Industrial Waste Materials as Alternative Fillers in Asphalt Mixtures
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Limestone Filler
2.1.2. Soda Sludge Powder
2.1.3. Calcium Sulfate Powder
2.1.4. Fly Ash Powder
2.1.5. Fly Ash with 10% Lime Powder
2.2. Laboratory Experiments
2.2.1. Particle Shape
2.2.2. Particle Size Distribution
2.2.3. Water Content
2.2.4. Particle Density
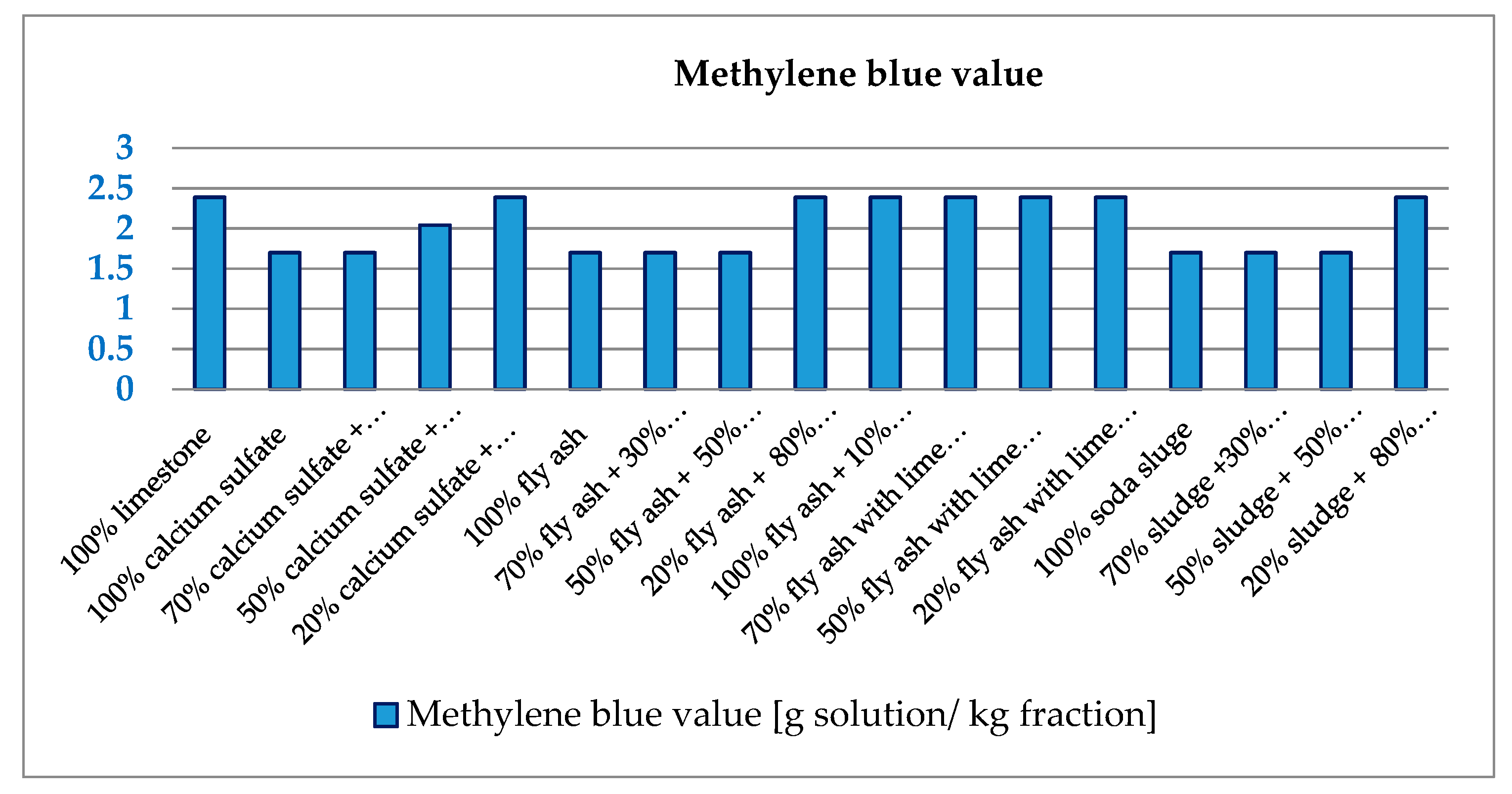
2.2.5. Methlene Blue Value
