The Monitoring of Selected Heavy Metals Content and Bioavailability in the Soil-Plant System and Its Impact on Sustainability in Agribusiness Food Chains
Abstract
:1. Introduction
1.1. Heavy Metals in Soil–Plant Systems
1.2. Chemical and Toxicological Aproperties of Selected Contaminants
2. Materials and Methods
2.1. Study Area
Monitored Sites (Localities I–III)-Arable Lands for Cultivating Cereals
2.2. Equipment and Analytical Procedure
2.3. Statistical Analysis
3. Results
3.1. Heavy Metals in Soil Units of Localities I–III
Correlations Analysis between Selected Heavy Metals Contents at Localities I–III
3.2. Influence of Selected Factors (pH and TOC) on the Mobility of Heavy Metals in Soil
3.2.1. pH Values in Soil Units of Localities I–III
3.2.2. Correlation Analysis between pH Values and Heavy Metal Contents
3.2.3. Analysis of Total Organic Carbon Content
Analysis of Differences between Seasons in pH(H2O), pH(KCl) Values and TOC Content
Analysis of Differences between Localities I–III in pH(H2O) and pH(KCl) Values and TOC Content in Soil
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, S.; Lin, H.; Deng, L.; Gong, G.; Jia, Y.; Xu, X.; Li, T.; Li, Y.; Chen, H. Cadmium tolerance and accumulation characteristics of Siegesbeckia orientalis L. Ecol. Eng. 2013, 51, 133–139. [Google Scholar] [CrossRef]
- Steffan, J.J.; Brevik, E.C.; Burgess, L.C.; Cerdàcet, A. The effect of soil on human health: An overview. Eur. J. Soil Sci. 2018, 69, 159–171. [Google Scholar] [CrossRef] [Green Version]
- Brevik, E.C. Soils and human health: An overview. In Soils and Human Health; Brevik, E.C., Burgess, L.C., Eds.; CRC Press: Boca Raton, FL, USA, 2013; pp. 29–56. [Google Scholar]
- Von Lindern, I.; Spalinger, S.; Stifelman, M.L.; Stanek, L.W.; Bartrem, C. Estimating Children’s Soil/Dust Ingestion Rates through Retrospective Analyses of Blood Lead Biomonitoring from the Bunker Hill Superfund Site in Idaho. Environ. Health Perspect. 2016, 124, 1462–1470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xing, W.; Zhao, Q.; Scheckel, K.; Zheng, L.; Li, L. Inhalation bioaccessibility of Cd, Cu, Pb and Zn and speciation of Pb in particulate matter fractions from areas with different pollution characteristics in Henan Province, China. Ecotoxicol. Environ. Saf. 2019, 175, 192–200. [Google Scholar] [CrossRef]
- Brevik, E.C.; Burgess, L.C. Soil: Influence on Human Health. In Encyclopedia of Environmental Management; Jorgensen, S.V., Ed.; CRC Press: Boca Raton, FL, USA, 2015; pp. 1–13. [Google Scholar]
- Steinnes, E. Soils and human health. In Sustaining Soil Productivity in Response to Global Climate Change: Science, Policy, and Ethics; Sauer, T., Norman, J.M., Sivakumar, M.V.K., Eds.; John Wiley & Sons. Inc.: Chichester, UK, 2011; pp. 79–86. [Google Scholar]
- Green, H.; Broun, P.; Cakmak, I.; Condon, L.; Fedoroff, N.; Gonzalez-Valero, J.; Graham, I.; Lewis, J.; Moloney, M.; Oniang’O, R.K.; et al. Planting seeds for the future of food. J. Sci. Food Agric. 2016, 96, 1409–1414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tóth, G.; Hermann, T.; Da Silva, M.; Montanarella, L. Heavy metals in agricultural soils of the European Union with implications for food safety. Environ. Int. 2016, 88, 299–309. [Google Scholar] [CrossRef]
- Zeng, Y.; Lai, Z.; Yang, W.; Li, H. Distribution of heavy metals in surface sediments from the Pearl River outlets. South China: Five-year monitoring program. Fresen. Environ. Bull. 2018, 27, 574–583. [Google Scholar]
- Rodríguez-Bocanegra, J.; Roca, N.; Febrero, A.; Bort, J. Assessment of heavy metal tolerance in two plant species growing in experimental disturbed polluted urban soil. J. Soil. Sediment. 2018, 18, 2305–2317. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, L.; Li, Y.; Li, H.; Wang, W.; Ye, B. Impacts of lead/zinc mining and smelting on the environment and human health in China. Environ. Monit. Assess. 2012, 184, 2261–2273. [Google Scholar] [CrossRef]
- Pepper, I.L. The Soil Health-Human Health Nexus. Crit. Rev. Environ. Sci. Technol. 2013, 43, 2617–2652. [Google Scholar] [CrossRef]
- Oliver, M.A.; Gregory, P.J. Soil, food security and human health: A review. Eur. J. Soil Sci. 2014, 66, 257–276. [Google Scholar] [CrossRef]
- Pandey, S.; Parvez, S.; Sayeed, I.; Haque, R.; Bin-Hafeez, B.; Raisuddin, S. Biomarkers of oxidative stress: A comparative study of river Yamuna fish Wallago attu (Bl. & Schn.). Sci. Total Environ. 2003, 309, 105–115. [Google Scholar] [CrossRef]
- Li, Z.; Ma, Z.; van der Kuijp, T.J.; Yuan, Z.; Huang, L. A review of soil heavy metal pollution from mines in China: Pollution and health risk assessment. Sci. Total Environ. 2014, 468-469, 843–853. [Google Scholar] [CrossRef]
- Satpathy, D.; Reddy, M.V.; Dhal, S.P. Risk Assessment of Heavy Metals Contamination in Paddy Soil, Plants, and Grains (Oryza sativa L.) at the East Coast of India. BioMed Res. Int. 2014, 2014, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, B.; Wang, J.; Jin, B.; Li, Y.; Shi, Z. Assessment of the potential health risks of heavy metals in soils in a coastal industrial region of the Yangtze River Delta. Environ. Sci. Pollut. Res. Int. 2017, 24, 19816–19826. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Liu, J.; Wang, Q.; Wang, Y. Source identification and availability of heavy metals in peri-urban vegetable soils: A case study from China. Hum. Ecol. Risk Assess. Int. J. 2016, 22, 1–14. [Google Scholar]
- Navarrete, I.A.; Gabiana, C.; Dumo, J.R.E.; Salmo Severino, S.G., III; Guzman, M.A.L.G.; Valera, N.S.; Espiritu, E.Q. Heavy metal concentrations in soils and vegetation in urban areas of Quezon City. Philippines. Environ. Monit. Assess. 2017, 189, 1–15. [Google Scholar] [CrossRef]
- Stocking, M.A. Tropical Soils and Food Security: The Next 50 Years. Science 2003, 302, 1356–1359. [Google Scholar] [CrossRef] [Green Version]
- Combs, G.F., Jr. Geological impacts on nutrition. In Essentials of Medical Geology; Selinus, O., Alloway, B., Centeno, J.A., Finkelman, R.B., Fuge, R., Lindh, U., Smedley, P., Eds.; Elsevier: Dordrecht, The Netherlands, 2005; pp. 161–177. [Google Scholar]
- Henry, J.M.; Cring, F.D. Geophagy: An anthropological perspective. In Soils and Human Health; Brevik, E.C., Burgess, L.C., Eds.; CRC Press: Boca Raton, FL, USA, 2013; pp. 179–198. [Google Scholar]
- Nagajyoti, P.C.; Lee, K.D.; Sreekanth, T.V.M.; Nagajyoti, P.C.; Lee, K.D.; Sreekanth, T.V.M. Heavy metals, occurrence and toxicity for plants: A review. Environ. Chem. Lett. 2010, 8, 199–216. [Google Scholar] [CrossRef]
- Rattan, R.; Datta, S.; Chhonkar, P.; Suribabu, K.; Singh, A. Long-term impact of irrigation with sewage effluents on heavy metal content in soils, crops and groundwater—A case study. Agric. Ecosyst. Environ. 2005, 109, 310–322. [Google Scholar] [CrossRef]
