [CrossRef]
- Nelson, D.C.; Giles, J.F. Implications of postemergence tillage on root injury and yields of potatoes. Am. Potato J. 1986, 63, 445–446. [Google Scholar]
- Gopu, V.; Meena, C.K.; Shetty, P.H. Quercetin influences quorum sensing in food borne bacteria: In-vitro and in-silico evi-dence. PLoS ONE 2015, 10, e0134684. [Google Scholar] [CrossRef]
- Mahmoud, A.W.M.; Abdeldaym, E.A.; Abdelaziz, S.M.; El-Sawy, M.B.I.; Mottaleb, S.A. Synergetic Effects of Zinc, Boron, Silicon, and Zeolite Nanoparticles on Confer Tolerance in Potato Plants Subjected to Salinity. Agronomy 2019, 10, 19. [Google Scholar] [CrossRef]
- Abuarab, M.E.; El-Mogy, M.M.; Hassan, A.M.; Abdeldaym, E.A.; Abdelkader, N.H.; El-Sawy, M.B.I. The Effects of Root Aeration and Different Soil Conditioners on the Nutritional Values, Yield, and Water Productivity of Potato in Clay Loam Soil. Agronomy 2019, 9, 418. [Google Scholar] [CrossRef]
- Zarzecka, K.; Gugała, M.; Mystkowska, I.; Baranowska, A.; Sikorska, A. Effect of herbicides on the content dry matter and sugars in edible potato tubers. Roman. Agric. Res. 2017, 23, 371–375. [Google Scholar]
- El-Sayed, S.F.; Hassan, H.A.; El-Mogy, M.M. Impact of Bio- and Organic Fertilizers on Potato Yield, Quality and Tuber Weight Loss After Harvest. Potato Res. 2014, 58, 67–81. [Google Scholar] [CrossRef]
- Arsenault, W.J.; Ivany, J.A. Response of several potato cultivars to metribuzin and diquat. Crop Prot. 2001, 20, 547–552. [Google Scholar] [CrossRef]
- Pedroso, R.M.; Al-Khatib, K.; Abdallah, I.; Alarcón-Reverte, R.; Fischer, A.J. Fischer. Resistance to propanil in ricefield bulrush (Schoenoplectus mucronatus) is conferred by a psba mutation, Val219 to Ile. Weed Sci. 2016, 64, 562–569. [Google Scholar] [CrossRef]
- Farooq, M.; Jabran, K.; Cheema, Z.A.; Wahid, A.; Siddique, K. The role of allelopathy in agricultural pest management. Pest. Manag. Sci. 2011, 67, 493–506. [Google Scholar] [CrossRef]
- Abuarab, M.F.; Hafez, S.M.; Shahein, M.M.; Hassan, A.M.; El-Sawy, M.B.; El-Mogy, M.M.; A Abdeldaym, E. Irrigation scheduling for green beans grown in clay loam soil under a drip irrigation system. Water SA 2020, 46, 573–582. [Google Scholar] [CrossRef]
- Banerjee, H.; Das, T.K.; Ray, K.; Laha, A.; Sarkar, S.; Pal, S. Herbicide ready-mixes effects on weed control efficacy, non-target and residual toxicities, productivity and profitability in sugarcane–green gram cropping system. Int. J. Pest. Manag. 2017, 64, 221–229. [Google Scholar] [CrossRef]
- Lagator, M.; Vogwill, T.; Mead, A.; Colegrave, N.; Neve, P. Herbicide mixtures at high doses slow the evolution of resistance in experimentally evolving populations of C hlamydomonas reinhardtii. New Phytol. 2013, 198, 938–945. [Google Scholar] [CrossRef] [PubMed]
- Abbas, T.; Nadeem, M.A.; Tanveer, A.; Matloob, A.; Zohaib, A.; Ehsan, M.; Safdar, H.H.A.; Farooq, N.; Javaid, M.M.; Tabassum, T.; et al. Herbicide Mixtures and Row Spacing Effects on Fenoxaprop Resistant Phalaris minor in Wheat. Int. J. Agric. Biol. 2018, 20, 2737–2744. [Google Scholar]
- Lichtenthaler, H.K.; Buschmann, C. Chlorophylls and Carotenoids: Measurement and Characterization by UV-VIS Spectroscopy. Curr. Protoc. Food Anal. Chem. 2001, 1, F4.3.1–F4.3.8. [Google Scholar] [CrossRef]
