Evaluation of Cowpea (Vigna unguiculata) in an Intercropping System as Pollinator Enhancer for Increased Crop Yield
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site and Experimental Plants
2.2. Experimental Design and Setup
2.3. Insect Sampling
2.3.1. Insect Sampling Using Direct Visual Counts
2.3.2. Insect Sampling Using Sticky Traps
2.3.3. Insect Sampling Using Pan Traps
2.4. Crop Yield Data Collection
2.5. Data Analysis
- pi = the proportion of individuals found in species i
- pi is estimated as pi = ni/N
- where ni = number of individuals in species i
- N = total number of individuals in the community
- H′ = Shannon–Weaver Diversity Index
- k = number of species/groups in the community
3. Results
3.1. Abundance and Diversity of Pollinators Associated with Cowpea Treatments and Controls
3.2. Pollinators on Cowpeas and Pollinator-Dependent Crops
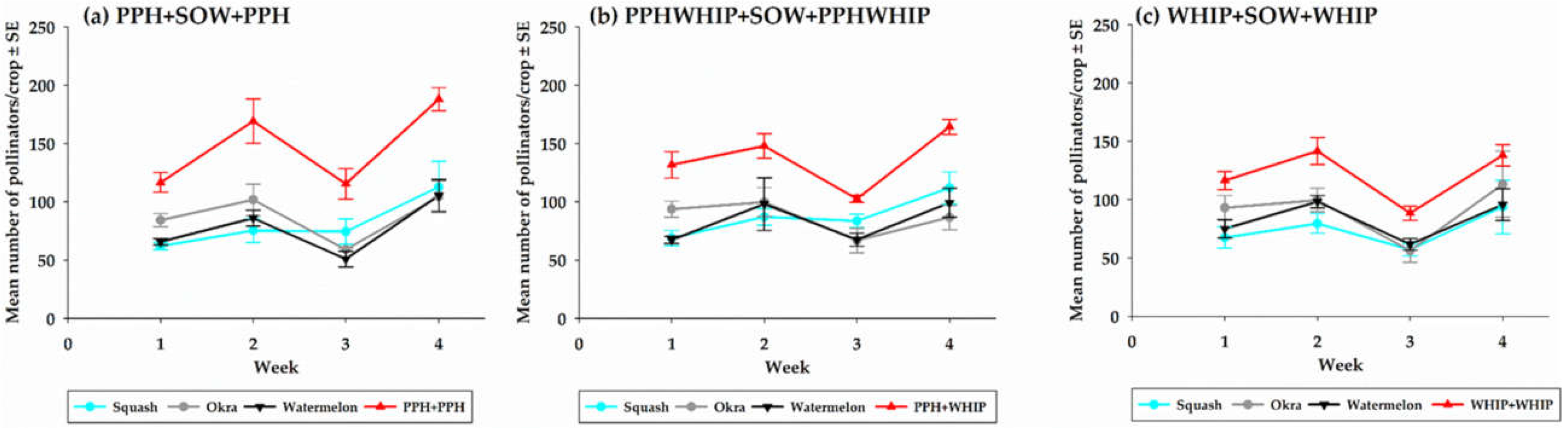
3.2.1. Pollinators on Cowpeas
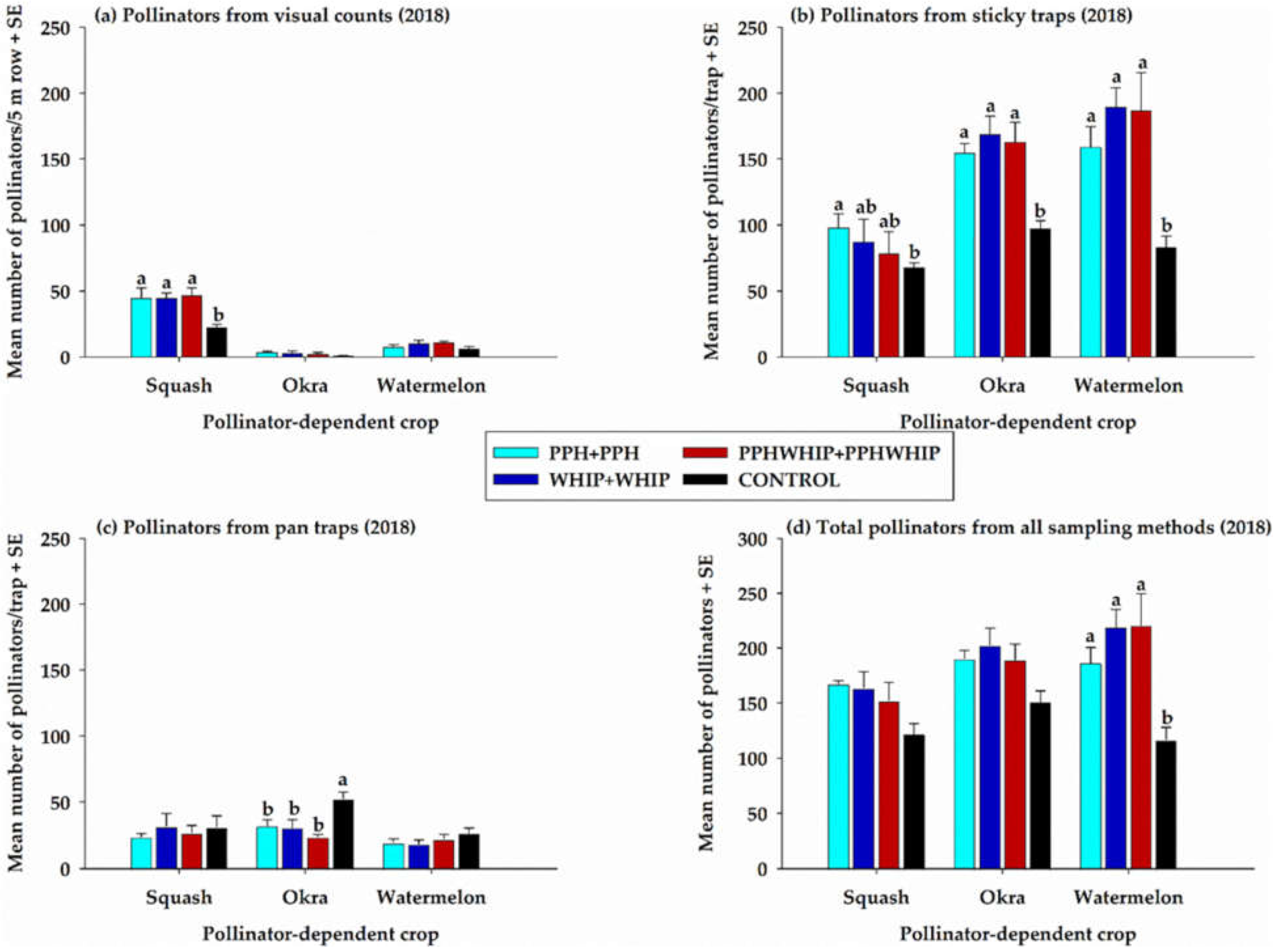
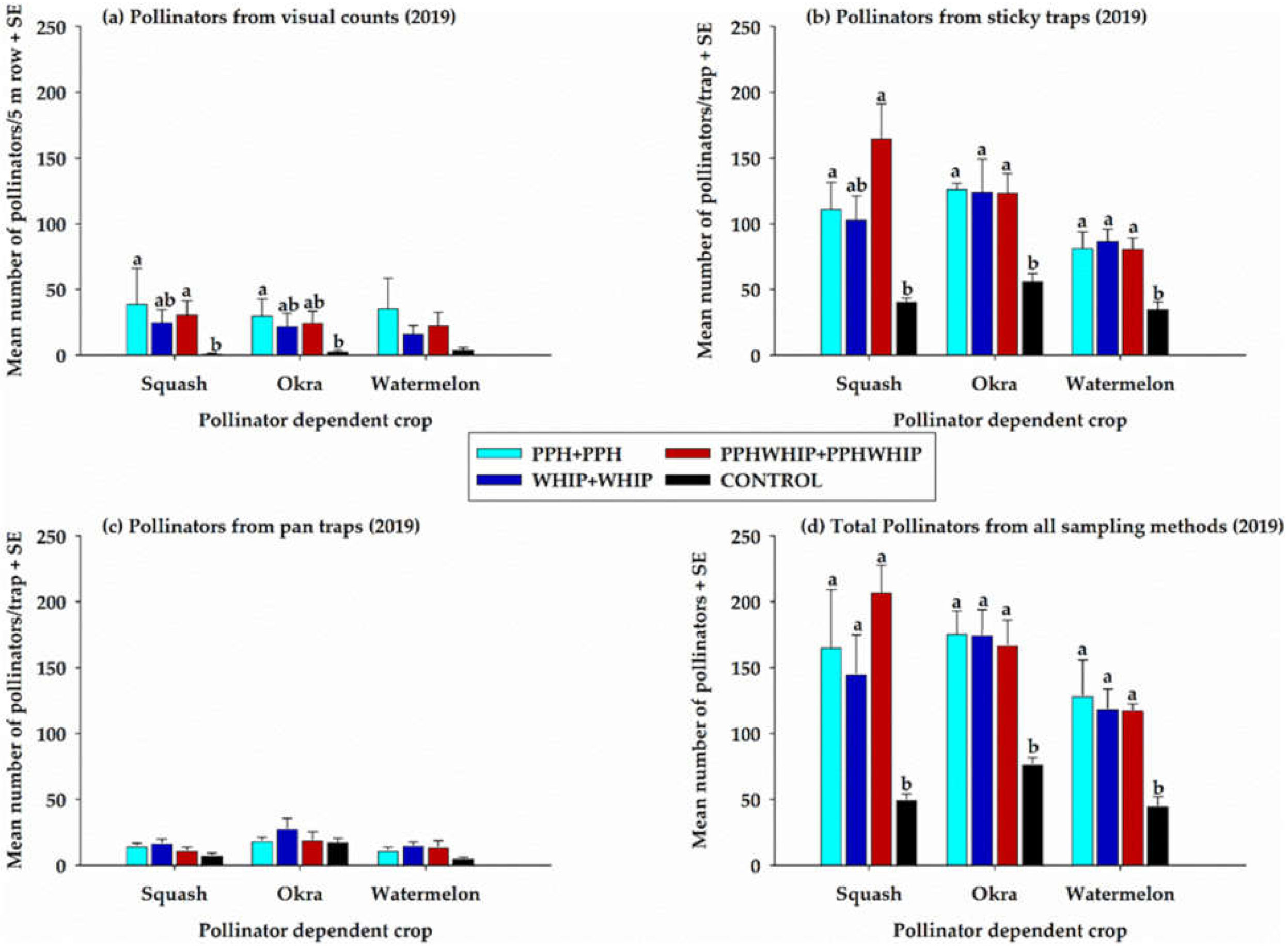
3.2.2. Pollinators on Pollinator-Dependent Crops (PDCs)
3.2.3. Total Pollinators on Cowpeas and on Pollinator-Dependent Crops and Controls
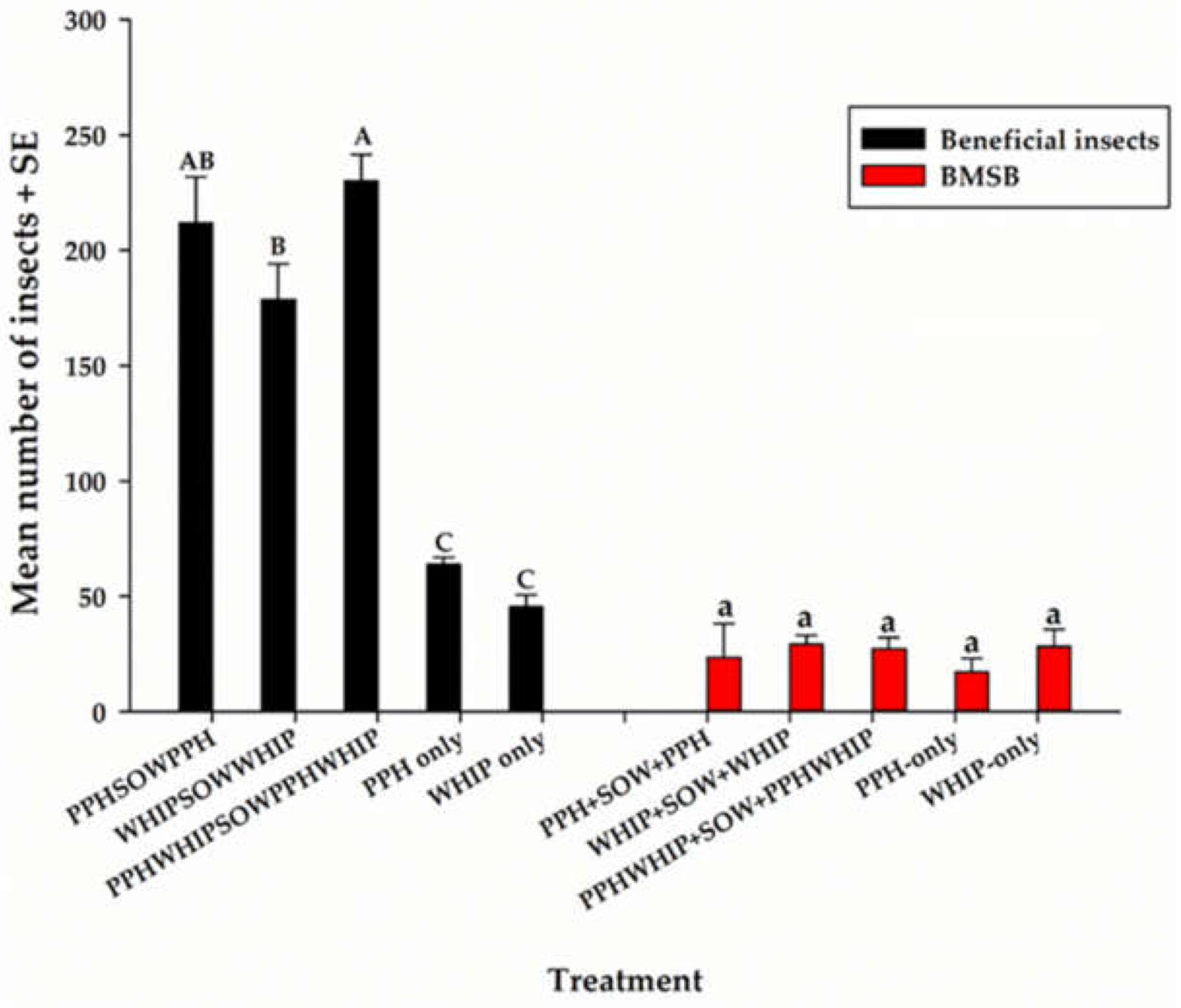
3.3. Other Beneficial Insects and H. halys on Cowpeas and PDCs
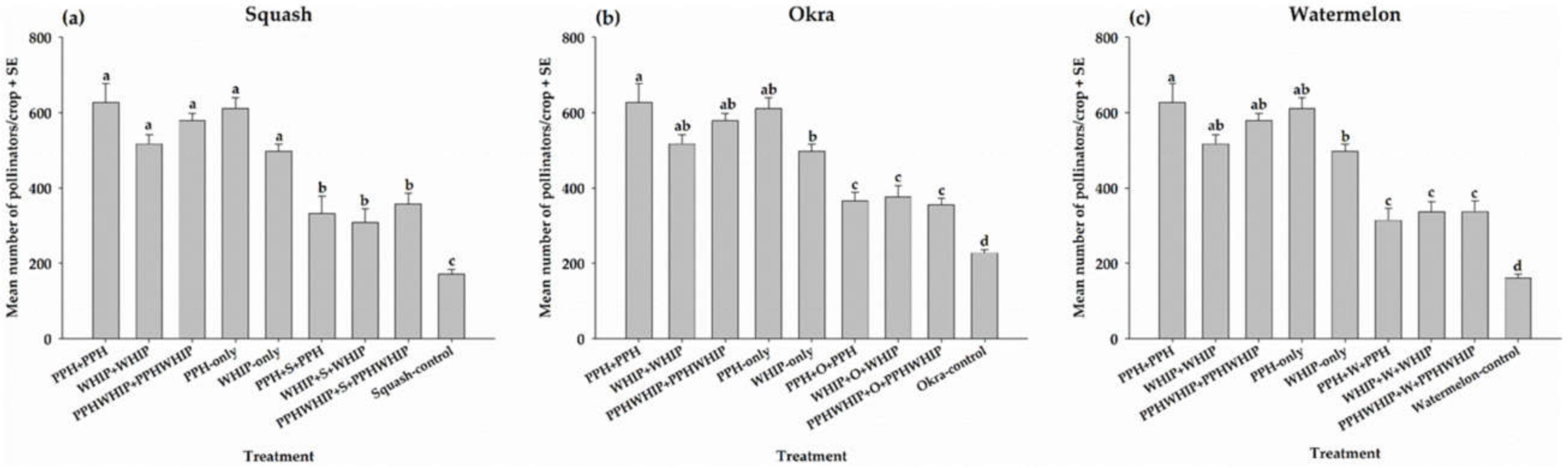
3.4. Crop Yield
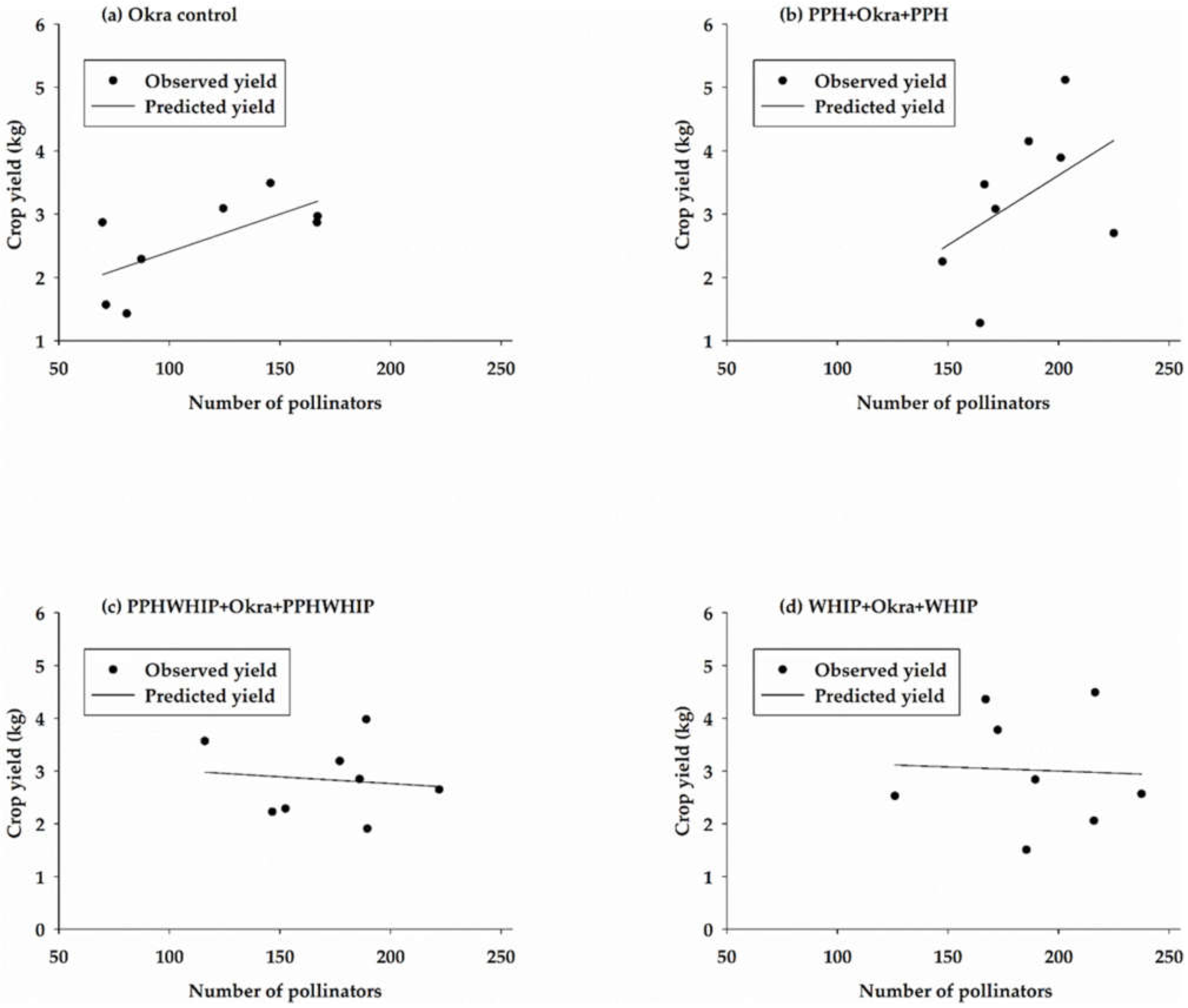
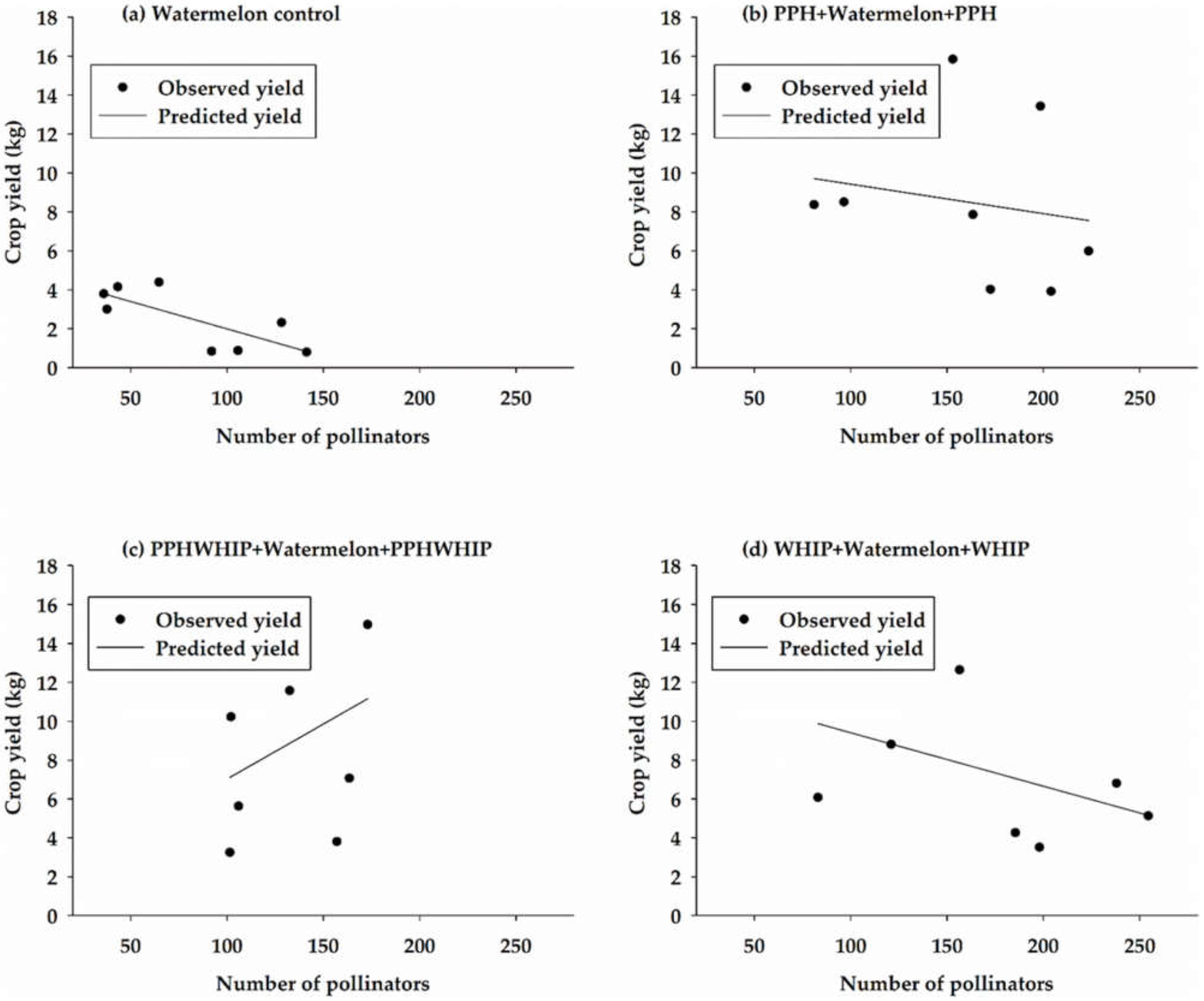
3.5. Relationship between Pollinator Abundance and Crop Yield
