Changes in Reserve Mobilization Caused by Salinity Could Interfere in the Initial Growth of Jatropha curcas
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Conditions, Harvesting and Ion Analysis
2.2. Carbohydrate Analysis
2.3. Analysis of Proteins, Amino Acids and Lipids
2.4. Statistical Analysis
3. Results
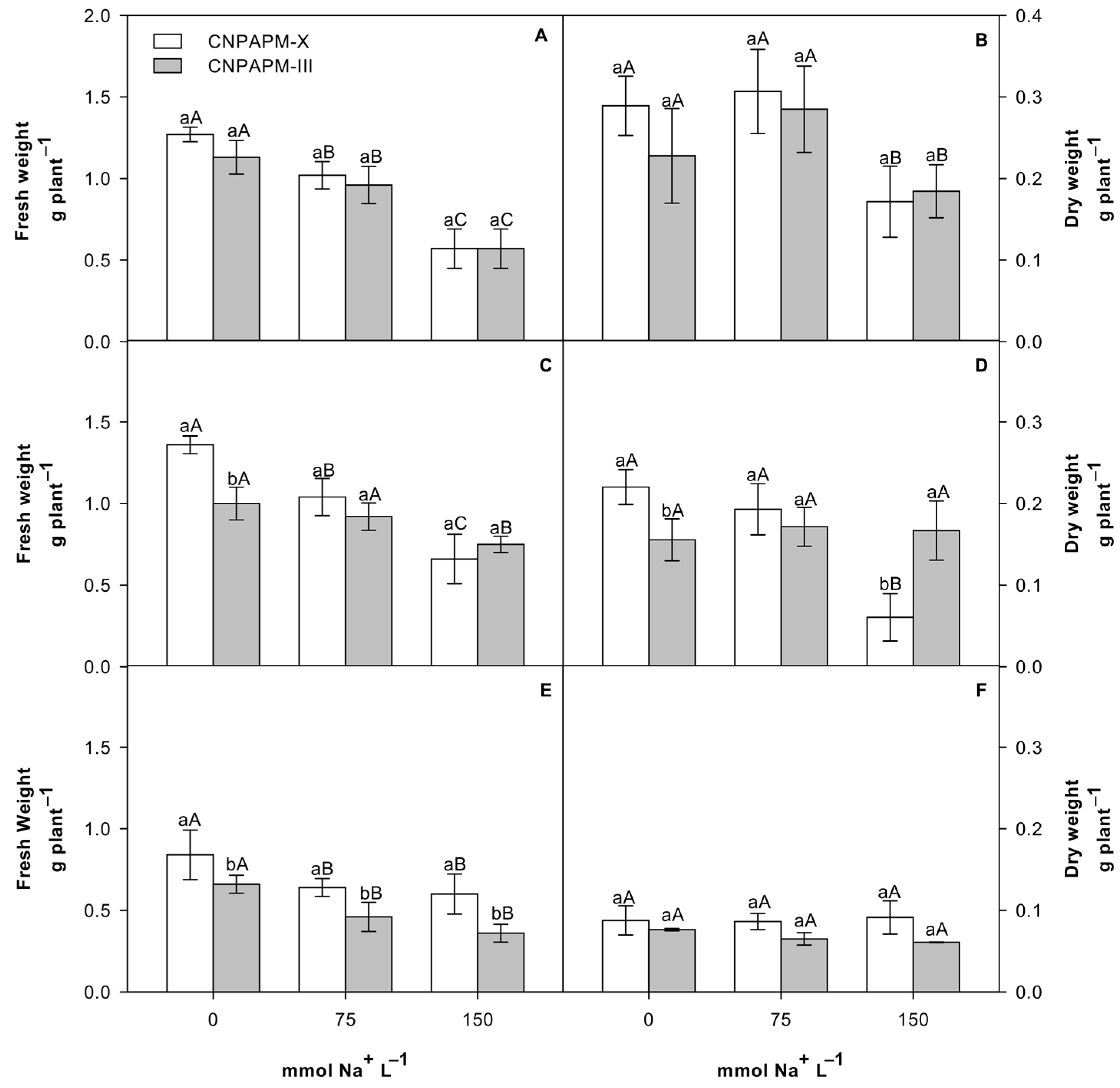
3.1. Effect of Salinity on Biomass and Ionic Partition
3.2. Changes in Carbohydrate Compartmentalization
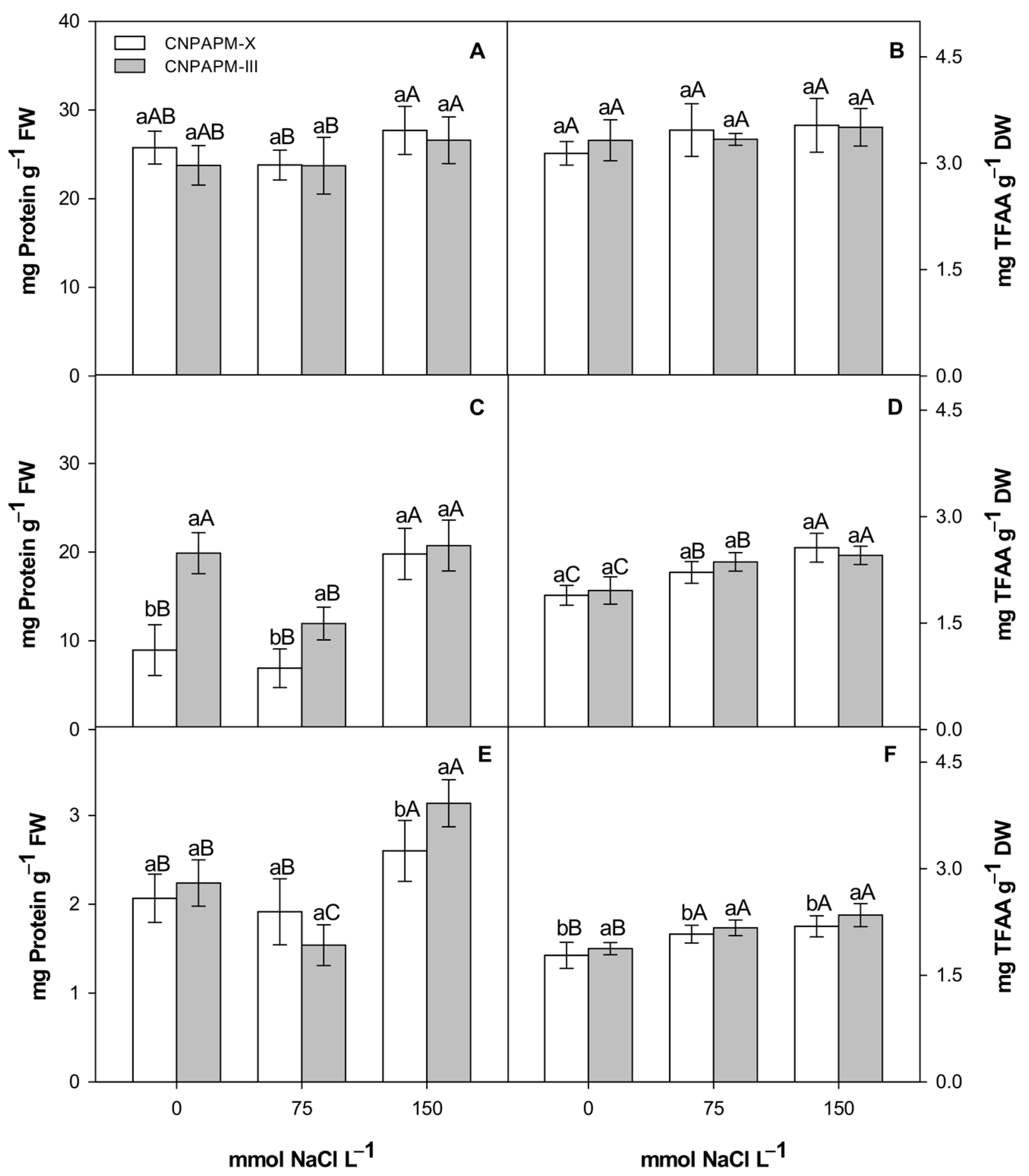
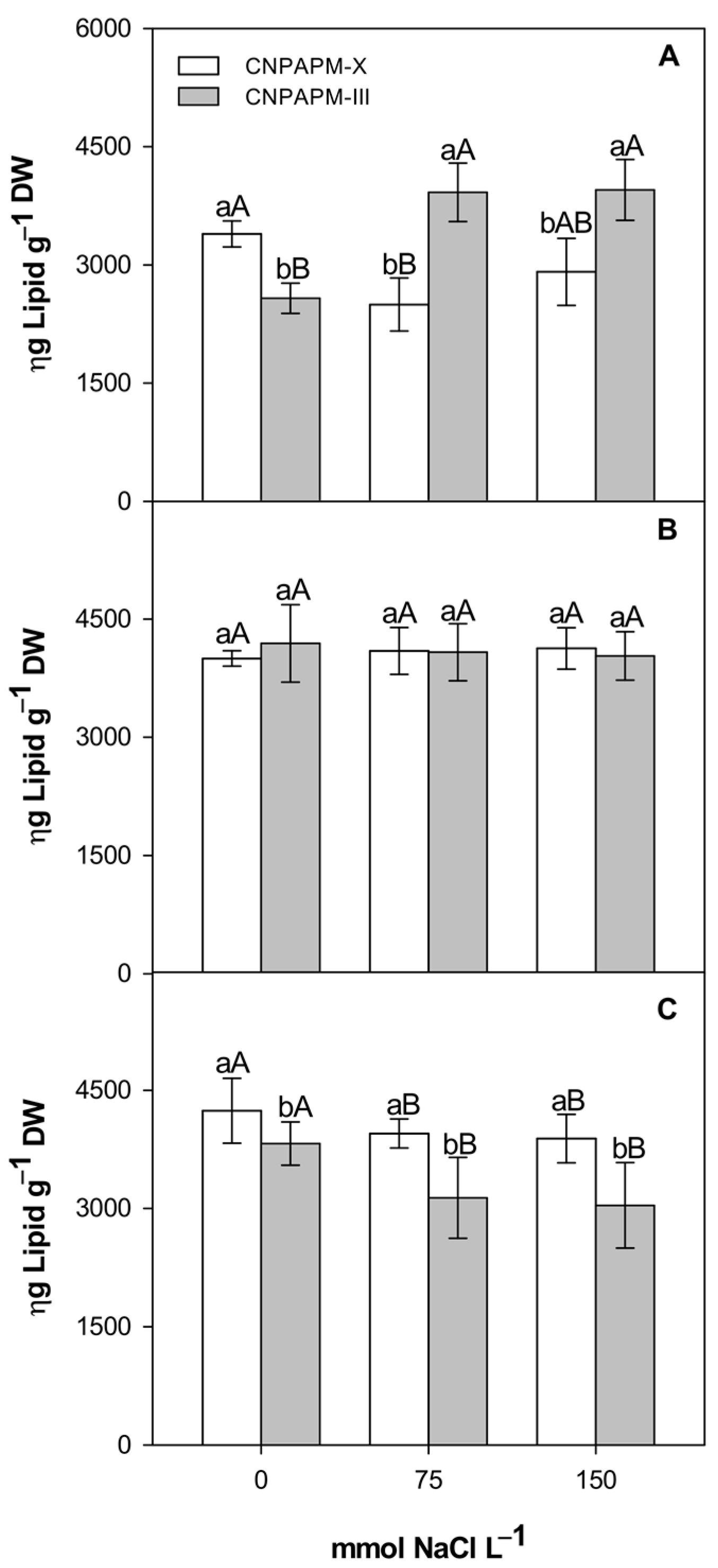
3.3. Changes in Protein, Aminoacids and Lipids Partition
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Mittler, R.; Blumwald, E. Genetic engineering for modern agriculture: Challenges and perspectives. Ann. Rev. Plant Biol. 2010, 61, 443–462. [Google Scholar] [CrossRef] [PubMed]
- Mudalkar, S.; Sreeharsha, R.V.; Reddy, A.R. Involvement of glyoxalases and glutathione reductase in conferring abiotic stress tolerance to Jatropha curcas L. Environ. Exp. Bot. 2017, 134, 141–150. [Google Scholar] [CrossRef]
- Coelho, D.S.; Simões, W.L.; Mendes, A.M.S.; Dantas, B.F.; Rodrigues, J.A.S.; Souza, M.A. Germination and initial growth of varieties of forage sorghum under saline stress. Rev. Bras. Eng. Agric. Ambient. 2014, 18, 25–30. [Google Scholar] [CrossRef]
- Pedrotti, A.; Chagas, R.M.; Ramos, V.C.; Prata, A.P.N.; Lucas, A.A.T.; Santos, P.B. Causes and consequences of the process of soil salinization. Rev. Eletr. Gest. Educ. Tecnol. Amb. 2015, 19, 1308–1324. [Google Scholar]
- Silva, E.N.; Vieira, S.A.; Ribeiro, R.V.; Ponte, L.F.; Ferreira-Silva, S.L.; Silveira, J.A. Contrasting physiological responses of Jatropha curcas plants to single and combined stresses of salinity and heat. J. Plant Growth Regul. 2013, 32, 159–169. [Google Scholar] [CrossRef]
- Singh, R.A.; Kumar, M.; Haider, E. Synergistic cropping of summer groundnut with Jatropha curcas—A new two-tier cropping system for Uttar Pradesh. J. SAT Agric. Res. 2007, 5, 1–2. [Google Scholar]
