Post-Anthesis Mobilization of Stem Assimilates in Wheat under Induced Stress
Abstract
1. Introduction
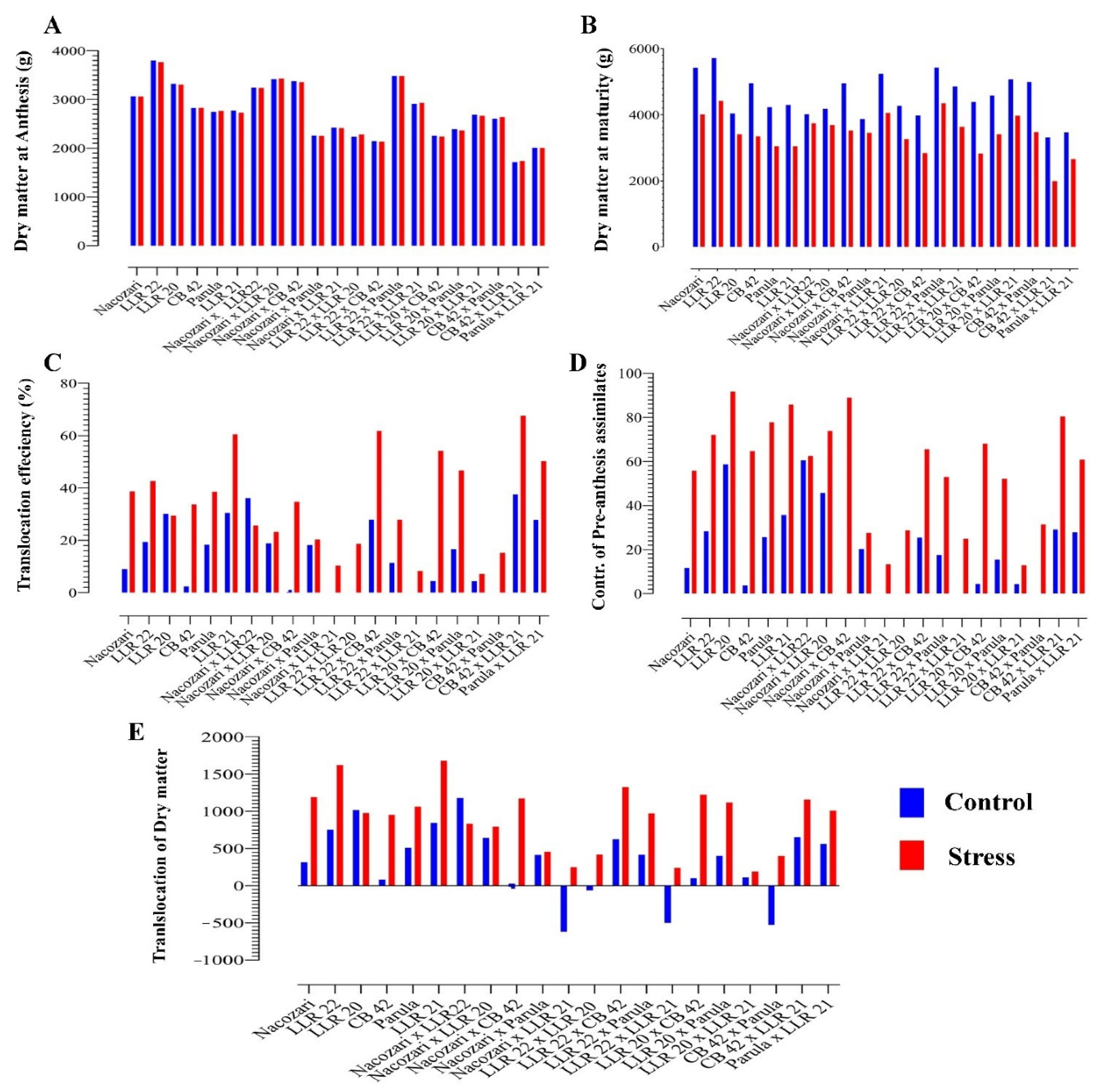
2. Results
Effect of Induced Stress
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Induced Senescence as Stress Stimuli
4.3. Data Collection
4.3.1. Areal Plant Biomass Treated
4.3.2. Areal Plant Biomass for Control
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chaudhry, Q.U.Z. Climate Change Profile of Pakistan; Asian Development Bank: Mandaluyong, Philippines, 2017. [Google Scholar]
- Piepho, H.P.; Nazir, M.F.; Qamar, M.; Rattu, A.U.R.; Hussain, M.; Ahmad, G.; Ahmad, J.; Laghari, K.B.; Vistro, I.A.; Kakar, M.S. Stability analysis for a countrywide series of wheat trials in Pakistan. Crop Sci. 2016, 56, 2465–2475. [Google Scholar] [CrossRef]
- Sarkar, D.; Kar, S.K.; Chattopadhyay, A.; Rakshit, A.; Tripathi, V.K.; Dubey, P.K.; Abhilash, P.C. Low input sustainable agriculture: A viable climate-smart option for boosting food production in a warming world. Ecol. Indic. 2020, 115, 106412. [Google Scholar] [CrossRef]
- Adnan, S.; Ullah, K.; Khan, A.H.; Gao, S. Meteorological impacts on evapotranspiration in different climatic zones of Pakistan. J. Arid Land 2017, 9, 938–952. [Google Scholar] [CrossRef]
- Mardeh, A.S.-S.; Ahmadi, A.; Poustini, K.; Mohammadi, V. Evaluation of drought resistance indices under various environmental conditions. Field Crops Res. 2006, 98, 222–229. [Google Scholar] [CrossRef]
- Kamal, K.; Raizada, M.N.; Navabi, A. Recent Progress in germplasm evaluation and gene mapping to enable breeding of drought-tolerant wheat. Front. Plant Sci. 2020, 11, 1149. [Google Scholar]
- Atlin, G.N.; Cairns, J.E.; Das, B. Rapid breeding and varietal replacement are critical to adaptation of cropping systems in the developing world to climate change. Glob. Food Secur. 2017, 12, 31–37. [Google Scholar] [CrossRef]
- Salem, K.F.M.; Röder, M.S.; Börner, A. Identification and mapping quantitative trait loci for stem reserve mobilisation in wheat (Triticum aestivum L.). Cereal Res. Commun. 2007, 35, 1367–1374. [Google Scholar] [CrossRef]
- Hussain, S.; Saleem, M.F.; Iqbal, J.; Ibrahim, M.; Atta, S.; Ahmad, T.; Rehmani, M.I.A. Exogenous application of abscisic acid may improve the growth and yield of sunflower hybrids under drought. Pak. J. Agric. Sci. 2014, 51, 49–58. [Google Scholar]