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Klein, A.-M.; Vaissière, B.E.; Cane, J.H.; Steffan-Dewenter, I.; Cunningham, S.A.; Kremen, C.; Tscharntke, T. Importance of pollinators in changing landscapes for world crops. Proc. Biol. Sci. 2007, 274, 303–313. [Google Scholar] [CrossRef] [Green Version]
- Vanengelsdorp, D.; Meixner, M.D. A historical review of managed honey bee populations in Europe and the United States and the factors that may affect them. J. Invertebr. Pathol. 2010, 103, S80–S95. [Google Scholar] [CrossRef]
- Vanbergen, A.J.; Baude, M.; Biesmeijer, J.C.; Britton, N.F.; Brown, M.J.F.; Brown, M.; Bryden, J.; Budge, G.E.; Bull, J.C.; Carvell, C.; et al. Threats to an ecosystem service: Pressures on pollinators. Front. Ecol. Environ. 2013, 11, 251–259. [Google Scholar] [CrossRef] [Green Version]
- Garibaldi, L.A.; Steffan-Dewenter, I.; Winfree, R.; Aizen, M.A.; Bommarco, R.; Cunningham, S.A.; Kremen, C.; Carvalheiro, L.G.; Harder, L.D.; Afik, O.; et al. Wild pollinators enhance fruit set of crops regardless of honey bee abundance. Science 2013, 339, 1608–1611. [Google Scholar] [CrossRef]
- Mandelik, Y.; Winfree, R.; Neeson, T.; Kremen, C. Complementary habitat use by wild bees in agro-natural landscapes. Ecol. Appl. 2012, 22, 1535–1546. [Google Scholar] [CrossRef] [PubMed]
- Winfree, R. The conservation and restoration of wild bees. Ann. N. Y. Acad. Sci. 2010, 1195, 169–197. [Google Scholar] [CrossRef] [PubMed]
- Blüthgen, N.; Klein, A.-M. Functional complementarity and specialisation: The role of biodiversity in plant–pollinator interactions. Basic Appl. Ecol. 2011, 12, 282–291. [Google Scholar] [CrossRef]