- Hodges, D.M.; Delong, J.M.; Forney, C.; Prange, R.K. Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta 1999, 207, 604–611. [Google Scholar] [CrossRef]
- Shabnam, N.; Tripathi, I.; Sharmila, P.; Pardha-Saradhi, P. A rapid, ideal, and eco-friendlier protocol for quantifying proline. Protoplasma 2015, 253, 1577–1582. [Google Scholar] [CrossRef]
- Rady, M.M. Effect of 24-epibrassinolide on growth, yield, antioxidant system and cadmium content of bean (Phaseolus vulgaris L.) plants under salinity and cadmium stress. Sci. Hortic. 2011, 129, 232–237. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in vitro. Method. Enzymol. 1984, 105, 121–126. [Google Scholar]
- Giannoccaro, E.; Wang, Y.-J.; Chen, P. Effects of Solvent, Temperature, Time, Solvent-to-Sample Ratio, Sample Size, and Defatting on the Extraction of Soluble Sugars in Soybean. J. Food Sci. 2006, 71, C59–C64. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Butron, W.G. The Potato; Chapman and Hall: London, UK, 1984; p. 319. [Google Scholar]
- Metsalu, T.; Vilo, J. ClustVis: A web tool for visualizing clustering of multivariate data using Principal Component Analysis and heatmap. Nucleic Acids Res. 2015, 43, W566–W570. [Google Scholar] [CrossRef] [PubMed]
- Klara, B.; Zvonimir, O. Control of weeds in potato using a mixture of flufenacet and metribuzin. Herbologia 2004, 5, 65–73. [Google Scholar]
- Oliveira, J.R.S. Seletividade de herbicidas para culturas e plantas daninhas. In Plantas Daninhas e Seu Manejo; Oliveira, R.S., Constantin, J., Eds.; Agropecuária: Guaíba, Brazil, 2001; pp. 291–314. [Google Scholar]
- Zarzecka, K.; Gugała, M.; Sikorska, A.; Grzywacz, K.; Niewęgłowski, M. Marketable Yield of Potato and Its Quantitative Parameters after Application of Herbicides and Biostimulants. Agriculture 2020, 10, 49. [Google Scholar] [CrossRef]
- Barbaś, P.; Sawicka, B.; Marczak, B.; Pszczółkowski, P. Effect of Mechanical and Herbicide Treatments on Weed Densities and Biomass in Two Potato Cultivars. Agriculture 2020, 10, 455. [Google Scholar] [CrossRef]
- Luz, J.M.Q.; Fonseca, L.F.; Duarte, I.N. Selectivity of pre-emergence herbicides in potato cv. Innovator. Hortic. Bras. 2018, 36, 223–228. [Google Scholar] [CrossRef]
- Abdallah, I.; Amer, A.; El-Hefny, D. Influence of herbicides under biofertilizer application on fennel (Foeniculum vulgare) yield and quality with special reference to herbicide residues. Bull. Natl. Res. Cent. 2021, 45, 1–13. [Google Scholar] [CrossRef]
- Soliman, I.E.; Morsi, A.R.; Khaffagy, A.E. Effect of competitive abilities of some soybean genotypes, plant densities and weed control treatments on soybean (Glycine max L.) and it’s associated weeds. J. Plant Prod. 2015, 6, 1413–1429. [Google Scholar] [CrossRef]
- Al Mahmud, A.; Hossain, M.; Kadian, M.S.; Hoque, A. Physiological and biochemical changes in potato under water stress condition. Indian J. Plant Physiol. 2015, 20, 297–303. [Google Scholar] [CrossRef]
- Arvin, M.J.; Donnelly, D.J. Screening potato cultivars and wild species to abiotic stresses using an electrolyte leakage bioassay. J. Agric. Sci. Technol. 2008, 10, 33–42. [Google Scholar]