- Pereira-Filho, J.L.; Gerônimo-Neto, P.S.; Monteiro, P.M.; Ferreira, J.M.S.; Pinheiro, A.A.; Rodrigues, C.D.P.; Vilanova, C.M. Technological prospection on the biological effects of plants of the Jatropha genus. Res. Soc. Dev. 2020, 9, 1–11. [Google Scholar] [CrossRef]
- Calvet, A.S.F.; Pinto, C.M.; Lima, R.E.M.; Maia-Joca, R.P.M.; Bezerra, M.A. Growth and solute accumulation in cowpea irrigated with waters rising salinity in different stages of development. Rev. Irrig. 2013, 18, 148–159. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, C.; Wu, P.; Chen, Y.; Li, M.; Jiang, H.; Wu, G. Global analysis of gene expression profiles in physic nut (Jatropha curcas L.) seedlings exposed to salt stress. PLoS ONE 2014, 9, e97878. [Google Scholar] [CrossRef] [PubMed]
- Cunha, P.C.; Mendes, B.S.S.; Oliveira Filho, R.A.; Camara, T.R.; Willadino, L.G. Growth, synthesis of organic solutes and ionic balance in seedlings of physic nut under saline stress. Rev. Caatinga 2013, 26, 46–52. [Google Scholar]
- Silva, E.N.; Ribeiro, R.V.; Ferreira-Silva, S.L.; Vieira, S.A.; Ponte, L.F.; Silveira, J.A. Coordinate changes in photosynthesis, sugar accumulation and antioxidative enzymes improve the performance of Jatropha curcas plants under drought stress. Biomed. Bioeng. 2012, 45, 270–279. [Google Scholar] [CrossRef]
- Marques, E.C.; Freitas, V.S.; Bezerra, M.A.; Prisco, J.T.; Gomes-Filho, E. Effects of salt stress on germination, emergence and establishment of dwarf-cashew seedling. Rev. Ciênc. Agron. 2011, 42, 993–999. [Google Scholar] [CrossRef]
- Silva, E.N.; Silveira, J.A.G.; Rodrigues, C.R.F.; Lima, C.S.; Viégas, R.A. Contribution of organic and inorganic solutes to osmotic adjustment of physic nut under salinity. Pesq. Agropec. Bras. 2009, 44, 437–445. [Google Scholar] [CrossRef]
- Galdino, L.S.; Lira, E.H.A.; Sousa, V.F.O.; Souza, J.M.; Maia, J.M.; Costa, P.O.C.; Arriel, N.H.C. Standardization of NaCl doses for saline stress induction in Jatropha curcas L. Rev. Ciênc. Agric. 2017, 40, 319–332. [Google Scholar] [CrossRef]
- Martins, C.C.; Machado, C.G.; Cavasini, R. Temperature and substrate for the germination test of physic nut seeds. Ciênc. Agrotec. 2008, 32, 863–868. [Google Scholar] [CrossRef]