- Nicholls, C.; Altieri, M. Plant biodiversity enhances bees and other insect pollinators in agroecosystems. A review. Agron. Sustain. Dev. 2013, 33, 257–274. [Google Scholar] [CrossRef] [Green Version]
- Vaudo, A.D.; Tooker, J.F.; Grozinger, C.M.; Patch, H.M. Bee nutrition and floral resource restoration. Curr. Opin. Insect Sci. 2015, 10, 133–141. [Google Scholar] [CrossRef] [Green Version]
- Free, J.B. Insect Pollination of Crops, 2nd ed.; Academic Press: London, UK, 1993; 684p. [Google Scholar]
- Garibaldi, L.A.; Carvalheiro, L.G.; Leonhardt, S.D.; Aizen, M.A.; Blaauw, B.R.; Isaacs, R.; Kuhlmann, M.; Kleijn, D.; Klein, A.M.; Kremen, C.; et al. From research to action: Enhancing crop yield through wild pollinators. Front. Ecol. Environ. 2014, 12, 439–447. [Google Scholar] [CrossRef] [Green Version]
- Blaauw, B.R.; Isaacs, R. Flower plantings increase wild bee abundance and the pollination services provided to a pollination-dependent crop. J. Appl. Ecol. 2014, 51, 890–898. [Google Scholar] [CrossRef]
- Williams, N.M.; Ward, K.L.; Pope, N.; Isaacs, R.; Wilson, J.K.; May, E.A.; Ellis, J.; Daniels, J.C.; Pence, A.; Ullmann, K.S.; et al. Native wildflower plantings support wild bee abundance and diversity in agricultural landscapes across the United States. Ecol. Appl. 2015, 25, 2119–2131. [Google Scholar] [CrossRef] [Green Version]