- Urminská, J.; Tóth, T.; Prokeinová, R.B.; Ondrišík, P. The effect of the selected remediation medium on the cadmium bioavailability in the selected ecosystem in the Southwestern locality of Slovakia. Ekológia 2019, 38, 214–224. [Google Scholar] [CrossRef] [Green Version]
- Wu, L.; Pan, X.; Chen, L.; Huang, Y.; Teng, Y.; Luo, Y.; Christie, P. Occurrence and distribution of heavy metals and tetracyclines in agricultural soils after typical land use change in east China. Environ. Sci. Pollut. Res. Int. 2013, 20, 8342–8354. [Google Scholar] [CrossRef] [PubMed]
- Ding, Q.; Cheng, G.; Wang, Y.; Zhuang, D. Effects of natural factors on the spatial distribution of heavy metals in soils surrounding mining regions. Sci. Total Environ. 2017, 578, 577–585. [Google Scholar] [CrossRef] [PubMed]
- Shen, F.; Liao, R.; Ali, A.; Mahar, A.; Guo, D.; Li, R.; Xining, S.; Awasthi, M.K.; Wang, Q.; Zhang, Z. Spatial distribution and risk assessment of heavy metals in soil near a Pb/Zn smelter in Feng County, China. Ecotoxico. Environ. Saf. 2017, 139, 254–262. [Google Scholar] [CrossRef]
- Lin, Y.-P.; Teng, T.-P.; Chang, T.-K. Multivariate analysis of soil heavy metal pollution and landscape pattern in Changhua county in Taiwan. Landsc. Urban Plan. 2002, 62, 19–35. [Google Scholar] [CrossRef]
- Shi, T.; Ma, J.; Wu, X.; Ju, T.; Lin, X.; Zhang, Y.; Li, X.; Gong, Y.; Hou, H.; Zhao, L.; et al. Inventories of heavy metal inputs and outputs to and from agricultural soils: A review. Ecotoxicol. Environ. Saf. 2018, 164, 118–124. [Google Scholar] [CrossRef]
- Hauptvogl, M.; Kotrla, M.; Prčík, M.; Pauková, Ž.; Kováčik, M.; Lošák, T. Phytoremediation Potential of Fast-Growing Energy Plants: Challenges and Perspectives—A Review. Pol. J. Environ. Stud. 2019, 29, 505–516. [Google Scholar] [CrossRef] [Green Version]
- Schwarz, K.; Pickett, S.T.A.; Lathrop, R.G.; Weathers, K.C.; Pouyat, R.V.; Cadenasso, M.L. The effects of the urban built environment on the spatial distribution of lead in residential soils. Environ. Pollut. 2012, 163, 32–39. [Google Scholar] [CrossRef]
- Djingova, R.; Kuleff, I. Instrumental Techniques for Trace Analysis. In Trace Elements—Their Distribution and Effects in the Environment; Vernet, J.P., Ed.; Elsevier: London, UK, 2000; pp. 137–185. [Google Scholar]
- Hu, W.; Huang, B.; Tian, K.; Holm, P.E.; Zhang, Y. Heavy metals in intensive greenhouse vegetable production systems along Yellow Sea of China: Levels, transfer and health risk. Chemosphere 2017, 167, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Viventsova, E.; Kumpiene, J.; Gunneriusson, L.; Holmgren, A. Changes in soil organic matter composition and quantity with distance to a nickel smelter—a case study on the Kola Peninsula, NW Russia. Geoderma 2005, 127, 216–226. [Google Scholar] [CrossRef]
- Park, J.H.; Lamb, D.; Paneerselvam, P.; Choppala, G.; Bolan, N.; Chung, J.-W. Role of organic amendments on enhanced bioremediation of heavy metal(loid) contaminated soils. J. Hazard. Mater. 2011, 185, 549–574. [Google Scholar] [CrossRef]
- Mahar, A.; Wang, P.; Li, R.; Zhang, Z. Immobilization of Lead and Cadmium in Contaminated Soil Using Amendments: A Review. Pedosphere 2015, 25, 555–568. [Google Scholar] [CrossRef]
- Wiszniewska, A.; Hanus-Fajerska, E.; Muszyńska, E.; Ciarkowska, K. Natural Organic Amendments for Improved Phytoremediation of Polluted Soils: A Review of Recent Progress. Pedosphere 2016, 26, 1–12. [Google Scholar] [CrossRef]
- Beesley, L.; Moreno-Jiménez, E.; Gomez-Eyles, J.L.; Harris, E.; Robinson, B.; Sizmur, T. A review of biochars’ potential role in the remediation. revegetation and restoration of contaminated soils. Environ. Pollut. 2011, 159, 3269–3282. [Google Scholar] [CrossRef] [PubMed]
- Komárek, M.; Vanek, A.; Ettler, V. Chemical stabilization of metals and arsenic in contaminated soils using oxides—A review. Environ. Pollut. 2013, 172, 9–22. [Google Scholar] [CrossRef]
- Bolan, N.; Kunhikrishnan, A.; Thangarajan, R.; Kumpiene, J.; Park, J.; Makino, T.; Kirkham, M.B.; Scheckel, K. Remediation of heavy metal(loid)s contaminated soils—To mobilize or to immobilize? J. Hazard. Mater. 2014, 266, 141–166. [Google Scholar] [CrossRef]
- Hernandez-Soriano, M.C.; Jimenez-Lopez, J.C. Effects of soil water content and organic matter addition on the speciation and bioavailability of heavy metals. Sci. Total Environ. 2012, 423, 55–61. [Google Scholar] [CrossRef]
- Jing, F.; Chen, C.; Chen, X.; Liu, W.; Wen, X.; Hu, S.; Yang, Z.; Guo, B.; Xu, Y.; Yu, Q. Effects of wheat straw derived biochar on cadmium availability in a paddy soil and its accumulation in rice. Environ. Pollut. 2020, 257, 113592. [Google Scholar] [CrossRef]
- Nordberg, G.F.; Fowler, B.; Nordberg, M. Cadmium. In Handbook on the Toxicology of Metals, 4th ed.; Nordberg, G.F., Fowler, B.A., Nordberg, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2015; Volume 2, pp. 667–716. [Google Scholar]
- Makovníková, J.; Barančíková, G.; Dlapa, P.; Dercová, K. Anorganické kontaminanty v pôdnom ekosystéme. Chem. Listy 2006, 100, 424–432. [Google Scholar]
- Halušková, L.; Valentovičová, K.; Huttová, J.; Mistrík, I.; Tamás, L. Effect of heavy metals on root growth and peroxidase activity in barley root tip. Acta Physiol. Plant. 2010, 32, 59–65. [Google Scholar] [CrossRef]
- Cobbett, C.; Goldsbrough, P. Phytochelatins and Metallothioneins: Roles in Heavy Metal Detoxification and Homeostasis. Annu. Rev. Plant Biol. 2002, 53, 159–182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chandrasekaran, A.; Ravisankar, R.; Harikrishnan, N.; Satapathy, K.; Prasad, M.; Kanagasabapathy, K. Multivariate statistical analysis of heavy metal concentration in soils of Yelagiri Hills, Tamilnadu, India—Spectroscopical approach. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 137, 589–600. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, W.; Wang, M.; Peng, C. Regional accumulation characteristics of cadmium in vegetables: Influencing factors, transfer model and indication of soil threshold content. Environ. Pollut. 2016, 219, 1036–1043. [Google Scholar] [CrossRef]
- Hunter, P. A toxic brew we cannot live without-Micronutrients give insights into the interplay between geochemistry and evolutionary biology. EMBO Rep. 2008, 9, 15–18. [Google Scholar] [CrossRef] [Green Version]
- Engström, A.; Michaëlsson, K.; Vahter, M.; Julin, B.; Wolk, A.; Åkesson, A. Associations between dietary cadmium exposure and bone mineral density and risk of osteoporosis and fractures among women. Bone 2012, 50, 1372–1378. [Google Scholar] [CrossRef]
- Ju, Y.-R.; Chen, W.-Y.; Liao, C.-M. Assessing human exposure risk to cadmium through inhalation and seafood consumption. J. Hazard. Mater. 2012, 227-228, 353–361. [Google Scholar] [CrossRef]