- Pascuali, L.C.; Silva, F.S.; Porto, A.G.; Silva Filho, A.; Meneghello, G.E. Germination of physic nut seeds in different temperatures, light and substrates. Semina Ciênc. Agric. 2012, 33, 1435–1440. [Google Scholar] [CrossRef][Green Version]
- Pimenta, A.C.; Zuffellato-Ribas, K.C.; Laviola, B.G. Morphology of fruits, seeds and seedlings of Jatropha curcas. Rev. Flor. 2014, 44, 73–80. [Google Scholar] [CrossRef]
- Morais, E.B.S.D. Standardization of the Germination Test and Qualify of Physic Nut (Jatropha curcas L.). Ph.D. Thesis, Universidade Estadual de Montes Claros, Montes Claros, Brazil, 2008. [Google Scholar]
- Malavolta, E.; Vitti, G.C.; Oliveira, A.S. Avaliação do Estado Nutricional das Plantas: Princípios e Aplicações, 1st ed.; Associação Brasileira para Pesquisa do Potássio e do Fosfato: Piracicaba, Brazil, 1989; pp. 115–143. [Google Scholar]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Morris, D.L. Quantitative determination of carbohydrates with Dreywood’s anthrone reagent. Science 1948, 107, 254–255. [Google Scholar] [CrossRef]
- Yemm, E.W.; Willis, A.J. The estimation of carbohydrates in plant extracts by anthrone. Biochem. J. 1954, 57, 508–514. [Google Scholar] [CrossRef]
- Passos, L.P. Métodos Analíticos e Laboratoriais em Fisiologia Vegetal, 1st ed.; Embrapa: Coronel Pacheco, Brazil, 1996; pp. 90–115. [Google Scholar]
- Mccready, R.M.; Gugoolz, J.; Silveira, V.; Owens, H.S. Determination of starch and amylose in vegetables: Application to peas. Anal. Chem. 1950, 22, 1156–1158. [Google Scholar] [CrossRef]
- Bradford, M.M.A. Rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Peoples, M.B.; Faizah, A.W.; Rerkasem, B.G.; Herridge, D.F. Methods for Evaluating Nitrogen Fixation by Nodulated Legumes in the Field, 1st ed.; Australian Center for International Agricultural Research: Camberra, Australia, 1989; pp. 210–235. [Google Scholar]
- Baumgärtner, T.R.S.; Burak, J.A.M.; Zanin, G.M.; Baumgärtner, D.; Sébastien, N.Y.; Arroyo, P.A. Different methods for extracting oil from the microalga Scenedesmus accuminatus for biodiesel production. Br. J. Anal. Chem. 2013, 10, 441–445. [Google Scholar]