- Amy, C.; Noël, G.; Hatt, S.; Uyttenbroeck, R.; Van de Meutter, F.; Genoud, D.; Francis, F. Flower Strips in Wheat Intercropping System: Effect on Pollinator Abundance and Diversity in Belgium. Insects 2018, 9, 114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pereira, A.L.C.; Taques, T.C.; Valim, J.O.S.; Madureira, A.P.; Campos, W.G. The management of bee communities by intercropping with flowering basil (Ocimum basilicum) enhances pollination and yield of bell pepper (Capsicum annuum). J. Insect Conserv. 2015, 19, 479–486. [Google Scholar] [CrossRef]
- Morandin, L.A.; Kremen, C. Hedgerow restoration promotes pollinator populations and exports native bees to adjacent fields. Ecol. Appl. 2013, 23, 829–839. [Google Scholar] [CrossRef] [Green Version]
- Musa, A.K.; Liadi, M.T.; Adegbite, O.R. Impact of honey bees (Apis mellifera adansonii) (Hymenoptera: Apidae) pollination on pod and seed set of cowpea (Vigna unguiculata L. Walp) in Ilorin, Southern Guinea Savanna of Nigeria. J. Agric. Res. 2013, 1, 83–87. [Google Scholar]
- Fohouo, F.-N.; Albert, N.; Kengni, B. Pollination and yield responses of cowpea (Vigna unguiculata L. Walp.) to the foraging activity of Apis mellifera adansonii (Hymenoptera: Apidae) at Ngaoundéré (Cameroon). Afr. J. Biotechnol. 2009, 8, 9. [Google Scholar]
- Dingha, B.N.; Jackai, L.E.; Amoah, B.A.; Akotsen-Mensah, C. Pollinators on Cowpea Vigna unguiculata: Implications for Intercropping to Enhance Biodiversity. Insects 2021, 12, 54. [Google Scholar] [CrossRef]