- Golan, K.; Jurado, I.; Kot, I.; Górska-Drabik, E.; Kmieć, K.; Łagowska, B.; Skwaryło-Bednarz, B.; Kopacki, M.; Jamiołkowska, A. Defense Responses in the Interactions between Medicinal Plants from Lamiaceae Family and the Two-Spotted Spider Mite Tetranychus urticae Koch (Acari: Tetranychidae). Agronomy 2021, 11, 438. [Google Scholar] [CrossRef]
- Alves, C.; Costa, E.; Sofiatti, J.R.; Forte, C.T.; Winter, F.L.; Holz, C.M.; Kaizer, R.R.; Galon, L. Effect of herbicides in the oxidative stress in crop winter species. SciELO 2018, 90, 1533–1542. [Google Scholar] [CrossRef]
- Radwan, D.; Mohamed, A.; Fayez, K.; Abdelrahman, A. Oxidative stress caused by Basagran® herbicide is altered by salicylic acid treatments in peanut plants. Heliyon 2019, 5, e01791. [Google Scholar] [CrossRef]
- Yaman, M.; Nalbantoğlu, B. Investigation of the Effects of the Fenoxaprop-p-Ethyl Herbicide and Salicylic Acid on the Ascorbic Acid and Vitamin B6 Vitamers in Wheat Leaves. J. Plant Growth Regul. 2019, 39, 729–737. [Google Scholar] [CrossRef]
- Lim, J.H.; Park, K.-J.; Kim, B.-K.; Jeong, J.-W.; Kim, H.-J. Effect of salinity stress on phenolic compounds and carotenoids in buckwheat (Fagopyrum esculentum M.) sprout. Food Chem. 2012, 135, 1065–1070. [Google Scholar] [CrossRef] [PubMed]
- Gill, S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef] [PubMed]
- Atia, M.A.; Abdeldaym, E.A.; Abdelsattar, M.; Ibrahim, D.S.; Saleh, I.; Elwahab, M.A.; Osman, G.H.; Arif, I.A.; Abdelaziz, M.E. Piriformospora indica promotes cucumber tolerance against Root-knot nematode by modulating photosynthesis and innate responsive genes. Saudi J. Biol. Sci. 2019, 27, 279–287. [Google Scholar] [CrossRef] [PubMed]
- Abdeldym, E.A.; El-Mogy, M.M.; Abdellateaf, H.R.L.; Atia, M.A.M. Genetic Characterization, Agro-Morphological and Physiological Evaluation of Grafted Tomato under Salinity Stress Conditions. Agronomy 2020, 10, 1948. [Google Scholar] [CrossRef]
- De Oliveira, T.K.; De Carvalho, G.J.; Moraes, R.N.D.S. Plantas de cobertura e seus efeitos sobre o feijoeiro em plantio direto. Fitotecnia 2002, 37, 1079–1087. [Google Scholar] [CrossRef]
- Gugała, M.; Zarzecka, K.; Dołęga, H.; Sikorska, A. Weed Infestation and Yielding of Potato Under Conditions of Varied Use of Herbicides and Bio-Stimulants. J. Ecol. Eng. 2018, 19, 191–196. [Google Scholar] [CrossRef]
- Vwioko, E.D.; El-Esawi, M.A.; Imoni, M.E.; Al-Ghamdi, A.A.; Ali, H.M.; El-Sheekh, M.M.; Abdeldaym, E.A.; Al-Dosary, M.A. Sodium Azide Priming Enhances Waterlogging Stress Tolerance in Okra (Abelmoschus esculentus L.). Agronomy 2019, 9, 679. [Google Scholar] [CrossRef]
- Demmig-Adams, B.; Adams, W.W. The role of xanthophyll cycle carotenoids in the protection of photosynthesis. Trends Plant Sci. 1996, 1, 21–26. [Google Scholar] [CrossRef]
- Zarzecka, K.; Gugala, M. The effect of herbicide applications on the content of ascorbic acid and glycoal-kaloids in potato tubers. Plant Soil Environ. 2003, 49, 237–240. [Google Scholar] [CrossRef]
- Fayez, K.; Kristen, U. The influence of herbicides on the growth and proline content of primary roots and on the ultrastructure of root caps. Environ. Exp. Bot. 1996, 36, 71–81. [Google Scholar] [CrossRef]