- Moayedi, A.A.; Boyce, A.N.; Barakbah, S.S. Influence of water deficit during different growth and developmental stages on the contribution of stored pre-anthesis assimilates to grain in selected durum and bread wheat genotypes. Aust. J. Basic Appl. Sci. 2009, 3, 4408–4415. [Google Scholar]
- Blum, A. Improving wheat grain filling under stress by stem reserve mobilisation. Euphytica 1998, 100, 77–83. [Google Scholar] [CrossRef]
- Bidinger, F.; Musgrave, R.; Fischer, R. Contribution of stored pre-anthesis assimilate to grain yield in wheat and barley. Nature 1977, 270, 431–433. [Google Scholar] [CrossRef]
- Saint Pierre, C.; Trethowan, R.; Reynolds, M. Stem solidness and its relationship to water-soluble carbohydrates: Association with wheat yield under water deficit. Funct. Plant Biol. 2010, 37, 166–174. [Google Scholar] [CrossRef]
- Piepho, H.-P.; Nazir, M.F.; Nawaz Shah, M.K. Design and analysis of a trial to select for stress tolerance. Commun. Biometry Crop Sci. 2016, 11, 1–9. [Google Scholar]
- Hussain, S.; Rivandi, A. Molecular breeding for drought tolerance in plants: Wheat perspective. Proc. Pak. Acad. Sci. 2007, 44, 35–62. [Google Scholar]
- Ding, L.; Wang, K.; Jiang, G.; Liu, M.; Niu, S.; Gao, L. Post-anthesis changes in photosynthetic traits of maize hybrids released in different years. Field Crops Res. 2005, 93, 108–115. [Google Scholar] [CrossRef]
- Shao, C.; Shen, L.; Qiu, C.; Wang, Y.; Qian, Y.; Chen, J.; Ouyang, Z.; Zhang, P.; Guan, X.; Xie, J. Characterizing the impact of high temperature during the grain filling on phytohormone levels, enzyme activities and metabolic profiles of the early indica-rice variety. Plant Biol. 2021. [Google Scholar] [CrossRef]
- Seleiman, M.F.; Al-Suhaibani, N.; Ali, N.; Akmal, M.; Alotaibi, M.; Refay, Y.; Dindaroglu, T.; Abdul-Wajid, H.H.; Battaglia, M.L. Drought stress impacts on plants and different approaches to alleviate its adverse effects. Plants 2021, 10, 259. [Google Scholar] [CrossRef]
- Rehmani, M.I.A.; Wei, G.; Hussain, N.; Ding, Q.; Li, G.; Liu, Z.; Wang, S.; Ding, D. Yield and quality responses of two indica rice hybrids to post-anthesis asymmetric day and night open-field warming in lower reaches of Yangtze River delta. Field Crops Res. 2014, 156, 231–241. [Google Scholar] [CrossRef]
- Nazir, M.F.; Jia, Y.; Ahmed, H.; He, S.; Iqbal, M.S.; Sarfraz, Z.; Ali, M.; Feng, C.; Raza, I.; Sun, G. Genomic insight into differentiation and selection sweeps in the improvement of upland cotton. Plants 2020, 9, 711. [Google Scholar] [CrossRef]
- Marascuilo, L.A.; Levin, J.R. The simultaneous investigation of interaction and nested hypotheses in two-factor analysis of variance designs. Am. Educ. Res. J. 1976, 13, 61–65. [Google Scholar] [CrossRef]