- Vaz, C.G.; De Oliveira, D.; Ohashi, O.S. Pollinator Contribution to the Production of Cowpea in the Amazon. HortScience 1998, 33, 1157–1159. [Google Scholar] [CrossRef] [Green Version]
- Jackai, L.E.N.; Daoust, R.A. Insect pests of cowpeas. Annu. Rev. Entomol. 1986, 1, 95–119. [Google Scholar] [CrossRef]
- Sreerama, Y.N.; Sashikala, V.B.; Pratape, V.M.; Singh, V. Nutrients and antinutrients in cowpea and horse gram flours in comparison to chickpea flour: Evaluation of their flour functionality. Food Chem. 2012, 131, 462–468. [Google Scholar] [CrossRef]
- Enyiukwu, D.N.; Amadioha, A.C.; Ononuju, C.C. Nutritional Significance of Cowpea Leaves for Human Consumption. Greener Trends Food Sci. Nutr. 2018, 1, 1–10. [Google Scholar] [CrossRef]
- Hall, A.E.; Frate, C.A. Blackeye Bean Production in California; University of California Division of Agriculture and Natural Resources: Oakland, CA, USA, 1996; 24p. [Google Scholar]
- Ehlers, J.D.; Hall, A.E. Cowpea (Vigna unguiculata L Walp). Field Crops Res. 1997, 53, 187–204. [Google Scholar] [CrossRef]
- Leskey, T.C.; Hamilton, G.C.; Nielsen, A.L.; Polk, D.F.; Rodriguez-Saona, C.; Bergh, J.C.; Herbert, D.A.; Kuhar, T.P.; Pfeiffer, D.; Dively, G.P.; et al. Pest Status of the Brown Marmorated Stink Bug, Halyomorpha Halys in the USA. Outlooks Pest Manag. 2012, 23, 218–226. [Google Scholar] [CrossRef] [Green Version]
- Jackai, L.E.; Dingha, B.N.; Worku, M. Cowpea—An Ancient Crop for Modern Challenges. Scientia. 2018. Available online: http://www.scientia.global/cowpea-an-ancient-crop-for-modern-challenges/ (accessed on 15 March 2020).