- Banzatto, D.A.; Kronka, S.D. Experimentação Agrícola, 4th ed.; FUNEP: Jaboticabal, Brazil, 2013. [Google Scholar]
- Gadelha, C.G.; Souza Miranda, R.; Alencar, N.L.M.; Costa, J.H.; Prisco, J.T.; Gomes-Filho, E. Exogenous nitric oxide improves salt tolerance during establishment of Jatropha curcas seedlings by ameliorating oxidative damage and toxic ion accumulation. J. Plant. Physiol. 2017, 212, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, H.; Yeasmin, R.; Kalemelawa, F.; Watanabe, T.; Aranami, M.; Nishihara, E. Evaluation of NaCl tolerance in the Physical Reduction of Jatropha curcas L. seedlings. Agric. Sci. 2014, 2, 23–35. [Google Scholar] [CrossRef][Green Version]
- Rodrigues, C.R.F.; Silveira, J.A.G.; Silva, E.N.; Dutra, A.T.B.; Viégas, R.A. Potassium transport and partitioning alleviates toxic effects of sodium on young physic nut plants. Rev. Bras. Ciênc. Sol. 2012, 36, 223–232. [Google Scholar] [CrossRef]
- Hishida, M.; Ascencio, F.; Fujiyama, H.; Orduno-Cruz, A.; Endo, T. Response to salt stress in growth water relations and ion content of Jatropha curcas and J. cinerea seedlings. Intercienc. J. 2013, 38, 289–304. [Google Scholar]
- Oliveira, M.D.M.; Bezerra, L.L.; Dantas, C.V.S.; Voigt, E.L.; Maia, J.M.; Macêdo, C.E.C. The role of xylopodium in Na+ exclusion and osmolyte accumulation in faveleira [Cnidoscolus phyllacanthus (M. Arg.) Pax et K. Hoffm] under salt stress. Acta Phys. Plant. 2014, 36, 2871–2882. [Google Scholar] [CrossRef]
- Lopes, J.C.; Macedo, C.M.P. Germination of Brassica pekinensis affected by seed moisture content, substrate and saline stress. Rev. Bras. Sem. 2008, 30, 79–85. [Google Scholar] [CrossRef]
- Lima, G.S.; Nobre, R.G.; Gheyi, H.R.; Soares, L.A.A.; Pinheiro, F.W.A.; Dias, A.S. Growth, content of sodium, chlorideand ion relation in castor bean under salt stress and nitrogen fertilization. Comun. Scient. 2015, 6, 212–223. [Google Scholar]