2.2.6. Voids of Dry Compacted Filler
2.2.7. Delta Ring and Ball
2.2.8. Blaine Specific Surface Area
3. Results and Discussion
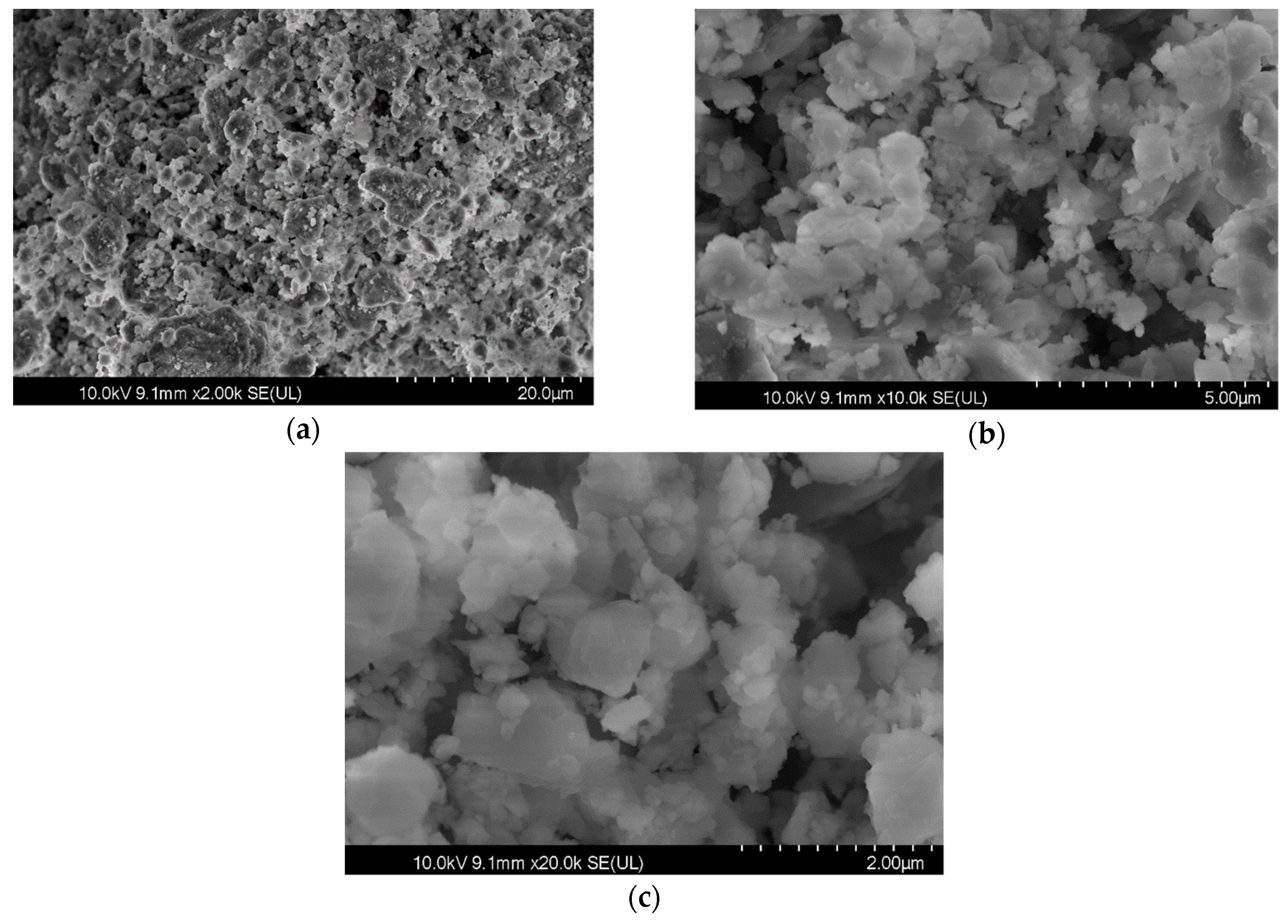
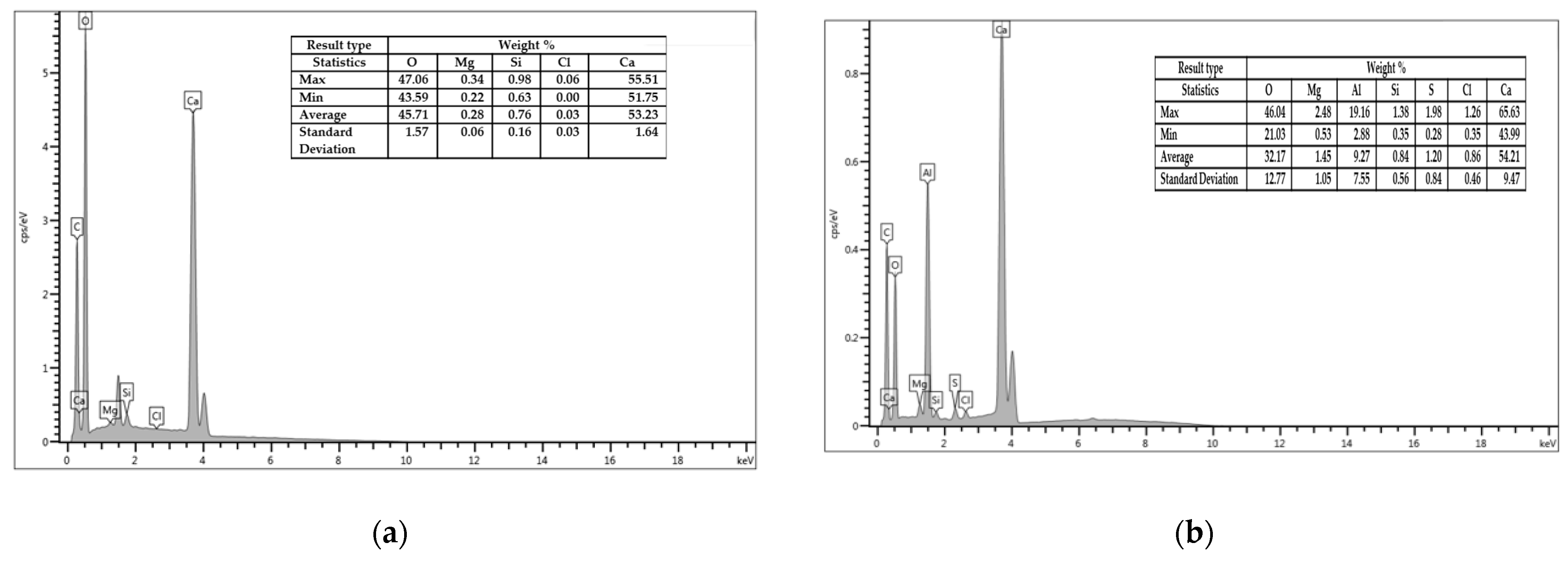
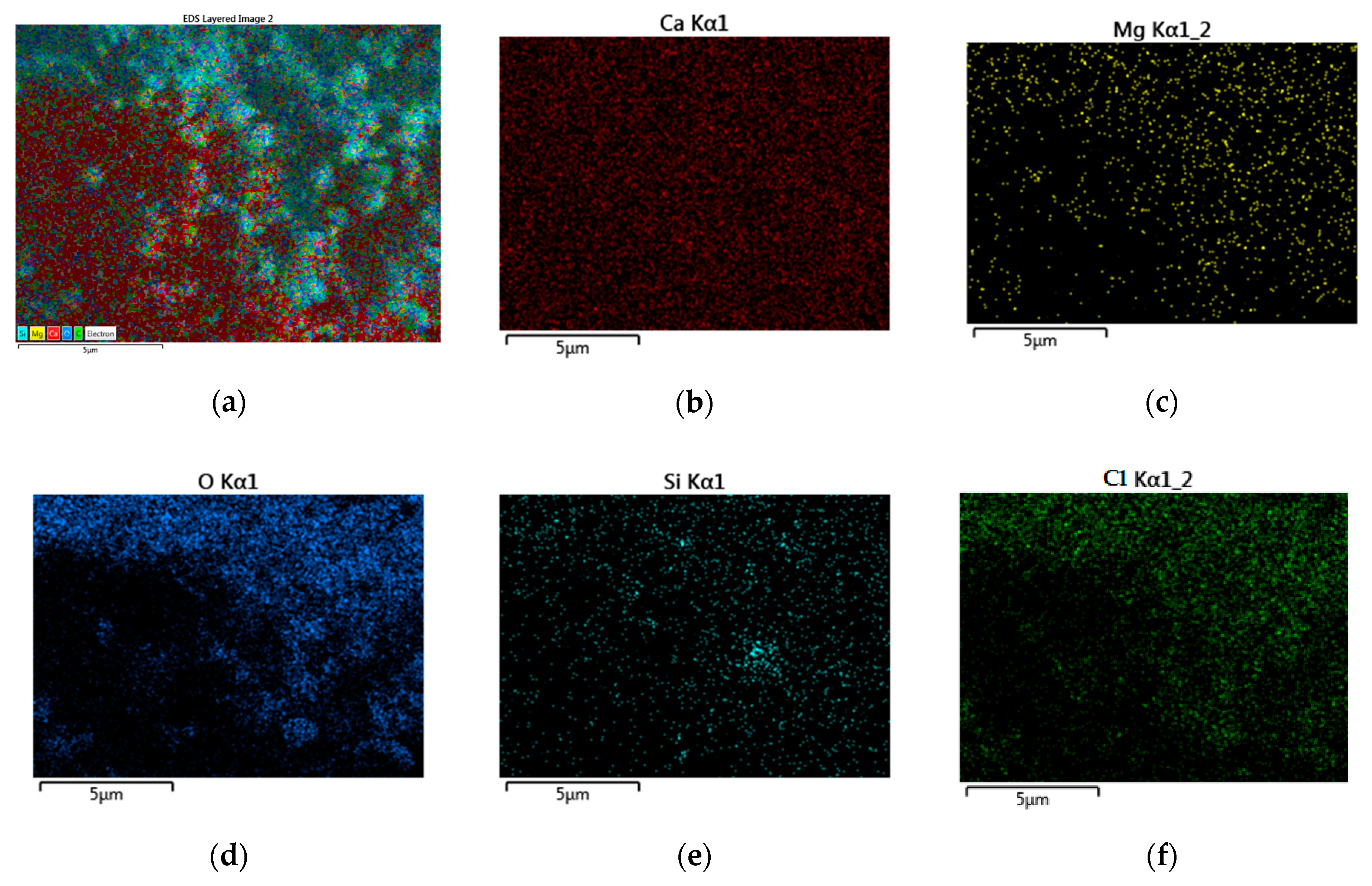
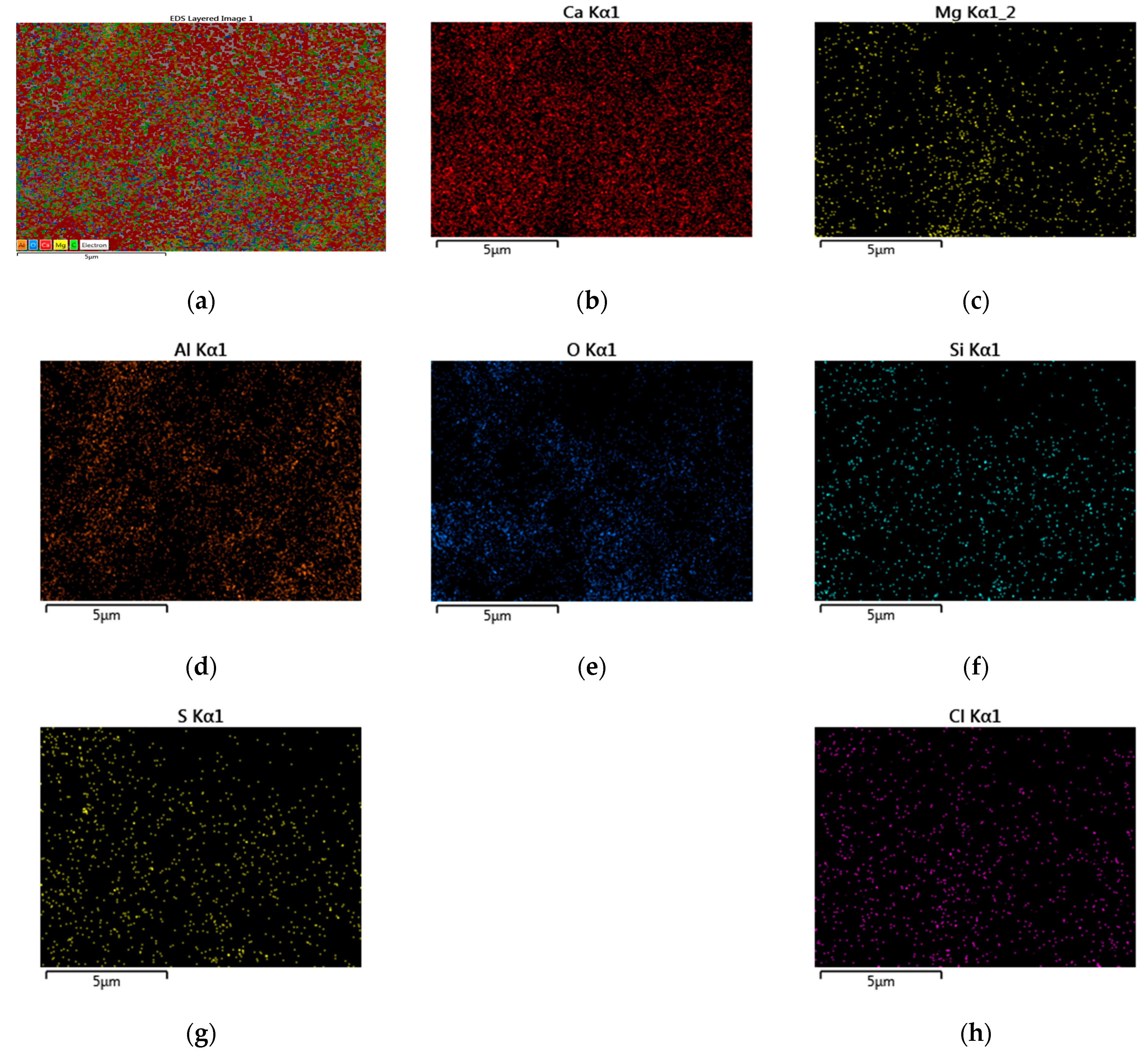
3.1. Morphological and Chemical Ccharacteristics
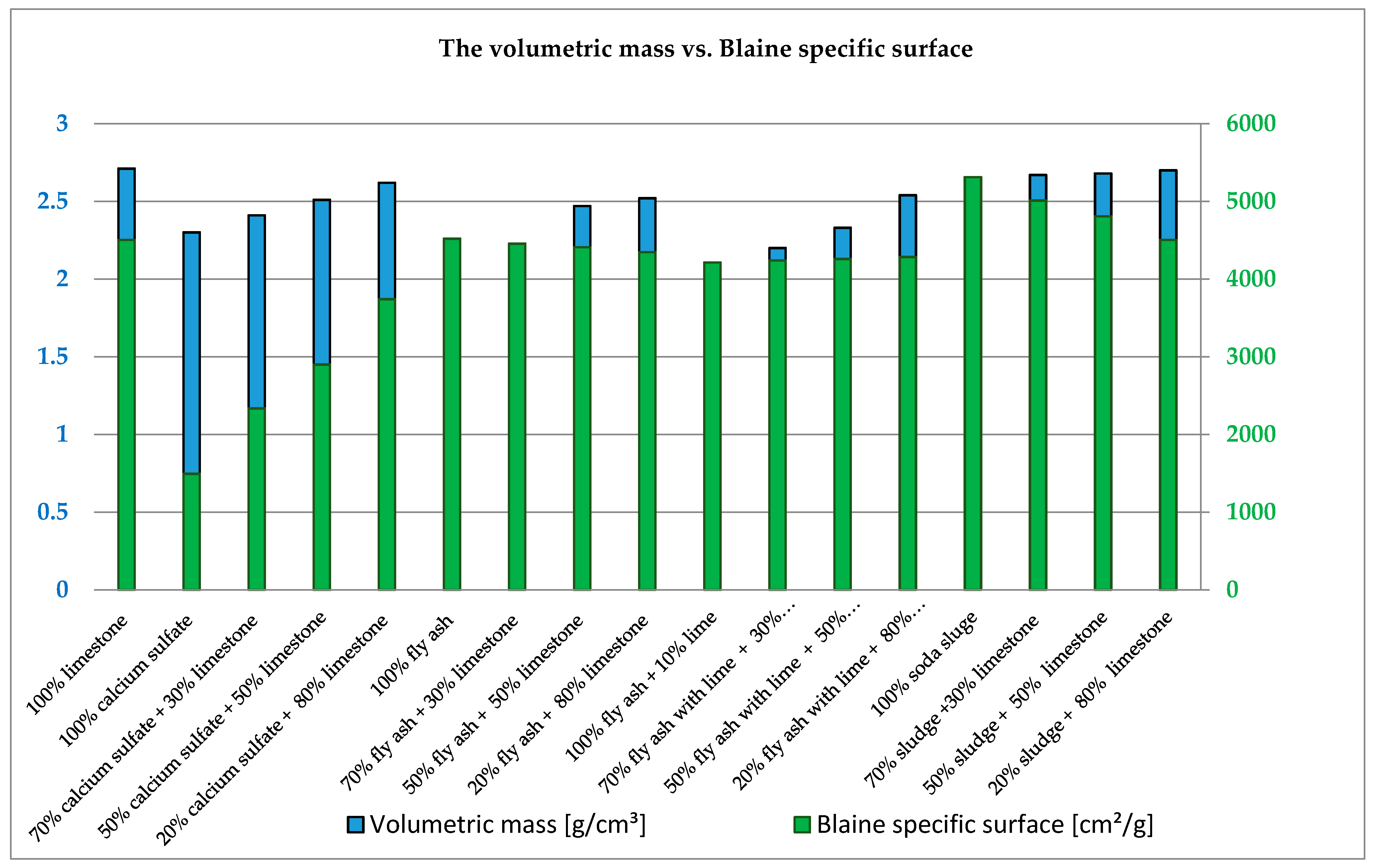
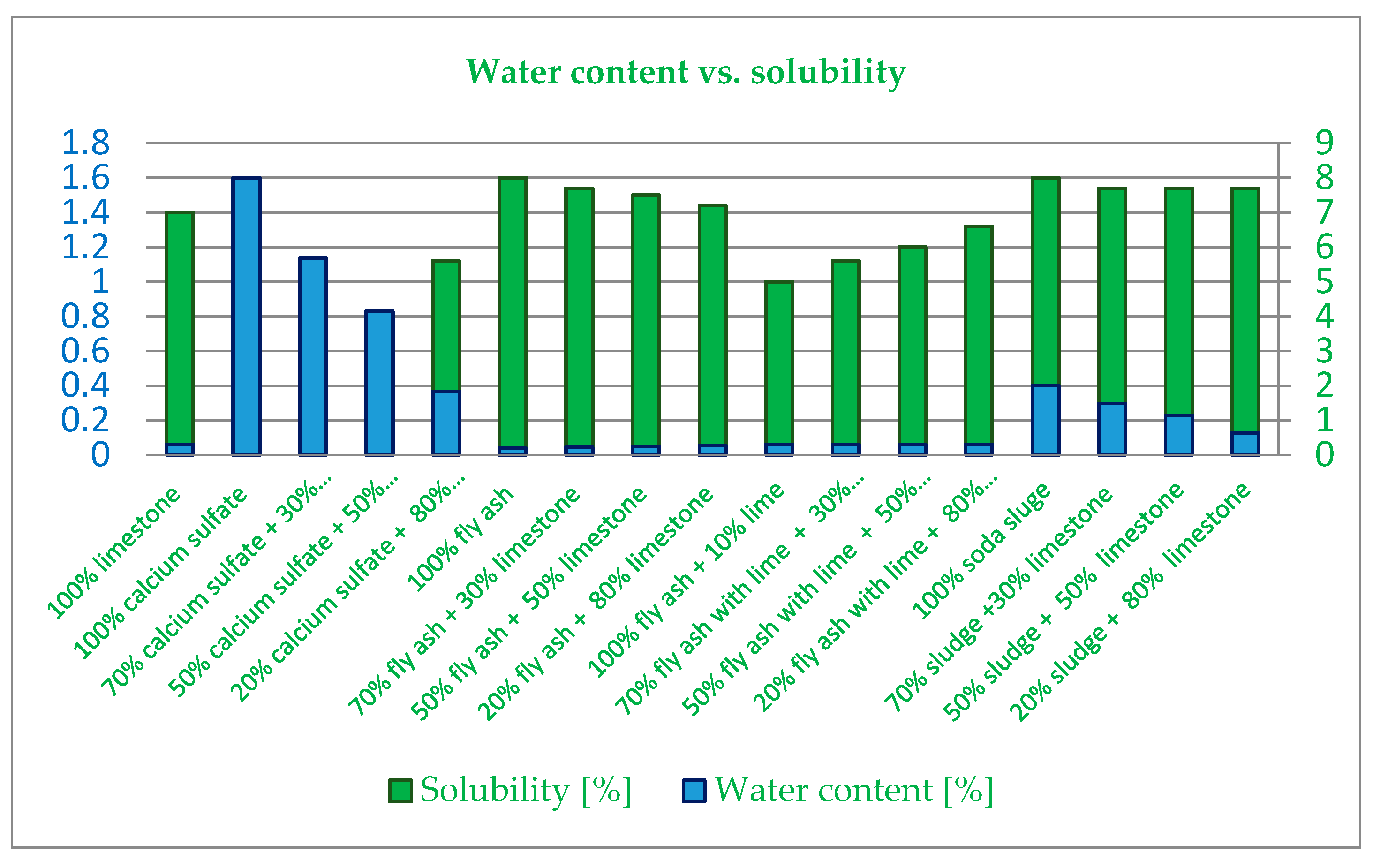
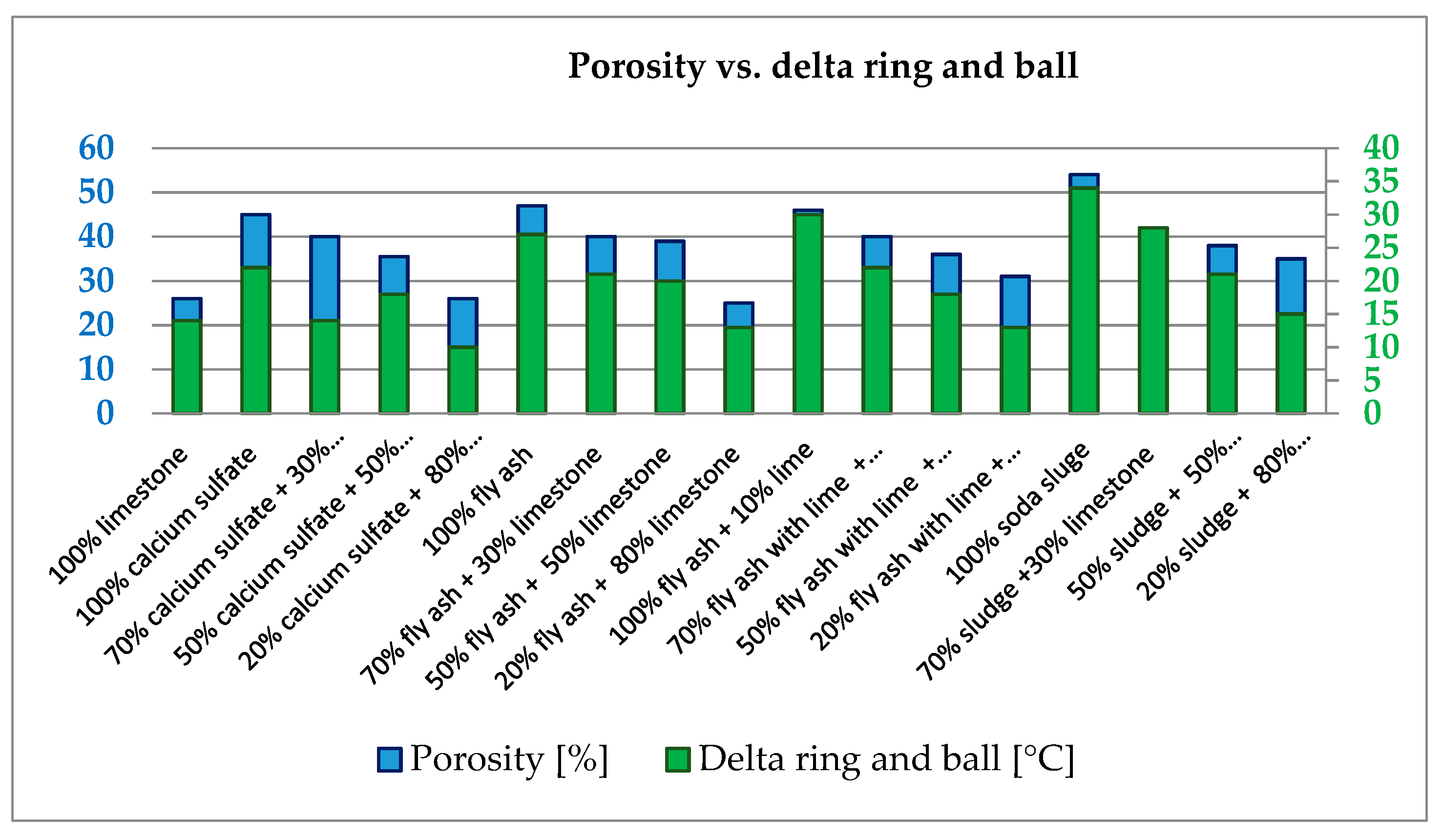
3.2. Geometrical and Physical Characteristics
Size Distribution
4. Conclusions
- The SEM and EDX chemical analyses present very important information, showing the similarities between the components and also in the distribution of all components in the soda sludge powder and the limestone filler. This important characteristic have a crucial role in time preparation and mixing, giving homogeneity to the composition of asphalt mixture.
- The EDS tests verified the presence of low quantities of silicon dioxide (SiO2) in the chemical composition of tested fillers. At the same time, the presence of calcium (Ca) is favorable, due to its good interaction with bitumen.
- According to the particle size distribution test results, we found that the calcium sulfate filler and the soda sludge filler can have an important role in the viscosity of asphaltic mixture composition, while the behavior of fly ash is similar with that of the control filler.
- The water content of limestone filler is comparable to the fly ash filler, but at the same time, the values of both the calcium sulfate filler and soda sludge fillers are above the values listed in SR EN 13043. Their water content values are influenced by the exposure to an environment with humidity during the storage process. It is highly recommended that humidity should be controlled in order to ensure that the process is correct for asphalt production in plants.