- Atlin, G.; Baker, R.; McRae, K.B.; Lu, X. Selection response in subdivided target regions. Crop Sci. 2000, 40, 7–13. [Google Scholar] [CrossRef]
- Ali, M.H.S.; Akhtar, N.; Saif-Ur-Rehman, A.S.; Nadeem, M.; Tanveer, M.H. Genetic analysis of Pakistani wheat germplasm for yield contributing traits under normal and heat stressed conditions. Pak. J. Agric. Sci. 2020, 57, 1503–1508. [Google Scholar]
- Sarfraz, Z.; Shah, M.M.; Iqbal, M.S.; Nazir, M.F.; Fatima, S.A. Identification of valuable traits through molecular and morphological markers in diploid wheat. Pak. J. Biotechnol. 2020, 17, 71–77. [Google Scholar] [CrossRef]
- Khan, I.; Lei, H.; Khan, A.; Muhammad, I.; Javeed, T.; Khan, A.; Huo, X. Yield gap analysis of major food crops in Pakistan: Prospects for food security. Environ. Sci. Pollut. Res. 2021, 28, 7994–8011. [Google Scholar] [CrossRef]
- Farrukh, M.U.; Bashir, M.K.; Hassan, S.; Adil, S.A.; Kragt, M.E. Mapping the food security studies in India, Pakistan and Bangladesh: Review of research priorities and gaps. Glob. Food Secur. 2020, 26, 100370. [Google Scholar] [CrossRef]
- Nazir, M.F.; Mahmood, T.; Shah, M.K.N.; Sarfraz, Z.; Ali, W.; Metlo, M.A.U.; Iqbal, M.S. Inheritance studies for morpho-physiological traits in wheat under rainfed condition. Pak. J. Biotechnol. 2019, 16, 105–113. [Google Scholar]
- Sun, Y.; Wang, C.; Chen, H.Y.; Ruan, H. Response of plants to water stress: A meta-analysis. Front. Plant Sci. 2020, 11, 978. [Google Scholar] [CrossRef]
- Yasir, T.A.; Khan, A.; Skalicky, M.; Wasaya, A.; Rehmani, M.I.A.; Sarwar, N.; Mubeen, K.; Aziz, M.; Hassan, M.M.; Hassan, F.A.S.; et al. Exogenous Sodium Nitroprusside Mitigates Salt Stress in Lentil (Lens culinaris Medik.) by Affecting the Growth, Yield, and Biochemical Properties. Molecules 2021, 26, 2576. [Google Scholar] [CrossRef]
- Samah, M.A.; Aiad, M.A.; Khatab, I.A. Genetic diversity and phenotypic association with salinity tolerance in Egyptian barley cultivars using SRAP markers. J. Environ. Agric. Sci. 2017, 13, 51–56. [Google Scholar]
- Shah, S.H.; Houborg, R.; McCabe, M.F. Response of chlorophyll, carotenoid and SPAD-502 measurement to salinity and nutrient stress in wheat (Triticum aestivum L.). Agronomy 2017, 7, 61. [Google Scholar] [CrossRef]
