Abstract
Research on natural zeolites (NZ) has increased over the years, showing potential in different areas, and many of them involve cation exchange (CE), considered one of the essential properties of NZ. This work aims to identify studies’ cognitive structure based on the cation exchange capacity (CEC) of NZ through bibliometric analysis to evaluate scientific production, growth trend, and visualization through bibliometric maps using the VOSviewer software. All types of documents and all languages indexed in Scopus from 1970 to 2020 were considered for the database, obtaining 703 documents. The results indicate an increasing trend in CE annual publications in NZ. This analysis shows the most influential authors such as Daković, Wang and Colella, while the countries that stand out are China, Turkey and the United States. Besides, the bibliometric maps made it possible to understand the intellectual structure of this academic discipline, identifying areas of current and potential interest in this field of studies such as its application in medicine, agriculture, catalysts, heavy metal removal, wastewater treatment (WWT), bioremediation and construction. Finally, these studies showed trends in science and technology studies favoring environmental remediation and human health.
1. Introduction
In 1756, the first natural zeolite was discovered. However, in the late 1950s, their commercial development began [1,2,3]. They were discovered in extensive and exploitable deposits, in tufaceous sedimentary rocks, formed due to the alteration of volcanic ash in marine and lake waters [3]. However, natural zeolites included in different geological environments and their classification varies according to the models and criteria of each author. Still, many agree on four: diagenetic, metamorphic, hydrothermal and magmatic [4]. NZ are hydrated aluminosilicate minerals [5,6,7,8] that have a porous structure with important physicochemical properties, such as CE, molecular sieving, catalysis [9] and high adsorption capacity (AD-C) [8]. They became beneficial industrial minerals with superficial and structural properties applied in industrial, agricultural [10], environmental [11] and biological technology [12].
NZ have microporous crystalline structure that allows the release and incorporation of water and cationic species because they consist of diameters adjusted through inlet ports of their internal structure, while the larger species are excluded, such as the ion sieving properties [13]. According to the studies in [8,14], zeolites conserve a structure in a three-dimensional framework of tetrahedra of SiO4 and AIO4, whose aluminum ion occupies the position in the center of the tetrahedron of four oxygen atoms, this substitution of AI3+ by Si4+ defines the negative charge in the network, which balanced with the exchangeable cation (Na, K and Ca, which are generally in a higher proportion than Mg, Ba, Sr, among others). These internal cations of zeolites can exchange with the cations in their environment and retain them in their internal network, causing the removal of cations such as copper, lead, cadmium, ammonium and certain radioactive cations. Different factors influence the IE behavior of NZ, such as the structure, the size and shape of the ions, the charge density of the anionic structure, the ionic charge and the concentration of the external electrolyte solution [15]. Due to the formation environment, zeolites present variability in their chemical composition and the CEC, between 0.6 and 2.3 meq/g [8]. The CEC and selectivity are specific according to the type of zeolite. A prior elemental analysis must perform to obtain the expected CEC to characterize synthetic zeolites [16].
There are around 70 types of NZ, and more than 260 synthetic zeolites registered [17]. The most common forms are clinoptilolite, mordenite, phillipsite, chabazite, stilbite, analcime and laumontite, while offretite, paulingite, barrerite and mazzite are not often occurring [8]. Among them, clinoptilolite is the most abundant natural zeolite in nature and is widely used worldwide [8,18,19].
Some countries, e.g., Cuba, China, the United States, Russia, Japan, Italy, South Africa, Hungary and Bulgaria, have significant reserves with excellent production potential. However, the total amount of this mineral in the world is not exactly known [10]. In the Ecuadorian coastal region, zeolites were described for the first time in 1994 in the Cayo Formation by a group of Cuban researchers as being composed of marine volcanoclastic rocks [20,21], and their application has had a high impact on agriculture as fertilizer carriers [22]. In the outcrop area of the Cayo Formation, clinoptilolite, heulandite, mordenite, laumontite, analcime, stilbite, epistilbite, chabazite, thomsonite and erionite-type zeolites have been identified [21].
Due to the significant development of the CE and AD-C properties of NZ, its commercialization has shown significant progress, and it was considered a product that has great potential [23]. NZ most prominent commercial applications remove heavy metal ions from wastewater [24,25,26]. The selectivity of cations for CE varies according to the type of zeolite, since in the case of studies with clinoptilolite [27,28,29], it maintains a higher selectivity for Pb2+, while in other cases, clinoptilolite shows a higher selectivity for Cu2+ [30,31] and Zn2+ [32]. Likewise, studies have evaluated other types of zeolites, such as scolecite, with higher selectivity for Cu2+ [33] and chabazite for Cu2+ [34]. These variations in selectivity for one or another NZ cation are due to the Si/Al ratio of their structure, following the variability of the concentrations of their exchange cations.
Likewise, NZ are used for the adsorption (AD) of ammonia in wastewater [35,36] and also of organic substances [37,38]. It is essential to highlight the reuse of NZ and its adsorbed components in agriculture as additional fertilizers to improve the quality and yield of crops [39]. Other possible uses of these minerals are in the construction industry [36,40,41], such as pozzolana cement [42], foamed geopolymers [43], oil spill cleaning [44], desiccants and gas-liquid separations [45], among others.
Furthermore NZ can be reused, where contaminants are first removed and then recycled as a pozzolan addition for Portland Clinker [46]. In addition, studies have been performed for the regeneration of zeolites after being used for the removal of NH4+, using solutions of 1 N NaCl or KCl, replacing the exchange site with Na+ or K+. This regeneration can increase its efficiency by raising the pH of the regeneration solution by adding lime [15].
Most zeolite applications have been implemented due to environmental concerns [47], mainly based on its CE properties, in areas such as nuclear wastewater [48], municipal and industrial wastewater [49,50], decontamination of mining effluents [51] and agricultural uses on contaminated soils [52].
Therefore, the CE of NZ could be an economical, environmentally compatible and effective way for pollutant removal [53]. Besides, in recent decades, the application of NZ in medicine has begun to be inserted [54]. In 2004, the first study of the effects of clinoptilolite supplementation to treat immunodeficiency diseases was given [55]. Similarly, good results have been obtained from the application of NZ for the treatment of gastrointestinal and cancerous affections [56].
Likewise, studies have been carried out, such as the potential use of zeolites modified with Cu and Zn for the removal of ethylene and delayed ripening of the tomato fruit, improving its postharvest quality [57], as well as the possible use of chemically modified natural zeolites in the production of biodiesel [58], and the potential use in the pharmaceutical industry [59]. In a previous study [60] regarding zeolite research areas through a citation network analysis, IE scored very high.
Therefore, it is essential to know how studies based on this field have developed over the years. However, there is no research from a bibliometric perspective on the CEC in NZ. Bibliometry gives the possibility of studying a specific research area to academics, using the analysis of citations, co-citations, geographical distribution and frequency of words to draw beneficial conclusions [61]. Bibliometric methods have been used to estimate scientific progress in various science and engineering disciplines as a standard research tool for systematic analysis [62]. Will it be possible to know, through the application of bibliometric analysis, the disciplines that had a more significant impact in this field of research? Who are the experts who stand out in this field of study? What has been the development of the intellectual structure of this field of research over time?
This work aims to identify the different research lines that make up the cognitive structure of studies based on the CEC of NZ, performing a bibliometric analysis to evaluate scientific production, growth trend and visualization through bibliometric maps using the VOSviewer software contributing to the development of this field of scientific research.
2. Materials and Methods
A literature review is very important for any research project [63]. The systematic review of the literature is a methodologically rigorous review of all the available research [64], making it possible to identify, evaluate and interpret the results with the most significant relevance of research for decision-making [65,66]. Bibliometric studies have a formal and rigorous process similar to the literature’s systematic review, which guarantees the quality of the information used [67].
Bibliometric analysis has become a necessary tool for measuring scientific progress in any study area [62]. Bibliometric studies evaluate scientific production’s quantity and quality [68,69,70]. Thus, sets of statistical and mathematical indices used are the so-called bibliometric indicators essential for the individual researcher and organizations [71,72]. These can be indicators of quantity, which evaluate productivity; quality indicators, which evaluate scientific production performance, and structural indicators, which evaluate the net between publications, authors and research areas [73,74]. Therefore, it is essential to establish a research methodology to carry out a bibliometric analysis in a research field, integrating knowledge and understanding its evolution and trends [75,76].
The methodology applied in this work developed in four phases: (1) Definition of search criteria; (2) Data collection; (3) Export and standardization of data; (4) Data analysis. Figure 1 details the graphic scheme of the phases above, which were implemented in this work.
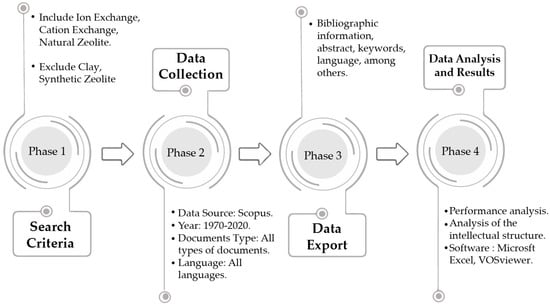
Figure 1.
Diagram of the methodology applied in this study.
2.1. Definition of Search Criteria
The terms ion exchange, cation exchange and natural zeolite were considered criteria for the search, which are part of the keywords, and in turn, the words clay and synthetic zeolite were excluded to perform a more specific search. These criteria made it possible to compile the database to evaluate this study, which was chosen based on the authors’ own research experience and bibliographic reviews which allowed excluding terms from similar topics (clay, synthetic zeolite) to define this field of study. Furthermore, through a previous study [60], cationic exchange in zeolites was proposed as a high impact research area.
2.2. Data Collection
Studies using bibliometric methods require the use of a quality database and consistent information [77]. Scopus was elected as a multidisciplinary database for the following reasons: (i) a vast collection of documents in most academic disciplines [78], including earth sciences [79]; (ii) contains documents that have undergone a rigorous content and quality selection process; (iii) the use of quality standards such as Scimago Journal Rank (SJR) [80] and (iv) the ability to view data, perform analyses and download information [81,82].
The analyzed database consisted of documents indexed by Scopus from 1970 to the present (22 September 2020). For this study, all types of documents and languages were included.
A search strategy was used in which the defined criteria was considered in the titles, abstract, and keywords. For this, the following search equation was used: (TITLE-ABS-KEY (“ion exchange”) OR TITLE-ABS-KEY (“cation exchange”) AND TITLE-ABS-KEY (“natural zeolit*”) AND NOT TITLE-ABS-KEY (clay) AND NOT TITLE-ABS-KEY (“synthetic zeolit*”)). The asterisk was implemented in “zeolit*” to allow all variants of the search term [83], which would include a “zeolite*” and “zeolitic*” in the database collected, [60], resulting in 710 documents found in total.
2.3. Data Export
Subsequently, the database obtained from Scopus exported in CSV format (comma-separated values), which included all bibliographic information, abstracts, years, keywords and language used for the bibliometric analysis [61,84].
Once this information was obtained, a data cleaning process is required since they usually contain errors or incomplete data [85,86]. A manual review of authors’ data, journal titles or affiliations, types of documents, languages and year of publication was performed using Microsoft Excel software. In this data normalization, records without the author’s name available, language and type of document were found, which restored with their corresponding information, and those without data were eliminated. Finally, 703 documents were obtained and processed.
2.4. Data Analysis and Results
In bibliometric studies, two types of analysis should be considered: the performance analysis of scientific production and the structure analysis [76,87].
The first allows evaluating the development of scientific production and its impact. The scientific production is examined based on the contribution of the most cited authors, countries, institutions, journals and cited documents [88,89]. Microsoft Excel was used for its versatility for the exploration and analysis of the information contained [90].
The second allows the analysis of the intellectual structure of the study field through recognized bibliometric networks, such as author occurrence maps, author citations and journals [91,92]. While the VOSviewer software was used to elaborate and visualize the bibliometric maps, it also allows the construction, exploration and graphic representation of two-dimensional maps of simple interpretation [93,94], a combination of three analyses used to understand the intellectual structure of this field of study. The analyses are: co-occurrence of author keywords, co-citation of cited authors and journals, which allow obtaining information at micro, meso and macro levels, respectively, of this structure [91,95]. These analyses require data pre-processing to eliminate errors and inconsistencies [85,86]. The VOSviewer has made a notable contribution to the development of bibliometric analyses in a wide variety of study areas: earth sciences [96,97], education [98,99], medicine [100,101] and food chemistry [102], among others.