- Yi, Z.; Lehto, N.J.; Robinson, B.H.; Cavanagh, J.-A.E. Environmental and edaphic factors affecting soil cadmium uptake by spinach, potatoes, onion and wheat. Sci. Total Environ. 2020, 713, 136694. [Google Scholar] [CrossRef]
- Braungardt, C.B.; Achterberg, E.P.; Axelsson, B.; Buffle, J.; Graziottin, F.; Howell, K.A.; Illuminati, S.; Scarponi, G.; Tappin, A.D.; Tercier-Waeber, M.-L.; et al. Analysis of dissolved metal fractions in coastal waters: An inter-comparison of five voltammetric in situ profiling (VIP) systems. Mar. Chem. 2009, 114, 47–55. [Google Scholar] [CrossRef]
- Vamerali, T.; Bandiera, M.; Mosca, G. Field crops for phytoremediation of metal-contaminated land. A review. Environ. Chem. Lett. 2009, 8, 1–17. [Google Scholar] [CrossRef]
- Yruela, I. Copper in plants: Acquisition, transport and interactions. Funct. Plant Biol. 2009, 36, 409–430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhattacharya, P.T.; Misra, S.R.; Hussain, M. Nutrition Aspects of Essential Trace Elements in Oral Health and Disease: An Extensive Rewiew. Scientifica 2016, 2016, 5464373. [Google Scholar] [CrossRef] [Green Version]
- Balabanova, B.; Stafilov, T.; Šajn, R.; Andonovska, K.B. Quantitative assessment of metal elements using moss species as biomonitors in downwind area of lead-zinc mine. J. Environ. Sci. Health Part A 2016, 52, 290–301. [Google Scholar] [CrossRef]
- Nabulo, G.; Oryem-Origa, H.; Diamond, M. Assessment of lead, cadmium, and zinc contamination of roadside soils, surface films, and vegetables in Kampala City, Uganda. Environ. Res. 2006, 101, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Beneš, S. Obsahy a Bilance Prvků ve Sférach Životního Prostředí, II. část; Ministerstvo Zemědelství ČR: Praha, Czech Republic, 1994; 184p. [Google Scholar]
- Matovic, V.; Buha, A.; Ðukić-Ćosić, D.; Bulat, Z. Insight into the oxidative stress induced by lead and/or cadmium in blood, liver and kidneys. Food Chem. Toxicol. 2015, 78, 130–140. [Google Scholar] [CrossRef] [PubMed]
- Hronec, O.; Tóth, J.; Tomáš, J. Cudzorodé Látky a Ich Riziká; Harlequin Quality Ltd.: Košice, Slovakia, 2002; 200p. [Google Scholar]
- Vallascas, E.; De Micco, A.; Deiana, F.; Banni, S.; Sanna, E. Adipose tissue: Another target organ for lead accumulation? A study on sardinian children (Italy). Am. J. Hum. Biol. 2013, 25, 789–794. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wu, S.; Xiang, Y.; Liang, X. An investigation of outpatient children’s blood lead level in Wuhan China. PLoS ONE 2014, 9, e95284. [Google Scholar] [CrossRef]
- Morgan, R. Soil. heavy metals, and human health. In Soils and Human Health; Brevik, E.C., Burgess, L.C., Eds.; CRC Press: Boca Raton, FL, USA, 2013; pp. 59–82. [Google Scholar]
- Sharma, V.K.; McDonald, T.J.; Sohn, M.; Anquandah, G.A.K.; Pettine, M.; Zboril, R. Biogeochemistry of selenium. A review. Environ. Chem. Lett. 2015, 13, 49–58. [Google Scholar] [CrossRef]
- Alloway, B.J. Zinc in Soil and Crop Nutrition, 2nd ed.; IZA and IFA: Brussels, Belgium; Paris, France, 2008; 135p. [Google Scholar]
- Baize, D.; Sterckeman, T. Of the necessity of knowledge of the natural pedo-geochemical background content in the evaluation of the contamination of soils by trace elements. Sci. Total Environ. 2001, 264, 127–139. [Google Scholar] [CrossRef]
- Liu, Y.-M.; Liu, D.-Y.; Zhao, Q.-Y.; Zhang, W.; Chen, X.-X.; Xu, S.-J.; Zou, C.-Q. Zinc fractions in soils and uptake in winter wheat as affected by repeated applications of zinc fertilizer. Soil Tillage Res. 2020, 200, 104612. [Google Scholar] [CrossRef]
- Linkeš, V.; Pestún, V.; Džatko, M. Príručka Pre Používanie Máp Bonitovaných Pôdno—Ekologických Jednotiek. Príručka Pre Bonitáciu Poľnohospodárskych Pôd, 3rd ed.; VÚPÚ: Bratislava, Slovakia, 1996; 104p. [Google Scholar]
- Bátora, M.; Zaťko, M. Zlaté Moravce, 1st ed.; Mestský úrad: Zlaté Moravce, Slovakia, 1998; 373p. [Google Scholar]
- Süle, P.; Šüle, P.M.L.; Adamová, M. Encyklopédia Miest a obcí Slovenska; PS-LINE. spol. s r. o.: Lučenec, Slovakia, 2005; 960p. [Google Scholar]
- Balkovič, J.; Bedrna, Z.; Bublinec, E.; Čurlík, J.; Dlapa, P.; Fulajtár, E.; Gömöryová, E.; Gregor, J.; Hanes, J.; Houšková, B.; et al. Morfogenetický Klasifikačný Systém Pôd Slovenska. In Bazálna Referenčná Taxonómia, 2nd ed.; SPS. NPPC VÚPOP: Bratislava, Slovakia, 2014; 96p. [Google Scholar]
- Decree of the National Council of the Slovak Republic No. 59/2013 Coll. Ministry of Agriculture and Rural Development of the Slovak Republic, which amends Decree of the Ministry of Agriculture of the Slovak Republic No. 508/2004 Coll., Which Implements § 27 of Act No. 220/2004 Coll. on the Protection and Use of Agricultural Land and Amending Act No. 245/2003 Coll. on Integrated Prevention and Control of Environmental Pollution and Amendments to Certain Acts. Available online: https://www.slov-lex.sk/vyhladavanie-pravnych-predpisov?text=59%2F2013%20 (accessed on 2 February 2021).
- Decree of the Ministry of Agriculture of the Slovak Republic No. 508/2004 Coll., Which Implements § 27 of Act No. 220/2004 Coll. on the Protection and Use of Agricultural Land and Amending Act No. 245/2003 Coll. on Integrated Prevention and Control of Environmental Pollution and Amendments to Certain Acts. Available online: https://www.slov-lex.sk/pravne-predpisy/SK/ZZ/2004/508/20130401 (accessed on 5 February 2021).
- Van Reeuwijk, L.P. Producer for Soil Analysis; International Soil reference and Information Centre: Wageningen, The Netherlands, 2002; 120p. [Google Scholar]
- Orlov, D.S.; Grišina, L.A. Praktikum po Chimiji Gumusa; Izdateľstvo Moskovskovo Uniresiteta: Moscow, Russia, 1981; 124p. [Google Scholar]
- Grišina, L.A. Gumusoobrazovanije i Gumusnoe Sostojanije Počv; Izd. MU: Moskva, Russia, 1986; 242p. [Google Scholar]
- Liu, W.-X.; Shen, L.-F.; Liu, J.-W.; Wang, Y.-W.; Li, S.-R. Uptake of Toxic Heavy Metals by Rice (Oryza sativa L.) Cultivated in the Agricultural Soil near Zhengzhou City, People’s Republic of China. Bull. Environ. Contam. Toxicol. 2007, 79, 209–213. [Google Scholar] [CrossRef]
- Markechová, D.; Stehlíková, B.; Tirpáková, A. Štatistické Metódy a ich Aplikácie; UKF: Nitra, Slovakia, 2011; 534p. [Google Scholar]
- Decree of the Ministry of Agriculture of the Slovak Republic and the Ministry of Health of the Slovak Republic of 29 October 2007 no. 14300/2007-OL. amending the Decree of the Ministry of Agriculture of the Slovak Republic and the Ministry of Health of the Slovak Republic of 11 September 2006 no. 18558/2006-SL. Issuing the Title of the Food Code of the Slovak Republic regulating Contaminants in Foodstuffs. Available online: https://www.mpsr.sk/index.php?navID=126&year=2007&ofs1=4 (accessed on 15 March 2021).
- State Veterinary and Food Administration of the Slovak Republic. Food Code of the Slovak Republic. Available online: https://www.svps.sk/legislativa/legislativa_kodex.asp (accessed on 15 February 2021).