- The use of filler improves the passive adhesion between the aggregates and the bitumen, leading to asphaltic mixtures with higher water resistance and thus higher durability. The porosity and also delta ring and ball tests show higher results compared with conventional filler, and these characteristics needs to be evaluated very closely in an asphaltic mixture composition.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- European Standard SR EN 13043:2003/AC:2004—Aggregates for Bituminous Mixtures and Surface Treatments for Roads, Airfields and Other Trafficked Areas; European Committee for Standardization: Brussels, Belgium, 2013.
- Bocci, M.; Giuliani, F. Caratterizzazione di filler per conglomerati bituminosi. In Proceedings of the XXIII Convegno Nazionale Stradale AIPCR, Verona, Italy, 1 January 1998; pp. 18–21. [Google Scholar]
- Kandhal, P.S.; Parker, F. Aggregate Tests Related to Asphalt Concrete Performance in Pavements; NCHRP Report 405; National Academy Press: Washington, DC, USA, 1998. [Google Scholar]
- Grabowski, W.; Wilanowicz, J. The structure of mineral filler and their stiffening properties in filler bitumen mastics. Mater. Struct. 2008, 41, 793–804. [Google Scholar] [CrossRef]
- Harris, B.M.; Stuart, K.D. Analysis of Mineral Filler and Mastics Used in Stone Matrix Asphalt. In Proceedings of the Association of Asphalt Paving Technologist (AAPT), Portland, OR, USA, 27–29 March 1995; Volume 64, pp. 27–29. [Google Scholar]
- Huang, B.; Shu, X.; Chen, X. Effect of mineral fillers on hot-mix aboratory measured properties. Int. J. Pavement Eng. 2007, 8, 1–9. [Google Scholar] [CrossRef]
- Zulkati, A.; Diew, W.Y.; Delai, D.S. Effects of fillers on properties of asphalt-concrete mixture. J. Transp. Eng. 2012, 138, 902–910. [Google Scholar] [CrossRef]
- Pasadin, A.R.; Perez, I. The influence of the mineral filler on the adhesion between aggregates and bitumen. Int. J. Adhes. Adhes. 2015, 58, 53–58. [Google Scholar] [CrossRef] [Green Version]
- Alvarez, A.E.; Ovalles, E.; Caro, S. Assessment of the effect of mineral filler on asphalt–aggregate interfaces based on thermodynamic properties. Constr. Build. Mater. 2012, 28, 599–606. [Google Scholar] [CrossRef]
- Little, D.N.; Petersen, J.C. Unique effects of hydrated lime filler on the performance-related properties of asphalt cements: Physical and chemical interactions revisited. J. Mater. Civ. Eng. 2005, 17, 207–218. [Google Scholar] [CrossRef]
- Dondi, G.; Mazzotta, F.; Sangiorgi, C.; Pettinari, M.; Simone, A.; Vignali, V.; Tataranni, P. Influence of cement and limestone filler on the rheological properties of mastic in cold bituminous recycled mixtures. In Proceedings of the 3rd International Conference on Trasportation Infrastructures (ICTI 2014), Pisa, Italy, 22–25 April 2014; pp. 22–25. [Google Scholar]
- Davis, C.; Castorena, C. Implications of physicoechemical interactions in asphalt mastics on asphalt microstructure. Constr. Build. Mater. 2015, 94, 83–89. [Google Scholar] [CrossRef]
- Cheng, Y.; Tao, J.; Jiao, Y.; Tan, G.; Guo, Q.; Wang, S.; Ni, P. Influence of the properties of filler on high and medium temperature performances of asphalt mastic. Constr. Build. Mater. 2016, 118, 268–275. [Google Scholar] [CrossRef]
- Dong, Z.; Liu, Z.; Wanga, P.; Gong, X. Nanostructure characterization of asphalt-aggregate interface through molecular dynamics simulation and atomic force microscopy. Fuel 2017, 189, 155–163. [Google Scholar] [CrossRef]
- Choudhary, J.; Kumar, B.; Gupta, A. Effect of filler on the bitumen-aggregate adhesion in asphalt mix. Int. J. Pavement Eng. 2018, 21, 1–9. [Google Scholar] [CrossRef]
- Antunes, V.; Freire, A.C.; Quaresma, L.; Micaelo, R. Effect of the chemical composition of fillers in the filler–bitumen interaction. Constr. Build. Mater. 2016, 104, 85–91. [Google Scholar] [CrossRef]
- Modarres, A.; Bengar, P.A. Investigating the indirect tensile stiffness, toughness and fatigue life of hot mix asphalt containing copper slag powder. Int. J. Pavement Eng. 2017. [Google Scholar] [CrossRef]
- Burlacu, A.; Răcănel, C. Reducing cost of infrastructure works using new technologies. In Proceedings of the 3rd International Conference on Road and Rail Infrastructure—CETRA, Dubrovnik, Split, 28–30 April 2014; pp. 189–194. [Google Scholar]
- Choudhary, J.; Kumar, B.; Gupta, A. Utilization of solid waste materials as alternative fillers in asphalt mixes: A review. Constr. Build. Mater. 2020, 234, 117271. [Google Scholar] [CrossRef]
- Matos, P.; Micaelo, R.; Duarte, C.; Quaresma, L. Influence of bitumen and filler on the selection of appropriate mixing and compaction temperatures. Int. J. Pavement Res. Technol. 2014, 7, 237–246. [Google Scholar]
- Micaelo, R.; Ribeiro, J.; Azevedo, M.; Azevedo, N. Asphalt compaction study: Micromechanical modelling of a simplified lab compaction procedure. Road Mater. Pavement Des. 2011, 12, 461–491. [Google Scholar] [CrossRef]
- Sangiorgi, C.; Tataranni, P.; Mazzotta, F.; Simone, A.; Vignali, V.; Lantieri, C. Alternative fillers for the production of bituminous mixtures: A screening investigation on waste powders. Coatings 2017, 7, 76. [Google Scholar] [CrossRef] [Green Version]
- Simone, A.; Mazzotta, F.; Eskandarsefat, F.; Sangiorgi, C.; Vignali, V.; Lantieri, C.; Dondi, G. Experimental application of waste glass powder filler in recycled dense-graded asphalt mixtures. Road Mater. Pavement Des. 2019, 20, 592–607. [Google Scholar] [CrossRef] [Green Version]