3. Results
3.1. Performance Analysis
3.1.1. Scientific Production Analysis
This analysis has been divided into four time periods: period I (1970–1990), period II (1991–2000), period III (2001–2010) and period IV (2011–2020) (Figure 2). It considered dividing the times into decades, as it granted the best representation to know the evolution of the field of study [103]. Likewise, two decades (1970–1990) have joined the beginning due to the small number of documents analyzed in these years. Additionally, Price’s Law, applied as an indicator in the analysis of this study’s productivity, reflects an elementary aspect of scientific production and exponential growth [104,105] (Table 1). A total of 703 documents were obtained, corresponding to 84.35% articles, 11.66% conference papers, 2.28% book chapters, 1.14% article reviews, 0.14% errata, 0.14% notes, 0.14% books and 0.14% editorials.
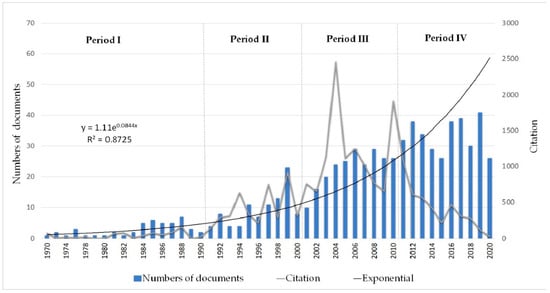
Figure 2.
Scientific productivity from 1970 to 2020 of the CE in NZ: Number of documents and citations.

Table 1.
Growth trajectory of the scientific production of studies based on CE of NZ.
- Period I (1970–1990): Opening of the CE of NZ
This period shows the beginning of the scientific production of this field of study with gradual growth, in which the first two decades grouped since between them, there was no notable growth in scientific production with a total of 48 documents, which represents 6.83% of the total, which included 40 articles, 7 conference papers and 1 book, where the highest production recorded was between 1984 and 1988. In this period, the citations obtained were 751 (3.61%), with articles standing out [106], in which they determined the importance of the conditioning procedure in zeolites, which influence the performance for the capacity and selectivity for metal ions. Likewise, at the beginning of this research area, other documents were recorded that cover topics such as the elimination of ammonia from wastewater using clinoptilolite through its selective IE process [107], and in the same way, the elimination of ammonium ions and phosphate and nutrient recovery in wastewater using clinoptilolite and Kastel A510, an anion resin that has AD properties [108], among others.
- 2.
- Period II (1991–2000): Development of CE of NZ
In this period, a notable increase was observed in a single decade with 93 documents (13.23%), a more significant number of article-type papers, including 85 articles, 7 conference papers and 1 review. In 1999 was the highest production of this period with 23 documents containing topics such as evaluating zeolites’ potential to remove heavy metals [109]. Likewise, the number of citations increased considerably to 4203 (20.23%), which indicates the beginning of interest in this field of study, highlighting the research in which AD experiments were carried out, where improved elimination of inorganic oxyanions from aqueous solution were obtained [6].
- 3.
- Period III (2001–2010): Progress of CE in NZ
In this following decade, the CE’s scientific production in NZ continues, with an exponential growth reaching 229 documents (32.57%), highlighting the number of article-type documents, including 196 articles, 25 conference papers, 4 book chapters, 3 reviews and 1 erratum. The most significant increase in citations was recorded in this period, obtaining 11,731 citations, representing 56.47% of the total, reaching the peak with 2457 citations in 2004. In 2010, the review document was published [8], which obtained the most significant impact in this decade with 1147 citations; in this document, the development of NZ as adsorbents used in water and WWT were reviewed. Other documents registered in this period were the article that deals with the use of agricultural and agrochemicals of clinoptilolite [52], and the importance of NZ applications for environmental use, which shows the potential of the zeolite as adsorbent material [110].
- 4.
- Period IV (2011–2020): The advance of CE in NZ
Finally, there is the highest scientific production in Period IV with 333 documents, which is equivalent to 47.37% of the total, with 272 articles, 12 book chapter, 43 conference papers, 4 reviews, 1 note and 1 editorial. While in the types of languages, the English language had the most significant influence with 313 documents. However, documents were also recorded in Chinese, Portuguese, Persian, Polish, Portuguese, Russian, Spanish and Turkish. Additionally, in the last decade, 4095 citations (19.71%) were registered, highlighting the article [11], which shows the importance of the different applications of NZ based on their CE properties, making a brief review of the literature on the application of NZ in environmental remediation.
3.1.2. Country Contribution
The activity, productivity and impact of scientific research can be promoted through research contribution. Therefore, it is necessary to have regular quantitative monitoring of supplies and results through bibliometric studies [111]. Seventy-one countries have developed a contribution to this field of study. Table 2 shows the scientific production of the 15 leading countries in this study area, where China leads the table with 70 documents, followed by Turkey and then the United States. In addition, these countries are among the largest producers of zeolites, with China standing out with 1,700,000 tons, the United States with 380,000–430,000 tons and Turkey with 150,000 tons [4]. However, according to the number of documents/number of citations [75], Australia, with 29 documents is the most cited country with 80.5 citations/documents, while the Russian Federation, with 24 documents is the least cited country with just 3 citations and 8 documents.

Table 2.
Top 15 countries with the highest scientific citations.
Moreover, this contribution of countries was visualized through a co-authorship network map (Figure 3) using VOSviewer software, where the nodes represent the countries that develop this field of study, and their size, according to the number of documents they need and the thickness of the lines they interconnect, represents the collaboration’s strength [92]. For elaborating the map, the countries with at least 5 contributions were used for better visualization, giving 38 countries. The nodes with the largest size, such as China, Turkey, United States and Italy, have a more significant contribution of documents: 70, 58, 54, 48, respectively. With the highest contribution, China is in cluster 2 (green color) and has a close relationship with South Korea, India and Jordan. Continuing with Turkey, which is in second place in scientific production, it is closer to Poland, Brazil and Slovakia; that is, it has a good relationship. While the United States, which is in cluster 1 (red), has a close relationship with Mexico, the United Kingdom and Slovakia. At the same time, Hungary has less research collaboration with other countries.
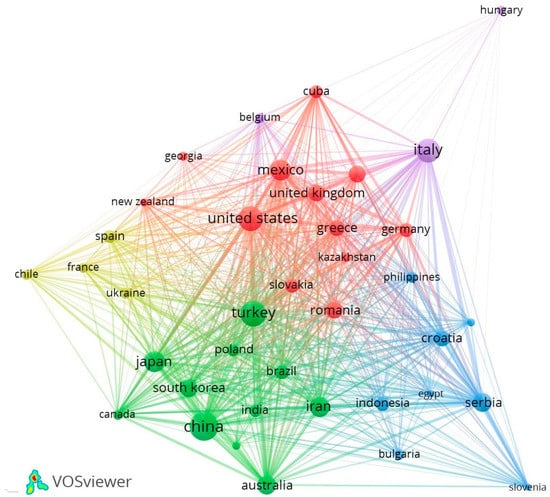
Figure 3.
The network of collaboration between countries in the field of study of CE of NZ.
3.1.3. Performance of Sources
For this analysis, the performance of sources of all types of documents were considered in this study. Table 3 shows the 15 most prominent sources based on the number of published documents and their percentage, with 184 documents representing 26.29% of the total. The number of citations and performance indicators were shown for each source, such as H-index, SJR 2019.

Table 3.
Top 15 of the most prominent sources by the number of documents in this field of study.
Leading the Top 15 was the Journal of Hazardous Materials with 30 documents representing 4.29%. It has an H 260 index, SJR 2.010, and the document that stands out the most in this journal corresponds to [112] with 287 citations related to the use of the NZ for the removal of ammonia from an aqueous solution. Continuing was the journal Microporous and Mesoporous Materials with 24 documents (3.43%); 966 citations present an H-index of 151 and SJR 0.999. Then, the sources that take possession are Studies in Surface Science and Catalysis, Science and Technology of Separation and Desalination and Water Treatment.
3.1.4. Author Contribution
In general, in 2054 documents, authors dedicated to studies related to the NZ CE were obtained. Table 3 shows the first 15 authors with the highest contribution of documents in the study area. Leading the investigation was Daković with 12 documents, followed by Colella and Gennaro with 10 and 9 documents, respectively. However, Stevens showed a higher number of citations than 262 with 8 documents in this Top 15 concerning the number of documents.
Figure 4 shows an author contribution map using the bibliographic coupling analysis. There is a bibliographic coupling between two publications when a third publication is cited by both publications [113]. This bibliographic coupling relationship between the two publications will be more significant than the number of references they have in common [93]. For this visualization, a minimum number of contributed documents were established, 5 for each author, resulting in 42 authors grouped in 6 clusters. The nodes represent the authors who contributed to this field of study; the size depends on the number of documents; the thickness of the line with which they interconnect represents the strength of collaboration. Daković, Colella, Gennaro, Rajić, Dong and Inglezakis, among others, are found in the most significant nodes, which coincide with the Top 15 of the authors in Table 4.
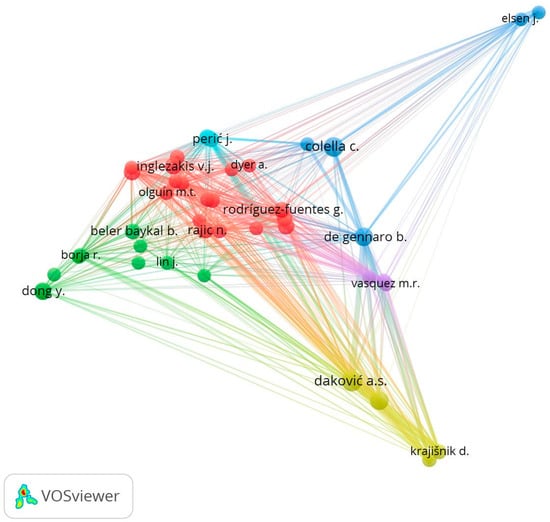
Figure 4.
Collaboration network between authors in the field of study of CE of NZ.

Table 4.
Top 15 of the most productive authors according to the number of documents.
Table S1 shows the Top 15 of the authors’ contributions from a different perspective, such as the number of citations, where new authors appear with fewer documents referring to the field of study but have a more significant influence on citations. Leading the investigation was Wang, with only 2 documents but 1431 citations. Then there is Peng and Donat, with 1147 and 1065 citations, respectively.
3.1.5. Frequently Cited Documents
Generally, the publications with the highest number of citations are more widely recognized and contribute to academic knowledge [114]. Hence, the number of citations is considered a measure of the impact, importance and influence of publications [115]. In this section, the 15 most cited documents (Table 5) of the 703 total documents obtained from the Scopus database about the field of study of the CE of NZ were analyzed. Heading the Top 15 is the review document [8] with 1147 citations, published in 2010, where it mentioned that NZ show great potential in the AD of ammonium and heavy metals in aqueous solution, for which they also have great potential in the treatment of water and wastewater.

Table 5.
Top 15 of the most cited documents.
Continuing the Top 15 is the article-type document based on a study about the removal of heavy metals using NZ [5], published in 2004, the year with the highest number of citations with 1065 citations. In this analysis, article-type documents have a more significant influence in this field of study.
3.2. Analysis of the Intellectual Structure
3.2.1. Author Keyword Co-Occurrence Network
Keyword analysis allows the deduction of critical new bibliometric approaches that drive the subject area’s development [120,121]. For this analysis of the author word co-occurrence network, information noise was first filtered, replacing all plural nouns into singular, synonymous words were grouped into a single term, acronyms were considered, and not relevant words were filtered [85]. A total of 1387 author keywords were obtained. Table 6 presents the 15 main words with the highest occurrence in this field of study, with the word natural zeolite leading with 174 occurrences, followed by ion exchange (IE) with 162 occurrences—these keywords being the center of this field of research. Continuing were zeolite and clinoptilolite with 142 and 141, respectively, corroborating that clinoptilolite is one of the types of zeolites most used in studies related to CE.

Table 6.
The 15 main words with the highest occurrence in CE studies in NZ.
For elaborating the bibliometric map, we considered the minimum number of co-occurrences to be 3. They are obtained through this procedure, where 103 keywords were represented in colored nodes (circles) and grouped into 7 clusters (Figure 5). The nodes represent the topics that appear in the intellectual structure, and their grouping (clusters) represents the main topics of this field of study. The analysis of each of the groups of this co-citation network were then carried out.
