Microalgal Production of Biofuels Integrated with Wastewater Treatment
Abstract
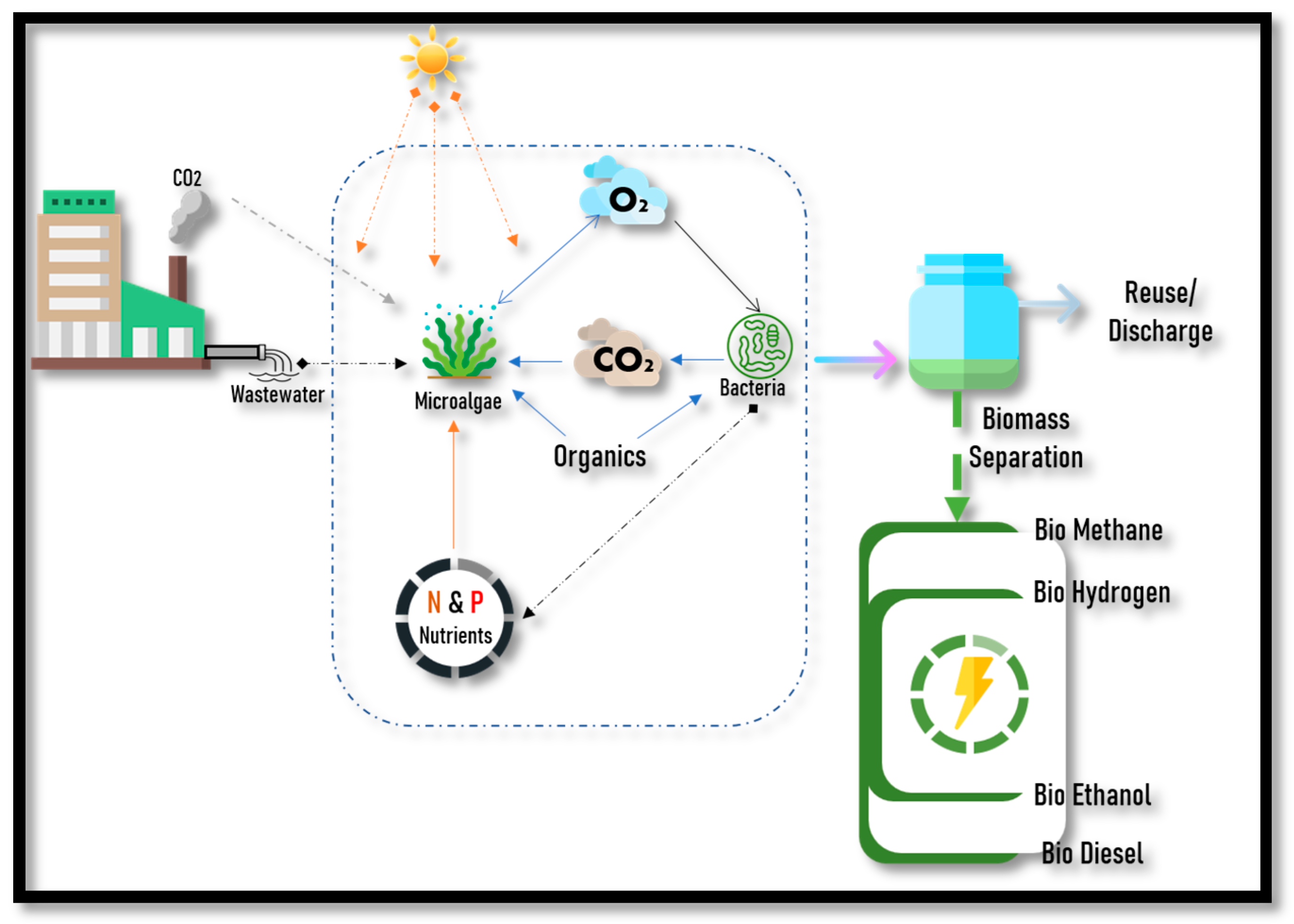
1. Introduction
2. Biofuel Production Pathways
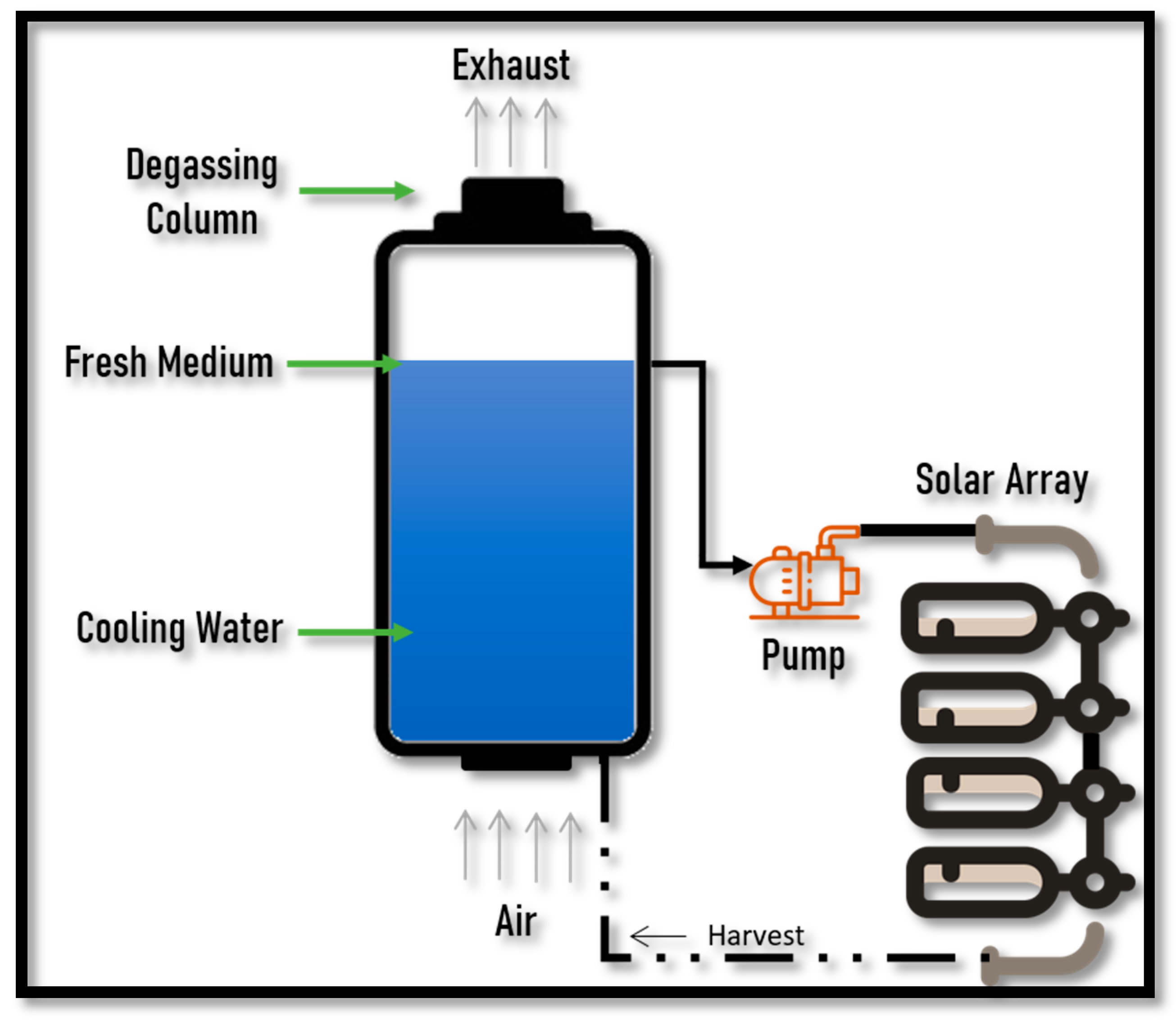
2.1. Biodiesel
2.2. Biomethane
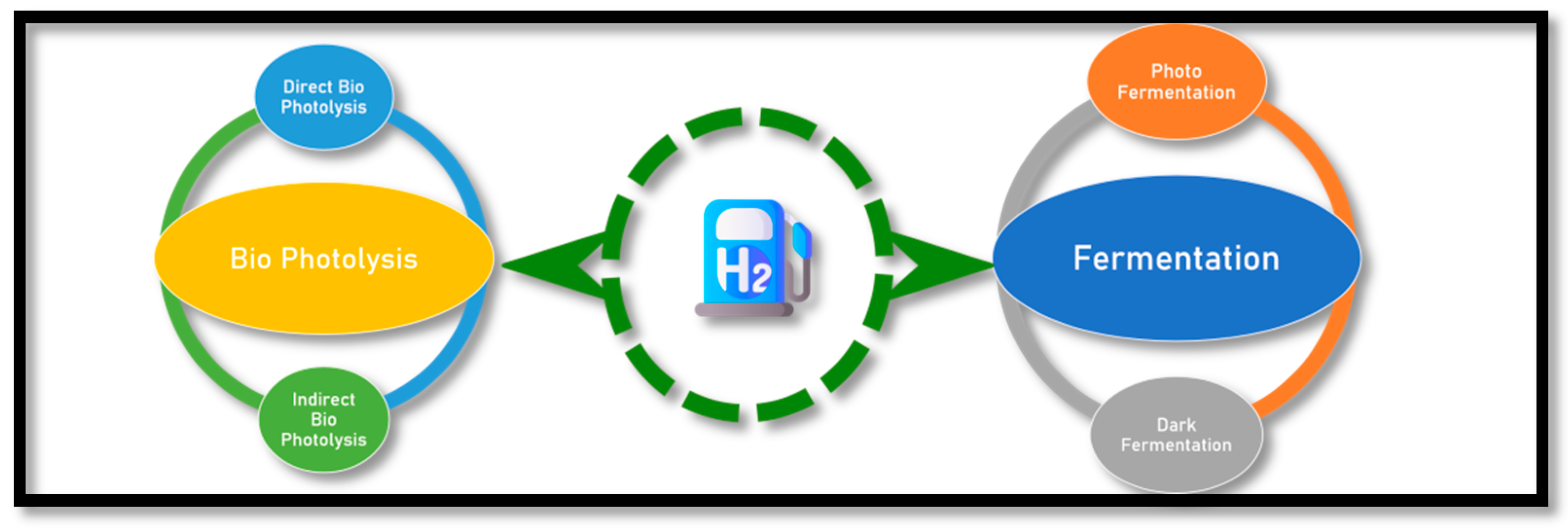
2.3. Biohydrogen
2.4. Bioethanol
2.4.1. Bioethanol by Hydrolysis and Fermentation
2.4.2. Bioethanol by Dark Fermentation
2.4.3. Bioethanol by Photofermentation
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Steinman, A.D.; Vymazal, J. Algae and element cycling in wetlands. J. N. Am. Benthol. Soc. 1996, 15, 138–140. [Google Scholar] [CrossRef]
- Larkum, A. Photosynthesis in Algae; Kluwer Academic Publisher: Dordrecht, The Netherlands, 2003. [Google Scholar]
- Cock, J.M. Introduction to Marine Genomics; Springer: Berlin, Germany, 2010. [Google Scholar]
- Lee, R.E. Phycology, 4th ed.; Cambridge University Press: Cambridge, UK, 2008. [Google Scholar]
- Brennan, L.; Owende, P. Biofuels from microalgae—A review of technologies for production, processing, and extractions of biofuels and co-products. Renew. Sustain. Energy Rev. 2010, 14, 557–577. [Google Scholar] [CrossRef]
- Feinberg, D.A.; Hill, A.M. Fuel from microalgae lipid products. Algae 1984, 4, 1–17. [Google Scholar]
- Packer, M. Algal capture of carbon dioxide; biomass generation as a tool for greenhouse gas mitigation with reference to New Zealand energy strategy and policy. Energy Policy 2009, 37, 3428–3437. [Google Scholar] [CrossRef]
- Ren, H.-Y.; Liu, B.-F.; Kong, F.; Zhao, L.; Ma, J.; Ren, N.-Q. Favorable energy conversion efficiency of coupling dark fermentation and microalgae production from food wastes. Energy Convers. Manag. 2018, 166, 156–162. [Google Scholar] [CrossRef]
- Wendt, L.M.; Kinchin, C.; Wahlen, B.D.; Davis, R.; Dempster, T.A.; Gerken, H. Assessing the stability and techno-economic implications for wet storage of harvested microalgae to manage seasonal variability. Biotechnol. Biofuels 2019, 12, 80. [Google Scholar] [CrossRef] [PubMed]
- Pritchard, H.N. Microalgae: Biotechnology and microbiology. Q. Rev. Biol. 1995, 70, 350–351. [Google Scholar] [CrossRef]
- Christenson, L.; Sims, R. Production and harvesting of microalgae for wastewater treatment, biofuels, and bioproducts. Biotechnol. Adv. 2011, 29, 686–702. [Google Scholar] [CrossRef] [PubMed]
- Pittman, J.K.; Dean, A.; Osundeko, O. The potential of sustainable algal biofuel production using wastewater resources. Bioresour. Technol. 2011, 102, 17–25. [Google Scholar] [CrossRef] [PubMed]
- de Alva, M.S.; Luna-Pabello, V.M.; Cadena, E.; Ortíz, E. Green microalga Scenedesmusacutus grown on municipal wastewater to couple nutrient removal with lipid accumulation for biodiesel production. Bioresour. Technol. 2013, 146, 744–748. [Google Scholar] [CrossRef] [PubMed]
- Prajapati, S.K.; Kaushik, P.; Malik, A.; Vijay, V.K. Phycoremediation coupled production of algal biomass, harvesting and anaerobic digestion: Possibilities and challenges. Biotechnol. Adv. 2013, 31, 1408–1425. [Google Scholar] [CrossRef] [PubMed]
- Nayak, M.; Karemore, A.; Sen, R. Performance evaluation of microalgae for concomitant wastewater bioremediation, CO2 biofixation and lipid biosynthesis for biodiesel application. Algal Res. 2016, 16, 216–223. [Google Scholar] [CrossRef]