- Hebbar, K.; Neethu, P.; Sukumar, P.A.; Sujithra, M.; Santhosh, A.; Ramesh, S.; Niral, V.; Hareesh, G.; Nameer, P.O.; Prasad, P. Understanding physiology and impacts of high temperature stress on the progamic phase of coconut (Cocos nucifera L.). Plants 2020, 9, 1651. [Google Scholar] [CrossRef] [PubMed]
- Sattar, A.; Iqbal, M.M.; Areeb, A.; Ahmed, Z.; Irfan, M.; Shabbir, R.N.; Aishia, G.; Hussain, S. Genotypic variations in wheat for phenology and accumulative heat unit under different sowing times. J. Environ. Agric. Sci. 2015, 2, 8. [Google Scholar]
- Zhang, Y.; Pan, J.; Huang, X.; Guo, D.; Lou, H.; Hou, Z.; Su, M.; Liang, R.; Xie, C.; You, M. Differential effects of a post-anthesis heat stress on wheat (Triticum aestivum L.) grain proteome determined by iTRAQ. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Schittenhelm, S.; Langkamp-Wedde, T.; Kraft, M.; Kottmann, L.; Matschiner, K. Effect of two-week heat stress during grain filling on stem reserves, senescence, and grain yield of European winter wheat cultivars. J. Agron. Crop Sci. 2020, 206, 722–733. [Google Scholar] [CrossRef]
- Ehdaie, B.; Alloush, G.; Waines, J. Genotypic variation in linear rate of grain growth and contribution of stem reserves to grain yield in wheat. Field Crops Res. 2008, 106, 34–43. [Google Scholar] [CrossRef]
- Borrell, A.K.; Incoll, L.; Dalling, M.J. The influence of the Rht1 and Rht2 alleles on the deposition and use of stem reserves in wheat. Ann. Bot. 1993, 71, 317–326. [Google Scholar] [CrossRef]
- Ehdaie, B.; Alloush, G.; Madore, M.; Waines, J. Genotypic variation for stem reserves and mobilization in wheat: II. Postanthesis changes in internode water-soluble carbohydrates. Crop Sci. 2006, 46, 2093–2103. [Google Scholar] [CrossRef]
- Setter, T.L.; Meller, V.H. Reserve carbohydrate in maize stem: [14C] glucose and [14C] sucrose uptake characteristics. Plant Physiol. 1984, 75, 617–622. [Google Scholar] [CrossRef]
- Andrade, F.H.; Ferreiro, M.A. Reproductive growth of maize, sunflower and soybean at different source levels during grain filling. Field Crops Res. 1996, 48, 155–165. [Google Scholar] [CrossRef]
- Wang, G.-Q.; Hao, S.-S.; Gao, B.; Chen, M.-X.; Liu, Y.-G.; Yang, J.-C.; Ye, N.-H.; Zhang, J.-H. Regulation of gene expression in the remobilization of carbon reserves in rice stems during grain filling. Plant Cell Physiol. 2017, 58, 1391–1404. [Google Scholar] [CrossRef]
- Fu, J.; Huang, Z.; Wang, Z.; Yang, J.; Zhang, J. Pre-anthesis non-structural carbohydrate reserve in the stem enhances the sink strength of inferior spikelets during grain filling of rice. Field Crops Res. 2011, 123, 170–182. [Google Scholar] [CrossRef]