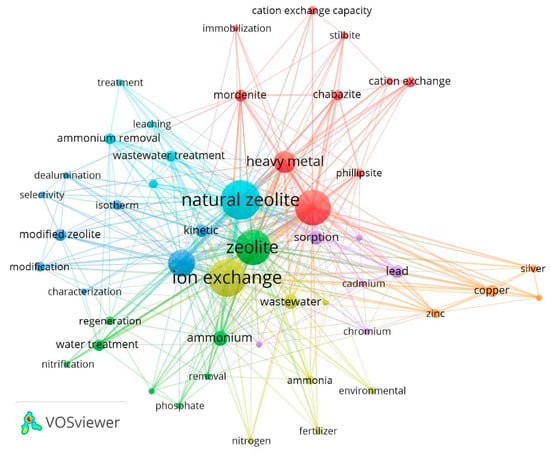
Figure 5.
Author keywordsco-occurrence map.
The first cluster (red color), “CEC in NZ”, was made up of 10 nodes, focused on the study of CE in NZ, highlighting clinoptilolite use. This type of zeolite has an ideal structure for AD and IE processes [52] either in different areas such as the removal of heavy metals [5,7,122], nutrient recovery [123,124] and kinetics in gas separation N2CH4 [125], among others. There is also CE application in other types of zeolites such as mordenite [126,127], chabazite [27,34] and phillipsite, where NH4+ exchanged preferably over Na+ [128]. The prominent terms in this cluster were clinoptilolite and heavy metal.
The second cluster (green color), “pollutant remotion with zeolite”, formed by 8 nodes, emphasizes the use of NZ in separation and purification processes due to its high CEC, either for the removal of ammonium through the use of zeolites for the improvement of environmental quality [129,130,131] and WWT [8,45,132]. The third group (blue color), “AD with modified zeolite (MZ)” was made up of 8 nodes; in this group, the term “adsorption” stands out with 83 occurrences. It is one of the most applied characteristics of NZ [124,130,133]. However, the zeolites’ AD-C varies, showing that their modification improves the CEC in various studies. Consequently, they improve their AD [134], among other properties, using the zeolites for eliminating metals such as lead, cadmium and copper, among others [14,135].
The fourth cluster (yellow color), “AD-C through IE”, where the theme that stands out in this group is the main characteristic of these studies, IE in NZ. This characteristic for zeolites have given it an AD_C that is widely used, gaining more significant interest in investigations with environmental fines [110]. Several NZ have demonstrated a variable ion exchange capacity (IEC) for cations such as ammonium and heavy metal ions, in addition to an AD-C of anions and organic substances in an aqueous solution [8]. The use of clinoptilolite has been evaluated for the removal of mercury from the industrial effluents of the copper smelter and refinery [24], improving the physical properties of the soil, the treatment of contaminated soils [52] and nutrient recovery [124].
The fifth cluster (purple color), “AD of metals”, has 6 nodes. It is one of the conglomerates with the minor occurrence, together with the seventh cluster (orange color) “MZ process”, constituted only with 4 nodes (copper, zinc, silver, nanoparticles). These clusters group investigations related to the DA of metals, such as zinc (Zn), copper (Cu) and lead (Pb) in aqueous solutions [117], as well as the removal of cadmium (Cd), chromium (Cr), nickel (Ni) and cobalt (Co) in contaminated effluents [119].
Continuing, the sixth cluster (light blue) “WWT with NZ”, was composed of 6 nodes. In this group, the NZ terms stand out, which is part of this study’s main topic, and WWT is one of the most used applications of NZ [5,135], either for the removal of metals [78] and non-ionic organic pollutants (benzene, toluene and phenol) [136].
Finally, the seventh cluster (orange color), “MZ process”, constituted only 4 nodes (copper, zinc, silver, nanoparticles), being the terms with the minor occurrences. Research related to removing metals demonstrates its removal effectiveness and improves IE by increasing temperature [14].
3.2.2. Cited Authors’ Co-Citation Network
The author co-citation network analysis is a bibliometric technique based on finding the co-occurrences in scientific documents’ references to use the co-cited authors to replace the concepts they represent and to be able to define the intellectual structure of some discipline [121,137], assuming that when more than two authors are cited together, the relationship between them is closer [138].
In this analysis, the VOS viewer software was used, obtaining 26,550 co-cited authors. Table 7 presents the 15 most co-cited authors, leading the table was Colella with 284 co-citations and 415 links, and Loizidou and Inglezakis with 208 and 185 co-citations, respectively. For the bibliometric map visualization, a minimum number of 20 citations per author was established, obtaining 295 authors. Figure 6 presents the 295 nodes that the authors represent, connected by co-citation links grouped into 7 clusters.

Table 7.
Top 15 of the authors co-cited from 1970 to 2020 in this field of study.
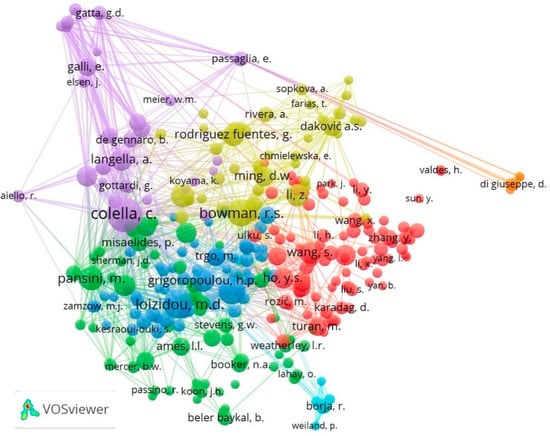
Figure 6.
Authors’ co-citation map during the period 1970 to 2020.
The first cluster (red color), “Environmental applications”, comprises 88 authors (nodes). Standing out are Wang, S. (116), Wang, Y. (107), Ho (102), Turan (80) and Mckay (78), with the corresponding co-citations. Several authors of this group propose the use of NZ for environmental applications due to their properties and significant presence, using NZ as adsorbents in separation and purification processes [8,139], due to their variable IEC for the ion of heavy metals [140,141], cations such as ammonium, anions and organic substances in an aqueous solution [45,142].
The second group (green color), “CE Applications and Characterization of NZ”, was composed of 64 authors, led by Pansini (136), Dyer (113), Breck (104), Barrer (97) and Ames (82). In this group, Pansini describes the state-of-the-art on the use of NZ for environmental conservation and review of the principles and procedures of CE used in industry, removal of ammonia in wastewater, removal of heavy metals in experiments in the laboratory, and finally, exposes NZ’s potential for decontamination of waters containing radionuclides [50]. Dyer details the classification, structural architecture, synthesis, and zeolites’ industrial and commercial importance. Meanwhile, Barrer has contributed to the research of zeolites and their synthesis and modification, sorption [143], IE [144], computational chemistry and catalysis [145], also including new types of zeolites, giving the name of Barrerite zeolite in his honor [146].
The third cluster (blue color), “Applications of clinoptilolite in CE”, has 57 authors, highlighting the authors Loizidou (208), Inglezakis (185), Grigoropoulou (118), Trgo (82) and Peric (75). This group is closely related to the second cluster. This group’s authors describe the clinoptilolite’s selectivity for heavy metals, such as Pb2+, Fe3+, Cr3+, Zn2+ and Cu2+ [117,147,148,149], and the impact of clinoptilolite pretreatment on its adequate capacity [150]. Similarly, Loizidou is part of studies comparing ammonia removal between synthetics and NZ [151].
The fourth cluster (yellow color), “Applications and regeneration of NZ”, was made up of 42 authors, such as Bowman (180), Mumpton (128), Li (124), Rodríguez Fuentes (117) and Ming (95). In one of Bowman’s outstanding works, he emphasizes the MZ’s application with surfactants for environmental remediation by removing contaminants from water [152]. However, the sorption capacity of MZ with surfactants is limited. Therefore, he proposes several methods for regenerating these zeolites with chromate and perchloroethylene [153]. Mumpton describes the morphology of six types of zeolites in sedimentary rocks using scanning electron microscopy [154]. Besides the application of NZ in zootechnics and aquaculture [155], pollution control, agriculture and biotechnology, advocating greater participation in surface, colloidal and biochemical investigations are for future applications of zeolites [3].
Continuing with the fifth cluster (purple color), “Properties and reactions of CE in NZ”, which groups 30 authors led by Colella (284), Langella (109), De Gennaro (103), Galli (83) and Armbruster (77). In this cluster, the author’s node, Colella, is more extensive since it has the highest number of co-citations and shows a close relationship with authors from the second and fourth cluster. This author carries out several investigations regarding zeolites, such as an analysis of their physical and chemical properties [156], a review of the use and potential of NZ as cation exchangers for environmental applications [26,157], and investigates the pozzolanic activity of zeolites [158]. Likewise, other authors analyze the AD of humic acid in zeolitic tuffs, analyzing their potential and selectivity [127]. In general, authors from this group evaluate the properties [159], reactions and equilibrium of the CE in NZ [160,161].
The sixth cluster (turquoise color), “NZ agricultural applications”, is one of the groups with the fewest nodes with seven authors. It consists of Borja (52), Sanchez (45), Milan (29), Montalvo (29), Weiland (22), Wajima (21) and Guerrero (20), with the number of co-citations, respectively. The authors of this research group discussed the application of zeolites as a tertiary treatment for the removal of nutrients by IE from swine waste [162], and the application of a bioreactor with biomass immobilized in zeolite for the study of the kinetics of the anaerobic digestion of cow manure [163,164]. They evaluated the performance of fluidized bed anaerobic reactors with NZ [165,166]. A review of the use of zeolites in biological processes has been carried out, such as aerobic processes, anaerobic digestion, composting and its use on an industrial scale to eliminate nitrogen [167].
Finally, the seventh cluster (orange color), “NZ application in soils”, was made up of only six authors, Di Giuseppe (33), Faccini (33), Coltorti (32), Ferretti (21), Colombani (20) and Mastrocicco (20). Members of this group corroborate the excellent use of zeolites to improve soil quality [168,169,170,171], and the variation of hydraulic properties in clay-silty soils with NZ enriched with ammonium, increasing their capacity for water retention [172].
3.2.3. Journal Co-Citation Network
In this analysis, each journal was distinguished by its specialties, either general or specific topics, with preferred methodological guidelines, among other conditions, that must be considered for the publication of articles [173,174]. In the journal co-citation network analysis, if two journals are co-cited, at least one article from each journal must be present in the references of a citing article [175].
Table 8 shows the 15 journals that lead to the highest number of co-citations. For elaborating the bibliometric map, a minimum number of 20 citations per journal was considered, obtaining a total of 67, which grouped into 5 clusters. Figure 7 shows the 68 nodes representing the journal names, grouped by color for each cluster, connected by co-citation links.

Table 8.
The Top 15 journals with the highest number of co-citations.
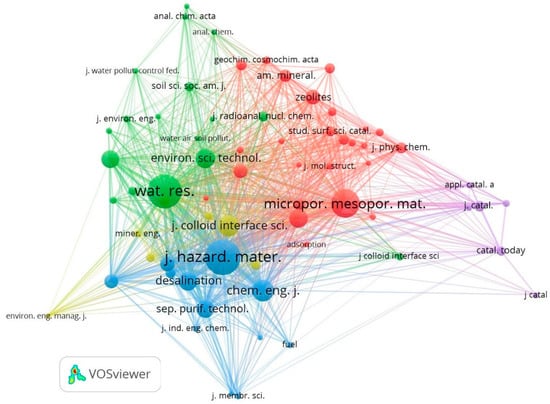
Figure 7.
Journals’ co-citation network map.
Cluster 1 (red color) “Synthesis and characterization of NZ” composed of 25 nodes, highlighting the journal Microporous and Mesoporous Materials (534 co-citations; H-index 151), mainly covering the topics of novel and distinctive aspects of porous solids, such as synthesis and physical-chemical characterization, among others. They are continuing with other representative journals such as Applied Clay Science (225 co-citations; H-index 119) and Zeolites (141 co-citations; H-index 43).
Cluster 2 (green color) “Applications in water quality”, groups 17 nodes; among the newspapers that stand out the most in this group is Water Research (737 co-citations; H-index 285), which includes topics of science and technology aspects about water quality and management. It was followed by Environmental Science & Technology (243 co-citations; H-index 373) and Water Science and Technology (206 co-citations; H-index 131).