- Jovović, Z.; Popović, T.; Velimirović, A.; Milić, V.; Dolijanović, P.; Šilj, M.; Poštić, D. Efficacy of Chemical Weed Control in Potato (Solanum tuberosum L.). Agro-Knowl. J. 2013, 14, 487–495. [Google Scholar] [CrossRef][Green Version]
- Deshi, K.E.; Nanbol, K.K.; Dawang, S.N.; Gushit, J.S. Response of two potato (Solanum tuberosum L.) plant varieties to different types of herbicides under field conditions. Direct Res. J. Agric. Food Sci. 2019, 7, 208–214. [Google Scholar] [CrossRef]
- Hussain, Z.; Munsif, F.; Marwat, K.B.; Ali, K.; Afridi, R.A.; Bibi, S. Studies on efficacy of different herbicides against weeds in potato crop in Peshawar. Pak. J. Bot. 2013, 45, 487–491. [Google Scholar]
- Barbaś, P.; Sawicka, B. Dependence of potato yield on weed infestation. Agron. Res. 2020, 18, 346–359. [Google Scholar]
- El-Quesni, F.E.M. Response of yield and chemical composition of soybean (Glycine max L.) seed to ox-yfluorfen and Gibberellic acid. Bull. Fac. Agric. Univ. Cairo 1993, 44, 859–872. [Google Scholar]
- Baranowska, A. Yield of dry matter and starch of edible potato tubers in conditions of application of growth biostimulators and herbicide. Acta Agrophysica 2018, 25, 397–407. [Google Scholar] [CrossRef]
- Arora, A.; Tomar, S.S.; Gole, M.K. Yield and quality of potato as influenced by weed management practices and their residual study in soil. Agric. Sci. Dig. 2009, 29, 39–41. [Google Scholar]
- Zhang, H.-H.; Xu, N.; Teng, Z.-Y.; Wang, J.-R.; Ma, S.; Wu, X.; Li, X.; Sun, G.-Y. 2-Cys Prx plays a critical role in scavenging H2O2 and protecting photosynthetic function in leaves of tobacco seedlings under drought stress. J. Plant Interact. 2018, 14, 119–128. [Google Scholar] [CrossRef]
- Hasan, M.; Rahman, A.; Skalicky, M.; Alabdallah, N.; Waseem, M.; Jahan, M.; Ahammed, G.; El-Mogy, M.; El-Yazied, A.; Ibrahim, M.; et al. Ozone Induced Stomatal Regulations, MAPK and Phytohormone Signaling in Plants. Int. J. Mol. Sci. 2021, 22, 6304. [Google Scholar] [CrossRef] [PubMed]
- Laxmi, S.; Chambhare, M.R.; Kadlag, S.; Ahire, M.L.; Nikam, T.D. Herbicide effects on pigments and anti-oxidant enzymes of Portulaca oleracea L. J. Pharmacogn. Phytochem. 2018, 7, 2024–2031. [Google Scholar]
- Youssef, S.A.; Safwat, G.; Shalaby, A.A.; El-Beltagi, H.S. Effect of phytoplasma infection on plant hormones, enzymes and their role in infected sesame. Fresenius Environ. Bull. 2018, 27, 5727–5735. [Google Scholar]
- Mohamed, H.I.; El-Beltagi, H.S.; Aly, A.A.; Latif, H.H. The role of systemic and non-systemic fungicides on the physiological and biochemical parameters in Gossypium hirsutum plant, implications for defense responses. Fresenius Environ. Bull. 2018, 27, 8585–8593. [Google Scholar]
- Soliman, A.M.; Alhudaib, K.A.; Rezk, A.A.; El-Beltagi, H.S.; El-Ganainy, S.M. Production of polyclonal an-tibodies to some potato viruses using synthetic peptides. Fresenius Environ. Bull. 2019, 28, 2957–2967. [Google Scholar]
- Nourian, F.; Ramaswamy, H.; Kushalappa, A. Kinetics of quality change associated with potatoes stored at different temperatures. LWT 2003, 36, 49–65. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).