- Rapant, S.; Cvečková, V.; Jurkovič, Ľ.; Macek, J. Aplikovaná Medicínska Geochémia; Univerzita Komenského v Bratislave: Bratislava, Slovakia, 2019; 163p. [Google Scholar]
- Essentials of Medical Geology: Impacts of the Natural Environment on Public Health; Selinus, O.; Alloway, B.J.; Centeno, J.A.; Finkelman, R.B.; Fuge, R.; Lindh, U.; Smedley, P. (Eds.) Elsevier Acadamic Press: Burlington, MA, USA, 2005; 793p. [Google Scholar]
- Wang, Z.; Xiao, J.; Wang, L.; Liang, T.; Guo, Q.; Guan, Y.; Rinklebe, J. Elucidating the differentiation of soil heavy metals under different land uses with geographically weighted regression and self-organizing map. Environ. Pollut. 2020, 260, 114065. [Google Scholar] [CrossRef]
- Yuanan, H.; He, K.; Sun, Z.; Chen, G.; Cheng, H. Quantitative source apportionment of heavy metal(loid)s in the agricultural soils of an industrializing region and associated model uncertainty. J. Hazard. Mater. 2020, 391, 122244. [Google Scholar] [CrossRef]
- Chen, T.; Wong, J.; Zhou, H.; Wong, M. Assessment of trace metal distribution and contamination in surface soils of Hong Kong. Environ. Pollut. 1997, 96, 61–68. [Google Scholar] [CrossRef]
- Huang, B.; Shi, X.; Yu, D.; Öborn, I.; Blombäck, K.; Pagella, T.; Wang, H.; Sun, W.; Sinclair, F.L. Environmental assessment of small-scale vegetable farming systems in peri-urban areas of the Yangtze River Delta Region, China. Agric. Ecosyst. Environ. 2006, 112, 391–402. [Google Scholar] [CrossRef]
- Duan, Q.; Lee, J.; Liu, Y.; Chen, H.; Hu, H. Distribution of Heavy Metal Pollution in Surface Soil Samples in China: A Graphical Review. Bull. Environ. Contam. Toxicol. 2016, 97, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.; Khan, S.; Khan, A.; Alam, M. Soil contamination with cadmium, consequences and remediation using organic amendments. Sci. Total Environ. 2017, 601–602, 1591–1605. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.; Guo, Z.; Peng, C.; Xiao, X.; Shi, L.; Zeng, P.; Ran, H.; Xue, Q. Atmospheric bulk deposition of heavy metal(loid)s in central south China: Fluxes, influencing factors and implication for paddy soils. J. Hazard. Mater. 2019, 371, 634–642. [Google Scholar] [CrossRef] [PubMed]
- Roca, N.; Pazos, M.S.; Bech, J. Background levels of potentially toxic elements in soils: A case study in Catamarca (a semiarid region in Argentina). Catena 2012, 92, 55–66. [Google Scholar] [CrossRef]
- Li, C.; Sun, G.; Wu, Z.; Zhong, H.; Wang, R.; Liu, X.; Guo, Z.; Cheng, J. Soil physiochemical properties and landscape patterns control trace metal contamination at the urban-rural interface in southern China. Environ. Pollut. 2019, 250, 537–545. [Google Scholar] [CrossRef]
- Ye, C.; Butler, O.M.; Du, M.; Liu, W.; Zhang, Q. Spatio-temporal dynamics, drivers and potential sources of heavy metal pollution in riparian soils along a 600 kilometre stream gradient in Central China. Sci. Total Environ. 2019, 651, 1935–1945. [Google Scholar] [CrossRef]
- Belon, E.; Boisson, M.; Deportes, I.; Eglin, T.; Feix, I.; Bispo, A.; Galsomies, L.; Leblond, S.; Guellier, C. An inventory of trace elements inputs to French agricultural soils. Sci. Total Environ. 2012, 439, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Wang, H.; Dong, L.; Huang, B.; Borggaard, O.K.; Hansen, H.C.B.; He, Y.; Holm, P.E. Source identification of heavy metals in peri-urban agricultural soils of southeast China: An integrated approach. Environ. Pollut. 2018, 237, 650–661. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Qiu, S.; He, P. Changes of heavy metals in soil and wheat grain under long-term environmental impact and fertilization practices in North China. J. Plant Nutr. 2018, 41, 1970–1979. [Google Scholar] [CrossRef]
- Shao, D.; Zhan, Y.; Zhou, W.; Zhu, L. Current status and temporal trend of heavy metals in farmland soil of the Yangtze River Delta Region: Field survey and meta-analysis. Environ. Pollut. 2016, 219, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Guo, S.; Zeng, G.-M.; Li, F.; Gu, Y.; Shi, Y.; Shi, L.; Liu, W.; Peng, S. A new exploration of health risk assessment quantification from sources of soil heavy metals under different land use. Environ. Pollut. 2018, 243, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Demková, L.; Bobulská, L.; Fazekášová, D. Toxicity of heavy metals to soil biological and chemical properties in conditions of environmentally polluted area middle Spiš (Slovakia). Carpath. J. Earth Env. 2015, 10, 193–201. [Google Scholar]
- Chai, Y.; Guo, J.; Chai, S.; Cai, J.; Xue, L.; Zhang, Q. Source identification of eight heavy metals in grassland soils by multivariate analysis from the Baicheng–Songyuan area, Jilin Province, Northeast China. Chemosphere 2015, 134, 67–75. [Google Scholar] [CrossRef]
- Lv, J.; Wang, J. Multi-scale analysis of heavy metals sources in soils of Jiangsu Coast. Eastern China. Chemosphere 2018, 212, 964–973. [Google Scholar] [CrossRef] [PubMed]
- Zupančič, N. Influence of climate factors on soil heavy metal content in Slovenia. J. Soils Sediments 2017, 17, 1073–1083. [Google Scholar] [CrossRef]
- Zhao, K.; Zhang, L.; Dong, J.; Wu, J.; Ye, Z.; Zhao, W.; Ding, L.; Fu, W. Risk assessment, spatial patterns and source apportionment of soil heavy metals in a typical Chinese hickory plantation region of southeastern China. Geoderma 2020, 360, 1–11. [Google Scholar] [CrossRef]
- Laurent, C.; Bravin, M.N.; Crouzet, O.; Pelosi, C.; Tillard, E.; Lecomte, P.; Lamy, I. Increased soil pH and dissolved organic matter after a decade of organic fertilizer application mitigates copper and zinc availability despite contamination. Sci. Total Environ. 2020, 709, 135927. [Google Scholar] [CrossRef]
