Abstract
The Breezhaler® dry powder inhaler (DPI) has a low carbon footprint compared with other inhalation therapies, consistent with the literature on other DPIs. This life-cycle assessment was conducted in France, Germany, the UK, and Japan using a “cradle-to-grave” technique to evaluate six environmental impact categories (global warming potential; acidification; ozone depletion; use of resource, minerals, and metals; eco-toxicity; and freshwater use) associated with the use of the Breezhaler®. Three variants of the Breezhaler® (30-day packs with and without the digital companion and a 90-day pack without the digital companion) were evaluated to identify major hotspots in the device life-cycle and to provide realistic solutions to reduce the environmental impact. Although no single life-cycle stage dominated the climate change impact of the 30-day device with the digital companion, the inhaler’s raw materials and packaging contributed to 96% of the resource depletion impact for the 30-day device without the digital companion. For the 90-day device without the digital companion, packaging contributed 42–62% of the impact across all categories. Overall, the Breezhaler® inhaler with the 90-day pack had the lowest environmental impact. The environmental impact of the device did not vary significantly among the considered markets. Further studies are needed to assess the impact of active pharmaceutical ingredients and improvement in clinical outcomes on the environment.
1. Introduction
Respiratory inhalers, which deliver medication directly to the lungs, are an integral part of the management of respiratory diseases. However, these inhalers contribute significantly to the environmental impact of healthcare, accounting for 3% of the carbon footprint (CFP), as reported by the National Health Service [1]. Among respiratory inhalers, pressurized metered-dose inhalers (pMDIs) have a much larger carbon footprint than dry powder inhalers (DPIs) [2,3,4]. Metered dose inhalers have hydrofluorocarbon propellants that are powerful greenhouse gases and persist in the atmosphere for between 14 and 260 years. Dry powder inhalers do not use these propellants and have substantially lower global warming potential, producing 20 g CO2 equivalent per dose compared with 500 g CO2 equivalent for metered dose inhalers. [5] A study by Jeswani and Azapagic showed that the use and end-of-life stages contribute significantly (~90%) to the carbon footprint of hydrofluoroalkane inhalers, which was estimated at 26.9 kg CO2/100 doses. The carbon footprint of DPIs is 10 times smaller (2.7 kg CO2/100 doses) [6]. A study by Wilkinson et al. demonstrated that changing from a pMDI device to a DPI could save 150–400 kg CO2 equivalent (eq) annually; this is roughly equivalent to installing wall insulation at home, and recycling or cutting out meat [7,8].
The Breezhaler® device (Novartis Pharmaceuticals) is a breath-actuated DPI that is used to deliver medications for chronic obstructive pulmonary disease and asthma. Recently, the fixed-dose once-daily combination of indacaterol acetate and mometasone furoate (IND/MF) and of indacaterol acetate, glycopyrronium bromide, and mometasone furoate (IND/GLY/MF) delivered using the Breezhaler® have received approval in many countries. In the European Union (EU), IND/MF is approved as a maintenance treatment for adults and adolescents aged ≥12 years in whom asthma is not adequately controlled with inhaled corticosteroids and inhaled short-acting beta2-agonists [9], and IND/GLY/MF is approved as a maintenance treatment for adult patients in whom asthma is not adequately controlled with a maintenance combination of a long-acting beta2-agonist and a high dose of an inhaled corticosteroid and for adults patients who have experienced one or more asthma exacerbations in the previous year [10].
The EU approval of IND/GLY/MF includes an optional sensor and an application (Propeller Health), making it the first digital companion that can be prescribed alongside a treatment for uncontrolled asthma. [11] The sensor delivers objective data on medication use to the Propeller app on the patient’s smartphone.
The CFP of the Breezhaler® has already been assessed in a previous study, which established that considering other inhalation therapies, the Breezhaler® portfolio has a low CFP, consistent with the literature on DPIs [12]. Our study was prompted by the findings of the study by Jeswani and Azapagic and uses the life-cycle assessment (LCA) technique to examine other environmental impacts and opportunities for improvement. LCA is a “cradle-to-grave” analysis technique to assess the environmental impact associated with all stages of a product’s life, from raw material extraction to processing, manufacturing, distribution, use, and disposal [13].
The objective of this streamlined LCA study was to evaluate six environmental impact categories (global warming potential; acidification; ozone depletion; use of resources, minerals, and metals; eco-toxicity; and freshwater use) of the Breezhaler® in four geographies (Germany, France, the UK, and Japan) to identify the major hotspots in the life-cycle of the device and to provide realistic solutions to these problems.