- Maathuis, F.J.M.; Amtmann, A. K+ nutrition and Na+ toxicity: The basis of cellular K+/Na+ rations. Ann. Bot. 1999, 84, 123–133. [Google Scholar] [CrossRef]
- Sousa, A.E.C.; Silveira, J.A.G.; Gheyi, H.R.; Lima Neto, M.C.; Lacerda, C.F.; Soares, F.A.L. Gas exchange and contents of carbohydrates and nitrogen compounds in physic nut irrigated with saline water and wastewater. Pesq. Agropec. Bras. 2012, 47, 1428–1435. [Google Scholar] [CrossRef]
- Souza, R.P.; Machado, E.C.; Silveira, J.A.G.; Ribeiro, R.V. Photosynthesis and accumulation of solutes in cowpea plants subjected to salinity. Pesq. Agropec. Bras. 2011, 46, 586–592. [Google Scholar] [CrossRef]
- Gomes, K.R.; Amorim, A.V.; Ferreira, F.J.; Filho, F.L.A.; Lacerda, C.F.; Gomes-Filho, E. Physiology and growth responses of maize subjected to salt stress in different cultiving spacings. Rev. Bras. Eng. Agríc. Ambient. 2011, 15, 365–370. [Google Scholar] [CrossRef]
- Lin, C.C.; Kao, C.H. NaCl stress in rice seedling: Starch mobilization and the influence of gibberellic acid on seedling growth. Bot. Bull. Acad. Sin. 1995, 36, 169–173. [Google Scholar]
- Adda, A.; Regagba, Z.; Latigui, A.; Merah, O. Effect of salt stress on α-amylase activity, sugars mobilization and osmotic potential of Phaseolus vulgaris L. Seeds var. ‘Cocorose’ and ‘Djadida’ during Germination. J. Biol. Sci. 2014, 14, 370–375. [Google Scholar] [CrossRef]
- Dkhil, B.B.; Denden, M. Salt stress induced changes in germination, sugars, starch and enzyme of carbohydrate metabolism in Abelmoschus esculentus L. (Moench.) seeds. Afr. J. Agric. Res. 2010, 5, 408–415. [Google Scholar]
- Reale, L.; Ricci, A.; Ferranti, F.; Torricelli, R.; Venanzoni, R.; Falcinelli, M. Cytohistological analysis and mobilization of reserves in Jatropha curcas L. Seed. Crop. Sci. 2012, 52, 830–835. [Google Scholar] [CrossRef]
- Munns, R.; Weir, R. Contribution of sugars to osmotic adjustment in elongating and expanded zones of wheat leaves during moderate water deficits at two light levels. Aust. J. Plant Phys. 1981, 8, 93–105. [Google Scholar] [CrossRef]