- Jones, J.B. Soil pH. Liming, and liming materials. In Agronomic Handbook Management of Crops, Soils and Their Fertility; CRC Press: Washington, DC, USA, 2002; pp. 237–251. [Google Scholar]
- Bołzan, B.D. Effect of pH and soil environment. World News Nat. Sci. 2017, 8, 50–60. [Google Scholar]
- Wilson, M.; Bell, N. Acid deposition and heavy metal mobilization. Appl. Geochem. 1996, 11, 133–137. [Google Scholar] [CrossRef]
- Kim, R.-Y.; Yoon, J.-K.; Kim, T.-S.; Yang, J.E.; Owens, G.; Kim, K.-R. Bioavailability of heavy metals in soils: Definitions and practical implementation—A critical review. Environ. Geochem. Health 2015, 37, 1041–1061. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.P.; Deng, W.; Yang, X.M. The background concentrations of 13 soil trace elements and their relationships to parent materials and vegetation in Xizang (Tibet), China. J. Asian Earth Sci. 2002, 21, 167–174. [Google Scholar] [CrossRef]
- Wang, Z.; Hong, C.; Xing, Y.; Wang, K.; Li, Y.; Feng, L.; Ma, S. Spatial distribution and sources of heavy metals in natural pasture soil around copper-molybdenum mine in Northeast China. Ecotoxicol. Environ. Saf. 2018, 154, 329–336. [Google Scholar] [CrossRef]
- Kosheleva, N.E.; Kasimov, N.S.; Vlasov, D.V. Factors of the accumulation of heavy metals and metalloids at geochemical barriers in urban soils. Eurasian Soil Sci. 2015, 48, 476–492. [Google Scholar] [CrossRef]
- Khorshid, M.S.H.; Thiele-Bruhn, S. Contamination status and assessment of urban and non-urban soils in the region of Sulaimani City, Kurdistan, Iraq. Environ. Earth Sci. 2016, 75, 1171. [Google Scholar] [CrossRef]
- Šimanský, V.; Bajčan, D.; Ducsay, L. The effect of organic matter on aggregation under different soil management practices in a vineyard in an extremely humid year. Catena 2013, 101, 108–113. [Google Scholar] [CrossRef]
- Purakayastha, T.; Rudrappa, L.; Singh, D.; Swarup, A.; Bhadraray, S. Long-term impact of fertilizers on soil organic carbon pools and sequestration rates in maize–wheat–cowpea cropping system. Geoderma 2008, 144, 370–378. [Google Scholar] [CrossRef]
- Cong, W.-F.; van Ruijven, J.; Mommer, L.; De Deyn, G.B.; Berendse, F.; Hoffland, E. Plant species richness promotes soil carbon and nitrogen stocks in grasslands without legumes. J. Ecol. 2014, 102, 1163–1170. [Google Scholar] [CrossRef]
- Sistla, S.A.; Moore, J.C.; Simpson, R.T.; Gough, L.; Shaver, G.R.; Schimel, J.P. Long-term warming restructures Arctic tundra without changing net soil carbon storage. Nature 2013, 497, 615–618. [Google Scholar] [CrossRef]
- Körschens, M.; Albert, E.; Armbruster, M.; Barkusky, D.; Baumecker, M.; Behle-Schalk, L.; Bischoff, R.; Čergan, Z.; Ellmer, F.; Herbst, F.; et al. Effect of mineral and organic fertilization on crop yield, nitrogen uptake, carbon and nitrogen balances, as well as soil organic carbon content and dynamics: Results from 20 European long-term field experiments of the twenty-first century. Arch. Agron. Soil Sci. 2013, 59, 1017–1040. [Google Scholar] [CrossRef]
- Von Lützow, M.; Kögel-Knaber, I. Temperature sensitivity of soil organic matter decomposition—What do we know? Biol. Fert. Soils 2009, 46, 1–15. [Google Scholar] [CrossRef]
- Tarnocai, C.; Canadell, J.; Schuur, E.A.G.; Kuhry, P.; Mazhitova, G.; Zimov, S. Soil organic carbon pools in the northern circumpolar permafrost region. Glob. Biogeochem. Cycles 2009, 23, 1–11. [Google Scholar] [CrossRef]
- Conant, R.T.; Ryan, M.G.; Agren, G.I.; Birge, H.E.; Davidson, E.A.; Eliasson, P.E.; Evans, S.E.; Frey, S.D.; Giardina, C.H.P.; Hopkins, F.M.; et al. Temperature and soil organic matter decomposition rates—synthesis of current knowledge and a way forward. Glob. Chang. Biol. 2011, 17, 3392–3404. [Google Scholar] [CrossRef]
- Scharlemann, J.; Tanner, E.V.; Hiederer, R.; Kapos, V. Global soil carbon: Understanding and managing the largest terrestrial carbon pool. Carbon Manag. 2014, 5, 81–91. [Google Scholar] [CrossRef]
- Wiesmeier, M.; Poeplau, C.; Sierra, C.; Maier, H.; Frühauf, C.; Hübner, R.; Kühnel, A.; Spoerlein, P.; Geuß, U.; Hangen, E.; et al. Projected loss of soil organic carbon in temperate agricultural soils in the 21st century: Effects of climate change and carbon input trends. Sci. Rep. 2016, 6, 1–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haring, V.; Fischer, H.; Cadisch, G.; Stahr, K. Implication of erosion on the assessment of decomposition and humification of soil organic carbon after land use change in tropical agricultural systems. Soil Biol. Biochem. 2013, 65, 158–167. [Google Scholar] [CrossRef]
- Jiang, G.; Xu, M.; He, X.; Zhang, W.; Huang, S.; Yang, X.; Liu, H.; Peng, C.; Shirato, Y.; Iizumi, T.; et al. Soil organic carbon sequestration in upland soils of northern China under variable fertilizer management and climate change scenarios. Glob. Biogeochem. Cycles 2014, 28, 319–333. [Google Scholar] [CrossRef]
- Nayak, A.; Gangwar, B.; Shukla, A.K.; Mazumdar, S.P.; Kumar, A.; Raja, R.; Kumar, A.; Kumar, V.; Rai, P.; Mohan, U. Long-term effect of different integrated nutrient management on soil organic carbon and its fractions and sustainability of rice–wheat system in Indo Gangetic Plains of India. Field Crops Res. 2012, 127, 129–139. [Google Scholar] [CrossRef]
- Lal, R.; Kimble, J.M.; Follett, R.F.; Cole, C.V. The Potential of U.S. Cropland to Sequester Carbon and Mitigate the Greenhouse Effect; Sleeping Bear Press: Chelsea, MI, USA, 1998; 128p. [Google Scholar]
- Liu, X.; Han, X.; Song, C.; Herbert, S.J.; Xing, B. Soil Organic Carbon Dynamics in Black Soils of China Under Different Agricultural Management Systems. Commun. Soil Sci. Plant Anal. 2003, 34, 973–984. [Google Scholar] [CrossRef]