- Dingha, B.N.; Nyaupane, S.; Jackai, L.E. Laboratory Assessment of Host Plant Selection of the Brown Marmorated Stink Bug (Halyomorpha halys). Am. J. Entomol. 2009, 4, 26–34. [Google Scholar] [CrossRef]
- Dahmardeh, M.; Ghanbari, A.; Syasar, B.; Ramrodi, M. Intercropping maize (Zea mays L.) and cowpea (Vigna unguiculata L.) as a whole-crop forage: Effects of planting ratio and harvest time on forage yield and quality. J. Food Agric. Environ. 2009, 7, 505–509. [Google Scholar]
- Adeniyan, O.N.; Ayoola, O.T.; Ogunleti, D.O. Evaluation of cowpea cultivars under maize and maize-cassava based in-tercropping systems. Afr. J. Plant Sci. 2011, 5, 570–574. [Google Scholar]
- Maduwanthi, A.K.M.R.B.; Karunarathna, B. Biological and economic benefit of okra (Abelmoschus. esculentus L.) cowpea (Vigna unguiculata L. Walp) intercropping in Sandy Regosol. Middle East J. Agri. Res. 2019, 8, 28–34. [Google Scholar]
- John, S.A.; Mini, C.B. Biological efficiency of intercropping in okra (Abelmoschus esculentus (L.) Moench). J. Trop. Agric. 2005, 43, 33–36. [Google Scholar]
- Ajayi, O.E.; Adeoye, B.I.; Shittu, A.O. Economics analysis of intercropping okra with legumes. J. Agric. Sci. 2017, 62, 193–202. [Google Scholar] [CrossRef]
- Olasantan, F.O. Response of tomato and okra to nitrogen fertilizer in sole cropping and intercropping with cowpea. J. Hortic. Sci. 1991, 66, 191–199. [Google Scholar] [CrossRef]
- Senaratne, R.; Liyanage, N.D.L.; Soper, R.J. Nitrogen fixation of and N transfer from cowpea, mungbean and groundnut when intercropped with maize. Fertil. Res. 1995, 40, 41–48. [Google Scholar] [CrossRef]
- Kemble, J.M.; Quesada-Ocampo, L.M.; Ivors, K.L.; Jennings, K.M.; Walgenbach, J.F. Southeastern United States Virginia Cooperative Extension, 2014 Vegetable Crop Handbook. 2014. Available online: https://vtechworks.lib.vt.edu/bitstream/handle/10919/55798/AREC-66.pdf?sequence=1 (accessed on 15 March 2020).
- Shannon, C.E.; Weaver, W.W. The Mathematical Theory of Communications; University of Illinois Press: Urbana, IL, USA, 1963; 117p. [Google Scholar]
- Spellerberg, I.F.; Fedor, P.J. A tribute to Claude Shannon (1916–2001) and a plea for more rigorous use of species richness, species diversity and the ‘Shannon-Wiener’ Index. Glob. Ecol. Biogeogr. 2003, 12, 177–179. [Google Scholar] [CrossRef] [Green Version]
- Dan, S.; Murungi, L.K.; Kioko, E. Diversity and abundance of insect pollinators and their effect on yield and quality of cowpea and cucumber in Makueni, Kenya. Afr. J. Hortic. Sci. 2019, 16, 43–54. [Google Scholar]
- Geroff, R.K.; Gibbs, J.; McCravy, K.W. Assessing bee (Hymenoptera: Apoidea) diversity of an Illinois restored tallgrass prairie: Methodology and conservation considerations. J. Insect Conserv. 2014, 18, 951–964. [Google Scholar] [CrossRef]
- Shapiro, L.H.; Tepedino, V.J.; Minckley, R.L. Bowling for bees: Optimal sample number for “bee bowl” sampling transects. J. Insect Conserv. 2014, 18, 1105–1113. [Google Scholar] [CrossRef]
- Campbell, J.W.; Hanula, J.L. Efficiency of Malaise traps and colored pan traps for collecting flower visiting insects from three forested ecosystems. J. Insect Conserv. 2007, 11, 399–408. [Google Scholar] [CrossRef]
- Wheelock, M.J.; O’Neal, M.E. Insect Pollinators in Iowa Cornfields: Community Identification and Trapping Method Analysis. PLoS ONE 2016, 11, e0143479. [Google Scholar] [CrossRef] [Green Version]
- Wousla, E.N.; Andargie, M.; Pasquet, R.S.; Mondon, M.; Menez, V.; Cochin, C.; Paul, L.; Pardon, L.; Roubaud, M. Is bigger better? Apidae (Xylocopinae), megachilidae and cowpea (Vigna unguiculata) pollination. Plant Breed. 2019, 139, 156–166. [Google Scholar] [CrossRef]
- Quinn, N.F.; Brainard, D.C.; Szendrei, Z. Floral strips attract beneficial insects but do not enhance yield in cucumber fields. J. Econ. Entomol. 2017, 110, 517–524. [Google Scholar] [CrossRef] [PubMed]
- Conner, J.K.; Rush, S. Effects of flower size and number on pollinator visitation to wild radish, Raphanus raphanistrum. Oecologia 1996, 105, 509–516. [Google Scholar] [CrossRef]
- Bell, G.; Hamilton, W.D. On the function of flowers. Proc. R. Soc. Lond. Ser. B Biol. Sci. Lond. 1985, 224, 223–265. [Google Scholar]
- Kawarasaki, S.; Hori, Y. Effect of flower number on the pollinator attractiveness and the threshold plant size for flowering in Pertya triloba (Asteraceae). Plant Species Biol. 1999, 14, 69–74. [Google Scholar] [CrossRef]
- Van Emden, H.F. Plant diversity and natural enemy efficiency in agroecosystems. In Critical Issues in Biological Control, 2nd ed.; Mackauer, M., Ehler, L.E., Roland, J., Eds.; Intercept Ltd.: Andover, UK, 1990; pp. 63–80. [Google Scholar]
- State Climate Office of North Carolina, NC State University. Cardinal [Data Retrieval Interface]. Available online: https://products.climate.ncsu.edu/cardinal/request (accessed on 14 May 2021).