Cluster 3 (blue color) “Applications in health and environment” with 12 nodes, contained the journal with the highest number of co-citations, Journal of Hazardous Materials (812 co-citations; H-index 260), which publishes issues related to the understanding, impact assessment, and mitigation of the dangers and risks that certain materials can generate for health and the environment. Likewise, other journals were Chemical Engineering Journal (312 co-citations; H-index 198) and Desalination (218 co-citations; H-index 169) stand out.
Then, cluster 4 (yellow color) “Technological applications”, was one of the minor groups made up of seven nodes, where the most prominent journals are Journal of Colloid and Interface Science (218 co-citations; H-index 169), Total Environmental Science (72 co-citations; H-index 224) and Applied Surface Science (56 co-citations; H 174 index).
Finally, cluster 5 (purple color) “NZ catalysis”, with only six nodes, is led by Journal of Catalysis (85 co-citations; H-index 231), Catalysis Today (58 co-citations; H-index 201) and Applied Catalysis B: Environmental (31 co-citations; H-index 229).
4. Discussion
This work shows an increase in scientific research on CE in NZ by the increased demand for adsorbent materials and low-cost IE, used for energy development, pollution control and metal removal, among other applications [27].
In the analysis of scientific production, a coefficient of de was obtained according to Price’s Law, and the field of study of the CE in NZ is exponential (Figure 2). In period I of the opening of the CE in NZ, two decades were analyzed (1970–1990), since there is a lower amount of production (6.83%), observing the beginning of investigations about the properties of IE in NZ and its different applications, as well as WWT, agricultural use, paper product and cement, among others [176]. However, the NZ did not achieve this success despite the different applications’ proposals because their development was affected by commercial efforts that tried to sell NZ without sufficient studies for their intended use. Due to this, Mumpton, in 1988, proposed the determination of properties of IE, AD, hydration, catalysis and reaction mechanisms for the implementation of marketing strategies for the company [177].
The increase in scientific research is reflected over the years, obtaining in period II (1991–2000) 13.23% in a single decade. The beginning of interest in this field of study shows the significant increase in the number of citations (20.23%), highlighting the importance of the ability to control the properties of NZ at the molecular level through the discovery of new materials and advances in technology that improve these processes [178]. Likewise, in period III (2001–2010), 32.57% of the production was recorded, and the highest number of citations was obtained (54.47%). Period IV (2011–2020), corresponding to the most recent time period, the highest amount of scientific production was obtained with 333 documents (47.37%), highlighting the application of NZ in environmental remediation [11]. Its application in medicine is beginning to have a greater interest [179], such as its benefit in nutrition due to improved supply of minerals [56].
In these 50 years of research in CE in NZ, it was observed that the most significant contribution to scientific production corresponds to articles (84.45%), and in terms of language, English (92.46%) dominates this study field. Furthermore, the potential of the different types of zeolites and their application, as well as clinoptilolite [180], mordenite, chabazite [181] and phillipsite [182] have been evaluated. Clinoptilolite stands out for its significant presence and ideal structure for the AD and IE processes [52].
A total of 71 countries obtained have contributed to this field of study (Figure 2), highlighting China with 70 documents and 1695 citations, where the use of zeolitic tuffs as cement additives is popular [183,184], with a production of 1,700,000 tons [4]. Followed by Turkey, whose presence is widespread, with an estimated 50 billion tons of NZ reserve, clinoptilolite being an essential mineral in this country [185], with a production of 150,000 tons [4]. China has had a greater collaboration with South Korea, Japan, Jordan and Turkey, corresponding to group 2, which are important producing countries of NZ [17]. Likewise, the commercial use of NZ has been developing in the United States, Italy, Mexico, Bulgaria and Germany [27]. The United States demonstrated a close relationship with Mexico and the United Kingdom (cluster 1), while Italy is a little further away in cluster 5. There is also Hungary, which is the country with a minor collaboration in research with others.
The analysis of the authors will verify the contribution of 2054 researchers, highlighting Daković with 12 documents, 374 citations and H-index 23; among their works, the study of the MZ predominates, evaluating its potentiality and application [186,187,188,189], demonstrating NZ’s higher efficacy in AD. However, it is curious to observe that among the authors that lead the Top 15 according to the number of citations, that they have a minimum value of documents, such as Wang, S., with 2 documents, 1431 citations and H- index 113, and Peng with 1 document, 1147 citations and H-index 18, standing out for his work related to the use of NZ as AD for the treatment of drinking water and wastewater [100]. The number of citations depends on several factors, such as the article’s quality, the impact factor of the journal, the author’s reputation, and the broad scope of the field of study [190]. Therefore, it can verify that the work of Wang, S., stands out for its relevant reputation in citations, despite having a minimal number of documents than Daković, who has had a more significant contribution to scientific research in this field of study.
Concerning the analysis of this field of study’s intellectual structure, we have found some relevant data exposed below.
The co-citation network analysis of cited authors (Figure 6) shows that these researchers grouped in clusters are related [152]. According to the number of co-citations, the most significant node represents Colella (284). Moreover, it has a more significant relationship with the other authors, not only with the members of cluster 5 (purple) “Properties and reactions of the CE in NZ”, but also with authors from other clusters such as Pansini who leads cluster 2 (green color) “Applications of CE and characterization of NZ”, contributing with an investigation of the use of chabazite for the elimination of lead from water [191]. That is, the close relationship not only implies belonging to the same cluster but also the size and proximity to which they are. Cluster 1 (red color) “Environmental applications” is grouped by Wang, S., Wang, Y., Ho and Turan, among others; their research shows a relationship for the use of NZ for removal of contaminants [139,140,141]. While cluster 2 (green color) presents a close relationship with cluster 3 (blue color), “Applications of clinoptilolite in CE”, in which topics related to the characterization and applications of NZ in CE stand out [50]. Cluster 7, “MZ process” (orange color), only shows a relationship with cluster 5 (purple color) “AD of metals”; these groups are the ones with a lower occurrence, showing studies related to the AD of zinc (Zn ), copper (Cu) and lead (Pb) by IE, giving a higher removal efficiency for Pb and Cu ions than for Zn ions [117].
In the analysis of the network of journal co-citations, the journals that have had the most significant influence in this field of study are evidenced (Table 8), highlighting the Journal of Hazardous Materials (812 co-citations; H-index 260), Water Research (737 co-citations; H-index 285) and Microporous and Mesoporous Materials (534 co-citations; H-index 151). Anthropogenic activities generate many pollutants to the environment related to environmental impact. NZ are presented as an alternative for the decontamination of the environment, using their properties, such as their high CEC, related to risk mitigation of hazardous materials. Additionally, it can show that the clusters are wholly differentiated because the analysis considers the number of co-citations obtained by each journal on the topic of CE in NZ (Figure 7). Moreover, in the analysis of sources’ performance, these journals’ influence in this scientific field can be corroborated due to their academic contribution, standing out in the Top 15 (Table 3).
One of the limitations of this study was using a single database (Scopus) since this could omit specific significant contributions in this field of study published in other databases. Furthermore, only NZ was used, excluding the various studies on CE in synthetic zeolites.
5. Conclusions
NZ have a wide field of applications due to their exceptional properties, mainly their high CEC. Through the analysis of the intellectual structure of this scientific field, it was possible to distinguish different lines of research related to CE in NZ, such as heavy metals removal [192,193], nutrient recovery [124,194], WWT [195,196], soil treatment [52,197], construction materials [43,198], nutrition and health [56] and feed additives [52], among others. Furthermore, NZ can be modified [14,134] and regenerated [199,200], increasing their efficiency in their CEC.
About the analysis of scientific production, in period II (1991–2000), a notable rebound in this scientific field’s development begins (Figure 3); this is mainly due to the continuous discovery of new materials that have allowed improvements in processes and the development of new technologies.
This field of study shows a growing trend in the scientific production of the CE in NZ, obtaining a total of 703 documents, which correspond primarily to articles (84.35%), conferences (11.66%), and to a lesser extent, other types of documents (3.98%). Most of these documents have been published in English. A total of 71 countries registered have contributed to this field of study, highlighting China with 70 documents, Turkey with 58 and the United States with 54, which are also part of the countries with the highest NZ production. Regarding the contribution of authors, a total of 2054 documents were obtained. According to the number of documents, Daković leads with 12 documents and 374 citations, while Wang, S., with 2 documents and 1431 citations stands out for the number of citations.
In the analysis of the intellectual structure, the researcher, Colella, obtained the highest number of co-citations (284) and has a more significant relationship with the other authors, contributing with research on the evaluation of the properties, reactions and equilibrium of the CE in NZ, and its various applications. Among the sources that had a more significant influence on the field of study of the CE in NZ are Journal of Hazardous Materials (812 co-citations; 30 documents), Water Research (737 co-citations; 11 documents) and Microporous and Mesoporous Materials (534 co-citations; 24 documents).
Regarding the analysis of future trends, the studies carried out by the CE on NZ have shown a trend in science and technology studies to benefit environmental sustainability and human health, considering NZ as economical, ecological, reusable and recyclable material. Such are the challenges of zeolites as catalysts, filter materials, medicines, pollutant removal, energy production, construction industry, agricultural and livestock uses.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/su13147751/s1, Table S1: Top 15 of the most cited documents.
