A Parametric Study on a Diesel Engine Fuelled Using Waste Cooking Oil Blended with Al2O3 Nanoparticle—Performance, Emission, and Combustion Characteristics
Abstract
:1. Introduction
2. Materials and Experimental Methodologies
2.1. Fuel Formulation
2.2. Engine Test Rig
3. Results and Discussion
3.1. Fuel Characterization
3.2. Variation in in-Cylinder Pressure with Respect to Crank Angle
3.3. Variation of Heat Release Rate
3.4. Analysis of Work Done
3.5. Speed Torque Characteristics of the Engine
3.6. Performance Characteristics
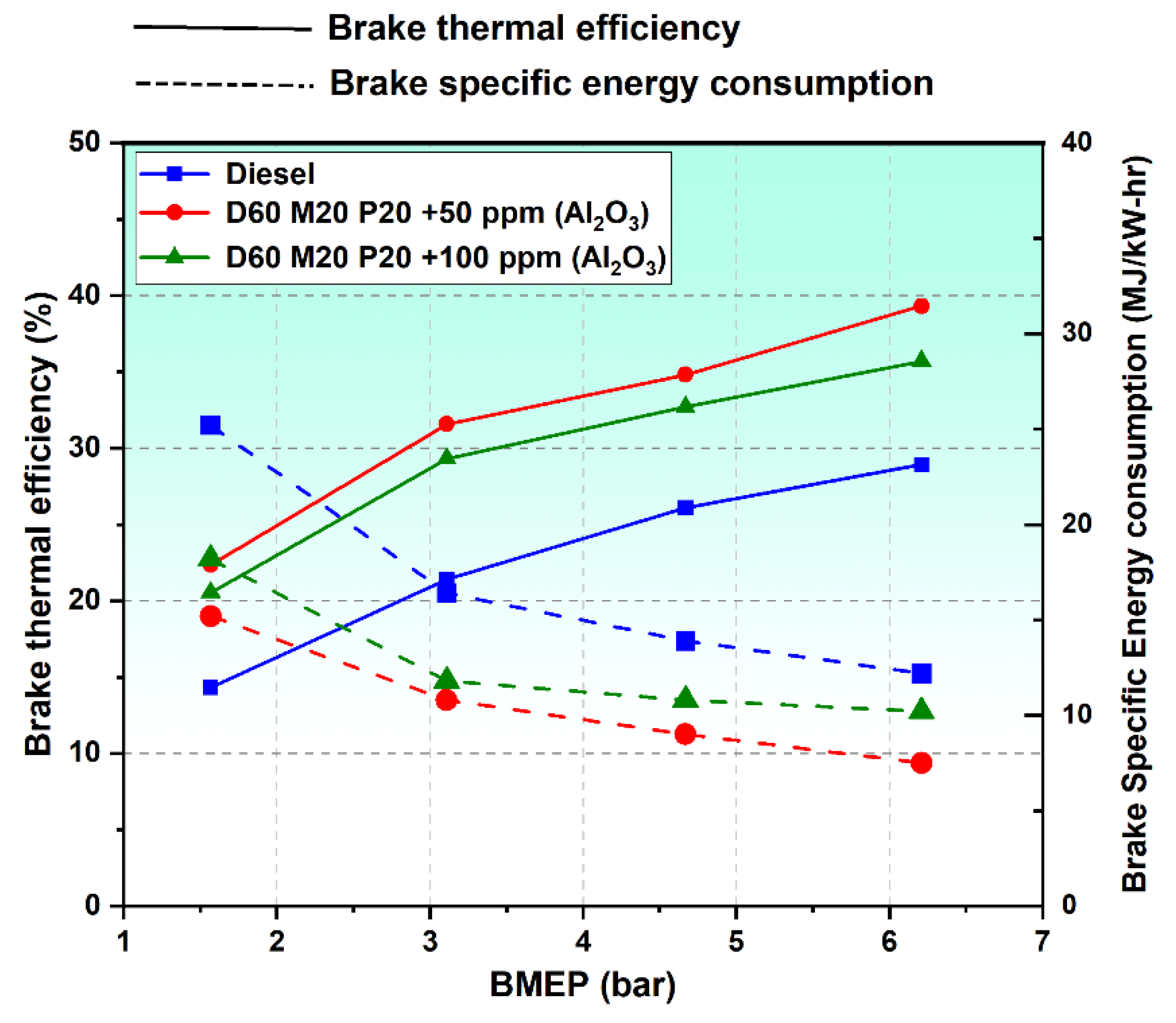
3.6.1. Brake Thermal Efficiency
3.6.2. Brake-Specific Energy Consumption
3.7. Emission Characteristics
3.7.1. Variation in Carbon Emissions