- Prandini, J.M.; da Silva, M.L.B.; Mezzari, M.P.; Pirolli, M.; Michelon, W.; Soares, H.M. Enhancement of nutrient removal from swine wastewater digestate coupled to biogas purification by microalgae Scenedesmus spp. Bioresour. Technol. 2016, 202, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Van Wagenen, J.; Pape, M.L.; Angelidaki, I. Characterization of nutrient removal and microalgal biomass production on an industrial waste-stream by application of the deceleration-stat technique. Water Res. 2015, 75, 301–311. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Li, C.; Yang, Z.-H.; Zeng, G.-M.; Feng, L.; Liu, J.-Z.; Liu, M.; Cai, H.-W. Continuous microalgae cultivation in aquaculture wastewater by a membrane photobioreactor for biomass production and nutrients removal. Ecol. Eng. 2016, 92, 55–61. [Google Scholar] [CrossRef]
- Caporgno, M.; Taleb, A.; Olkiewicz, M.; Font, J.; Pruvost, J.; Legrand, J.; Bengoa, C. Microalgae cultivation in urban wastewater: Nutrient removal and biomass production for biodiesel and methane. Algal Res. 2015, 10, 232–239. [Google Scholar] [CrossRef]
- Choudhary, P.; Prajapati, S.K.; Malik, A. Screening native microalgal consortia for biomass production and nutrient removal from rural wastewaters for bioenergy applications. Ecol. Eng. 2016, 91, 221–230. [Google Scholar] [CrossRef]
- Chinnasamy, S.; Bhatnagar, A.; Hunt, R.W.; Das, K. Microalgae cultivation in a wastewater dominated by carpet mill effluents for biofuel applications. Bioresour. Technol. 2010, 101, 3097–3105. [Google Scholar] [CrossRef]
- Wu, L.F.; Chen, P.C.; Huang, A.P.; Lee, C.M. The feasibility of biodiesel production by microalgae using industrial wastewater. Bioresour. Technol. 2012, 113, 14–18. [Google Scholar] [CrossRef]
- Wang, L.; Min, M.; Li, Y.; Chen, P.; Chen, Y.; Liu, Y.; Wang, Y.; Ruan, R. Cultivation of green algae Chlorella sp. in different wastewaters from municipal wastewater treatment plant. Appl. Biochem. Biotechnol. 2009, 162, 1174–1186. [Google Scholar] [CrossRef]
- Gao, F.; Li, C.; Yang, Z.-H.; Zeng, G.-M.; Mu, J.; Liu, M.; Cui, W. Removal of nutrients, organic matter, and metal from domestic secondary effluent through microalgae cultivation in a membrane photobioreactor. J. Chem. Technol. Biotechnol. 2016, 91, 2713–2719. [Google Scholar] [CrossRef]
- Prajapati, S.K.; Choudhary, P.; Malik, A.; Vijay, V.K. Algae mediated treatment and bioenergy generation process for handling liquid and solid waste from dairy cattle farm. Bioresour. Technol. 2014, 167, 260–268. [Google Scholar] [CrossRef]
- Mobin, S.; Alam, F. Biofuel production from algae utilizing wastewater. In Proceedings of the 19th Australasian Fluid Mechanics Conference, Melbourne, Australia, 8–11 December 2014. [Google Scholar]
- Kiran, B.; Pathak, K.; Kumar, R.; Deshmukh, D. Cultivation of Chlorella sp. IM-01 in municipal wastewater for simultaneous nutrient removal and energy feedstock production. Ecol. Eng. 2014, 73, 326–330. [Google Scholar] [CrossRef]
- Escapa, C.; Coimbra, R.; Paniagua, S.; García, A.; Otero, M. Nutrients and pharmaceuticals removal from wastewater by culture and harvesting of Chlorella sorokiniana. Bioresour. Technol. 2015, 185, 276–284. [Google Scholar] [CrossRef]
- Khanzada, Z.T. Phosphorus removal from landfill leachate by microalgae. Biotechnol. Rep. 2020, 25, 419. [Google Scholar] [CrossRef]