- Brady, N.C.; Weil, R.R. The Nature and Properties of Soils, 14th ed.; Pearson Education Inc.: Upper Saddle River, NJ, USA, 2008; 965p. [Google Scholar]
- Syswerda, S.; Corbin, A.; Mokma, D.; Kravchenko, A.; Robertson, G. Agricultural Management and Soil Carbon Storage in Surface vs. Deep Layers. Soil Sci. Soc. Am. J. 2011, 75, 92–101. [Google Scholar] [CrossRef] [Green Version]
- Rumpel, C.; Kögel-Knabner, I. Deep soil organic matter—a key but poorly understood component of terrestrial C cycle. Plant Soil 2011, 338, 143–158. [Google Scholar] [CrossRef]
- Zhang, W.; Xu, M.; Wang, B.; Wang, X. Soil organic carbon, total nitrogen and grain yields under long-term fertilizations in the upland red soil of southern China. Nutr. Cycl. Agroecosystems 2008, 84, 59–69. [Google Scholar] [CrossRef]
- Zhang, J.; Balkovič, J.; Azevedo, L.B.; Skalský, R.; Bouwman, A.F.; Xu, G.; Wang, J.; Xu, M.; Yu, C. Analyzing and modelling the effect of long-term fertilizer management on crop yield and soil organic carbon in China. Sci. Total Environ. 2018, 627, 361–372. [Google Scholar] [CrossRef]
- Galati, A.; Crescimanno, M.; Gristina, L.; Keesstra, S.; Novara, A. Actual provision as an alternative criterion to improve the efficiency of payments for ecosystem services for C sequestration in semiarid vineyards. Agric. Syst. 2016, 144, 58–64. [Google Scholar] [CrossRef]
- Guo, J.H.; Liu, X.J.; Zhang, Y.; Shen, J.L.; Han, W.X.; Zhang, W.F.; Christie, P.; Goulding, K.; Vitousek, P.M.; Zhang, F.; et al. Significant Acidification in Major Chinese Croplands. Science 2010, 327, 1008–1010. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paľove-Balang, P. Príjem a Transport Minerálnych Látok v Rastlinách; Univerzita Pavla Jozefa Šafárika: Košice, Slovakia, 2012; pp. 11–12. [Google Scholar]
- Filippi, P.; Cattle, S.R.; Bishop, T.; Odeh, I.O.; Pringle, M.J. Digital soil monitoring of top- and sub-soil pH with bivariate linear mixed models. Geoderma 2018, 322, 149–162. [Google Scholar] [CrossRef]
- Marchant, B.P.; Crawford, D.M.; Robinson, N.J. What can legacy datasets tell us about soil quality trends? Soil acidity in Victoria. In Proceeding of the IOP Conference Series: Earth and Environmental Science, Bendigo, Australia, 24–27 March 2014. [Google Scholar]
- Bowman, W.D.; Cleveland, C.C.; Halada, Ĺ.; Hreško, J.; Baron, J.S. Negative impact of nitrogen deposition on soil buffering capacity. Nat. Geosci. 2008, 1, 767–770. [Google Scholar] [CrossRef]
- Bielek, P. Kompendium Praktického Pôdoznalectva, 1st ed.; Slovenská poľnohospodárska Univerzita: Nitra, Slovakia, 2014; 244p. [Google Scholar]
- Xing, W.; Zhang, H.; Scheckel, K.G.; Li, L. Heavy metal and metalloid concentrations in components of 25 wheat (Triticum aestivum) varieties in the vicinity of lead smelters in Henan province, China. Environ. Monit. Assess. 2016, 188, 1–10. [Google Scholar] [CrossRef]
- Kumpiene, J.; Antelo, J.; Brännvall, E.; Carabante, I.; Ek, K.; Komárek, M.; Söderberg, C.; Wårell, L. In situ chemical stabilization of trace element-contaminated soil—Field demonstrations and barriers to transition from laboratory to the field—A review. Appl. Geochem. 2019, 100, 335–351. [Google Scholar] [CrossRef]
- Gao, X.; Brown, K.R.; Racz, G.J.; Grant, C.A. Concentration of cadmium in durum wheat as affected by time, source and placement of nitrogen fertilization under reduced and conventional-tillage management. Plant Soil 2010, 337, 341–354. [Google Scholar] [CrossRef]
- McLaughlin, M.J.; Smolders, E.; Degryse, F.; Rietra, R. Uptake of Metals from Soil into Vegetables. In Dealing with Contaminated Sites; Swartjes, F., Ed.; Springer: Dordrecht, The Netherland, 2011; pp. 325–367. [Google Scholar]
- Shaheen, S.; Rinklebe, J. Impact of emerging and low cost alternative amendments on the (im)mobilization and phytoavailability of Cd and Pb in a contaminated floodplain soil. Ecol. Eng. 2015, 74, 319–326. [Google Scholar] [CrossRef]
- Chaney, R.L. Food Safety Issues for Mineral and Organic Fertilizers. Adv. Agron. 2012, 117, 51–116. [Google Scholar] [CrossRef]
- Soltani, S.M.; Hanafi, M.M.; Wahid, S.A.; Kharidah, S.M.S. Zinc fractionation of tropical paddy soils and their relationships with selected soil properties. Chem. Speciat. Bioavailab. 2015, 27, 53–61. [Google Scholar] [CrossRef]
- Antoniadis, V.; Shaheen, S.; Tsadilas, C.; Selim, M.H.; Rinklebe, J. Zinc sorption by different soils as affected by selective removal of carbonates and hydrous oxides. Appl. Geochem. 2018, 88, 49–58. [Google Scholar] [CrossRef]
- Cakmak, I.; Kutman, U.B. Agronomic biofortification of cereals with zinc: A review. Eur. J. Soil Sci. 2018, 69, 172–180. [Google Scholar] [CrossRef] [Green Version]
- Peijnenburg, W.J.; Zablotskaja, M.; Vijver, M.G. Monitoring metals in terrestrial environments within a bioavailability framework and a focus on soil extraction. Ecotoxicol. Environ. Saf. 2007, 67, 163–179. [Google Scholar] [CrossRef]
- Norouzi, S.; Khademi, H.; Cano, A.F.; Acosta, J. Using plane tree leaves for biomonitoring of dust borne heavy metals: A case study from Isfahan, Central Iran. Ecol. Indic. 2015, 57, 64–73. [Google Scholar] [CrossRef]
- Demková, L.; Árvay, J.; Bobulská, L.; Tomáš, J.; Stanovič, R.; Lošák, T.; Harangozo, L.; Vollmannová, A.; Bystrická, J.; Musilová, J.; et al. Accumulation and environmental risk assessment of heavy metals in soil and plants of four different ecosystems in a former polymetallic ores mining and smelting area (Slovakia). J. Environ. Sci. Health Part A 2017, 52, 479–490. [Google Scholar] [CrossRef] [PubMed]