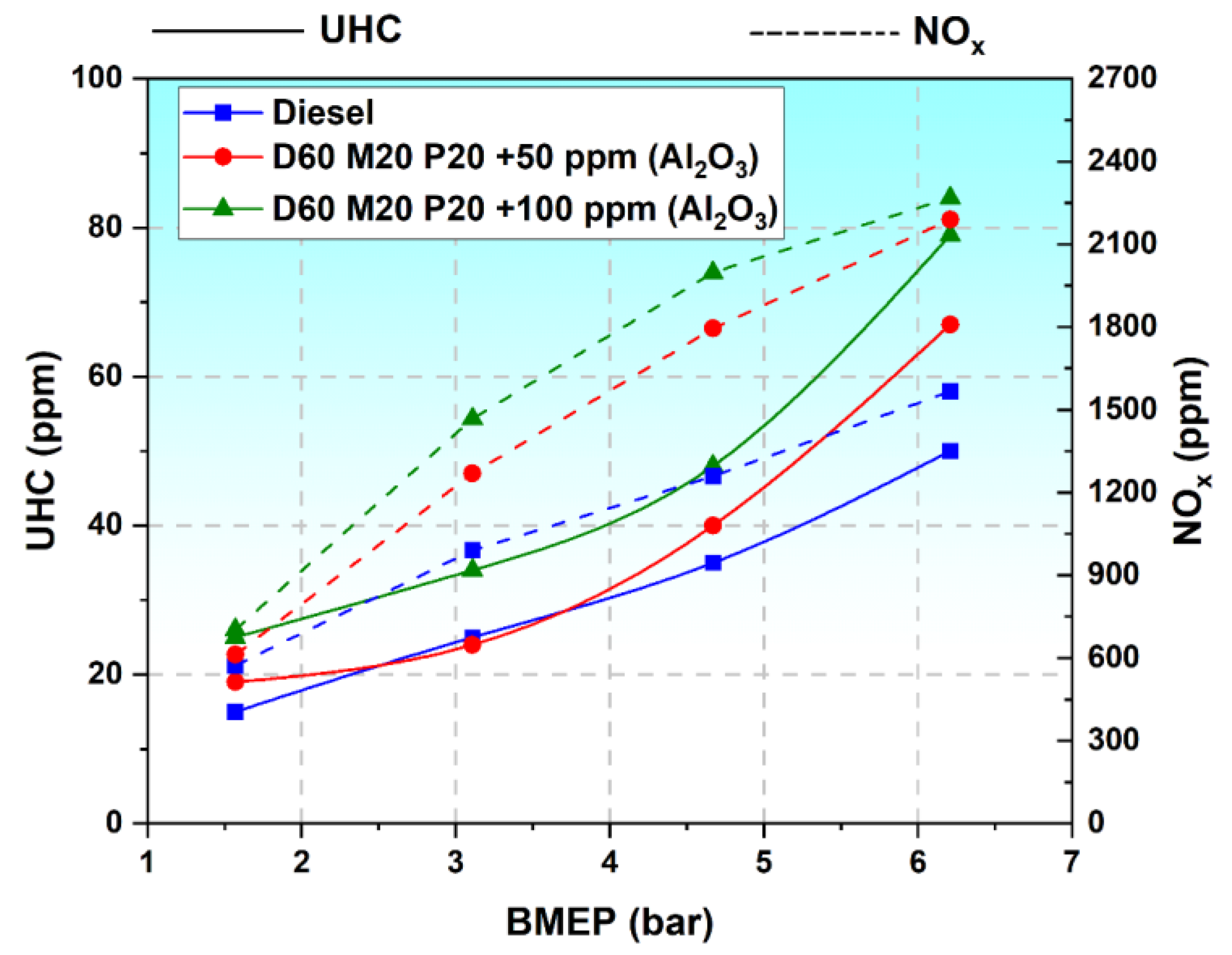
3.7.2. Variation in Oxides of Nitrogen
3.7.3. Variation in Unburnt Hydrocarbon Emissions
4. Conclusions
- Usage of the oxygenator in the form of nanoparticles will considerably increase the performance when compared with pure biodiesel blends.
- The biodiesel will increase the ignition delay of the fuel.
- The work done from biodiesel will be more when compared with diesel, but the restriction in internal energy of the system reduces the work done in biodiesel.
- The addition of metal oxides such as nanoparticles in smaller extents can achieve better performance.
- The emission for the blends with nanoparticles has been found to be increased.