- Kumar, G.; Huy, M.; Bakonyi, P.; Bélafi-Bakó, K.; Kim, S.-H. Evaluation of gradual adaptation of mixed microalgae consortia cultivation using textile wastewater via fed batch operation. Biotechnol. Rep. 2018, 20, 289. [Google Scholar] [CrossRef]
- Mar, C.C.; Fan, Y.; Li, F.-L.; Hu, G.-R. Bioremediation of wastewater from edible oil refinery factory using oleaginous microalga Desmodesmus sp. S1. Int. J. Phytoremediat. 2016, 18, 1195–1201. [Google Scholar] [CrossRef]
- Choi, H.-J. Dairy wastewater treatment using microalgae for potential biodiesel application. Environ. Eng. Res. 2016, 21, 393–400. [Google Scholar] [CrossRef]
- Green, F.B.; Bernstone, L.S.; Lundquist, T.J.; Oswald, W.J. Advanced integrated wastewater pond systems for nitrogen removal. Water Sci. Technol. 1996, 33. [Google Scholar] [CrossRef]
- Spolaore, P.; Joannis-Cassan, C.; Duran, E.; Isambert, A. Commercial applications of microalgae. J. Biosci. Bioeng. 2006, 101, 87–96. [Google Scholar] [CrossRef]
- Sialve, B.; Bernet, N.; Bernard, O. Anaerobic digestion of microalgae as a necessary step to make microalgal biodiesel sustainable. Biotechnol. Adv. 2009, 27, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Kapdan, I.K.; Kargi, F. Bio-hydrogen production from waste materials. Enzym. Microb. Technol. 2006, 38, 569–582. [Google Scholar] [CrossRef]
- Fedorov, A.; Kosourov, S.; Ghirardi, M.L.; Seibert, M. Continuous hydrogen photoproduction by Chlamydomonas reinhardtii: Using a novel two-stage, sulfate-limited chemostat system. Appl. Biochem. Biotechnol. 2005, 121, 0403–0412. [Google Scholar] [CrossRef]
- Dexter, J.; Fu, P. Metabolic engineering of cyanobacteria for ethanol production. Energy Environ. Sci. 2009, 2, 857–864. [Google Scholar] [CrossRef]
- Choi, S.P.; Nguyen, M.-T.; Sim, S.J. Enzymatic pretreatment of Chlamydomonas reinhardtii biomass for ethanol production. Bioresour. Technol. 2010, 101, 5330–5336. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Miao, X.; Wu, Q. High quality biodiesel production from a microalga Chlorella protothecoides by heterotrophic growth in fermenters. J. Biotechnol. 2006, 126, 499–507. [Google Scholar] [CrossRef]
- Banerjee, A.; Sharma, R.; Chisti, Y.; Banerjee, U.C. Botryococcus braunii: A renewable source of hydrocarbons and other chemicals. Crit. Rev. Biotechnol. 2002, 22, 245–279. [Google Scholar] [CrossRef] [PubMed]
- Miao, X.; Wu, Q.; Yang, C. Fast pyrolysis of microalgae to produce renewable fuels. J. Anal. Appl. Pyrolysis 2004, 71, 855–863. [Google Scholar] [CrossRef]
- Sawayama, S.; Inoue, S.; Dote, Y.; Yokoyama, S.-Y. CO2 fixation and oil production through microalga. Energy Convers. Manag. 1995, 36, 729–731. [Google Scholar] [CrossRef]
- Nagle, N.; Lemke, P. Production of methyl ester fuel from microalgae. Appl. Biochem. Biotechnol. 1990, 24–25, 355–361. [Google Scholar] [CrossRef]
- Koberg, M.; Cohen, M.; Ben-Amotz, A.; Gedanken, A. Bio-diesel production directly from the microalgae biomass of Nannochloropsis by microwave and ultrasound radiation. Bioresour. Technol. 2011, 102, 4265–4269. [Google Scholar] [CrossRef] [PubMed]
- Chisti, Y. Biodiesel from microalgae. Biotechnol. Adv. 2007, 25, 294–306. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.B.; Wen, Z. Production of biodiesel fuel from the microalga Schizochytrium limacinum by direct transesterification of algal biomass. Energy Fuels 2009, 23, 5179–5183. [Google Scholar] [CrossRef]