2. Materials and Methods
2.1. Selection of Impact Assessment Methods and Indicators
The streamlined LCA was modeled using the SimaPro LCA software and analyzed using the International Reference Life-cycle Data (ILCD) environmental impact categories (v1.11). This study was conducted in accordance with the International Standards Organization (ISO) 14040 and ISO 14044 standards and per the Greenhouse Gas (GHG) Protocol Sector Guidance for Pharmaceutical Products and Medical Devices, and this study was subject to critical review [14]. The study was streamlined and limited to those impacts that were of interest to the study commissioner. The environmental impact categories considered in this study are presented in Table 1. Other methods and impact categories are available, such as RECIPE 2016 (v1.04) [15]. For additional insights and to allow comparison with the findings of Jeswani and Azapagic, the results of the assessment using this method are shown in Table S1. The LCA results are relative expressions and do not predict impacts on category endpoints, the exceedance of thresholds, safety margins, or risks. Normalization, grouping, and weighting of the results were not undertaken in this study.

Table 1.
Description, with references, of each impact category used.
Product Systems
Breezhaler® devices are sold as 30-day or 90-day packages. The main advantage of the 90-day package is that only one inhaler device is used over the 90-day period, whereas in the 30-day package, three inhaler devices are used over the same 90-day period. For the 30-day products, an optional digital companion (sensor, Propeller Health) for the inhaler is offered. The markets assessed were France, Germany, the UK, and Japan.
2.2. Functional Unit
A functional unit is a unit of analysis that typically encompasses the specific function of the product, the duration over which this function is fulfilled, and the expected quality of the function, if applicable. In our study, the functional unit was defined as follows: an inhaler device, excluding active pharmaceutical ingredients (APIs), used for 30 days to treat a patient with asthma differentiated according to the characteristics of the site in France, Germany, the UK, or Japan.
The 90-day device was analyzed for 30 days of use (i.e., employing the same functional unit as for the 30-day inhalers).
2.3. System Boundary
Figure 1 outlines the system boundaries for the product with the digital companion and shows each step in the life-cycle, the location where each step takes place, and the end market locations. Details on the life-cycle inventory analysis are presented in Table S2.
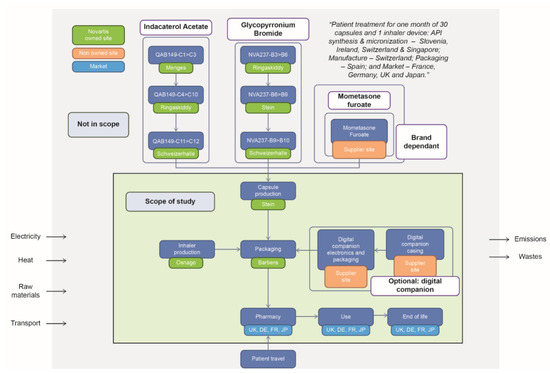
Figure 1.
System boundaries for devices with the digital companion (green box indicates the location of the production site).
2.4. Inclusions
Capsule/inhaler/digital companion production: this aspect included the production of raw materials (excluding the API), the transport of raw materials to the production site and their use in manufacture, the transport to the packaging facility in Spain, and the energy required during manufacture.
Packaging and distribution: the processes included production of packaging materials (e.g., card, paper) used directly for packaging production; the transport of raw materials to the site and their use in the packaging of the Breezhaler® device, digital companion, and capsules; and the energy used in the packaging and distribution of the Breezhaler® device, digital companion, and capsules.
Other inclusions: these aspects included the energy used at the pharmacy during storage, the use of the inhaler by patients, and the disposal of the Breezhaler® device, digital companion, and packaging.
2.5. Exclusions
API production was excluded as this study was a streamlined LCA of the Breezhaler® inhaler design and was independent of the APIs that might be delivered using this device. The inhaler design is flexible and can be used in combination with many APIs that are delivered via the capsule system.
The digital companion is used to remind the patient to take their medicine, and is, thus, expected to improve medication adherence. Although these benefits have been proven in studies using various sensor models, improved patient outcomes, specifically for users of the Breezhaler® device, have not yet been fully defined; therefore, these aspects were not included in this study. As the digital companion may have clinical benefits that are not considered in the study’s functional unit, direct comparisons between the environmental impact of the devices with and without the digital companion should not be made.