- Galati, A.; Schifani, G.; Crescimanno, M.; Vrontis, D.; Migliore, G. Innovation strategies geared toward the circular economy: A case study of the organic olive-oil industry. RivISTA. Studi Sulla Sostenibilita 2018, 2018, 137–158. [Google Scholar] [CrossRef]
- Galati, A.; Gristina, L.; Crescimanno, M.; Barone, E.; Novara, A. Towards More Efficient Incentives for Agri-environment Measures in Degraded and Eroded Vineyards. Land Degrad. Dev. 2015, 26, 557–564. [Google Scholar] [CrossRef]
- International Organization for Standardization. ISO 22000: 2018 Food Safety Management Systems—Requirements for Any Organization in the Food Chain, 2nd ed.; International Organization for Standardization: Geneva, Switzerland, 2018. [Google Scholar]
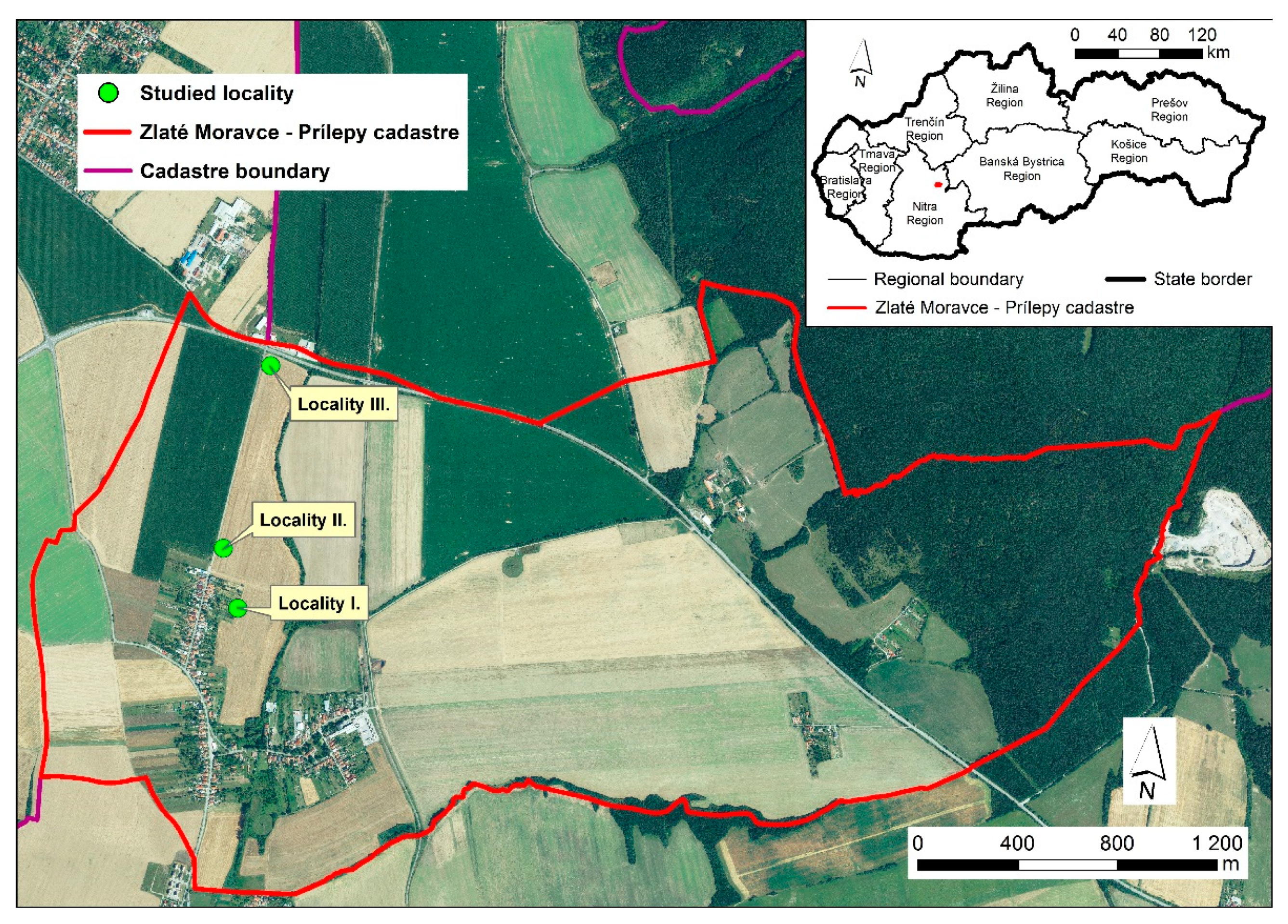
| Localities | Coordinates | Type of Land | Use of Sites | Soil Unit Designation According to BPEJ 1 |
|---|---|---|---|---|
| Locality I | 48°21′53.5″ N 18°24′59.4″ E | Arable land | For growing wheats | Brown soils typical, on loess, medium (moderate)-heavy |
| Locality II | 48°22′01.1″ N 18°24′55.6″ E | Arable land | For growing wheats | Brown soils (type) on loess clays, heavy |
| Locality III | 48°22′25.3″ N 18°25′01.8″ E | Arable land | For growing wheats | Brown soils typical, on loess, medium (moderate)-heavy |
| Metal Concentration | 2017 | 2018 | 2019 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Spring | Ave ± SD | Min | Max | Ave ± SD | Min | Max | Ave ± SD | Min | Max |
| Cd | 1.32 ± 0.59 | 0.20 | 1.80 | 0.96 ± 0.48 | 0.20 | 1.40 | 1.72 ± 0.48 | 1.20 | 2.40 |
| Cu | 16.00 ± 1.39 | 14.40 | 17.60 | 19.68 ± 0.57 | 19.00 | 20.60 | 19.04 ± 2.82 | 16.00 | 24.40 |
| Pb | 45.60 ± 6.12 | 36.00 | 54.00 | 56.76 ± 1.82 | 54.94 | 59.94 | 65.68 ± 3.01 | 62.52 | 70.56 |
| Zn | 51.91 ± 2.37 | 48.52 | 54.82 | 71.20 ± 5.88 | 64.00 | 80.00 | 63.20 ± 16.33 | 38.00 | 80.00 |
| Autumn | Ave ± SD | Min | Max | Ave ± SD | Min | Max | Ave ± SD | Min | Max |
| Cd | 2.32 ± 0.48 | 1.40 | 2.80 | 1.52 ± 0.10 | 1.40 | 1.60 | 0.92 ± 0.39 | 0.40 | 1.60 |
| Cu | 20.48 ± 2.33 | 18.40 | 25.00 | 19.88 ± 1.72 | 18.20 | 22.60 | 18.92 ± 0.49 | 18.40 | 19.80 |
| Pb | 59.42 ± 14.72 | 47.70 | 87.90 | 58.64 ± 8.01 | 49.50 | 70.92 | 44.9 ± 7.83 | 34.96 | 52.40 |
| Zn | 55.20 ± 14.73 | 46.00 | 84.00 | 60.00 ± 11.10 | 40.00 | 74.00 | 52.40 ± 9.91 | 40.00 | 66.00 |
| Metal Concentration | 2017 | 2018 | 2019 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Spring | Ave ± SD | Min | Max | Ave ± SD | Min | Max | Ave ± SD | Min | Max |
| Cd | 1.56 ± 0.15 | 1.40 | 1.80 | 1.48 ± 0.16 | 1.20 | 1.60 | 0.80 ± 0.67 | 0.20 | 1.80 |
| Cu | 16.96 ± 0.70 | 16.00 | 18.00 | 21.28 ± 0.92 | 20.00 | 22.80 | 20.96 ± 0.82 | 20.00 | 22.00 |
| Pb | 46.00 ± 6.32 | 38.00 | 56.00 | 54.80 ± 7.76 | 46.00 | 66.00 | 79.60 ± 2.94 | 76.00 | 84.00 |
| Zn | 54.692 ± 2.02 | 52.36 | 56.94 | 62.05 ± 3.49 | 57.06 | 67.18 | 63.56 ± 2.65 | 60.28 | 66.54 |
| Autumn | Ave ± SD | Min | Max | Ave ± SD | Min | Max | Ave ± SD | Min | Max |
| Cd | 2.48 ± 0.32 | 2.00 | 2.80 | 1.68 ± 0.10 | 1.60 | 1.80 | 1.16 ± 0.23 | 0.80 | 1.40 |
| Cu | 21.60 ± 0.66 | 20.40 | 22.40 | 19.68 ± 0.81 | 18.60 | 20.40 | 19.72 ± 1.12 | 18.00 | 21.00 |
| Pb | 47.20 ± 3.25 | 42.00 | 52.00 | 65.60 ± 14.11 | 38.00 | 78.00 | 70.40 ± 4.96 | 66.00 | 80.00 |
| Zn | 61.80 ± 5.89 | 54.76 | 72.58 | 65.22 ± 8.04 | 54.92 | 74.66 | 55.24 ± 6.94 | 45.04 | 63.20 |
| Metal Concentration | 2017 | 2018 | 2019 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Spring | Ave ± SD | Min | Max | Ave ± SD | Min | Max | Ave ± SD | Min | Max |
| Cd | 1.44 ± 0.41 | 1.00 | 2.20 | 1.44 ± 0.27 | 1.00 | 1.80 | 1.04 ± 0.23 | 0.80 | 1.40 |
| Cu | 14.56 ± 12.60 | 12.60 | 16.00 | 19.40 ± 2.90 | 15.60 | 22.40 | 18.60 ± 1.99 | 15.80 | 20.60 |
| Pb | 41.60 ± 3.88 | 36.00 | 46.00 | 52.00 ± 4.90 | 44.00 | 58.00 | 77.60 ± 1.96 | 76.00 | 80.00 |
| Zn | 48.30 ± 2.69 | 44.36 | 52.44 | 55.10 ± 4.77 | 49.28 | 61.12 | 57.20 ± 2.81 | 52.46 | 60.86 |
| Autumn | Ave ± SD | Min | Max | Ave ± SD | Min | Max | Ave ± SD | Min | Max |
| Cd | 2.52 ± 0.27 | 2.00 | 2.80 | 1.60 ± 0.13 | 1.40 | 1.80 | 1.16 ± 0.39 | 0.80 | 1.80 |
| Cu | 17.52 ± 0.98 | 16.40 | 18.80 | 19.36 ± 1.20 | 17.40 | 21.00 | 18.44 ± 2.29 | 15.20 | 21.00 |
| Pb | 41.60 ± 4.96 | 32.00 | 46.00 | 67.60 ± 4.08 | 62.00 | 74.00 | 65.60 ± 5.57 | 60.00 | 76.00 |
| Zn | 52.10 ± 6.42 | 44.64 | 62.70 | 58.08 ± 4.74 | 50.78 | 63.86 | 56.44 ± 18.67 | 33.84 | 86.66 |
| Season | 2017–2019 (Spring) | 2017–2019 (Autumn) | ||||||
|---|---|---|---|---|---|---|---|---|
| Metal cont. 1 | Cu | Cd | Pb | Metal cont. 1 | Cu | Cd | Pb | |
| Zn | 0.5903 | −0.0277 | 0.6613 | Zn | 0.7037 | 0.0072 | 0.5885 | |
| Locality I | Cu | - | −0.4586 | 0.8088 | Cu | - | −0.0363 | 0.6697 |
| Cd | - | −0.4947 | Cd | - | - | −0.3004 | ||
| Locality II | Zn | 0.9272 | −0.5375 | 0.5655 | Zn | 0.3516 | 0.1402 | 0.2478 |
| Cu | - | −0.4793 | 0.4919 | Cu | - | 0.5262 | −0.2480 | |
| Cd | - | −0.3224 | Cd | - | −0.5971 | |||
| Locality III | Zn | 0.8704 | −0.2138 | 0.5653 | Zn | 0.8853 | −0.4627 | 0.4758 |
| Cu | - | −0.2929 | 0.4018 | Cu | - | −0.5501 | 0.5829 | |
| Cd | - | - | −0.2977 | Cd | - | - | −0.7186 | |
| (a) | ||||||||||||
| Localities | 2017 | 2018 | 2019 | |||||||||
| Spring | Ave ± SD | Min | Max | Median | Ave ± SD | Min | Max | Median | Ave ± SD | Min | Max | Median |
| Locality I | 6.41 ± 0.11 | 6.26 | 6.60 | 6.40 | 6.21 ± 0.17 | 5.96 | 6.46 | 6.15 | 6.78 ± 0.05 | 6.71 | 6.86 | 6.76 |
| Locality II | 7.09 ± 0.05 | 7.00 | 7.15 | 7.08 | 7.38 ± 0.05 | 7.32 | 7.45 | 7.37 | 7.64 ± 0.05 | 7.56 | 7.71 | 7.63 |
| Locality III | 7.22 ± 0.07 | 7.12 | 7.31 | 7.22 | 7.56 ± 0.08 | 7.46 | 7.66 | 7.52 | 7.55 ± 0.09 | 7.46 | 7.70 | 7.50 |