- Trindade, S.C. Nanotech biofuels and fuel additives. In Biofuel’s Engineering Process Technology; IntechOpen: London, UK, 2011. [Google Scholar]
- Wahlen, B.; Willis, R.M.; Seefeldt, L. Biodiesel production by simultaneous extraction and conversion of total lipids from microalgae, cyanobacteria, and wild mixed-cultures. Bioresour. Technol. 2011, 102, 2724–2730. [Google Scholar] [CrossRef]
- AshokKumar, V.; Agila, E.; Sivakumar, P.; Salam, Z.; Rengasamy, R.; Ani, F.N. Optimization and characterization of biodiesel production from microalgae Botryococcus grown at semi-continuous system. Energy Convers. Manag. 2014, 88, 936–946. [Google Scholar] [CrossRef]
- Lewis, T.; Nichols, P.D.; McMeekin, T.A. Evaluation of extraction methods for recovery of fatty acids from lipid-producing microheterotrophs. J. Microbiol. Methods 2000, 43, 107–116. [Google Scholar] [CrossRef]
- Danielo, O. An Algae-Based Fuel; Biofuture: Venelles, France, 2005. [Google Scholar]
- Metzger, P.; Largeau, C. Botryococcus braunii: A rich source for hydrocarbons and related ether lipids. Appl. Microbiol. Biotechnol. 2004, 66, 486–496. [Google Scholar] [CrossRef]
- Knothe, G. The Biodiesel Handbook; AOCS Press: Urbana, IL, USA, 2005. [Google Scholar]
- Kumar, M.; Sharma, M.P. Selection of potential oils for biodiesel production. Renew. Sustain. Energy Rev. 2016, 56, 1129–1138. [Google Scholar] [CrossRef]
- Ribeiro, N.M.; Pinto, A.C.; Quintella, C.; da Rocha, G.O.; Teixeira, L.; Guarieiro, L.L.N.; Rangel, M.D.C.; Veloso, M.C.C.; Rezende, M.J.C.; da Cruz, R.S.; et al. The role of additives for diesel and diesel blended (ethanol or biodiesel) fuels: A review. Energy Fuels 2007, 21, 2433–2445. [Google Scholar] [CrossRef]
- Bhale, P.V.; Deshpande, N.; Thombre, S.B. Improving the low temperature properties of biodiesel fuel. Renew. Energy 2009, 34, 794–800. [Google Scholar] [CrossRef]
- Zabed, H.M.; Boyce, A.N.; Sahu, J.N.; Faruq, G. Evaluation of the quality of dried distiller’s grains with solubles for normal and high sugary corn genotypes during dry–grind ethanol production. J. Clean. Prod. 2017, 142, 4282–4293. [Google Scholar] [CrossRef]
- Ayala-Parra, P.; Liu, Y.; Field, J.; Sierra-Alvarez, R. Nutrient recovery and biogas generation from the anaerobic digestion of waste biomass from algal biofuel production. Renew. Energy 2017, 108, 410–416. [Google Scholar] [CrossRef]
- Zamalloa, C.; Boon, N.; Verstraete, W. Anaerobic digestibility of Scenedesmus obliquus and Phaeodactylum tricornutum under mesophilic and thermophilic conditions. Appl. Energy 2012, 92, 733–738. [Google Scholar] [CrossRef]
- Saratale, R.G.; Kumar, G.; Banu, R.; Xia, A.; Periyasamy, S.; Saratale, G.D. A critical review on anaerobic digestion of microalgae and macroalgae and co-digestion of biomass for enhanced methane generation. Bioresour. Technol. 2018, 262, 319–332. [Google Scholar] [CrossRef]
- Ward, A.; Lewis, D.; Green, F. Anaerobic digestion of algae biomass: A review. Algal Res. 2014, 5, 204–214. [Google Scholar] [CrossRef]