- Sturm, A.; Tang, G.Q. The sucrose-cleaving enzymes of plants are crucial for development, growth and carbon partitioning. Trends Plant Sci. 1999, 4, 401–407. [Google Scholar] [CrossRef]
- Lin, J.; Li, X.; Zhang, Z.; Yu, X.; Gao, Z.; Wang, Y.; Wang, J.; Li, Z.; Mu, C. Salinity-alkalinity tolerance in wheat: Seed germination, early seedling growth, ion relations and solute accumulation. Afr. J. Agric. Res. 2012, 7, 467–474. [Google Scholar] [CrossRef]
- Hussain, S.; Khaliq, A.; Tanveer, M.; Matloob, A.; Hussain, H.A. Aspirin priming circumvents the salinity-induced effects on wheat emergence and seedling growth by regulating starch metabolism and antioxidant enzyme activities. Acta Physiol. Plant. 2018, 40, 68. [Google Scholar] [CrossRef]
- Rocha, J.G.; Ferreira, L.M.; Tavares, O.C.H.; Santos, A.M.; Souza, S.R. Nitrogen absorption kinetics and accumulation of soluble nitrogenous fractions and sugars in sunflower. Pesq. Agropec. Trop. 2014, 44, 381–390. [Google Scholar] [CrossRef]
- Silveira, J.A.G.; Viégas, R.D.; Rocha, I.M.A.; Moreira, A.C.D.M.; Moreira, R.D.; Oliveira, J.T.A. Proline accumulation and glutamine synthetase activity are increased by salt-induced proteolysis in cashew leaves. J. Plant Phys. 2003, 160, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Lobo, A.K.M.; Martins, M.O.; Lima-Neto, M.C.; Bonifácio, A.; Silveira, J.A.G. Nitrogenous compounds and carbohydrates in sorghum subjected to salinity and combinations of nitrate and ammonium. Rev. Ciênc. Agron. 2011, 42, 390–397. [Google Scholar] [CrossRef]
- Mohammadkhani, N.; Heidari, R. Effects of drought stress on soluble proteins in two maize varieties. Turk. J. Biol. 2008, 32, 23–30. [Google Scholar]
- Benko-Iseppon, A.M.; Cavalcanti, N.M.S.; Berlarmino, L.C.; Bezerra-Neto, J.P.; Amorim, L.L.B.; Ferreira-Neto, J.R.; Pandolfi, P.; Azevedo, H.M.S.A.; Silva, R.L.O.; Santos, M.G.; et al. Exploration of Genes for Resistance to Drought and Salinity in Native and Cultivated Plants. Rev. Bras. Geogr. Fís. 2011, 6, 1112–1134. [Google Scholar]
- Nascimento, H.H.C.; Santos, C.A.; Freire, C.S.; Silva, M.A.; Nogueira, R.J.M.C. Osmotic adjustment in jatobá seedlings subjected to salinity in hydroponic medium. Rev. Árv. 2015, 39, 641–653. [Google Scholar] [CrossRef]