This study used the recycled content method. In this method, the impact of recycling or recovering energy from waste is allocated to the end user of the material or recovered energy, not the party generating the waste. Hence, there was no contribution to the environmental impact of the product from the recycling or energy recovery processes. This method was chosen using guidance from the GHG Protocol Product Standard, as the product contained some recycled input (aluminum in packaging). but no recycling happened downstream (all was incinerated).
The downside of choosing this method is that energy recovery essentially has no burden within the system boundary. Thus, the impact of incinerating the inhaler and its packaging and the displacement of virgin fuels were not accounted for.
Other exclusions from the scope of the study were capital goods and infrastructure, research and development, app energy use, patient travel to the pharmacy, employee commute, site waste, and refrigerants.
2.6. Cutoff Criteria
The ISO standards on LCA suggest using a percentage of total mass, total energy, and total environmental impacts as the “cutoff” criteria (ISO 14044:2006). The criteria that were used are as follows:
Mass: if the flow is <1% of the total mass of all inputs and outputs, it may be excluded, provided its environmental relevance is not a concern.
Energy: if the flow is <1% of the total energy inputs, it may be excluded, provided its environmental relevance is not a concern.
Economic/environmental/social relevance: if the flow meets the above criteria for exclusion and yet is considered to potentially have a significant economic/environmental/social impact, it must be included. Material flows that leave the system (products, emissions, waste) and whose environmental impact is >1% of the whole impact of an impact category that is considered in the assessment must be covered. This judgment is made based on experience and documented, as necessary.
The sum of the excluded flows must not exceed 5% of the mass, energy, or environmental relevance.
Cutoff criteria applied to background data (energy and materials) were taken from the ecoinvent 3.6 database and are documented online.
3. Results and Discussion
3.1. 30-Day Device without the Digital Companion
For the 30-day device a without digital companion, inhaler production energy, inhaler raw materials, and packaging were the top three contributors to all the environmental impact categories studied, except for freshwater eco-toxicity, where distribution was more impactful than production energy. For all categories, either packaging or inhaler production energy was the highest impacting life-cycle stage. Inhaler raw materials and packaging contributed to 96% of the impact on resource depletion. The production of steel, used in the manufacture of the Breezhaler® inhaler, was found to contribute to 33% of the overall impact. The geographical variation in impact was minimal and resulted from only the difference in downstream activities (distribution and end of life). For example, the use of ocean freight results in an increased impact on acidification potential for the Japanese market.
Figure 2 is a graphical depiction of the percentage breakdown of each life-cycle stage for all countries for the 30-day device without the digital companion.
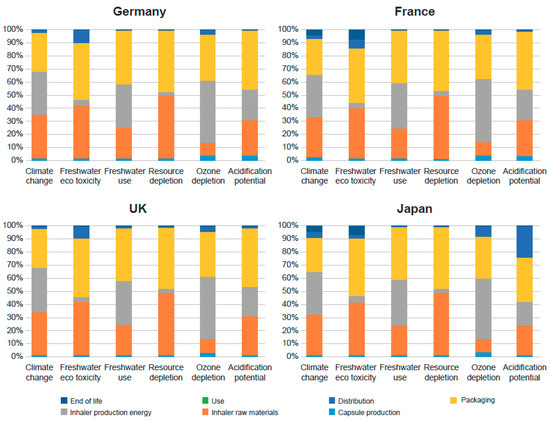
Figure 2.
Life-cycle stage breakdown for the 30-day device without the digital companion across all geographies.
3.2. 30-Day Device with the Digital Companion
No single life-cycle stage was found to dominate the climate change impact of the 30-day device with the digital companion. Inhaler raw materials, inhaler production energy, digital companion raw materials, and packaging all contributed to between 21% and 26% of the total impact. Acrylonitrile-butadiene-styrene, used in the Breezhaler® inhaler, accounted for 13% of the overall climate change impact. A similar pattern was seen in freshwater use impact: none of the life-cycle stages dominated and the four stages influencing the impact the most were inhaler raw materials, inhaler production energy, digital companion raw materials, and packaging. Freshwater use was closely associated with the freshwater used in electricity production rather than its direct use in the production of the inhaler (Figure 3).