- Park, J.; Craggs, R.; Shilton, A. Wastewater treatment high rate algal ponds for biofuel production. Bioresour. Technol. 2011, 102, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Sivakumar, G.; Xu, J.; Thompson, R.W.; Yang, Y.; Randol-Smith, P.; Weathers, P.J. Integrated green algal technology for bioremediation and biofuel. Bioresour. Technol. 2012, 107, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Zhao, Y.; Zhao, G.; Zhang, H. Nutrient removal and biogas upgrading by integrating freshwater algae cultivation with piggery anaerobic digestate liquid treatment. Appl. Microbiol. Biotechnol. 2015, 99, 6493–6501. [Google Scholar] [CrossRef]
- Ehimen, E.; Sun, Z.; Carrington, C.; Birch, E.; Eaton-Rye, J. Anaerobic digestion of microalgae residues resulting from the biodiesel production process. Appl. Energy 2011, 88, 3454–3463. [Google Scholar] [CrossRef]
- Sforza, E.; Barbera, E.; Girotto, F.; Cossu, R.; Bertucco, A. Anaerobic digestion of lipid-extracted microalgae: Enhancing nutrient recovery towards a closed loop recycling. Biochem. Eng. J. 2017, 121, 139–146. [Google Scholar] [CrossRef]
- Gonzalez, L.M.; Correa, D.F.; Ryan, S.; Jensen, P.D.; Pratt, S.; Schenk, P.M. Integrated biodiesel and biogas production from microalgae: Towards a sustainable closed loop through nutrient recycling. Renew. Sustain. Energy Rev. 2018, 82, 1137–1148. [Google Scholar] [CrossRef]
- Golueke, C.G.; Oswald, W.J.; Gotaas, H.B. Anaerobic digestion of algae. Appl. Microbiol. 1957, 5, 47–55. [Google Scholar] [CrossRef]
- Klassen, V.; Blifernez-Klassen, O.; Wobbe, L.; Schlüter, A.; Kruse, O.; Mussgnug, J.H. Efficiency and biotechnological aspects of biogas production from microalgal substrates. J. Biotechnol. 2016, 234, 7–26. [Google Scholar] [CrossRef]
- Klassen, V.; Blifernez-Klassen, O.; Hoekzema, Y.; Mussgnug, J.H.; Kruse, O. A novel one-stage cultivation/fermentation strategy for improved biogas production with microalgal biomass. J. Biotechnol. 2015, 215, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Huang, Y.; Wu, B.; Zhang, X.; Zhang, S. Biogas upgrading technologies: Energetic analysis and environmental impact assessment. Chin. J. Chem. Eng. 2015, 23, 247–254. [Google Scholar] [CrossRef]
- Krischan, J.; Makaruk, A.; Harasek, M. Design and scale-up of an oxidative scrubbing process for the selective removal of hydrogen sulfide from biogas. J. Hazard. Mater. 2012, 215–216, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wan, W. Factors influencing fermentative hydrogen production: A review. Int. J. Hydrogen Energy 2009, 34, 799–811. [Google Scholar] [CrossRef]
- Argun, H.; Kargi, F.; Kapdan, I.K.; Oztekin, R. Biohydrogen production by dark fermentation of wheat powder solution: Effects of C/N and C/P ratio on hydrogen yield and formation rate. Int. J. Hydrogen Energy 2008, 33, 1813–1819. [Google Scholar] [CrossRef]
- Ferreira, A.F.; Marques, A.C.; Batista, A.P.; Marques, P.A.; Gouveia, L.; Silva, C. Biological hydrogen production by Anabaena sp.—Yield, energy and CO2 analysis including fermentative biomass recovery. Int. J. Hydrogen Energy 2012, 37, 179–190. [Google Scholar] [CrossRef]