Author Contributions
Conceptualization: F.M.-C., N.M.-B., P.C.-M., and N.E.-S.; methodology: N.M.-B., F.M.-C., and N.E.-S.; investigation: N.M.-B., N.E.-S., and F.M.-C.; writing—original draft preparation: N.M.-B. and N.E.-S.; writing—reviewing and editing: N.M.-B., F.M.-C., P.C.-M., and N.E.-S.; supervision: F.M.-C. and P.C.-M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
This work has been made possible by the valuable collaboration of the ESPOL research project: “Register of geological and mining heritage and its incidence in the defense and preservation of geodiversity in Ecuador” under grant number CIPAT-01-2018, and the support of NOVA Science Research Associates.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Flanigen, E.M. Chapter 2 Zeolites and Molecular Sieves an Historical Perspective. Stud. Surf. Sci. Catal. 1991, 58, 13–34. [Google Scholar] [CrossRef]
- Lew, C.M.; Cai, R.; Yan, Y. Zeolite thin films: From computer chips to space stations. Acc. Chem. Res. 2010, 43, 210–219. [Google Scholar] [CrossRef]
- Mumpton, F.A. La roca magica: Uses of natural zeolites in agriculture and industry. Proc. Natl. Acad. Sci. USA 1999, 96, 3463–3470. [Google Scholar] [CrossRef] [PubMed]
- Costafreda, J.L.; Martín Sánchez, D.A.; Costafreda Velázquez, J.L.; Prado Govea, R.; Iván Tobón, J.; Álvarez Gutiérrez, Y.; Bello Vasquez, L.A.; Vattuone, M.E.; Gargiulo, M.F.; Crosta, S.; et al. Las Zeolitas Naturales de Iberoamérica; Fundación Gómez Pardo: Madrid, Spain, 2018; p. 201. ISBN 978-84-09-00125-5. [Google Scholar]
- Erdem, E.; Karapinar, N.; Donat, R. The removal of heavy metal cations by natural zeolites. J. Colloid Interface Sci. 2004, 280, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Haggerty, G.M.; Bowman, R.S. Sorption of Chromate and Other Inorganic Anions by Organo-Zeolite. Environ. Sci. Technol. 1994, 28, 452–458. [Google Scholar] [CrossRef]
- Mier, M.V.; Callejas, R.L.; Gehr, R.; Cisneros, B.E.J.; Alvarez, P.J.J. Heavy metal removal with mexican clinoptilolite: Multi-component ionic exchange. Water Res. 2001, 35, 373–378. [Google Scholar] [CrossRef]
- Wang, S.; Peng, Y. Natural zeolites as effective adsorbents in water and wastewater treatment. Chem. Eng. J. 2010, 156, 11–24. [Google Scholar] [CrossRef]
- Zhang, Q.; Yu, J.; Corma, A. Applications of Zeolites to C1 Chemistry: Recent Advances, Challenges, and Opportunities. Adv. Mater. 2020, 32, 2002927. [Google Scholar] [CrossRef]
- Polat, E.; Karaca, M.; Demir, H.; Onus, A.N. Use of Natural Zeolite (Clinoptilolite) in Agriculture. J. Fruit Ornam. Plant. Res. 2004, 12, 183–189. [Google Scholar]
- Misaelides, P. Application of natural zeolites in environmental remediation: A short review. Microporous Mesoporous Mater. 2011, 144, 15–18. [Google Scholar] [CrossRef]
- Bacakova, L.; Vandrovcova, M.; Kopova, I.; Jirka, I. Applications of zeolites in biotechnology and medicine-a review. Biomater. Sci. 2018, 6, 974–989. [Google Scholar] [CrossRef] [PubMed]
- Calvo, B.; Canoira, L.; Morante, F.; Martínez-Bedia, J.M.; Vinagre, C.; García-González, J.E.; Elsen, J.; Alcantara, R. Continuous elimination of Pb2+, Cu2+, Zn2+, H+ and NH4+ from acidic waters by ionic exchange on natural zeolites. J. Hazard. Mater. 2009, 166, 619–627. [Google Scholar] [CrossRef]
- Ćurković, L.; Cerjan-Stefanović, Š.; Filipan, T. Metal ion exchange by natural and modified zeolites. Water Res. 1997, 31, 1379–1382. [Google Scholar] [CrossRef]
- Kalló, D. Applications of natural zeolites in water and wastewater treatment. Rev. Mineral. Geochem. 2001, 45, 518–550. [Google Scholar] [CrossRef]
- Dyer, A. Ion Exchange Capacity. In Verified Syntheses of Zeolitic Materials; Elsevier: Amsterdam, The Netherlands, 2001; pp. 67–68. [Google Scholar]
- Cadar, O.; Senila, M.; Hoaghia, M.-A.; Scurtu, D.; Miu, I.; Levei, E.A. Effects of Thermal Treatment on Natural Clinoptilolite-Rich Zeolite Behavior in Simulated Biological Fluids. Molecules 2020, 25, 2570. [Google Scholar] [CrossRef]
- Hay, R.L.; Sheppard, R.A. Occurrence of zeolites in sedimentary rocks: An overview. Rev. Mineral. Geochem. 2001, 45, 217–234. [Google Scholar] [CrossRef]
- Uzal, B.; Turanli, L.; Yücel, H.; Göncüoǧlu, M.C.; Çulfaz, A. Pozzolanic activity of clinoptilolite: A comparative study with silica fume, fly ash and a non-zeolitic natural pozzolan. Cem. Concr. Res. 2010, 40, 398–404. [Google Scholar] [CrossRef]
- Machiels, L.; Morante, F.; Snellings, R.; Calvo, B.; Canoira, L.; Paredes, C.; Elsen, J. Zeolite mineralogy of the Cayo formation in Guayaquil, Ecuador. Appl. Clay Sci. 2008, 42, 180–188. [Google Scholar] [CrossRef]
- Machiels, L.; Garcés, D.; Snellings, R.; Vilema, W.; Morante, F.; Paredes, C.; Elsen, J. Zeolite occurrence and genesis in the Late-Cretaceous Cayo arc of Coastal Ecuador: Evidence for zeolite formation in cooling marine pyroclastic flow deposits. Appl. Clay Sci. 2014, 87, 108–119. [Google Scholar] [CrossRef]
- Morante, F.; Costafreda, J.; Carrión, P.; Calvo, B.; Garcés, D.; Machiels, L. Zeolitas Naturales del Ecuador: Geología, Caracterización y Aplicaciones; ESPOL: Guayaquil, Ecuador, 2011; ISBN 978-9978-310-90-8. [Google Scholar]
- Chmielewská, E. An update of zeolitic and other traditional adsorption and ion exchange materials in water cleanup processes. In Handbook of Natural Zeolites; Bentham Science Publishers Ltd.: Bratislava, Slovakia, 2012; pp. 436–452. ISBN 9781608054466. [Google Scholar]
- Chojnacki, A.; Chojnacka, K.; Hoffmann, J.; Górecki, H. The application of natural zeolites for mercury removal: From laboratory tests to industrial scale. Miner. Eng. 2004, 17, 933–937. [Google Scholar] [CrossRef]
- Ibrahim, K.M.; NasserEd-Deen, T.; Khoury, H. Use of natural chabazite-phillipsite tuff in wastewater treatment from electroplating factories in Jordan. Environ. Geol. 2002, 41, 547–551. [Google Scholar] [CrossRef]
- Colella, C. Natural zeolites in environmentally friendly processes and applications. Stud. Surf. Sci. Catal. 1999, 125, 641–655. [Google Scholar] [CrossRef]
- Kesraoui-Ouki, S.; Cheeseman, C.R.; Perry, R. Natural zeolite utilisation in pollution control: A review of applications to metals’ effluents. J. Chem. Technol. Biotechnol. 1994, 59, 121–126. [Google Scholar] [CrossRef]
- Oter, O.; Akcay, H. Use of Natural Clinoptilolite to Improve Water Quality: Sorption and Selectivity Studies of Lead(II), Copper(II), Zinc(II), and Nickel(II). Water Environ. Res. 2007, 79, 329–335. [Google Scholar] [CrossRef]
- Llanes-Monter, M.M.; Olguín, M.T.; Solache-Ríos, M.J. Lead sorption by a Mexican, clinoptilolite-rich tuff. Environ. Sci. Pollut. Res. 2007, 14, 397–403. [Google Scholar] [CrossRef] [PubMed]
- Sprynskyy, M.; Buszewski, B.; Terzyk, A.P.; Namieśnik, J. Study of the selection mechanism of heavy metal (Pb2+, Cu2+, Ni2+, and Cd2+) adsorption on clinoptilolite. J. Colloid Interface Sci. 2006, 304, 21–28. [Google Scholar] [CrossRef]
- Cincotti, A.; Mameli, A.; Locci, A.M.; Orrù, R.; Cao, G. Heavy metals uptake by Sardinian natural zeolites: Experiment and modeling. Ind. Eng. Chem. Res. 2006, 45, 1074–1084. [Google Scholar] [CrossRef]
- Turkman, A.; Aslan, S.; Ege, I. Treatment of metal containing wastewaters by natural zeolites. Fresenius Environ. Bull 2004, 13, 574–580. [Google Scholar]
- Bosso, S.T.; Enzweiler, J. Evaluation of heavy metal removal from aqueous solution onto scolecite. Water Res. 2002, 36, 4795–4800. [Google Scholar] [CrossRef]
- Caputo, D.; Pepe, F. Experiments and data processing of ion exchange equilibria involving Italian natural zeolites: A review. Microporous Mesoporous Mater. 2007, 105, 222–231. [Google Scholar] [CrossRef]
- Mercer, B.W.; Ames, L.L.; Touhill, C.J.; Van Slyke, W.J.; Dean, R.B. Ammonia Removal from Secondary Effluents by Selective Ion Exchange. J. Water Pollut. Control. Fed. 1970, 42, R95–R107. [Google Scholar]
- Fragoulis, D.; Chaniotakis, E.; Stamatakis, M.G. Zeolitic tuffs of Kimolos Island, Aegean Sea, Greece and their industrial potential. Cem. Concr. Res. 1997, 27, 889–905. [Google Scholar] [CrossRef]
- Cibulić, V.V.; Stamenković, L.J.; Veljković, N.D.; Staletović, N.M. Dinamika procesa adsorpcije boje iz otpadnih voda od bojenja tekstilnih vlakana na prirodnim zeolitima. Hem. Ind. 2013, 67, 41–49. [Google Scholar] [CrossRef]
- Chung, Y.C.; Son, D.H.; Ahn, D.H. Nitrogen and organics removal from industrial wastewater using natural zeolite media. Water Sci. Technol. 2000, 42, 127–134. [Google Scholar] [CrossRef]
- Kochan, R.; Pohrebennyk, V.; Hyvlyud, A.; Ruda, M.; Witos, K. Complex evaluation of efficiency of compatible application spent zeolite and mycorhizae on the kinetics of plants growth. In Proceedings of the International Multidisciplinary Scientific GeoConference Surveying Geology and Mining Ecology Management, SGEM, International Multidisciplinary Scientific Geoconference, Varna, Bulgaria, 28 June–7 July 2019; Volume 19, pp. 633–641. [Google Scholar]
- Özpinar, Y. Use of zeolitic tuffs as cement additives, building stone and removal of heavy metal cations. Carpathian J. Earth Environ. Sci. 2011, 6, 147–158. [Google Scholar]
- Cornejo, M.H.; Elsen, J.; Paredes, C.; Baykara, H. Thermomechanical treatment of two Ecuadorian zeolite-rich tuffs and their potential usage as supplementary cementitious materials. J. Therm. Anal. Calorim. 2014, 115, 309–321. [Google Scholar] [CrossRef]
- Presa, L.; Costafreda, J.L.; Martín, D.A.; Díaz, I. Natural Mordenite from Spain as Pozzolana. Molecules 2020, 25, 1220. [Google Scholar] [CrossRef]
- Lynch, J.L.V.; Baykara, H.; Cornejo, M.; Soriano, G.; Ulloa, N.A. Preparation, characterization, and determination of mechanical and thermal stability of natural zeolite-based foamed geopolymers. Constr. Build. Mater. 2018, 172, 448–456. [Google Scholar] [CrossRef]
- Adebajo, M.O.; Frost, R.L.; Kloprogge, J.T.; Carmody, O.; Kokot, S. Porous Materials for Oil Spill Cleanup: A Review of Synthesis and Absorbing Properties. J. Porous Mater. 2003, 10, 159–170. [Google Scholar] [CrossRef]
- Wang, S.; Zhu, Z.H. Characterisation and environmental application of an Australian natural zeolite for basic dye removal from aqueous solution. J. Hazard. Mater. 2006, 136, 946–952. [Google Scholar] [CrossRef] [PubMed]
- Albino, V.; Cioffi, R.; Pansini, M.; Colella, C. Disposal of lead-containing zeolite sludges in cement matrix. Environ. Technol. 1995, 16, 147–156. [Google Scholar] [CrossRef]
- Samardzioska, T.; Jovanovski, M.; Lepitkova, S. Zeolites—Sustainable Building Material. In Proceedings of the 1st International Conference on Construction materials for sustainable future (CoMS_2017), Zadar, Croatia, 19–21 April 2017. [Google Scholar]