- Blum, A.; Mayer, J.; Golan, G. Chemical desiccation of wheat plants as a simulator of post-anthesis stress: II. Relations to drought stress. Field Crops Res. 1983, 6, 149–155. [Google Scholar] [CrossRef]
- Salem, K.F.; Elabsawy, E.A.; Aldahak, L.; Elshamy, H. Evaluation of stem reserve mobilization in Egyptian bread wheat (Triticum aestivum L.) genotypes and F1 hybrids under post-anthesis chemical desiccation stress. J. Genet. Environ. Resour. Conserv. 2021, 9, 176–182. [Google Scholar]
- Tarawneh, R.A.; Szira, F.; Monostori, I.; Behrens, A.; Alqudah, A.M.; Thumm, S.; Lohwasser, U.; Röder, M.S.; Börner, A.; Nagel, M. Genetic analysis of drought response of wheat following either chemical desiccation or the use of a rain-out shelter. J. Appl. Genet. 2019, 60, 137–146. [Google Scholar] [CrossRef]
- Gare, S.; Wagh, R.; Ingle, A.; Soni, N. Effect of temperature on stem reserve mobilization for grain development in wheat. J. Pharmacogn. Phytochem. 2018, 7, 1119–1123. [Google Scholar]
- Dodig, D.; Kandić, V.; Zorić, M.; Nikolić-Đorić, E.; Nikolić, A.; Mutavdžić, B.; Perović, D.; Šurlan-Momirović, G. Comparative kernel growth and yield components of two-and six-row barley (Hordeum vulgare) under terminal drought simulated by defoliation. Crop Pasture Sci. 2019, 69, 1215–1224. [Google Scholar] [CrossRef]
- Resende, M.D.V.d. Software Selegen-REML/BLUP: A useful tool for plant breeding. Crop Breed. Appl. Biotechnol. 2016, 16, 330–339. [Google Scholar] [CrossRef]
- Papakosta, D.K.; Gagianas, A. Nitrogen and dry matter accumulation, remobilization, and losses for Mediterranean wheat during grain filling. Agron. J. 1991, 83, 864–870. [Google Scholar] [CrossRef]
- D’Steel, R.G.; Torrie, J.H. Principles and Procedures of Statistics: A Biometrical Approach; McGraw-Hill: New York, NY, USA, 1986. [Google Scholar]
- Stehlik, M.; Wagner, H. Exact likelihood ratio testing for homogeneity of the exponential distribution. Commun. Stat. Simul. Comput. 2011, 40, 663–684. [Google Scholar] [CrossRef]
| SOV | Df | DMA | DMM | TDM | TE | CPA |
|---|---|---|---|---|---|---|
| Replication | 2 | 2334.381 ns | 2971.341 ns | 9122.198 ns | 3.133 ns | 7.41 ns |
| Treatment (T) | 1 | 243.056 ** | 38,006,177.7 ** | 10,549,093 ** | 11,600.643 ** | 43,260.51 ** |
| Error | 2 | 1811.937 | 278.167 | 2468.865 | 7.198 | 0.872 |
| Genotype (G) | 20 | 1,835,737.2 ** | 2,096,902.058 ** | 1,045,662 ** | 1160.817 ** | 2246.031 ** |
| T × G | 41 | 748.772 ** | 212,916.902 ** | 242,712.4 ** | 322.716 ** | 639.084 ** |