- The addition of metallic oxides nanoparticle will also account for nanometals emission, which is unregulated.
5. Future Research
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Atabani, A.; Silitonga, A.; Ong, H.; Mahlia, T.M.I.; Masjuki, H.; Badruddin, I.A.; Fayaz, H. Non-edible vegetable oils: A critical evaluation of oil extraction, fatty acid compositions, biodiesel production, characteristics, engine performance and emissions production. Renew. Sustain. Energy Rev. 2013, 18, 211–245. [Google Scholar] [CrossRef]
- Uner, A.; Ebinc, F.A.; Akyurek, N.; Unsal, D.; Mentes, B.B.; Dursun, A. Vascular endothelial growth factor, C-ERBB-2 and C-ERBB-3 expression in colorectal adenoma and adenocarcinoma. Exp. Oncol. 2005, 27, 225–228. [Google Scholar]
- Enweremadu, C.C.; Rutto, H.L. Combustion, emission and engine performance characteristics of used cooking oil bio-diesel—A review. Renew. Sustain. Energy Rev. 2010, 14, 2863–2873. [Google Scholar] [CrossRef]
- Enweremadu, C.; Mbarawa, M. Technical aspects of production and analysis of biodiesel from used cooking oil—A review. Renew. Sustain. Energy Rev. 2009, 13, 2205–2224. [Google Scholar] [CrossRef]
- Xue, J.; Grift, T.E.; Hansen, A.C. Effect of biodiesel on engine performances and emissions. Renew. Sustain. Energy Rev. 2011, 15, 1098–1116. [Google Scholar] [CrossRef]
- Sudhir, C.; Sharma, N.Y. Potential of UCOs as Biodiesel Feed Stock. Engineering 2007, 12, 69–75. [Google Scholar]
- Demirbas, A. Biodiesel from UCO via base-catalytic and supercritical methanol transesterification. Energy Convers. Manag. 2009, 50, 923–927. [Google Scholar] [CrossRef]
- Demirbas, A. Biodiesel fuels from vegetable oils via catalytic and non-catalytic supercritical alcohol transesterifications and other methods: A survey. Energy Convers. Manag. 2003, 44, 2093–2109. [Google Scholar] [CrossRef]
- Kannan, G.; Anand, R. Effect of injection pressure and injection timing on DI diesel engine fuelled with biodiesel from waste cooking oil. Biomass Bioenergy 2012, 46, 343–352. [Google Scholar] [CrossRef]
- Berchmans, H.J.; Hirata, S. Biodiesel production from crude Jatropha curcas L. seed oil with a high content of free fatty acids. Bioresour. Technol. 2008, 99, 1716–1721. [Google Scholar] [CrossRef]
- Hwang, J.; Qi, D.; Jung, Y.; Bae, C. Effect of injection parameters on the combustion and emission characteristics in a common-rail direct injection diesel engine fueled with waste cooking oil biodiesel. Renew. Energy 2014, 63, 9–17. [Google Scholar] [CrossRef]
- Encinar, J.M.; González, J.F.; Rodríguez-Reinares, A. Ethanolysis of used frying oil. Biodiesel preparation and charac-terization. Fuel Process. Technol. 2007, 88, 513–522. [Google Scholar] [CrossRef]
- Xue, J. Combustion characteristics, engine performances and emissions of waste edible oil biodiesel in diesel engine. Renew. Sustain. Energy Rev. 2013, 23, 350–365. [Google Scholar] [CrossRef]
- Muralidharan, K.; Vasudevan, D.; Sheeba, K. Performance, emission and combustion characteristics of biodiesel fuelled variable compression ratio engine. Energy 2011, 36, 5385–5393. [Google Scholar] [CrossRef]
- Meher, L.; Sagar, D.V.; Naik, S. Technical aspects of biodiesel production by transesterification—A review. Renew. Sustain. Energy Rev. 2006, 10, 248–268. [Google Scholar] [CrossRef]
- Raqeeb, A.; Bhargavi, R. Biodiesel production from UCO. J. Chem. Pharm. Res. 2015, 7, 670–681. [Google Scholar]
- Math, M.C.; Kumar, S.P.; Chetty, S.V. Technologies for biodiesel production from used cooking oil—A review. Energy Sustain. Dev. 2010, 14, 339–345. [Google Scholar] [CrossRef]