- Muniandy, R.; Aburkaba, E.; Taha, R. Effect of mineral filler type and particle size on the engineering properties of stone mastic asphalt pavements. J. Eng. Res. 2013, 10, 13–32. [Google Scholar] [CrossRef] [Green Version]
- Chen, M.; Lin, J.; Wu, S. Potential of recycled fine aggregates powder as filler in asphalt mixture. Constr. Build. Mater. 2011, 25, 3909–3914. [Google Scholar] [CrossRef]
- Ekblad, J.; Lundström, R.; Simonsen, E. Water susceptibility of asphalt mixtures as influenced by hydraulically active fillers. Mater. Struct. 2015, 48, 1135–1147. [Google Scholar] [CrossRef]
- Androjić, I.; Dimter, S. Properties of hot mix asphalt with substituted waste glass. Mater. Struct. 2016, 49, 249–259. [Google Scholar] [CrossRef]
- Chen, M.; Lin, J.; Wu, S.; Liu, C. Utilization of recycled brick powder as alternative filler in asphalt mixture. Constr. Build. Mater. 2011, 25, 1532–1536. [Google Scholar] [CrossRef]
- Al-Hdabi, A. Laboratory investigation on the properties of asphalt concrete mixture with Rice Husk Ash as filler. Constr. Build. Mater. 2016, 126, 544–551. [Google Scholar] [CrossRef]
- Modarres, A.; Rahmanzadeh, M. Application of coal waste powder as filler in hot mix asphalt. Constr. Build. Mater. 2016, 66, 476–483. [Google Scholar] [CrossRef]
- Azzam, M.O.; Al-Ghazawi, Z. Evaluation of incorporating oil shale filler aggregate into hot mix asphalt using Superpave mix design. Constr. Build. Mater. 2015, 10, 359–379. [Google Scholar] [CrossRef]
- Sangiorgi, C.; Tataranni, P.; Simone, A.; Vignali, V.; Lantieri, C.; Dondi, G. Assessment of waste bleaching clay as alternative filler for the production of porous asphalts. Constr. Build. Mater. 2016, 109, 1–7. [Google Scholar] [CrossRef]
- Pasandin, A.R.; Perez, I.; Ramırez, A.; Cano, M.M. Moisture damage resistance of hot-mix asphalt made with paper industry wastes as filler. J. Clean. Prod. 2016, 112, 853–862. [Google Scholar] [CrossRef] [Green Version]
- Do, H.S.; Mun, P.H.; Keun, R.S. A study on engineering characteristics of asphalt concrete using filler with recycled waste lime. Waste Manag. 2008, 28, 191–199. [Google Scholar]
- Katamine, N.M. Phosphate waste in mixtures to improve their deformation. J. Transp. Eng. 2000, 126, 382–389. [Google Scholar] [CrossRef]
- Yongjie, X.; Haobo, H.; Shujing, Z.; Jin, Z. Utilization of municipal solid waste incineration ash in stone mastic asphalt mixture: Pavement performance and environmental impact. Constr. Build. Mater. 2009, 23, 989–996. [Google Scholar]
- Rutkowska, G.; Wichowski, P.; Fronczyk, J.; Franus, M.; Chalecki, M. Use of fly ashes from municipal sewage soda sludge combustion in production of ash concretes. Constr. Build. Mater. 2018, 188, 874–883. [Google Scholar] [CrossRef]
- Suchorab, Z.; Barnat-Hunek, D.; Franus, M.; Łagód, G. Mechanical and physical properties of hydrophobized lightweight Aggregate concrete with sewage soda sludge. Materials 2016, 9, 317. [Google Scholar] [CrossRef] [Green Version]
- Deng-Fong, L.; Jyh-Dong, L.; Shun-Hsing, C. The application of baghouse fines in Taiwan. Resour. Conserv. Recycl. 2006, 46, 281–301. [Google Scholar]
- Ibrahim, A.; Assa’ad, A. Effect of Jordanian oil shale fly ash on asphalt mixes. J. Mater. Civ. Eng. 2005, 17, 553–559. [Google Scholar]
- Baoshan, H.; Qiao, D.; Burdette, E.G. Laboratory evaluation of incorporating waste ceramic materials into Portland cement and asphaltic concrete. Constr. Build. Mater. 2009, 23, 3451–3456. [Google Scholar]
- Bilgin, N.; Yeprem, H.A.; Arslan, S.; Bilgin, A.; Gunay, E.; Marşoglu, M. Use of waste marble powder in brick industry. Constr. Build. Mater. 2012, 29, 449–457. [Google Scholar] [CrossRef]
- Karasahin, M.; Terzi, S. Evaluation of marble waste dust in the mixture of asphaltic concrete. J. Sci. Direct. 2007, 21, 616–620. [Google Scholar] [CrossRef]
- West, R.C.; James, R.S. Evaluation of a lime kiln dust as a mineral filler for stone matrix asphalt. In Proceedings of the 85th Annual Meeting of the Transportation Research Board, Washington, DC, USA, 22–26 January 2008. [Google Scholar]
- Tuncan, M.; Tuncan, A.; Cetin, A. The use of waste materials in asphalt concrete mixtures. J. Waste Manag. Res. 2005, 23, 550–559. [Google Scholar] [CrossRef]
- Punyaslok, R.; Joshua, E.L.; William, G.B.; Henrique, R. Performance analysis of asphalt mixtures modified with ground tire rubber modifiers and recycled materials. Sustainability 2019, 11, 1792. [Google Scholar] [CrossRef] [Green Version]
- Racanel, C.; Burlacu, A. Using crumb rubber in warm asphalt concrete. In Proceedings of the 15th Geo Conference—“International Multidisciplinary Scientific Geo Conference SGEM”, Albena, Bulgaria, 18–24 June 2015; pp. 795–802. [Google Scholar] [CrossRef]
- Diab, A.; Enieb, M. Investigating influence of mineral filler at asphalt mixture and mastic scales. Int. J. Pavement Res. Technol. 2018, 11, 213–224. [Google Scholar] [CrossRef]
- Lesueur, D.; Teixeira, A.; Lazaro, M.M.; Andaluz, D.; Ruiz, A. A simple test method in order to assess the effect of mineral fillers on bitumen ageing. Constr. Build. Mater. 2016, 117, 182–189. [Google Scholar] [CrossRef]
- European Standard SR EN 933-10:2009—Tests for Geometrical Properties of Aggregates Assessment of Fines. Grading of Filler Aggregates (Air Jet Sieving); European Committee for Standardization: Brussels, Belgium, 2009.