- Baxter, S.G.; Berghauser, D.C. The selection and performance of the natural zeolite clinoptilolite in British Nuclear Fuels’ site ion exchange effluent plant, SIXEP. In Waste Management 86; British Nuclear Fuels: Daresbury, UK, 1986. [Google Scholar]
- Kalló, D. Wasterwater purification in Hungary using natural zeolites. In Natural Zeolites ’93 Occurrence, Properties, Use; Intern. Committee on Natural Zeolites: Brockport, NY, USA, 1995. [Google Scholar]
- Pansini, M. Natural zeolites as cation exchangers for environmental protection. Miner. Depos. 1996, 31, 563–575. [Google Scholar] [CrossRef]
- Curi, A.; Granda, W.J.V.; Lima, H.M.; Sousa, W.T. Las zeolitas y su aplicación en la descontaminación de efluentes mineros. Inf. Tecnol. 2006, 17, 111–118. [Google Scholar] [CrossRef]
- Reháková, M.; Čuvanová, S.; Dzivák, M.; Rimár, J.; Gaval’Ová, Z. Agricultural and agrochemical uses of natural zeolite of the clinoptilolite type. Curr. Opin. Solid State Mater. Sci. 2004, 8, 397–404. [Google Scholar] [CrossRef]
- Widiastuti, N.; Wu, H.; Ang, M.; Zhang, D. The potential application of natural zeolite for greywater treatment. Desalination 2008, 218, 271–280. [Google Scholar] [CrossRef]
- Pavelić, K.; Hadžija, M.; Bedrica, L.; Pavelić, J.; Crossed, D.; Signikić, I.; Katić, M.; Kralj, M.; Bosnar, M.H.; Kapitanović, S.; et al. Natural zeolite clinoptilolite: New adjuvant in anticancer therapy. J. Mol. Med. 2000, 78, 708–720. [Google Scholar] [CrossRef] [PubMed]
- Ivkovic, S.; Deutsch, U.; Silberbach, A.; Walraph, E.; Mannel, M. Dietary supplementation with the tribomechanically activated zeolite clinoptilolite in immunodeficiency: Effects on the immune system. Adv. Ther. 2004, 21, 135–147. [Google Scholar] [CrossRef] [PubMed]
- Smical, I. Properties of natural zeolites in benefit of nutrition and health. Hum. Vet. Med. 2011, 3, 51–57. [Google Scholar]
- de Bruijn, J.; Gómez, A.; Loyola, C.; Melín, P.; Solar, V.; Abreu, N.; Azzolina-Jury, F.; Valdés, H. Use of a copper-and zinc-modified natural zeolite to improve ethylene removal and postharvest quality of tomato fruit. Crystals 2020, 10, 471. [Google Scholar] [CrossRef]
- Otieno, S.O.; Kowenje, C.O.; Okoyo, A.; Onyango, D.M.; Amisi, K.O.; Nzioka, K.M. Optimizing production of biodiesel catalysed by chemically tuned natural zeolites. In Materials Today Proceedings; Elsevier Ltd.: Amsterdam, The Netherlands, 2018; Volume 5, pp. 10561–10569. [Google Scholar]
- de Gennaro, B.; Mercurio, M.; Cappelletti, P.; Catalanotti, L.; Daković, A.; De Bonis, A.; Grifa, C.; Izzo, F.; Kraković, M.; Monetti, V.; et al. Use of surface modified natural zeolite (SMNZ) in pharmaceutical preparations. Part 2. A new approach for a fast functionalization of zeolite-rich carriers. Microporous Mesoporous Mater. 2016, 235, 42–49. [Google Scholar] [CrossRef]
- Ogawa, T.; Iyoki, K.; Fukushima, T.; Kajikawa, Y. Landscape of Research Areas for Zeolites and Metal-Organic Frameworks Using Computational Classification Based on Citation Networks. Materials 2017, 10, 1428. [Google Scholar] [CrossRef] [PubMed]
- Liao, H.; Tang, M.; Luo, L.; Li, C.; Chiclana, F.; Zeng, X.-J. A Bibliometric Analysis and Visualization of Medical Big Data Research. Sustainability 2018, 10, 166. [Google Scholar] [CrossRef]
- van Raan, A.F.J. For Your Citations Only? Hot Topics in Bibliometric Analysis. Meas. Interdiscip. Res. Perspect. 2005, 3, 50–62. [Google Scholar] [CrossRef]
- Tranfield, D.; Denyer, D.; Smart, P. Towards a Methodology for Developing Evidence-Informed Management Knowledge by Means of Systematic Review. Br. J. Manag. 2003, 14, 207–222. [Google Scholar] [CrossRef]
- Kitchenham, B.; Brereton, O.P.; Budgen, D.; Turner, M.; Bailey, J.; Linkman, S. Systematic literature reviews in software engineering—A systematic literature review. Inf. Softw. Technol. 2009, 51, 7–15. [Google Scholar] [CrossRef]
- Kitchenham, B. Procedures for Performing Systematic Reviews; Keele University: Keele, UK, 2004. [Google Scholar]
- Mulrow, C.D. Rationale for systematic reviews. Br. Med. J. 1994, 309, 597–599. [Google Scholar] [CrossRef] [PubMed]
- Fahimnia, B.; Sarkis, J.; Davarzani, H. Green supply chain management: A review and bibliometric analysis. Int. J. Prod. Econ. 2015, 162, 101–114. [Google Scholar] [CrossRef]
- Wei, M.; Wang, W.; Zhuang, Y. Worldwide research productivity in the field of spine surgery: A 10-year bibliometric analysis. Eur. Spine J. 2016, 25, 976–982. [Google Scholar] [CrossRef] [PubMed]
- Wallin, J.A. Bibliometric methods: Pitfalls and possibilities. Basic Clin. Pharmacol. Toxicol. 2005, 97, 261–275. [Google Scholar] [CrossRef] [PubMed]
- Luukkonen, T. Invited review article: Bibliometrics and evaluation of Research performance. Ann. Med. 1990, 22, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Joshi, M.A. Bibliometric indicators for evaluating the quality of scientific publications. J. Contemp. Dent. Pract. 2014, 15, 258–262. [Google Scholar] [CrossRef]
- Valérie, D.; Pierre, A.G. Bibliometric idicators: Quality masurements of sientific publication. Radiology 2010, 255, 342–351. [Google Scholar]
- Zurita, G.; Shukla, A.K.; Pino, J.A.; Merigó, J.M.; Lobos-Ossandón, V.; Muhuri, P.K. A bibliometric overview of the Journal of Network and Computer Applications between 1997 and 2019. J. Netw. Comput. Appl. 2020, 165, 102695. [Google Scholar] [CrossRef]
- Herrera-Franco, G.; Montalván-Burbano, N.; Carrión-Mero, P.; Jaya-Montalvo, M.; Gurumendi-Noriega, M. Worldwide research on geoparks through bibliometric analysis. Sustainability 2021, 13, 1175. [Google Scholar] [CrossRef]
- Briones-Bitar, J.; Carrión-Mero, P.; Montalván-Burbano, N.; Morante-Carballo, F. Rockfall research: A bibliometric analysis and future trends. Geosciences 2020, 10, 403. [Google Scholar] [CrossRef]
- Cobo, M.J.; López-Herrera, A.G.; Herrera-Viedma, E.; Herrera, F. Science mapping software tools: Review, analysis, and cooperative study among tools. J. Am. Soc. Inf. Sci. Technol. 2011, 62, 1382–1402. [Google Scholar] [CrossRef]
- Andres, A. Measuring Academic Research; Chandos Publishing: Hull, UK, 2009. [Google Scholar] [CrossRef]
- Baas, J.; Schotten, M.; Plume, A.; Côté, G.; Karimi, R. Scopus as a curated, high-quality bibliometric data source for academic research in quantitative science studies. Quant. Sci. Stud. 2020, 1, 377–386. [Google Scholar] [CrossRef]
- Ruban, D.A.; Ponedelnik, A.A.; Yashalova, N.N. Megaclasts: Term use and relevant biases. Geosciences 2019, 9, 14. [Google Scholar] [CrossRef]
- del Río-Rama, M.D.L.C.; Maldonado-Erazo, C.P.; Álvarez-García, J.; Durán-Sánchez, A. Cultural and Natural Resources in Tourism Island: Bibliometric Mapping. Sustainability 2020, 12, 724. [Google Scholar] [CrossRef]
- Meseguer-Sánchez, V.; Abad-Segura, E.; Belmonte-Ureña, L.J.; Molina-Moreno, V. Examining the research evolution on the socio-economic and environmental dimensions on university social responsibility. Int. J. Environ. Res. Public Health 2020, 17, 4729. [Google Scholar] [CrossRef] [PubMed]
- Harzing, A.W.; Alakangas, S. Google Scholar, Scopus and the Web of Science: A longitudinal and cross-disciplinary comparison. Scientometrics 2016, 106, 787–804. [Google Scholar] [CrossRef]
- Nieuwenhuis, J. Publication bias in the neighbourhood effects literature. Geoforum 2016, 70, 89–92. [Google Scholar] [CrossRef]
- Montalván-Burbano, N.; Velastegui-Montoya, A.; Gurumendi-Noriega, M.; Morante-Carballo, F.; Adami, M. Worldwide Research on Land Use and Land Cover in the Amazon Region. Sustainability 2021, 13, 6039. [Google Scholar] [CrossRef]
- Najmi, A.; Rashidi, T.H.; Abbasi, A.; Travis Waller, S. Reviewing the transport domain: An evolutionary bibliometrics and network analysis. Scientometrics 2017, 110, 843–865. [Google Scholar] [CrossRef]
- León-Castro, M.; Rodríguez-Insuasti, H.; Montalván-Burbano, N.; Victor, J.A. Bibliometrics and Science Mapping of Digital Marketing. In Marketing and Smart Technologies; Rocha, Á., Reis, J.L., Peter, M.K., Cayolla, R., Loureiro, S., Bogdanović, Z., Eds.; Springer: Singapore, 2021; pp. 95–107. [Google Scholar]
- Noyons, E.C.M.; Moed, H.F.; Van Raan, A.F.J. Integrating research performance analysis and science mapping. Scientometrics 1999, 46, 591–604. [Google Scholar] [CrossRef]
- Cobo, M.J.; Martínez, M.A.; Gutiérrez-Salcedo, M.; Fujita, H.; Herrera-Viedma, E. 25 years at Knowledge-Based Systems: A bibliometric analysis. Knowl. Based Syst. 2015, 80, 3–13. [Google Scholar] [CrossRef]
- Pico-Saltos, R.; Carrión-Mero, P.; Montalván-Burbano, N.; Garzás, J.; Redchuk, A. Research Trends in Career Success: A Bibliometric Review. Sustainability 2021, 13, 4625. [Google Scholar] [CrossRef]
- Kovačević, J.; Hallinger, P. Finding Europe’s niche: Science mapping the knowledge base on educational leadership and management in Europe, 1960–2018. Sch. Eff. Sch. Improv. 2019, 31, 405–425. [Google Scholar] [CrossRef]
- Chandra, Y. Mapping the evolution of entrepreneurship as a field of research (1990–2013): A scientometric analysis. PLoS ONE 2018, 13, e0190228. [Google Scholar] [CrossRef] [PubMed]
- Montalván-Burbano, N.; Pérez-Valls, M.; Plaza-Úbeda, J. Analysis of scientific production on organizational innovation. Cogent Bus. Manag. 2020, 7, 1745043. [Google Scholar] [CrossRef]
- van Eck, N.J.; Waltman, L. Visualizing Bibliometric Networks. In Measuring Scholarly Impact; Springer International Publishing: New York, NY, USA, 2014; pp. 285–320. [Google Scholar]
- van Eck, N.J.; Waltman, L. Software survey: VOSviewer, a computer program for bibliometric mapping. Scientometrics 2010, 84, 523–538. [Google Scholar] [CrossRef] [PubMed]
- Herrera-Franco, G.; Montalván-Burbano, N.; Carrión-Mero, P.; Bravo-Montero, L. Worldwide Research on Socio-Hydrology: A Bibliometric Analysis. Water 2021, 13, 1283. [Google Scholar] [CrossRef]
- Carrión-Mero, P.; Montalván-Burbano, N.; Paz-Salas, N.; Morante-Carballo, F. Volcanic Geomorphology: A Review of Worldwide Research. Geosciences 2020, 10, 347. [Google Scholar] [CrossRef]
- Maldonado-Erazo, C.P.; Álvarez-García, J.; Río-Rama, M.D.L.C.D.; Durán-Sánchez, A. Scientific Mapping on the Impact of Climate Change on Cultural and Natural Heritage: A Systematic Scientometric Analysis. Land 2021, 10, 76. [Google Scholar] [CrossRef]
- Durán-Sánchez, A.; Río-Rama, M.D.L.C.D.; Álvarez-García, J.; García-Vélez, D.F. Mapping of scientific coverage on education for Entrepreneurship in Higher Education. J. Enterprising Communities 2019, 13, 84–104. [Google Scholar] [CrossRef]
- Hallinger, P.; Kovačević, J. Science mapping the knowledge base in educational leadership and management: A longitudinal bibliometric analysis, 1960 to 2018. Educ. Manag. Adm. Leadersh. 2021, 49, 5–30. [Google Scholar] [CrossRef]