- Ruiz-Marin, A.; Canedo-López, Y.; Chávez-Fuentes, P. Biohydrogen production by Chlorella vulgaris and Scenedesmus obliquus immobilized cultivated in artificial wastewater under different light quality. AMB Express 2020, 10, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Nobre, B.; Villalobos, F.; Barragán-Huerta, B.E.; Oliveira, A.; Batista, A.P.; Marques, P.; Mendes, R.; Sovova, H.; Palavra, A.; Gouveia, L. A biorefinery from Nannochloropsis sp. microalga—Extraction of oils and pigments. Production of biohydrogen from the leftover biomass. Bioresour. Technol. 2013, 135, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Batyrova, K.; Hallenbeck, P.C. Hydrogen production by a Chlamydomonas reinhardtii strain with inducible expression of photosystem II. Int. J. Mol. Sci. 2017, 18, 647. [Google Scholar] [CrossRef] [PubMed]
- Juantorena, A.; Sebastian, P.; Santoyo, E.; Gamboa, S.; Lastres, O.; Sánchez-Escamilla, D.; Bustos, A.; Eapen, D. Hydrogen production employing Spirulina maxima 2342: A chemical analysis. Int. J. Hydrogen Energy 2007, 32, 3133–3136. [Google Scholar] [CrossRef]
- Batista, A.P.; Moura, P.; Marques, P.A.; Ortigueira, J.; Alves, L.; Gouveia, L. Scenedesmus obliquus as feedstock for biohydrogen production by Enterobacter aerogenes and Clostridium butyricum. Fuel 2014, 117, 537–543. [Google Scholar] [CrossRef]
- Wieczorek, N.; Kucuker, M.A.; Kuchta, K. Fermentative hydrogen and methane production from microalgal biomass (Chlorella vulgaris) in a two-stage combined process. Appl. Energy 2014, 132, 108–117. [Google Scholar] [CrossRef]
- Yang, Z.; Guo, R.; Xu, X.; Fan, X.; Li, X. Enhanced hydrogen production from lipid-extracted microalgal biomass residues through pretreatment. Int. J. Hydrogen Energy 2010, 35, 9618–9623. [Google Scholar] [CrossRef]
- Vijayaraghavan, K.; Karthik, R.; Nal, S.K. Hydrogen generation from algae: A review. J. Plant Sci. 2009, 5, 1–19. [Google Scholar] [CrossRef][Green Version]
- Rupprecht, J.; Hankamer, B.; Mussgnug, J.H.; Ananyev, G.; Dismukes, C.; Kruse, O. Perspectives and advances of biological H2 production in microorganisms. Appl. Microbiol. Biotechnol. 2006, 72, 442–449. [Google Scholar] [CrossRef]
- Kosourov, S.; Patrusheva, E.; Ghirardi, M.L.; Seibert, M.; Tsygankov, A. A comparison of hydrogen photoproduction by sulfur-deprived Chlamydomonas reinhardtii under different growth conditions. J. Biotechnol. 2007, 128, 776–787. [Google Scholar] [CrossRef]
- Gaffron, H.; Rubin, J. Fermentative and photochemical production of hydrogen in algae. J. Gen. Physiol. 1942, 26, 219–240. [Google Scholar] [CrossRef] [PubMed]
- Kessler, E. Hydrogenase, photoreduction, and anaerobic growth. In Algal Physiology and Biochemistry; University of California Press: Berkeley, CA, USA, 1974. [Google Scholar]
- Hallenbeck, P. Fundamentals of the fermentative production of hydrogen. Water Sci. Technol. 2005, 52, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Tam, N.; Wong, Y. Effect of immobilized microalgal bead concentrations on wastewater nutrient removal. Environ. Pollut. 2000, 107, 145–151. [Google Scholar] [CrossRef]
- Azwar, M.; Hussain, M.A.; Abdul-Wahab, A. Development of biohydrogen production by photobiological, fermentation and electrochemical processes: A review. Renew. Sustain. Energy Rev. 2014, 31, 158–173. [Google Scholar] [CrossRef]
- Rashid, N.; Lee, K.; Han, J.-I.; Gross, M. Hydrogen production by immobilized Chlorella vulgaris: Optimizing pH, carbon source and light. Bioprocess Biosyst. Eng. 2012, 36, 867–872. [Google Scholar] [CrossRef]
- Razzak, S.; Hossain, M.M.; Lucky, R.A.; Bassi, A.S.; de Lasa, H. Integrated CO2 capture, wastewater treatment and biofuel production by microalgae culturing—A review. Renew. Sustain. Energy Rev. 2013, 27, 622–653. [Google Scholar] [CrossRef]