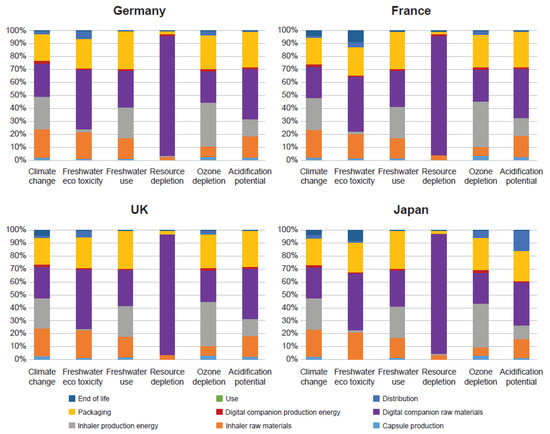
Figure 3.
Life-cycle stage breakdown for the 30-day device with the digital companion across all geographies.
The upstream activities associated with the production of the raw materials for the digital companion dominated the impact of resource depletion and, to a lesser extent, the freshwater eco-toxicity and acidification potential impacts, accounting for 94%, 46%, and 39% of the total impact, respectively. The circuit board (the electronics within the digital companion) accounted for 80% of the total resource depletion impact, with the gold used in the board accounting for 70%.
The ozone depletion impact was heavily influenced by upstream activities associated with the energy and raw materials required for inhaler production. The packaging, the digital companion raw materials, and the inhaler production energy accounted for 85% of the impact. Three compounds, namely, bromochlorodifluoromethane (halon 1211), bromotrifluoromethane (halon 1301), and carbon tetrachloride (CFC-10), accounted for 75% of the impact on ozone depletion. Impacts varied little across most geographies, and the differences were caused by variation in the downstream activities (distribution and end of life).
3.3. 90-Day Device without Digital Companion
Packaging was the major contributor to all environmental impact categories for the 90-day device without the digital companion. Packaging contributed between 42% and 62% of the impact in all categories (Figure 4).
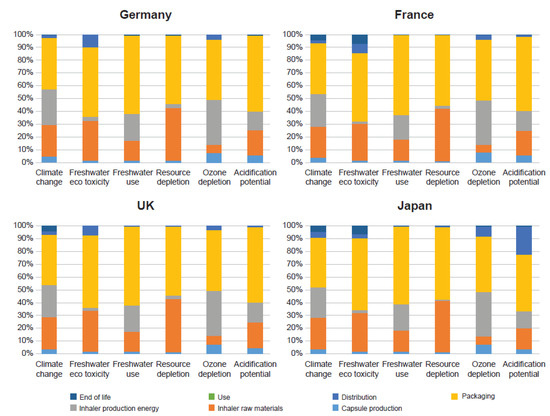
Figure 4.
Life-cycle stage breakdown for the 90-day device without the digital companion across all geographies.
The 90-day product is used for three months, which lowers the environmental impact for all life-cycle stages by a factor of three, except for the capsule production and packaging stages, where the impact is largely unchanged. The functional unit for the 90-day device still contains 30 capsules and three blister packs, but only one-third of an inhaler, thus reducing the impact associated with inhaler production, distribution, and end of life.
The upstream activities for all geographies were the same, and the differences in impact resulted from variation in the downstream activities.
3.4. Major Contributors to Each Environmental Impact Category
The contributors to each of the six different impact categories for the 30-day device with and without the digital companion and for the 90-day device without the digital companion are detailed below:
- Climate change: for all devices, the upstream activities associated with raw material and production energy dominated the climate change impact. The impact was split evenly among inhaler raw materials, production energy, packaging, and digital companion raw materials (if the device in question included a companion). These three or four life-cycle stages contributed to 96% of the overall impact for the 30-day devices and to 93% of the impact for the 90-day device.
- Freshwater eco-toxicity: when included, the digital companion was found to be the major freshwater eco-toxicity hotspot, contributing to 46% of the overall impact. A high proportion of this contribution was associated with the circuit board. When the digital companion was not included, the packaging and the inhaler raw materials were the major hotspots, contributing to over 85% of the overall impact for the 30-day device.
- Freshwater use: most of the impact arising from freshwater use was associated with the upstream activities involved in the production and manufacturing of parts, with a high proportion of the impact originating from the freshwater used to generate the electricity needed to produce the raw materials and the devices. Packaging was the major hotspot for all devices, contributing to 62% of the overall impact for the 90-day device and to 42% of the impact for the 30-day device without the companion. When included, the digital companion was the next major hotspot, contributing to 29% of the impact, with a high proportion of the contribution being attributable to the circuit board. Inhaler production energy was the next biggest hotspot, as the national grid in Italy (where the device is made) uses hydroelectric power.