- Xie, L.; Chen, Z.; Wang, H.; Zheng, C.; Jiang, J. Bibliometric and Visualized Analysis of Scientific Publications on Atlantoaxial Spine Surgery Based on Web of Science and VOSviewer. World Neurosurg. 2020, 137, 435–442.e4. [Google Scholar] [CrossRef]
- Yang, G.; Wu, L. Trend in H2S Biology and Medicine Research—A Bibliometric Analysis. Molecules 2017, 22, 2087. [Google Scholar] [CrossRef] [PubMed]
- Kamdem, J.P.; Duarte, A.E.; Lima, K.R.R.; Rocha, J.B.T.; Hassan, W.; Barros, L.M.; Roeder, T.; Tsopmo, A. Research trends in food chemistry: A bibliometric review of its 40 years anniversary (1976–2016). Food Chem. 2019, 294, 448–457. [Google Scholar] [CrossRef] [PubMed]
- Batistič, S.; Kaše, R. The organizational socialization field fragmentation: A bibliometric review. Scientometrics 2015, 104, 121–146. [Google Scholar] [CrossRef]
- López-Muñoz, F.; Vieta, E.; Rubio, G.; García-García, P.; Alamo, C. Bipolar disorder as an emerging pathology in the scientific literature: A bibliometric approach. J. Affect. Disord. 2006, 92, 161–170. [Google Scholar] [CrossRef]
- Andreo-Martínez, P.; Ortiz-Martínez, V.M.; García-Martínez, N.; de los Ríos, A.P.; Hernández-Fernández, F.J.; Quesada-Medina, J. Production of biodiesel under supercritical conditions: State of the art and bibliometric analysis. Appl. Energy 2020, 264, 114753. [Google Scholar] [CrossRef]
- Semmens, M.J.; Martin, W.P. The influence of pretreatment on the capacity and selectivity of clinoptilolite for metal ions. Water Res. 1988, 22, 537–542. [Google Scholar] [CrossRef]
- Jorgensen, T.C.; Weatherley, L.R. Ammonia removal from wastewater by ion exchange in the presence of organic contaminants. Water Res. 2003, 37, 1723–1728. [Google Scholar] [CrossRef]
- Liberti, L.; Boari, G.; Petruzzelli, D.; Passino, R. Nutrient removal and recovery from wastewater by ion exchange. Water Res. 1981, 15, 337–342. [Google Scholar] [CrossRef]
- Matheickal, J.T.; Yu, Q. Biosorption of lead(II) and copper(II) from aqueous solutions by pre-treated biomass of Australian marine algae. Bioresour. Technol. 1999, 69, 223–229. [Google Scholar] [CrossRef]
- Englert, A.H.; Rubio, J. Characterization and environmental application of a Chilean natural zeolite. Int. J. Miner. Process. 2005, 75, 21–29. [Google Scholar] [CrossRef]
- Glänzel, W.; Schubert, A. Analysing Scientific Networks Through Co-Authorship. In Handbook of Quantitative Science and Technology Research; Kluwer Academic Publishers: Berlin/Heidelberg, Germany, 2006; pp. 257–276. [Google Scholar]
- Saltali, K.; Sari, A.; Aydin, M. Removal of ammonium ion from aqueous solution by natural Turkish (Yi{dotless}ldi{dotless}zeli) zeolite for environmental quality. J. Hazard. Mater. 2007, 141, 258–263. [Google Scholar] [CrossRef]
- Kessler, M.M. Bibliographic coupling between scientific papers. Am. Doc. 1963, 14, 10–25. [Google Scholar] [CrossRef]
- Moed, H.F. Citation Analysis in Research Evaluation; Springer: Berlin/Heidelberg, Germany, 2005; Volume 2, ISBN 9789171403391. [Google Scholar]
- Costas, R.; Bordons, M. The h-index: Advantages, limitations and its relation with other bibliometric indicators at the micro level. J. Informetr. 2007, 1, 193–203. [Google Scholar] [CrossRef]
- Bissen, M.; Frimmel, F.H. Arsenic—A review. Part II: Oxidation of arsenic and its removal in water treatment. Acta Hydrochim. Hydrobiol. 2003, 31, 97–107. [Google Scholar] [CrossRef]
- Perić, J.; Trgo, M.; Medvidović, N.V. Removal of zinc, copper and lead by natural zeolite—A comparison of adsorption isotherms. Water Res. 2004, 38, 1893–1899. [Google Scholar] [CrossRef] [PubMed]
- Wingenfelder, U.; Hansen, C.; Furrer, G.; Schulin, R. Removal of heavy metals from mine waters by natural zeolites. Environ. Sci. Technol. 2005, 39, 4606–4613. [Google Scholar] [CrossRef] [PubMed]
- Ouki, S.K.; Kavannagh, M. Performance of natural zeolites for the treatment of mixed metal- contaminated effluents. Waste Manag. Res. 1997, 15, 383–394. [Google Scholar] [CrossRef]
- Wang, M.; Chai, L. Three new bibliometric indicators/approaches derived from keyword analysis. Scientometrics 2018, 116, 721–750. [Google Scholar] [CrossRef]
- Herrera-Franco, G.; Montalván-Burbano, N.; Carrión-Mero, P.; Apolo-Masache, B.; Jaya-Montalvo, M. Research Trends in Geotourism: A Bibliometric Analysis Using the Scopus Database. Geosciences 2020, 10, 379. [Google Scholar] [CrossRef]
- Abusafa, A.; Yücel, H. Removal of 137Cs from aqueous solutions using different cationic forms of a natural zeolite: Clinoptilolite. Sep. Purif. Technol. 2002, 28, 103–116. [Google Scholar] [CrossRef]
- Komarowski, S.; Yu, Q. Ammonium ion removal from wastewater using australian natural zeolite: Batch equilibrium and kinetic studies. Environ. Technol. 1997, 18, 1085–1097. [Google Scholar] [CrossRef]
- Lind, B.B.; Ban, Z.; Bydén, S. Nutrient recovery from human urine by struvite crystallization with ammonia adsorption on zeolite and wollastonite. Bioresour. Technol. 2000, 73, 169–174. [Google Scholar] [CrossRef]
- Ackley, M.W.; Giese, R.F.; Yang, R.T. Clinoptilolite: Untapped potential for kinetics gas separations. Zeolites 1992, 12, 780–788. [Google Scholar] [CrossRef]
- Sprynskyy, M.; Lebedynets, M.; Zbytniewski, R.; Namieśnik, J.; Buszewski, B. Ammonium removal from aqueous solution by natural zeolite, Transcarpathian mordenite, kinetics, equilibrium and column tests. Sep. Purif. Technol. 2005, 46, 155–160. [Google Scholar] [CrossRef]
- Loizidou, M.; Townsend, R.P. Ion-exchange properties of natural clinoptilolite, ferrierite and mordenite: Part 2. Lead-sodium and lead-ammonium equilibria. Zeolites 1987, 7, 153–159. [Google Scholar] [CrossRef]
- Dwairi, I.M. Evaluation of jordanian zeolite tuff as a controlled slow-release fertilizer for NH4+. Environ. Geol. 1998, 34, 1–4. [Google Scholar] [CrossRef]
- Leyva-Ramos, R.; Monsivais-Rocha, J.E.; Aragon-Piña, A.; Berber-Mendoza, M.S.; Guerrero-Coronado, R.M.; Alonso-Davila, P.; Mendoza-Barron, J. Removal of ammonium from aqueous solution by ion exchange on natural and modified chabazite. J. Environ. Manag. 2010, 91, 2662–2668. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Lei, Z.; Wang, L.; Liu, X.; Zhang, Y.; Wan, C.; Lee, D.J.; Tay, J.H. Adsorption mechanisms of high-levels of ammonium onto natural and NaCl-modified zeolites. Sep. Purif. Technol. 2013, 103, 15–20. [Google Scholar] [CrossRef]
- Bernal, M.P.; Lopez-Real, J.M. Natural zeolites and sepiolite as ammonium and ammonia adsorbent materials. Bioresour. Technol. 1993, 43, 27–33. [Google Scholar] [CrossRef]
- Miladinovic, N.; Weatherley, L.R. Intensification of ammonia removal in a combined ion-exchange and nitrification column. Chem. Eng. J. 2008, 135, 15–24. [Google Scholar] [CrossRef]
- Castaldi, P.; Santona, L.; Enzo, S.; Melis, P. Sorption processes and XRD analysis of a natural zeolite exchanged with Pb2+, Cd2+ and Zn2+ cations. J. Hazard. Mater. 2008, 156, 428–434. [Google Scholar] [CrossRef]
- Sun, X.; Xi, C.; Hou, Z. Study on modification and fluoride-adsorption capacity of zeolite. In Proceedings of the International Conference on Challenges in Environmental Science and Computer Engineering, CESCE, Wuhan, China, 6–7 March 2010; Volume 1, pp. 354–357. [Google Scholar]
- Panayotova, M.I. Kinetics and thermodynamics of copper ions removal from wastewater by use of zeolite. Waste Manag. 2001, 21, 671–676. [Google Scholar] [CrossRef]
- Ghiaci, M.; Abbaspur, A.; Kia, R.; Seyedeyn-Azad, F. Equilibrium isotherm studies for the sorption of benzene, toluene, and phenol onto organo-zeolites and as-synthesized MCM-41. Sep. Purif. Technol. 2004, 40, 217–229. [Google Scholar] [CrossRef]
- Sircar, S.; Nerur, S.P.; Mahapatra, R. Revolution or evolution? A comparison of object-oriented and structured systems development methods. MIS Quarterly. 2001, 25, 457–471. [Google Scholar] [CrossRef]
- White, H.D.; Griffith, B.C. Author cocitation: A literature measure of intellectual structure. J. Am. Soc. Inf. Sci. 1981, 32, 163–171. [Google Scholar] [CrossRef]
- Du, Q.; Liu, S.; Cao, Z.; Wang, Y. Ammonia removal from aqueous solution using natural Chinese clinoptilolite. Sep. Purif. Technol. 2005, 44, 229–234. [Google Scholar] [CrossRef]
- Wang, S.; Terdkiatburana, T.; Tadé, M.O. Adsorption of Cu(II), Pb(II) and humic acid on natural zeolite tuff in single and binary systems. Sep. Purif. Technol. 2008, 62, 64–70. [Google Scholar] [CrossRef]
- Turan, M.; Mart, U.; Yüksel, B.; Çelik, M.S. Lead removal in fixed-bed columns by zeolite and sepiolite. Chemosphere 2005, 60, 1487–1492. [Google Scholar] [CrossRef]
- Karadag, D.; Akgul, E.; Tok, S.; Erturk, F.; Kaya, M.A.; Turan, M. Basic and reactive dye removal using natural and modified zeolites. J. Chem. Eng. Data 2007, 52, 2436–2441. [Google Scholar] [CrossRef]
- Barrer, R.M. Zeolites and clay minerals as sorbents and molecular sieves. Mineral. Mag. 1980, 43, 829–830. [Google Scholar] [CrossRef]
- Barrer, R.M.; Townsend, R.P. Transition metal ion exchange in zeolites. Part 1.-Thermodynamics of exchange of hydrated Mn2+, Co2+, Ni2+, Cu 2+ and Zn2+ ions in ammonium mordenite. J. Chem. Soc. Faraday Trans. 1 Phys. Chem. Condens. Phases 1976, 72, 661–673. [Google Scholar] [CrossRef]
- Vaughan, D.E.W. Contributions of R. M. Barrer to zeolite synthesis. In Studies in Surface Science and Catalysis; Elsevier Inc.: Amsterdam, The Netherlands, 2007; Volume 170, pp. 87–95. ISBN 0444530681. [Google Scholar]
- Rees, L.V.C. Richard Maling Barrer. 16 June 1910–12 September 1996. Biogr. Mem. Fellows R. Soc. 1998, 44, 37–49. [Google Scholar] [CrossRef][Green Version]
- Inglezakis, V.J.; Zorpas, A.A.; Loizidou, M.D.; Grigoropoulou, H.P. The effect of competitive cations and anions on ion exchange of heavy metals. Sep. Purif. Technol. 2005, 46, 202–207. [Google Scholar] [CrossRef]
- Stylianou, M.A.; Hadjiconstantinou, M.P.; Inglezakis, V.J.; Moustakas, K.G.; Loizidou, M.D. Use of natural clinoptilolite for the removal of lead, copper and zinc in fixed bed column. J. Hazard. Mater. 2007, 143, 575–581. [Google Scholar] [CrossRef] [PubMed]
- Inglezakis, V.J.; Loizidou, M.D.; Grigoropoulou, H.P. Equilibrium and kinetic ion exchange studies of Pb2+, Cr3+, Fe3+ and Cu2+ on natural clinoptilolite. Water Res. 2002, 36, 2784–2792. [Google Scholar] [CrossRef]
- Inglezakis, V.J.; Hadjiandreou, K.J.; Loizidou, M.D.; Grigoropoulou, H.P. Pretreatment of natural clinoptilolite in a laboratory-scale ion exchange packed bed. Water Res. 2001, 35, 2161–2166. [Google Scholar] [CrossRef]