- Canakci, M.; Van Gerpen, J. Biodiesel production from oils and fats with high free fatty acids. Trans. ASAE 2001, 44, 1429–1436. [Google Scholar] [CrossRef]
- Kulkarni, M.G.; Dalai, A.K. UCO—An economical source for biodiesel: A review. Ind. Eng. Chem. Res. 2006, 45, 2901–2913. [Google Scholar] [CrossRef]
- Lam, M.K.; Lee, K.T.; Mohamed, A.R. Homogeneous, heterogeneous and enzymatic catalysis for transesterification of high free fatty acid oil (UCO) to biodiesel: A review. Biotechnol. Adv. 2010, 28, 500–518. [Google Scholar] [CrossRef]
- Al-Widyan, M.I.; Al-Shyoukh, A.O. Experimental evaluation of the transesterification of waste palm oil into biodiesel. Bioresour. Technol. 2002, 85, 253–256. [Google Scholar] [CrossRef]
- Singh, S.P.; Singh, D. Biodiesel production through the use of different sources and characterization of oils and their esters as the substitute of diesel: A review. Renew. Sustain. Energy Rev. 2010, 14, 200–216. [Google Scholar] [CrossRef]
- Lapuerta, M.; Herreros, J.M.; Lyons, L.L.; García-Contreras, R.; Briceño, Y. Effect of the alcohol type used in the production of waste cooking oil biodiesel on diesel performance and emissions. Fuel 2008, 87, 3161–3169. [Google Scholar] [CrossRef]
- Lapuerta, M.; Fernández, J.R.; Agudelo, J. Diesel particulate emissions from used cooking oil biodiesel. Bioresour. Technol. 2008, 99, 731–740. [Google Scholar] [CrossRef]
- Dorado, M.P.; Ballesteros, E.; Arnal, J.M.; Gómez, J.; López, F.J. Exhaust emissions from a Diesel engine fueled with transesterified waste olive oil. Fuel 2003, 82, 1311–1315. [Google Scholar] [CrossRef]
- Pugazhvadivu, M.; Jeyachandran, K. Investigations on the performance and exhaust emissions of a diesel engine using preheated waste frying oil as fuel. Renew. Energy 2005, 30, 2189–2202. [Google Scholar] [CrossRef]
- Can, Ö. Combustion characteristics, performance and exhaust emissions of a diesel engine fueled with a UCO biodiesel mixture. Energy Convers. Manag. 2014, 87, 676–686. [Google Scholar] [CrossRef]
- Valente, O.S.; Pasa, V.M.D.; Belchior, C.; Sodré, J.R. Exhaust emissions from a diesel power generator fuelled by waste cooking oil biodiesel. Sci. Total. Environ. 2012, 431, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Chen, G.; Wang, Y. Biodiesel production from UCO via alkali catalyst and its engine test. Fuel Process. Technol. 2008, 89, 851–857. [Google Scholar] [CrossRef]
- Di, Y.; Cheung, C.S.; Huang, Z. Experimental investigation of particulate emissions from a diesel engine fueled with ultralow-sulfur diesel fuel blended with diglyme. Atmos. Environ. 2010, 44, 55–63. [Google Scholar] [CrossRef]
- Zhang, Y.; Dubé, M.A.; Mclean, D.D.; Kates, M. Biodiesel production from UCO: 1. Process design and technological assessment. Bioresour. Technol. 2003, 89, 1–16. [Google Scholar] [CrossRef]
- Zhang, Y.; Dubé, M.; McLean, D.; Kates, M. Biodiesel production from waste cooking oil: 2. Economic assessment and sensitivity analysis. Bioresour. Technol. 2003, 90, 229–240. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, S.O.; Zhang, Z. Preparation of biodiesel from UCO via two-step catalyzed process. Energy Convers. Manag. 2007, 48, 184–188. [Google Scholar] [CrossRef]
- Wang, Y.; Ou, S.; Liu, P.; Xue, F.; Tang, S. Comparison of two different processes to synthesize biodiesel by waste cooking oil. J. Mol. Catal. A Chem. 2006, 252, 107–112. [Google Scholar] [CrossRef]
- Utlu, Z.; Koçak, M.S. The effect of biodiesel fuel obtained from waste frying oil on direct injection diesel engine performance and exhaust emissions. Renew. Energy 2008, 33, 1936–1941. [Google Scholar] [CrossRef]