- Klein, U.; Betz, A. Fermentative metabolism of hydrogen-evolving Chlamydomonas moewusii. Plant Physiol. 1978, 61, 953–956. [Google Scholar] [CrossRef] [PubMed]
- Jurado-Oller, J.L.; Dubini, A.; Galván, A.; Fernández, E.; González-Ballester, D. Low oxygen levels contribute to improve photohydrogen production in mixotrophic non-stressed Chlamydomonas cultures. Biotechnol. Biofuels 2015, 8, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Dale, B.E. Thinking clearly about biofuels: Ending the irrelevant ‘net energy’ debate and developing better performance metrics for alternative fuels. Biofuels Bioprod. Biorefin. 2007, 1, 14–17. [Google Scholar] [CrossRef]
- Demirbaş, A. Conversion of biomass using glycerin to liquid fuel for blending gasoline as alternative engine fuel. Energy Convers. Manag. 2000, 41, 1741–1748. [Google Scholar] [CrossRef]
- Naik, S.; Goud, V.V.; Rout, P.K.; Dalai, A.K. Production of first and second generation biofuels: A comprehensive review. Renew. Sustain. Energy Rev. 2010, 14, 578–597. [Google Scholar] [CrossRef]
- Harun, R.; Singh, M.; Forde, G.M.; Danquah, M.K. Bioprocess engineering of microalgae to produce a variety of consumer products. Renew. Sustain. Energy Rev. 2010, 14, 1037–1047. [Google Scholar] [CrossRef]
- Radakovits, R.; Jinkerson, R.; Darzins, A.; Posewitz, M.C. Genetic engineering of algae for enhanced biofuel production. Eukaryot. Cell 2010, 9, 486–501. [Google Scholar] [CrossRef]
- González-Fernández, C.; Ballesteros, M. Linking microalgae and cyanobacteria culture conditions and key-enzymes for carbohydrate accumulation. Biotechnol. Adv. 2012, 30, 1655–1661. [Google Scholar] [CrossRef]
- Ueno, Y.; Kurano, N.; Miyachi, S. Ethanol production by dark fermentation in the marine green alga, Chlorococcum littorale. J. Ferment. Bioeng. 1998, 86, 38–43. [Google Scholar] [CrossRef]
- Hernández, D.; Riaño, B.; Coca, M.; González, M.C.G. Saccharification of carbohydrates in microalgal biomass by physical, chemical and enzymatic pre-treatments as a previous step for bioethanol production. Chem. Eng. J. 2015, 262, 939–945. [Google Scholar] [CrossRef]
- Magneschi, L.; Catalanotti, C.; Subramanian, V.; Dubini, A.; Yang, W.; Mus, F.; Posewitz, M.C.; Seibert, M.; Perata, P.; Grossman, A.R. A mutant in the ADH1 gene of Chlamydomonas reinhardtii elicits metabolic restructuring during anaerobiosis. Plant Physiol. 2012, 158, 1293–1305. [Google Scholar] [CrossRef]
- Dexter, J.; Armshaw, P.; Sheahan, C.; Pembroke, J.T. The state of autotrophic ethanol production in cyanobacteria. J. Appl. Microbiol. 2015, 119, 11–24. [Google Scholar] [CrossRef] [PubMed]
- Saad, M.G.; Dosoky, N.S.; Zoromba, M.S.; Shafik, H.M. Algal Biofuels: Current Status and Key Challenges. Energies 2019, 12, 1920. [Google Scholar] [CrossRef]
- Deng, M.-D.; Coleman, J.R. Ethanol synthesis by genetic engineering in cyanobacteria. Appl. Environ. Microbiol. 1999, 65, 523–528. [Google Scholar] [CrossRef]
- Piven, I.; Friedrich, A.; Dühring, U.; Uliczka, F.; Baier, K.; Inaba, M. Cyanobacterium sp. for Production of Compounds. U.S. Patent US20140178958A1, 26 June 2012. [Google Scholar]
- Hellingwerf, K.; De Mattos, M.T. Alternative routes to biofuels: Light-driven biofuel formation from CO2 and water based on the ‘photanol’ approach. J. Biotechnol. 2009, 142, 87–90. [Google Scholar] [CrossRef]