| Error | 80 | 1661.159 | 636.312 | 5210.865 | 4.009 | 5.298 |
| SOV | Df | TGW (g) | DMA (g) | DMM (g) | TDM (g) | TE% | CPA | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F | p | F | p | F | p | F | p | F | p | F | p | |||
| Cont. | Rep | 2 | 1.02 | ns | 0.07 | ns | 5.26 | ** | 1.77 | ns | 3.4 | 0.043 | 3.21 | ns |
| Gen | 20 | 66.75 | *** | 1254.2 | *** | 2782.4 | *** | 478.6 | *** | 490.1 | *** | 658.4 | *** | |
| GCA | 5 | 100.62 | *** | 1561.53 | *** | 1702.4 | *** | 171.21 | *** | 136.55 | *** | 379.5 | *** | |
| SCA | 15 | 55.46 | *** | 1151.82 | *** | 3142.5 | *** | 581.16 | *** | 608.03 | *** | 751.42 | *** | |
| St | Rep | 2 | 0.1 | ns | 1.59 | ns | 0.98 | ns | 1 | ns | 0.98 | 0.3849 | 0.34 | ns |
| Geno | 20 | 2609.16 | *** | 351.94 | *** | 1252.9 | *** | 63.84 | *** | 140 | *** | 202.06 | *** | |
| GCA | 5 | 3986.5 | *** | 447.01 | *** | 2143 | *** | 16.03 | *** | 83.52 | *** | 58.63 | *** | |
| SCA | 15 | 2150.05 | *** | 320.25 | *** | 956.01 | *** | 79.78 | *** | 158.9 | *** | 249.87 | *** | |
| Experiment | TGW (g) | DMA (g) | DMM (g) | TDM (g) | TE% | CPA | |
|---|---|---|---|---|---|---|---|
| Control | 0.68 | 739.47 | 467.69 | 1501.93 | 1.0299 | 1.6321 | |
| Induced Stress | 0.0201 | 2582.85 | 804.93 | 8919.8 | 6.989 | 8.9604 | |
| LR-Test for Homogeneity of Variance | x2 | 87.74 | 14.72 | 2.91 | 28.26 | 32.14 | 26.05 |
| p | <0.0001 | 0.0001 | 0.0879 | <0.0001 | <0.0001 | <0.0001 | |
| SOV | Df | TGW | DMA (g) | DMM (g) | TDM (g) | TE% | CPA | |
|---|---|---|---|---|---|---|---|---|
| Replicate-Treatment | 5 | F | 897.38 | 0.68 | 11779.9 | 399.78 | 571.44 | 1610.19 |
| p | <0.0001 | 0.64 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | ||
| Genotype | 20 | F | 250.6 | 1105.09 | 3295.4 | 200.67 | 289.55 | 424.08 |
| p | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | ||
| GCA | 5 | F | 402.6 | 1389.95 | 3430.09 | 23.87 | 77.72 | 50.77 |
| p | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | ||
| SCA | 15 | F | 199.94 | 1010.14 | 3250.52 | 259.68 | 360.16 | 548.52 |
| p | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | ||
| Genotype-Treatment | 20 | F | 28.87 | 0.45 | 334.61 | 46.58 | 80.49 | 120.67 |
| p | <0.0001 | 0.9749 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | ||
| GCA-Treatment | 5 | F | 21.75 | 0.2 | 532 | 52.95 | 102.95 | 165.38 |
| p | <0.0001 | 0.9631 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | ||
| SCA-Treatment | 15 | F | 31.25 | 0.54 | 268.81 | 44.47 | 73 | 105.77 |