- Mansour, M.M.F. Nitrogen containing compounds and adaptation of plants to salinity stress. Biol. Plant. 2000, 43, 491–500. [Google Scholar] [CrossRef]
- Handa, S.; Bressan, R.A.; Handa, A.K.; Carpita, N.C.; Hasegawa, M. Solutes contributing to osmotic adjustment to plant cells adapted to water stress. Plant. Physiol. 1983, 73, 834–843. [Google Scholar] [CrossRef]
- Melo, Y.L. Agronomic Performance and Characterization of Sunflower (Helianthus annuus L.) Genotypes for Phenological, Physiological and Biochemical Markers in Two Edaphoclimate Microregions of Rio Grande do Norte. Ph.D. Thesis, Universidade Federal Rural do Semi-Árido, Mossoró, Brazil, 2012. [Google Scholar]
- Krasensky, J.; Jonak, C. Drought, salt, and temperature stress-induced metabolic rearrangements and regulatory networks. J. Exp. Bot. 2012, 63, 1593–1608. [Google Scholar] [CrossRef] [PubMed]
- Gomes, A.D.V.; Leite, M.J.H.; Freire, A.L.O. Accumulation of organic solutes in plants munquêm (Albizia inundala (Mart)) subjected to salt stress at different levels of sodium chloride. Rev. Verde Agroecol. Desenv. Sustent. 2012, 7, 54–56. [Google Scholar] [CrossRef]
- Aghaleh, M.; Niknam, V.; Ebrahimzadeh, H.; Razavi, K. Effect of salt stress on phisyological and antioxidative responses in two species of Saliconia (S. persica and S. europaea). Acta Physiol. Plant. 2011, 33, 1261–1270. [Google Scholar] [CrossRef]
- Alencar, N.L.M. Mobilização de Reservas Endospérmicas de Pinhão-Manso Durante a Germinação e Desenvolvimento da Plântula sob Condições de Estresse Salino. Master’s Thesis, Universidade Federal do Ceará, Fortaleza, Brazil, 2014. [Google Scholar]
- Ghosh, D.; Xu, J. Abiotic stress responses in plant roots: A proteomics perspective. Front. Plant. Sci 2014, 5, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Tester, M.; Davenport, R. Na+ tolerance and Na+ transport in higher plants. Ann. Bot. 2003, 91, 502–527. [Google Scholar] [CrossRef]


| CNPAPM-X | CNPAPM-III | ||||||
|---|---|---|---|---|---|---|---|