- Metropoulos, K.; Maliou, E.; Loizidou, M.; Spyrellis, N. Comparative studies between synthetic and natural zeolites for ammonium uptake. J. Environ. Sci. Health Part A Environ. Sci. Eng. Toxicol. 1993, 28, 1507–1518. [Google Scholar] [CrossRef]
- Bowman, R.S. Applications of surfactant-modified zeolites to environmental remediation. Microporous Mesoporous Mater. 2003, 61, 43–56. [Google Scholar] [CrossRef]
- Li, Z.; Bowman, R.S. Regeneration of surfactant-modified zeolite after saturation with chromate and perchloroethylene. Water Res. 2001, 35, 322–326. [Google Scholar] [CrossRef]
- Mumpton, F.A.; Ormsby, W.C. Morphology of zeolites in sedimentary rocks by scanning electron microscopy. Clays Clay Miner. 1976, 24, 1–23. [Google Scholar] [CrossRef]
- Mumpton, F.A.; Fishman, P.H. The Application of Natural Zeolites in Animal Science and Aquaculture. J. Anim. Sci. 1977, 45, 1188–1203. [Google Scholar] [CrossRef]
- Colella, C. Natural zeolites. In Zeolite and Ordered Mesoporous Materials: Progress and Prospects: The 1st FEZA School on Zeolites; Elsevier Inc.: Amsterdam, The Netherlands, 2005; Volume 157, pp. 13–40. [Google Scholar]
- Colella, C. Environmental Applications of Natural Zeolitic Materials Based on Their Ion Exchange Properties. In Natural Microporous Materials in Environmental Technology; Springer: Dordrecht, The Netherlands, 1999; pp. 207–224. [Google Scholar]
- Caputo, D.; Liguori, B.; Colella, C. Some advances in understanding the pozzolanic activity of zeolites: The effect of zeolite structure. Cem. Concr. Compos. 2008, 30, 455–462. [Google Scholar] [CrossRef]
- Pabalan, R.T.; Bertetti, F.P. Cation-exchange properties of natural zeolites. Rev. Mineral. Geochem. 2001, 45, 453–517. [Google Scholar] [CrossRef]
- Colella, C. Ion exchange equilibria in zeolite minerals. Miner. Depos. 1996, 31, 554–562. [Google Scholar] [CrossRef]
- Torracca, E.; Galli, P.; Pansini, M.; Colella, C. Cation exchange reactions of a sedimentary chabazite. Microporous Mesoporous Mater. 1998, 20, 119–127. [Google Scholar] [CrossRef]
- Sánchez, E.; Milán, Z.; Borja, R.; Weiland, P.; Rodriguez, X. Piggery waste treatment by anaerobic digestion and nutrient removal by ionic exchange. Resour. Conserv. Recycl. 1995, 15, 235–244. [Google Scholar] [CrossRef]
- Borja, R.; Sánchez, E.; Weiland, P.; Travieso, L.; Martín, A. Effect of natural zeolite support on the kinetics of cow manure anaerobic digestion. Biomass Bioenergy 1993, 5, 395–400. [Google Scholar] [CrossRef]
- Borja, R.; Sánchez, E.; Weiland, P.; Travieso, L.; Martín, A. Kinetics of anaerobic degestion of cow manure with biomass immobilized on zeolite. Chem. Eng. J. Biochem. Eng. J. 1994, 54, B9–B14. [Google Scholar] [CrossRef]
- Fernández, N.; Montalvo, S.; Borja, R.; Guerrero, L.; Sánchez, E.; Cortés, I.; Colmenarejo, M.F.; Travieso, L.; Raposo, F. Performance evaluation of an anaerobic fluidized bed reactor with natural zeolite as support material when treating high-strength distillery wastewater. Renew. Energy 2008, 33, 2458–2466. [Google Scholar] [CrossRef]
- Fernández, N.; Montalvo, S.; Fernández-Polanco, F.; Guerrero, L.; Cortés, I.; Borja, R.; Sánchez, E.; Travieso, L. Real evidence about zeolite as microorganisms immobilizer in anaerobic fluidized bed reactors. Process. Biochem. 2007, 42, 721–728. [Google Scholar] [CrossRef]
- Montalvo, S.; Huiliñir, C.; Borja, R.; Sánchez, E.; Herrmann, C. Application of zeolites for biological treatment processes of solid wastes and wastewaters—A review. Bioresour. Technol. 2020, 301, 122808. [Google Scholar] [CrossRef]
- Malferrari, D.; Laurora, A.; Brigatti, M.F.; Coltorti, M.; Di Giuseppe, D.; Faccini, B.; Passaglia, E.; Vezzalini, M.G. Open-field experimentation of an innovative and integrated zeolitite cycle: Project definition and material characterization. Rend. Lincei 2013, 24, 141–150. [Google Scholar] [CrossRef]
- Ferretti, G.; Keiblinger, K.M.; Di Giuseppe, D.; Faccini, B.; Colombani, N.; Zechmeister-Boltenstern, S.; Coltorti, M.; Mastrocicco, M. Short-Term Response of Soil Microbial Biomass to Different Chabazite Zeolite Amendments. Pedosphere 2018, 28, 277–287. [Google Scholar] [CrossRef]
- Ferretti, G.; Di Giuseppe, D.; Faccini, B.; Coltorti, M. Mitigation of sodium risk in a sandy agricultural soil by the use of natural zeolites. Environ. Monit. Assess. 2018, 190, 646. [Google Scholar] [CrossRef] [PubMed]
- Faccini, B.; Di Giuseppe, D.; Malferrari, D.; Coltorti, M.; Abbondanzi, F.; Campisi, T.; Laurora, A.; Passaglia, E. Ammonium-exchanged zeolitite preparation for agricultural uses: From laboratory tests to large-scale application in ZeoLIFE project prototype. Period. Mineral. 2015, 84, 303–321. [Google Scholar] [CrossRef]
- Colombani, N.; Mastrocicco, M.; Di Giuseppe, D.; Faccini, B.; Coltorti, M. Variation of the hydraulic properties and solute transport mechanisms in a silty-clay soil amended with natural zeolites. Catena 2014, 123, 195–204. [Google Scholar] [CrossRef]
- Carrión-Mero, P.; Montalvan-Burbano, N.; Herrera-Narvá, E.G.; Morante-Carballo, F. Geodiversity and Mining Towards the Development of Geotourism: A Global Perspective. Int. J. Des. Nat. Ecodynamics 2021, 16, 191–201. [Google Scholar] [CrossRef]
- Zupic, I.; Čater, T. Bibliometric Methods in Management and Organization. Organ. Res. Methods 2015, 18, 429–472. [Google Scholar] [CrossRef]
- McCain, K.W. Mapping economics through the journal literature: An experiment in journal cocitation analysis. J. Am. Soc. Inf. Sci. 1991, 42, 290–296. [Google Scholar] [CrossRef]
- Dyer, A. Uses of Natural Zeolites. Chem. Ind. 1984, 7, 241–245. [Google Scholar]
- Mumpton, F.A. Development of uses for natural zeolites: A critical commentary. In Proceedings of the International Conference on the Occurrence, Properties, and Utilization of Natural Zeolites, Budapest, Hungary, 12–16 August 1988; pp. 333–366. [Google Scholar]
- Davis, M.E. New Vistas in Zeolite and Molecular Sieve Catalysis. Acc. Chem. Res. 1993, 26, 111–115. [Google Scholar] [CrossRef]
- Selvam, T.; Schwieger, W.; Dathe, W. Natural Cuban zeolites for medical use and their histamine binding capacity. Clay Miner. 2014, 49, 501–512. [Google Scholar] [CrossRef]
- Saiapina, O.Y.; Dzyadevych, S.V.; Walcarius, A.; Jaffrezic-Renault, N. A Novel Highly Sensitive Zeolite-Based Conductometric Microsensor for Ammonium Determination. Anal. Lett. 2012, 45, 1467–1484. [Google Scholar] [CrossRef]
- Montagna, G.; Bigi, S.; Kónya, P.; Szakáll, S.; Vezzalini, G. Chabazite-Mg: A new natural zeolite of the chabazite series. Am. Mineral. 2010, 95, 939–945. [Google Scholar] [CrossRef]
- Pepe, F.; de Gennaro, B.; Aprea, P.; Caputo, D. Natural zeolites for heavy metals removal from aqueous solutions: Modeling of the fixed bed Ba2+/Na+ ion-exchange process using a mixed phillipsite/chabazite-rich tuff. Chem. Eng. J. 2013, 219, 37–42. [Google Scholar] [CrossRef]
- Colella, C.; De Gennaro, M.; Aiello, R. Use of zeolitic tuff in the building industry. Rev. Mineral. Geochem. 2001, 45, 551–587. [Google Scholar] [CrossRef]
- Poon, C.S.; Lam, L.; Kou, S.C.; Lin, Z.S. A study on the hydration rate of natural zeolite blended cement pastes. Constr. Build. Mater. 1999, 13, 427–432. [Google Scholar] [CrossRef]
- Ozaydin, S.; Kocer, G.; Hepbasli, A. Natural zeolites in energy applications. Energy Sources Part A Recover. Util. Environ. Eff. 2006, 28, 1425–1431. [Google Scholar] [CrossRef]
- Kragović, M.; Daković, A.; Marković, M.; Krstić, J.; Gatta, G.D.; Rotiroti, N. Characterization of lead sorption by the natural and Fe(III)-modified zeolite. Appl. Surf. Sci. 2013, 283, 764–774. [Google Scholar] [CrossRef]
- Krajišnik, D.; Daković, A.; Malenović, A.; Kragović, M.; Milić, J. Ibuprofen sorption and release by modified natural zeolites as prospective drug carriers. Clay Miner. 2015, 50, 11–22. [Google Scholar] [CrossRef]
- Marković, M.; Daković, A.; Rottinghaus, G.E.; Petković, A.; Kragović, M.; Krajišnik, D.; Milić, J. Ochratoxin A and zearalenone adsorption by the natural zeolite treated with benzalkonium chloride. Colloids Surf. A Physicochem. Eng. Asp. 2017, 529, 7–17. [Google Scholar] [CrossRef]
- Krajišnik, D.; Daković, A.; Milojević, M.; Malenović, A.; Kragović, M.; Bogdanović, D.B.; Dondur, V.; Milić, J. Properties of diclofenac sodium sorption onto natural zeolite modified with cetylpyridinium chloride. Colloids Surf. B Biointerfaces 2011, 83, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Tahamtan, I.; Afshar, A.S.; Ahamdzadeh, K. Factors affecting number of citations: A comprehensive review of the literature. Scientometrics 2016, 107, 1195–1225. [Google Scholar] [CrossRef]
- Pansini, M.; Colella, C. Dynamic data on lead uptake from water by chabazite. Desalination 1990, 78, 287–295. [Google Scholar] [CrossRef]
- Liu, X.; Tian, R.; Ding, W.; He, Y.; Li, H. Adsorption selectivity of heavy metals by Na-clinoptilolite in aqueous solutions. Adsorption 2019, 25, 747–755. [Google Scholar] [CrossRef]
- Kragović, M.; Daković, A.; Sekulić, Ž.; Trgo, M.; Ugrina, M.; Perić, J.; Gatta, G.D. Removal of lead from aqueous solutions by using the natural and Fe(III)-modified zeolite. Appl. Surf. Sci. 2012, 258, 3667–3673. [Google Scholar] [CrossRef]
- Kocatürk, N.P.; Baykal, B.B. Recovery of Plant Nutrients from Dilute Solutions of Human Urine and Preliminary Investigations on Pot Trials. Clean Soil Air Water 2012, 40, 538–544. [Google Scholar] [CrossRef]
- Mousavi, H.Z.; Asghari, A. Removal of heavy metal ions in wastewater by Semnan natural zeolite. Asian J. Chem. 2009, 21, 2881–2886. [Google Scholar]
- Elysabeth, T.; Ramayanti, G. Modification of Lampung and Bayah natural zeolite to enhance the efficiency of removal of ammonia from wastewater. Asian J. Chem. 2019, 31, 873–878. [Google Scholar] [CrossRef]
- Ferretti, G.; Faccini, B.; Antisari, L.V.; Di Giuseppe, D.; Coltorti, M. 15N natural abundance, nitrogen and carbon pools in soil-sorghum system amended with natural and NH4+-enriched zeolitites. Appl. Sci. 2019, 9, 4524. [Google Scholar] [CrossRef]
- Terzić, A.; Pezo, L.; Andrić, L. Chemometric assessment of mechano-chemically activated zeolites for application in the construction composites. Compos. Part B Eng. 2017, 109, 30–44. [Google Scholar] [CrossRef]
- Rahmani, A.R.; Samadi, M.T.; Ehsani, H.R. Investigation of clinoptilolite natural zeolite regeneration by air stripping followed by ion exchange for removal of ammonium from aqueous solutions. Iran. J. Environ. Health Sci. Eng. 2009, 6, 167–172. [Google Scholar]
- Ashrafizadeh, S.N.; Khorasani, Z.; Gorjiara, M. Ammonia removal from aqueous solutions by Iranian natural zeolite. Sep. Sci. Technol. 2008, 43, 960–978. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).