- European Standard SR EN 1097-5:2008—Tests for Mechanical and Physical Properties of Aggregates Determination of the Water Content by Drying in a Ventilated Oven; European Committee for Standardization: Brussels, Belgium, 2008.
- European Standard SR EN 1097-7:2001—Test for Mechanical and Physical Properties of Aggregates. Part 7: Determination of the Particle Density of Filler—Pycnometer Method; European Committee for Standardization: Brussels, Belgium, 2001.
- European Standard SR EN 933-9+A1:2013—Tests for Geometrical Properties of Aggregates Assessment of Fines. Methylene Blue Test; European Committee for Standardization: Brussels, Belgium, 2013.
- European Standard SR EN 1097-4:2001—Test for Mechanical and Physical Properties of Aggregates. Part 4: Determination of the Voids of Dry Compacted Filler; European Committee for Standardization: Brussels, Belgium, 2001.
- European Standard SR EN 1427:2007—Bitumen and Bituminous Binders—Determination of the Softening Point—Ring and Ball Method; European Committee for Standardization: Brussels, Belgium, 2007.
- European Standard SR EN 13179-1:2013—Tests for Filler Aggregate Used in Bituminous Mixtures—Part 1: Delta Ring and Ball Test; European Committee for Standardization: Brussels, Belgium, 2013.
- European Standard SR EN 196-6:2010—Methods of Testing Cement. Part 6: Determination of Fineness; European Committee for Standardization: Brussels, Belgium, 2010.
- Ashfaq, M.Y.; Al-Ghouti, M.A.; Al Disi, Z.A.; Zouari, N. Investigating the microorganisms-calcium sulfate interaction in reverse osmosis systems using SEM-EDX technique. J. Environ. Chem. Eng. 2020, 8, 103963. [Google Scholar] [CrossRef]
- Blissett, R.; Rowson, N. A review of the multi-component utilisation of coal fly ash. Fuel 2012, 97, 1–23. [Google Scholar] [CrossRef]
- Assi, A.; Bilo, F.; Zanoletti, A.; Ponti, J.; Valsesia, A.; La Spina, R.; Depero, L.E.; Bontempi, E. Review of the reuse possibilities concerning ash residues from thermal process in a medium-sized urban system in Northern Italy. Sustainability 2020, 12, 4193. [Google Scholar] [CrossRef]
- Yong-Rak, K.; Ingrid, P.; Seong-Wan, P. Experimental evaluation of anti- stripping additives in bituminous mixtures through multiple scale laboratory test results. Constr. Build. Mater. 2012, 29, 383–393. [Google Scholar] [CrossRef] [Green Version]
- Epps, J.; Berger, E.; Anagnos, J.N. Treatments. In Proceedings of the National Seminar on Moisture Sensitivity of Asphalt Pavements, San Diego, CA, USA, 4–6 February 2003. [Google Scholar]

| Sieves Size | Mass of Material that Remains on the Sieve | Percentage of Material that Remains on the Sieve | Total Percentage of the Material Passing through the Sieve | Lower and Upper Limits According to SR EN 13043: 2003 and AND 605:2016 |
|---|---|---|---|---|
| Limestone filler | ||||
| mm | g | % | % | % |
| 2 | 0 | 0 | 100 | 100 |
| 0.125 | 3 | 6 | 94 | 85–100 |
| 0.063 | 10.3 | 20.6 | 79 | 70–100 |
| Soda sludge filler | ||||
| mm | g | % | % | % |
| 2 | 0 | 0 | 100 | 100 |
| 0.125 | 8 | 16 | 84 | 85–100 |
| 0.063 | 18.1 | 36.2 | 64 | 70–100 |
| Calcium sulfate filler | ||||
| mm | g | % | % | % |
| 2 | 0 | 0 | 100 | 100 |
| 0.125 | 0 | 0 | 100 | 85–100 |
| 0.063 | 0.1 | 0.2 | 100 | 70–100 |
| Fly ash with 10% lime filler | ||||
| mm | g | % | % | % |
| 2 | 0 | 0 | 100 | 100 |
| 0.125 | 2.5 | 5 | 95 | 85–100 |
| 0.063 | 10.1 | 20 | 80 | 70–100 |
| Fly ash filler | ||||
| mm | g | % | % | % |
| 2 | 0 | 0 | 100 | 100 |
| 0.125 | 5 | 10 | 90 | 85... 100 |
| 0.063 | 13.3 | 26.6 | 73 | 70... 100 |
| Voids of Dry Compacted Filler | Category |
|---|---|
| 100% limestone | V28/38 |
| 100% fly ash | V44/45 |
| 100% fly ash with lime | |
| 100% soda sludge | |
| 20–100% calcium sulfate | V28/45 |
| 20–80% fly ash | |
| 20–80% fly ash with lime | |
| 20–80% soda sludge |
| Delta Ring and Ball (°C) | Category ΔR&B |
|---|---|
| 100% limestone | ΔR&B8/16 |
| 100% fly ash | ΔR&B25 |
| 100% fly ash with lime | |
| 70% and 100% soda sludge | |
| 20–100% calcium sulfate | ΔR&B8/25 |
| 20–80% fly ash | |
| 20–80% fly ash with lime | |
| 20–80% soda sludge |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dimulescu, C.; Burlacu, A. Industrial Waste Materials as Alternative Fillers in Asphalt Mixtures. Sustainability 2021, 13, 8068. https://doi.org/10.3390/su13148068
Dimulescu C, Burlacu A. Industrial Waste Materials as Alternative Fillers in Asphalt Mixtures. Sustainability. 2021; 13(14):8068. https://doi.org/10.3390/su13148068
Chicago/Turabian StyleDimulescu, Catalina, and Adrian Burlacu. 2021. "Industrial Waste Materials as Alternative Fillers in Asphalt Mixtures" Sustainability 13, no. 14: 8068. https://doi.org/10.3390/su13148068
APA StyleDimulescu, C., & Burlacu, A. (2021). Industrial Waste Materials as Alternative Fillers in Asphalt Mixtures. Sustainability, 13(14), 8068. https://doi.org/10.3390/su13148068