- Bommarco, R.; Kleijn, D.; Potts, S.G. Ecological intensification: Harnessing ecosystem services for food security. Trends Ecol. Evol. 2013, 28, 230–238. [Google Scholar] [CrossRef] [PubMed]
- Tscharntke, T.; Clough, Y.; Wanger, T.C.; Jackson, L.; Motzke, I.; Perfecto, I.; Vandermeer, J.; Whitbread, A. Global food security, biodiversity conservation and the future of agricultural intensification. Biol. Conserv. 2012, 151, 53–59. [Google Scholar] [CrossRef]
- Angbanyere, M.A.; Baidoo, P.K. The Effect of Pollinators and Pollination on Fruit Set and Fruit Yield of Okra (Abelmoschus esculentus (L.) Moench) in the Forest Region of Ghana. Am. J. Exp. Agric. 2014, 4, 985–995. [Google Scholar] [CrossRef]
- Stanghellini, M.S.; Ambrose, J.T.; Schulthesis, J.R. Seed production in watermelon: A comparison between two commercially available pollinators. HortScience 1998, 33, 28–30. [Google Scholar] [CrossRef] [Green Version]
- Campbell, J.W.; Stanley-Stahr, C.; Bammer, M.; Daniels, J.C.; Ellis, J.D. Contribution of bees and other pollinators to watermelon (Citrullus lanatus Thunb.) pollination. J. Apic. Res. 2019, 58, 597–603. [Google Scholar] [CrossRef]
- Shuler, R.E.; Roulston, T.H.; Farris, G.E. Farming practices influence wild pollinator populations on squash and pumpkin. J. Econ. Entomol. 2005, 98, 790–795. [Google Scholar] [CrossRef] [PubMed]
- Songa, J.M.; Jiang, N.; Schulthess, F.; Omwega, C. The role of intercropping different cereal species in controlling lepidopteran stemborers on maize in Kenya. J. Appl. Entomol. 2007, 131, 40–49. [Google Scholar] [CrossRef]
- Srinivasarao, M.; Ca, R.; Karlapudi, S.; Gudapaty, P.; Sekhar, S.M.; Vani, G.; Venkateswarlu, B. Intercropping for Management of Insect Pests of Castor, Ricinus communis, in the Semi—Arid Tropics of India. J. Insect Sci. 2012, 12, 14. [Google Scholar]
- Matteson, P.C. The effects of intercropping with cereals and minimal permethrin applications on insect pests of cowpea and their natural enemies in Nigeria. Trop. Pest Manag. 1982, 28, 372–380. [Google Scholar] [CrossRef]
- McPherson, R.M.; Pitis, J.R.; Newsom, L.D.; Chapin, J.B.; Herzog, D.C. Incidence of Tachinid Parasitism of Several Stink Bug (Heteroptera: Pentatomidae) Species Associated with Soybean. J. Econ. Entomol. 1982, 75, 783–786. [Google Scholar] [CrossRef]
- Tillman, P.G.; Carpenter, J.E. Milkweed (Gentianales: Apocynaceae): A Farmscape Resource for Increasing Parasitism of Stink Bugs (Hemiptera: Pentatomidae) and Providing Nectar to Insect Pollinators and Monarch Butterflies. Environ. Entomol. 2014, 43, 370–376. [Google Scholar] [CrossRef] [PubMed]
- Cornelius, M.L.; Vinyard, B.T.; Gates, M.W. Use of Flowering Plants to Enhance Parasitism and Predation Rates on Two Squash Bug Species Anasa tristis and Anasa armigera (Hemiptera: Coreidae). Insects 2019, 10, 318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stopbmsb.org Management of Brown Marmorated Stink Bug in US Specialty Crops. Available online: http://www.stopbmsb.org/where-is-bmsb/ (accessed on 20 April 2021).




| Order | Pollinator Type (Common Name) | Treatments and Number of Pollinators (2018) | Treatments and Number of Pollinators (2019) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PPH + SOW + PPH | WHIP + SOW + WHIP | PPHWHIP + SOW + PPHWHIP | PPH-Only | WHIP-Only | SOW | PPH + SOW + PPH | WHIP + SOW + WHIP | PPHWHIP + SOW + PPHWHIP | PPH-Only | WHIP-Only | SOW | ||
| Apidae | Bumble bee | 526 | 322 | 405 | 298 | 40 | 227 | 145 | 108 | 199 | 62 | 63 | 10 |
| Carpenter bee | 36 | 24 | 16 | 8 | 11 | 8 | 4 | 1 | 4 | 0 | 1 | 0 | |
| Honeybee | 203 | 182 | 220 | 197 | 103 | 100 | 589 | 342 | 495 | 259 | 232 | 42 | |
| Hymenoptera | Wasps | 260 | 74 | 135 | 233 | 80 | 9 | 1289 | 814 | 957 | 538 | 417 | 18 |
| Lepidoptera | Butterfly and moth | 134 | 143 | 121 | 159 | 182 | 20 | 215 | 175 | 209 | 94 | 101 | 34 |
| Total | 1159 | 745 | 897 | 895 | 416 | 364 | 2242 | 1440 | 1864 | 953 | 814 | 104 | |
| (H′) | 1.36 | 1.36 | 1.33 | 1.40 | 1.35 | 0.98 | 1.08 | 1.12 | 1.19 | 1.08 | 1.17 | 1.26 | |
| (E) | 0.84 | 0.85 | 0.83 | 0.87 | 0.84 | 0.61 | 0.67 | 0.70 | 0.74 | 0.78 | 0.72 | 0.91 | |