- Bhatia, S.K.; Gurav, R.; Choi, T.-R.; Kim, H.J.; Yang, S.-Y.; Song, H.-S.; Park, J.Y.; Park, Y.-L.; Han, Y.-H.; Choi, Y.-K.; et al. Conversion of waste cooking oil into biodiesel using heterogenous catalyst derived from cork biochar. Bioresour. Technol. 2020, 302, 122872. [Google Scholar] [CrossRef] [PubMed]
- Khounani, Z.; Hosseinzadeh-Bandbafha, H.; Nizami, A.-S.; Sulaiman, A.; Goli, S.A.H.; Tavassoli-Kafrani, E.; Ghaffari, A.; Rajaeifar, M.A.; Kim, K.-H.; Talebi, A.F.; et al. Unlocking the potential of walnut husk extract in the production of waste cooking oil-based biodiesel. Renew. Sustain. Energy Rev. 2020, 119, 109588. [Google Scholar] [CrossRef]
- Chong, C.T.; Tian, B.; Ng, J.-H.; Fan, L.; Ni, S.; Wong, K.Y.; Hochgreb, S. Quantification of carbon particulates produced under open liquid pool and prevaporised flame conditions: Waste cooking oil biodiesel and diesel blends. Fuel 2020, 270, 117469. [Google Scholar] [CrossRef]











| Name | Kirloskar |
|---|---|
| Number of cylinder | 1 |
| Displacement Volume | 622cc |
| Bore × Stroke | 87.5 mm × 110 mm |
| Compression ratio | 17.4:1 |
| Operating speed | 1500 rpm |
| Injection Timing | 23 deg BTDC |
| Injection Pressure | 200 bar |
| Power | 4.41 kW |
| Property | Diesel | Palm Oil Biodiesel | D60 M20 P20 +50 ppm (Al2O3) | D60 M20 P20 +100 ppm (Al2O3) |
|---|---|---|---|---|
| Density (kg/m3) | 855 | 846 | 866 | 878 |
| Kinematic Viscosity (Cst) (at 40 °C) | 2.95 | 3.10 | 3.28 | 3.36 |
| Flash Point (°C) | 60–80 | 35 | 36 | 39 |
| Fire Point (°C) | 96 | 38 | 40 | 43 |
| Calorific Value (MJ/Kg) | 43.000 | 39.252 | 41.111 | 41.189 |
| Peak No | Retention Time | Description of Esters | Name of the Acid | Molecular Structure |
|---|---|---|---|---|
| 1 | 16.215 | Methyl tetradecanoate | Tetradecanoic Acid |  |
| 2 | 18.140 | 9-hexadecenoic acid, methyl ester | Palmitic acid |  |
| 3 | 18.407 | Hexadecanolic acid, methyl ester | Palmitic acid |  |
| 4 | 20.014 | 9,12-octadecadienoic acid, methyl ester | Linoleic acid |  |
| 5 | 20.216 | 11-octadecenoic acid, methyl ester | Oleic acid |  |
| 6 | 20.303 | Methyl stearate | Stearic acid |  |
| 7 | 21.661 | Oxlraneoctanoic acid, methyl ester | Octanoic acid |  |
| 8 | 21.784 | Oxlraneoctanoic acid, methyl ester | Octanoic acid |
| Type of Bond | Transmittance (cm−1) | Intensity |
|---|---|---|
| C–O | 1054 | strong |
| 1075 | ||
| 1237 | ||
| C–N | 1115 | medium |
| 1186 | ||
| C=O | 1738 | strong |
| O–H (ACID) | 2858 | strong, very broad |
| 2956 | ||
| N-H | 3344 | medium, broad |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ponnusamy, M.; Ramani, B.; Sathyamruthy, R. A Parametric Study on a Diesel Engine Fuelled Using Waste Cooking Oil Blended with Al2O3 Nanoparticle—Performance, Emission, and Combustion Characteristics. Sustainability 2021, 13, 7195. https://doi.org/10.3390/su13137195
Ponnusamy M, Ramani B, Sathyamruthy R. A Parametric Study on a Diesel Engine Fuelled Using Waste Cooking Oil Blended with Al2O3 Nanoparticle—Performance, Emission, and Combustion Characteristics. Sustainability. 2021; 13(13):7195. https://doi.org/10.3390/su13137195
Chicago/Turabian StylePonnusamy, Muruganantham, Bharathwaaj Ramani, and Ravishankar Sathyamruthy. 2021. "A Parametric Study on a Diesel Engine Fuelled Using Waste Cooking Oil Blended with Al2O3 Nanoparticle—Performance, Emission, and Combustion Characteristics" Sustainability 13, no. 13: 7195. https://doi.org/10.3390/su13137195
APA StylePonnusamy, M., Ramani, B., & Sathyamruthy, R. (2021). A Parametric Study on a Diesel Engine Fuelled Using Waste Cooking Oil Blended with Al2O3 Nanoparticle—Performance, Emission, and Combustion Characteristics. Sustainability, 13(13), 7195. https://doi.org/10.3390/su13137195