| Autumn | Ave ± SD | Min | Max | Median | Ave ± SD | Min | Max | Median | Ave ± SD | Min | Max | Median |
| Locality I Locality II Locality III | 6.40 ± 0.10 | 6.24 | 6.53 | 6.41 | 6.84 ± 0.09 | 6.75 | 7.01 | 6.81 | 7.03 ± 0.06 | 6.95 | 7.10 | 7.03 |
| 6.59 ± 0.17 | 6.41 | 6.80 | 6.52 | 7.76 ± 0.05 | 7.71 | 7.84 | 7.77 | 7.18 ± 0.24 | 6.76 | 7.43 | 7.31 | |
| 6.81 ± 0.29 | 6.41 | 7.15 | 6.83 | 7.59 ± 0.14 | 7.40 | 7.85 | 7.49 | 7.65 ± 0.09 | 7.50 | 7.76 | 7.66 | |
| (b) | ||||||||||||
| Localities | 2017 | 2018 | 2019 | |||||||||
| Spring | Ave ± SD | Min | Max | Median | Ave ± SD | Min | Max | Median | Ave ± SD | Min | Max | Median |
| Locality I | 6.06 ± 0.07 | 5.95 | 6.15 | 6.08 | 5.15 ± 0.28 | 4.81 | 5.52 | 5.05 | 5.29 ± 0.31 | 4.80 | 5.65 | 5.31 |
| Locality II | 6.64 ± 0.23 | 6.25 | 6.96 | 6.63 | 6.33 ± 0.18 | 6.11 | 6.55 | 6.30 | 6.35 ± 0.33 | 6.08 | 6.92 | 6.11 |
| Locality III | 6.80 ± 0.13 | 6.61 | 6.95 | 6.82 | 6.71 ± 0.19 | 6.42 | 6.89 | 6.82 | 6.65 ± 0.07 | 6.55 | 6.72 | 6.67 |
| Autumn | Ave ± SD | Min | Max | Median | Ave ± SD | Min | Max | Median | Ave ± SD | Min | Max | Median |
| Locality I Locality II Locality III | 5.09 ± 0.13 | 4.89 | 5.24 | 5.05 | 5.59 ± 0.27 | 5.24 | 5.95 | 5.57 | 5.57 ± 0.26 | 5.14 | 5.81 | 5.73 |
| 5.49 ± 0.05 | 5.43 | 5.56 | 5.47 | 6.48 ± 0.30 | 6.10 | 6.83 | 6.47 | 6.37 ± 0.19 | 6.06 | 6.61 | 6.34 | |
| 6.05 ± 0.10 | 5.87 | 6.14 | 6.11 | 6.76 ± 0.08 | 6.64 | 6.87 | 6.76 | 6.64 ± 0.08 | 6.53 | 6.76 | 6.62 | |
| Metal Contaminant | Locality I | Locality II | Locality III | |||
|---|---|---|---|---|---|---|
| pH | spring | autumn | spring | autumn | spring | autumn |
| Cd | 0.3915 | −0.7265 | −0.3368 | −0.3103 | −0.0481 | −0.6234 |
| Cu | −0.2023 | −0.0859 | 0.5558 | −0.6894 | 0.2543 | −0.0484 |
| Pb | −0.0744 | 0.2617 | 0.8420 | 0.3324 | 0.6637 | 0.5633 |
| Zn | 0.5594 | −0.2750 | 0.6440 | 0.1895 | 0.2272 | −0.2466 |
| TOC Content | Localities | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| I | II | III | |||||||
| Years | 2017 | 2018 | 2019 | 2017 | 2018 | 2019 | 2017 | 2018 | 2019 |
| TOC spring | 1.32 | 0.87 | 0.88 | 1.03 | 1.34 | 0.90 | 1.23 | 1.60 | 1.43 |
| TOC spring Ave. | 1.02 | 1.09 | 1.42 | ||||||
| TOC autumn | 1.04 | 1.10 | 1.18 | 1.08 | 0.95 | 1.31 | 1.24 | 1.46 | 1.22 |
| TOC autumn Ave. | 1.11 | 1.22 | 1.31 | ||||||
| Monitor Character | Spring–Autumn | |
|---|---|---|
| Z | p-Value | |
| pH(H2O) | −4.238 | 0.000 1 |
| pH(KCl) | −1.397 | 0.162 |
| TOC | 0.437 | 0.662 |
| Localities | pH(H2O) | pH(KCl) | ||||
|---|---|---|---|---|---|---|
| Locality I | Locality II | Locality III | Locality I | Locality II | Locality III | |
| Locality I | - | 0.2880 | 0.0003 1 | - | 0.1576 | 0.0010 1 |
| Locality II | - | 1.0000 | - | 1.0000 | ||
| Locality III | - | - | ||||
| Localities | pH(H2O) | pH(KCl) | ||||
|---|---|---|---|---|---|---|
| Locality I | Locality II | Locality III | Locality I | Locality II | Locality III | |
| Locality I | - | 1.0000 | 0.0200 1 | - | 0.4844 | 0.0080 1 |
| Locality II | - | 1.0000 | - | 1.0000 | ||
| Locality III | - | - | ||||
| Localities | Seasons | Cd 1 | Cu 1 | Pb 1 | Zn 1 |
|---|---|---|---|---|---|
| (mg·kg−1) | (mg·kg−1) | (mg·kg−1) | (mg·kg−1) | ||
| Locality I | Spring | 0.08 | 0.64 | 0.25 | 1.28 |
| Autumn | 0.06 | 0.63 | 0.71 | 1.25 | |
| Locality II | Spring | 0.04 | 0.67 | 0.26 | 1.34 |
| Autumn | 0.08 | 0.87 | 0.99 | 1.22 | |
| Locality III | Spring | 0.04 | 0.66 | 0.35 | 1.32 |
| Autumn | 0.07 | 0.67 | 1.05 | 1.33 |
| Localities | Part of Plant | Cd | Cu | Pb | Zn |
|---|---|---|---|---|---|
| Locality I | root | 0.30 | 15.50 | 2.40 | 61.95 |
| stalk | 0.00 | 2.00 | 0.60 | 8.14 | |
| leaf | 0.00 | 2.90 | 1.70 | 3.54 | |
| grain | 0.00 | 4.40 | 0.00 | 25.47 | |
| Locality II | root | 0.30 | 22.10 | 2.60 | 85.41 |
| stalk | 0.00 | 1.80 | 0.75 | 5.65 | |
| leaf | 0.00 | 3.60 | 1.80 | 4.00 | |
| grain | 0.00 | 4.10 | 0.00 | 18.38 | |
| Locality III | root | 0.20 | 18.00 | 2.90 | 44.40 |
| stalk | 0.00 | 7.20 | 0.85 | 13.66 | |
| leaf | 0.00 | 6.40 | 1.85 | 16.38 | |
| grain | 0.00 | 4.20 | 0.00 | 21.98 |
| Arable Soils | Seasons | Bioaccumulation Coefficient | |||
|---|---|---|---|---|---|
| Cd | Cu | Pb | Zn | ||
| Locality I | Spring | 4.67 | 0.73 | 0.09 | 0.63 |
| Autumn | 0.00 | 0.79 | 0.08 | 0.53 | |
| Locality II | Spring | 6.00 | 0.63 | 0.06 | 0.53 |
| Autumn | 3.33 | 0.66 | 0.07 | 0.56 | |
| Locality III | Spring | 4.00 | 0.56 | 0.07 | 0.61 |
| Autumn | 4.00 | 0.59 | 0.09 | 0.89 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feszterová, M.; Porubcová, L.; Tirpáková, A. The Monitoring of Selected Heavy Metals Content and Bioavailability in the Soil-Plant System and Its Impact on Sustainability in Agribusiness Food Chains. Sustainability 2021, 13, 7021. https://doi.org/10.3390/su13137021
Feszterová M, Porubcová L, Tirpáková A. The Monitoring of Selected Heavy Metals Content and Bioavailability in the Soil-Plant System and Its Impact on Sustainability in Agribusiness Food Chains. Sustainability. 2021; 13(13):7021. https://doi.org/10.3390/su13137021
Chicago/Turabian StyleFeszterová, Melánia, Lýdia Porubcová, and Anna Tirpáková. 2021. "The Monitoring of Selected Heavy Metals Content and Bioavailability in the Soil-Plant System and Its Impact on Sustainability in Agribusiness Food Chains" Sustainability 13, no. 13: 7021. https://doi.org/10.3390/su13137021
APA StyleFeszterová, M., Porubcová, L., & Tirpáková, A. (2021). The Monitoring of Selected Heavy Metals Content and Bioavailability in the Soil-Plant System and Its Impact on Sustainability in Agribusiness Food Chains. Sustainability, 13(13), 7021. https://doi.org/10.3390/su13137021