| Leaves | Hypocotyl | Root | Leaves | Hypocotyl | Root | ||
| 0 mM | 611.30aC | 652.06aB | 862.62aA | 468.67bC | 570.55bC | 883.00aB | |
| Na+ | 75 mM | 801.49aB | 978.09aA | 835.45bA | 692.81bB | 903.37bB | 937.34aB |
| 150 mM | 910.17aA | 1005.26aA | 686.02bB | 774.32bA | 1059.60aA | 1175.07aA | |
| 0 mM | 1149.45aA | 1045.75aA | 734.63bA | 1101.59bA | 814.40bB | 981.93aA | |
| K+ | 75 mM | 1141.47aA | 1053.72aA | 726.65bA | 1037.77bA | 1141.47aA | 989.90aA |
| 150 mM | 918.11aB | 1141.47aA | 439.47bB | 942.04aB | 1213.27aA | 1037.77aA | |
| 0 mM | 1.8878bA | 0.1612aA | 0.8514bA | 2.3685aA | 0.1561aA | 1.1126aA | |
| K+/Na+ | 75 mM | 1.4293aB | 0.1168aB | 0.8700bA | 1.5003aB | 0.1264aB | 1.0777aA |
| 150 mM | 1.0093bC | 0.1139aB | 0.6909bB | 1.2201aC | 0.1142aB | 0.8832aB | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lira, E.; Souza, J.; Galdino, L.; Macêdo, C.; Silva, A.; Melo, Y.; Santos, I.; Arriel, N.; Meneses, C.; Maia, J. Changes in Reserve Mobilization Caused by Salinity Could Interfere in the Initial Growth of Jatropha curcas. Sustainability 2021, 13, 7446. https://doi.org/10.3390/su13137446
Lira E, Souza J, Galdino L, Macêdo C, Silva A, Melo Y, Santos I, Arriel N, Meneses C, Maia J. Changes in Reserve Mobilization Caused by Salinity Could Interfere in the Initial Growth of Jatropha curcas. Sustainability. 2021; 13(13):7446. https://doi.org/10.3390/su13137446
Chicago/Turabian StyleLira, Emannuella, Joilma Souza, Lucas Galdino, Cristiane Macêdo, Anselmo Silva, Yuri Melo, Ivanice Santos, Nair Arriel, Carlos Meneses, and Josemir Maia. 2021. "Changes in Reserve Mobilization Caused by Salinity Could Interfere in the Initial Growth of Jatropha curcas" Sustainability 13, no. 13: 7446. https://doi.org/10.3390/su13137446
APA StyleLira, E., Souza, J., Galdino, L., Macêdo, C., Silva, A., Melo, Y., Santos, I., Arriel, N., Meneses, C., & Maia, J. (2021). Changes in Reserve Mobilization Caused by Salinity Could Interfere in the Initial Growth of Jatropha curcas. Sustainability, 13(13), 7446. https://doi.org/10.3390/su13137446