| p | <0.0001 | 0.9099 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| Genotype | TGW (g) | DMA (g) | DMM (g) | TE% | TDM | CPA | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SRi | p | SRi | p | SRi | p | SRi | p | SRi | p | SRi | p | |
| Nacozari | −0.3 | 1.0 | 1.3 | 1.0 | 333.1 | <0.0001 | −312.9 | <0.0001 | −11.1 | <0.0001 | −7.4 | 0.0 |
| Nacozari × LLR22 | −1.0 | 0.6 | 4.8 | 1.0 | −869.9 | <0.0001 | 969.9 | <0.0001 | 31.0 | <0.0001 | 36.8 | <0.0001 |
| Nacozari × LLR 20 | 4.5 | <0.0001 | −16.9 | 1.0 | −643.8 | <0.0001 | 452.2 | <0.0001 | 15.5 | <0.0001 | 9.3 | <0.0001 |
| Nacozari × CB 42 | 0.2 | 1.0 | 15.3 | 1.0 | 349.6 | <0.0001 | −637.7 | <0.0001 | −16.1 | <0.0001 | −54.5 | <0.0001 |
| Nacozari × Paula | −0.6 | 1.0 | 3.0 | 1.0 | −713.1 | <0.0001 | 559.7 | <0.0001 | 17.9 | <0.0001 | 31.2 | <0.0001 |
| Nacozari × LLR21 | −2.7 | <0.0001 | 7.2 | 1.0 | 85.0 | 0.0 | −305.2 | <0.0001 | 9.3 | <0.0001 | 24.9 | <0.0001 |
| LLR 22 | −4.9 | <0.0001 | 31.0 | 1.0 | 211.0 | <0.0001 | −304.8 | <0.0001 | −4.4 | 0.2 | −7.1 | 0.0 |
| LLR 22 × LLR 20 | 1.4 | 0.1 | −53.3 | 0.9 | −95.3 | 0.0 | 102.6 | 0.8 | 0.6 | 1.0 | 8.7 | 0.0 |
| LLR 22 × CB 42 | 0.4 | 1.0 | 11.4 | 1.0 | 42.3 | 0.6 | −129.1 | 0.5 | −15.4 | <0.0001 | −3.3 | 0.8 |
| LLR 22 × Paula | −0.9 | 0.7 | −4.3 | 1.0 | −20.8 | 1.0 | 20.7 | 1.0 | 2.8 | 0.9 | 1.7 | 1.0 |
| LLR 22 × LLR 21 | 4.8 | <0.0001 | −22.5 | 1.0 | 127.3 | <0.0001 | −169.0 | 0.1 | 11.5 | <0.0001 | 12.7 | <0.0001 |
| LLR 20 | −0.2 | 1.0 | 13.9 | 1.0 | −495.4 | <0.0001 | 647.5 | <0.0001 | 21.0 | <0.0001 | 4.3 | 0.4 |
| LLR 20 × CB 42 | −0.5 | 1.0 | 14.6 | 1.0 | 482.9 | <0.0001 | −571.5 | <0.0001 | −32.1 | <0.0001 | −27.9 | <0.0001 |
| LLR 20 × Paula | 3.9 | <0.0001 | 21.6 | 1.0 | 76.2 | 0.0 | −144.5 | 0.3 | −11.4 | <0.0001 | 0.3 | 1.0 |
| LLR 20 × LLR 21 | −1.7 | 0.0 | 18.8 | 1.0 | 0.3 | 1.0 | 521.5 | <0.0001 | 17.3 | <0.0001 | 29.9 | <0.0001 |
| CB 42 | 5.6 | <0.0001 | −4.3 | 1.0 | 537.2 | <0.0001 | −307.6 | <0.0001 | −12.8 | <0.0001 | −25.1 | <0.0001 |
| CB 42 × Paula | −0.6 | 1.0 | −36.5 | 1.0 | 435.7 | <0.0001 | −364.3 | <0.0001 | 4.1 | 0.3 | 5.9 | 0.1 |
| CB 42 × LLR 21 | −2.1 | 0.0 | −28.5 | 1.0 | 228.5 | <0.0001 | 76.3 | 1.0 | −11.5 | <0.0001 | −15.0 | <0.0001 |
| Paula | −0.3 | 1.0 | −22.9 | 1.0 | 80.4 | 0.0 | 23.8 | 1.0 | −1.3 | 1.0 | −16.0 | <0.0001 |
| Paula × LLR 21 | −3.5 | <0.0001 | 1.3 | 1.0 | −305.0 | <0.0001 | 138.6 | 0.4 | −3.3 | 0.6 | 4.3 | 0.4 |
| LLR 21 | −1.5 | 0.1 | 45.0 | 1.0 | 153.9 | <0.0001 | −266.3 | 0.0 | −11.3 | <0.0001 | −13.7 | <0.0001 |