- Gimpel, J.; Specht, E.A.; Georgianna, D.R.; Mayfield, S.P. Advances in microalgae engineering and synthetic biology applications for biofuel production. Curr. Opin. Chem. Biol. 2013, 17, 489–495. [Google Scholar] [CrossRef]
- Sforza, E.; Gris, B.; De Farias Silva, C.E.; Morosinotto, T.; Bertucco, A. Effects of light on cultivation of scenedesmus obliquus in batch and continuous flat plate photobioreactor. Chem. Eng. Trans. 2014, 38, 211–216. [Google Scholar] [CrossRef]
- Peccia, J.; Haznedaroglu, B.Z.; Gutierrez, J.; Zimmerman, J.B. Nitrogen supply is an important driver of sustainable microalgae biofuel production. Trends Biotechnol. 2013, 31, 134–138. [Google Scholar] [CrossRef] [PubMed]
- Kuhad, R.C.; Gupta, R.; Khasa, Y.P.; Singh, A.; Zhang, Y.-H.P. Bioethanol production from pentose sugars: Current status and future prospects. Renew. Sustain. Energy Rev. 2011, 15, 4950–4962. [Google Scholar] [CrossRef]
| Microalgae Species | Protein | Carbohydrates | Fatty Acids |
|---|---|---|---|
| Anabaena cylindrica | 43–56 | 25–30 | 4–7 |
| Aphanizomenonflos-aquae | 62 | 23 | 3 |
| Chlamydomonas rheinhardii | 48 | 17 | 21 |
| Chlorella pyrenoidosa | 57 | 26 | 2 |
| Chlorella vulgaris | 51–58 | 12–17 | 14–22 |
| Dunaliella salina | 57 | 32 | 6 |
| Euglena gracilis | 39–61 | 14–18 | 14–20 |
| Porphyridiumcruentum | 28–39 | 40–57 | 9–14 |
| Scenedesmus obliquus | 50–56 | 10–17 | 12–14 |
| Spirogyra sp. | 6–20 | 33–64 | 11–21 |
| Arthrospira maxima | 60–71 | 13–16 | 6–7 |
| Spirulina platensis | 46–63 | 8–14 | 4–9 |
| Synechococcus sp. | 63 | 15 | 11 |
| Wastewater | Strain | Removal of Total Nitrogen (%) | Removal of Total Phosphorus (%) | Reference |
|---|---|---|---|---|
| Municipal sewage water | Chlorella | 97.81 | 89.39 | [27] |
| Pharmaceutical wastewater | Chlorella sorokiniana | 70 | 89 | [28] |
| Landfill Leachate | Chlorella vulgaris | 69 | 100 | [29] |
| Aquaculture wastewater | Chlorella vulgaris and Scenedesmus obliquus | 86.1 | 82.7 | [18] |
| Textile wastewater | Mixed consortia of Microalga | 95 | 70 | [30] |
| Edible Oil Refinery wastewater | Desmodesmussp | 96 | 53 | [31] |
| Dairy wastewater | Chlorella vulgaris | 85.47 | 65.96 | [32] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jayaseelan, M.; Usman, M.; Somanathan, A.; Palani, S.; Muniappan, G.; Jeyakumar, R.B. Microalgal Production of Biofuels Integrated with Wastewater Treatment. Sustainability 2021, 13, 8797. https://doi.org/10.3390/su13168797
Jayaseelan M, Usman M, Somanathan A, Palani S, Muniappan G, Jeyakumar RB. Microalgal Production of Biofuels Integrated with Wastewater Treatment. Sustainability. 2021; 13(16):8797. https://doi.org/10.3390/su13168797
Chicago/Turabian StyleJayaseelan, Merrylin, Mohamed Usman, Adishkumar Somanathan, Sivashanmugam Palani, Gunasekaran Muniappan, and Rajesh Banu Jeyakumar. 2021. "Microalgal Production of Biofuels Integrated with Wastewater Treatment" Sustainability 13, no. 16: 8797. https://doi.org/10.3390/su13168797
APA StyleJayaseelan, M., Usman, M., Somanathan, A., Palani, S., Muniappan, G., & Jeyakumar, R. B. (2021). Microalgal Production of Biofuels Integrated with Wastewater Treatment. Sustainability, 13(16), 8797. https://doi.org/10.3390/su13168797