| Pollinator Family | Treatments and Number of Pollinators (2018) | Treatments and Number of Pollinators (2019) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PPH + SOW + PPH | WHIP + SOW + WHIP | PPHWHIP + SOW + PPHWHIP | PPH-Only | WHIP-Only | SOW | PPH + SOW + PPH | WHIP + SOW + WHIP | PPHWHIP + SOW + PPHWHIP | PPH-Only | WHIP-Only | SOW | |
| Apidae | 14 | 9 | 23 | 7 | 2 | 21 | 12 | 15 | 9 | 8 | 7 | 2 |
| Crabronidae | 550 | 673 | 579 | 476 | 383 | 392 | 134 | 169 | 127 | 72 | 68 | 34 |
| Halictidae | 1019 | 1018 | 1114 | 642 | 654 | 679 | 606 | 644 | 662 | 369 | 377 | 190 |
| Pyralidae | 25 | 22 | 34 | 15 | 16 | 45 | 66 | 77 | 77 | 29 | 34 | 153 |
| Tachinidae | 4995 | 4444 | 4636 | 3234 | 2276 | 1757 | 4577 | 3951 | 4595 | 3397 | 2566 | 1182 |
| Vespidae | 47 | 74 | 78 | 41 | 34 | 86 | 167 | 160 | 236 | 192 | 179 | 20 |
| Total | 6650 | 6240 | 6464 | 4415 | 3365 | 2980 | 5562 | 5016 | 5706 | 4067 | 3231 | 1581 |
| (H′) | 0.78 | 0.86 | 0.86 | 0.82 | 0.91 | 1.12 | 0.66 | 0.76 | 0.71 | 0.63 | 0.74 | 0.84 |
| (E) | 0.43 | 0.48 | 0.48 | 0.46 | 0.51 | 0.62 | 0.37 | 0.42 | 0.40 | 0.35 | 0.41 | 0.47 |
| Pollinator Family | Treatments and Number of Pollinators (2018) | Treatments and Number of Pollinators (2019) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PPH + SOW + PPH | WHIP + SOW + WHIP | PPHWHIP + SOW + PPHWHIP | PPH-Only | WHIP-Only | SOW | PPH + SOW + PPH | WHIP + SOW + WHIP | PPHWHIP + SOW + PPHWHIP | PPH-Only | WHIP-Only | SOW | |
| Andrenidae | 16 | 20 | 19 | 8 | 15 | 23 | 1 | 6 | 1 | 1 | 0 | 3 |
| Apidae | 77 | 72 | 76 | 25 | 30 | 82 | 22 | 34 | 24 | 14 | 15 | 7 |
| Crabronidae | 138 | 165 | 112 | 79 | 73 | 20 | 68 | 93 | 73 | 68 | 68 | 19 |
| Formicidae | 37 | 33 | 38 | 21 | 20 | 119 | 1 | 5 | 3 | 3 | 2 | 4 |
| Halictidae | 669 | 787 | 740 | 407 | 465 | 556 | 410 | 489 | 420 | 228 | 254 | 118 |
| Tachinidae | 400 | 649 | 535 | 210 | 328 | 501 | 377 | 397 | 378 | 216 | 215 | 197 |
| Vespidae | 79 | 63 | 44 | 52 | 50 | 31 | 249 | 227 | 178 | 190 | 174 | 43 |
| Total | 1416 | 1789 | 1564 | 802 | 981 | 1332 | 1128 | 1251 | 1077 | 720 | 728 | 391 |
| (H′) | 1.40 | 1.32 | 1.30 | 1.35 | 1.32 | 1.34 | 1.33 | 1.38 | 1.32 | 1.41 | 1.39 | 1.25 |
| (E) | 0.72 | 0.68 | 0.67 | 0.69 | 0.68 | 0.69 | 0.68 | 0.71 | 0.70 | 0.72 | 0.77 | 0.64 |
| Treatment | Yield of Pollinator-Dependent Crop (kg) | |||
|---|---|---|---|---|
| Squash | Okra | Watermelon | ||
| 2018 | PPH + PPH | 29.8 ± 5.8 | 4.1 ± 0.4 | 5.5 ± 1.0 a |
| WHIP + WHIP | 34.6 ± 1.8 | 3.6 ± 0.5 | 4.9 ± 0.7 a | |
| PPH + WHIP | 38.3 ± 4.8 | 3.0 ± 0.4 | 5.7 ± 1.6 a | |
| CONTROL | 21.2 ± 4.4 | 3.1 ± 0.1 | 1.2 ± 0.4 b | |
| 2019 | PPH + PPH | 4.6 ± 0.6 a | 2.4 ± 0.5 | 11.5 ± 1.9 a |
| WHIP + WHIP | 4.4 ± 0.5 a | 2.5 ± 0.5 | 10.1 ± 1.6 a | |
| PPH + WHIP | 5.8 ± 0.6 a | 2.6 ± 0.4 | 12.2 ± 1.9 a | |
| CONTROL | 1.6 ± 0.2 b | 2.0 ± 0.3 | 3.8 ± 0.3 b | |
| Mean of both years | PPH + PPH | 17.2 ± 3.1 | 3.2 ± 0.3 | 8.5 ± 1.2 a |
| WHIP + WHIP | 19.5 ± 1.0 | 3.0 ± 0.5 | 7.5 ± 1.1 a | |
| PPH + WHIP | 22.0 ± 2.5 | 2.8 ± 0.4 | 9.0 ± 0.2 a | |
| CONTROL | 11.4 ± 2.2 | 2.6 ± 0.2 | 2.5 ± 0.1 b | |
| Percent change in intercrop vs. control | +26.5% | +7.1% | +53.7% | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dingha, B.N.; Omaliko, P.C.; Amoah, B.A.; Jackai, L.E.; Shrestha, D. Evaluation of Cowpea (Vigna unguiculata) in an Intercropping System as Pollinator Enhancer for Increased Crop Yield. Sustainability 2021, 13, 9612. https://doi.org/10.3390/su13179612
Dingha BN, Omaliko PC, Amoah BA, Jackai LE, Shrestha D. Evaluation of Cowpea (Vigna unguiculata) in an Intercropping System as Pollinator Enhancer for Increased Crop Yield. Sustainability. 2021; 13(17):9612. https://doi.org/10.3390/su13179612
Chicago/Turabian StyleDingha, Beatrice N., Paul C. Omaliko, Barbara A. Amoah, Louis E. Jackai, and Deepak Shrestha. 2021. "Evaluation of Cowpea (Vigna unguiculata) in an Intercropping System as Pollinator Enhancer for Increased Crop Yield" Sustainability 13, no. 17: 9612. https://doi.org/10.3390/su13179612
APA StyleDingha, B. N., Omaliko, P. C., Amoah, B. A., Jackai, L. E., & Shrestha, D. (2021). Evaluation of Cowpea (Vigna unguiculata) in an Intercropping System as Pollinator Enhancer for Increased Crop Yield. Sustainability, 13(17), 9612. https://doi.org/10.3390/su13179612