| SE | 0.5 | 34.1 | 21.1 | 60.4 | 1.7 | 1.9 | ||||||
| SOV | TGW (g) | DMA (g) | DMM (g) | TE% | TDM | CPA |
|---|---|---|---|---|---|---|
| 14.9024 | 308,943.45 | 433,260.52 | 239,132.1 | 167.9183 | 357.66478 | |
| 17.4721 | 302,552.56 | 335,651.14 | 186,835.69 | 323.94253 | 600.51176 | |
| 0.8016 | 1 | 0.8226291 | 0.6330897 | 0.5989808 | 0.5778774 | |
| 0.6800 | 696.74361 | 467.69211 | 1501.9254 | 1.0298581 | 1.632095 | |
| 0.02010 | 2162.5795 | 804.93264 | 8919.8047 | 6.9890113 | 8.9604112 | |
| 0.9850 | 0.9992488 | 0.9996403 | 0.9979108 | 0.9979598 | 0.9984812 | |
| 0.9996 | 0.9976231 | 0.9992013 | 0.9843355 | 0.9928597 | 0.9950509 | |
| 0.7957 | 1.0008145 | 0.8228098 | 0.6374404 | 0.6005172 | 0.5788727 | |
| 1.4321 | 13,408 | 5930.75 | 885.84 | 0.00000 | 0.00 | |
| 10.0279 | 13,927 | 63,706 | 79,752 | 146.22 | 282.74 | |
| 0 | 6673.19 | 591.43 | 1541.84 | 0.0000 | 16.7078 | |
| 2.1136 | 53,856 | 38,929 | 45,475 | 24.2590 | 56.1866 | |
| 0 | 4532.98 | 4049.25 | 146.24 | 8.3914 | 0.00 | |
| 4.3910 | 46,997 | 13,982 | 18,935 | 167.32 | 315.65 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nazir, M.F.; Sarfraz, Z.; Mangi, N.; Nawaz Shah, M.K.; Mahmood, T.; Mahmood, T.; Iqbal, M.S.; Ishaq Asif Rehmani, M.; El-Sharnouby, M.; Shabaan, M.K.A.; et al. Post-Anthesis Mobilization of Stem Assimilates in Wheat under Induced Stress. Sustainability 2021, 13, 5940. https://doi.org/10.3390/su13115940
Nazir MF, Sarfraz Z, Mangi N, Nawaz Shah MK, Mahmood T, Mahmood T, Iqbal MS, Ishaq Asif Rehmani M, El-Sharnouby M, Shabaan MKA, et al. Post-Anthesis Mobilization of Stem Assimilates in Wheat under Induced Stress. Sustainability. 2021; 13(11):5940. https://doi.org/10.3390/su13115940
Chicago/Turabian StyleNazir, Mian Faisal, Zareen Sarfraz, Naimatullah Mangi, Muhammad Kausar Nawaz Shah, Talat Mahmood, Tahir Mahmood, Muhammad Shahid Iqbal, Muhammad Ishaq Asif Rehmani, Mohamed El-Sharnouby, Mohamed Khamees Aly Shabaan, and et al. 2021. "Post-Anthesis Mobilization of Stem Assimilates in Wheat under Induced Stress" Sustainability 13, no. 11: 5940. https://doi.org/10.3390/su13115940
APA StyleNazir, M. F., Sarfraz, Z., Mangi, N., Nawaz Shah, M. K., Mahmood, T., Mahmood, T., Iqbal, M. S., Ishaq Asif Rehmani, M., El-Sharnouby, M., Shabaan, M. K. A., Sorour, S. G. R., & EL Sabagh, A. (2021). Post-Anthesis Mobilization of Stem Assimilates in Wheat under Induced Stress. Sustainability, 13(11), 5940. https://doi.org/10.3390/su13115940