- Resource depletion: the impacts associated with resource depletion were found to arise from production stages, specifically from the use of rare metals either in the device itself or in the steps upstream of production. When the digital companion was included, it was associated with 94% of the emissions owing to the depletion of gold used in the circuit board. In the absence of the digital companion, the packaging and inhaler raw materials accounted for 96% for both the 30- and 90-day devices.
- Ozone depletion: five species of gas contributed to 92% of the ozone depletion impact, namely, bromochlorodifluoromethane, bromotrifluoromethane, carbon tetrafluoride, dichlorodifluoromethane, and 1,2-dichloro-1,1,2,2-tetrafluoroethane. For all 30-day devices, inhaler production energy contributed the most to ozone depletion, accounting for 34–48% of the emissions, followed by packaging (or digital companion, if included).
- Acidification: sulfur dioxide was the main contributor to the acidification impact of all devices, accounting for 65% of the overall impact. If the digital companion was used, it was the major hotspot, contributing to 49% of the overall impact. In the absence of the digital companion, packaging was the major hotspot, accounting for 45% of the impact for the 30-day device and 58% of the impact for the 90-day device.
The impacts of the three devices on each category were consistent across all four geographies. Figure 5 represents the results for Germany, where the devices were plotted using a percentile of the device with the highest impact, which has been set to 100. Details on the results for impact categories in all the geographies can be found in Table S3. Details on the life-cycle heat map breakdown for each device in the four geographies are presented in Figure S1.
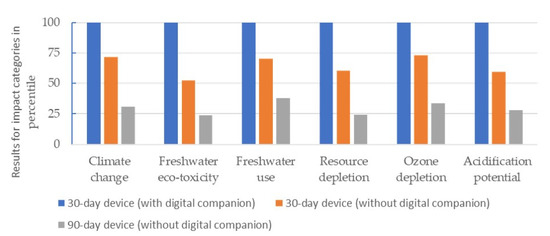
Figure 5.
Results for the 30-day device (with and without the digital companion) and 90-day device for each impact category expressed as a percentile of the device with the highest impact (Germany).
3.5. Sensitivity Analysis
A sensitivity analysis was conducted to explore the potential contribution of patient travel toward the environmental impact of the devices.
A study undertaken by Pharmacy2U suggests the average distance travelled to a pharmacy in the England is 2.3 miles (3.7 km, one way) [21]. Figure 6 shows the comparison of the environmental impact of the 30-day device without the digital companion both with and without patient travel for all markets, based on UK pharmacy travel assumptions (that the patient travels by car for the whole journey). The impact of the increase resulting from freshwater use and resource depletion was minimal. However, for all other categories, the Breezhaler® device increased to over 350% of the impact without patient travel. The climate change and ozone depletion impacts increased by 550% and 900%, respectively.
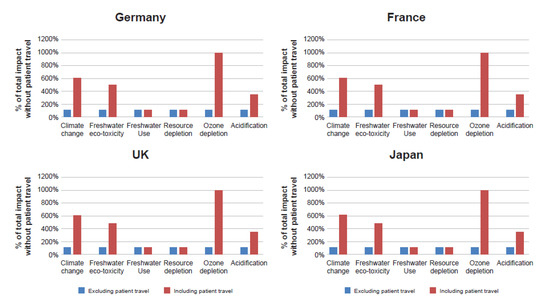
Figure 6.
Impact of patient travel on each impact category.
It is important to note that this is considered to be the worst-case scenario. Active travel (e.g., walking/cycling) has very little environmental impact, and the use of public transport would also significantly decrease the contribution of patient travel to the total impact. However, the effect of promoting the 90-day device would be to reduce the contribution made by patient travel to the CFP of the device by two-thirds, regardless of its scale. It was also suggested that the device could be mailed directly to the patient, avoiding the impact of travel altogether. If postal delivery is not practical, the use of public transport such as buses, which have a CFP one-fifth that of a car for the same traveled distance, could be encouraged.
4. Conclusions
A cradle-to-grave, streamlined LCA study was used to assess the contribution to six environmental impact categories by the Breezhaler® device used to treat asthma, excluding APIs.
Owing to its extended life and a supply of 90 days of capsules, the Breezhaler® inhaler device with the 90-day pack was found to have the lowest environmental impact. The environmental impact of the inhaler did not vary significantly among the considered markets.
While no single life-cycle stage dominated the climate change impact of the 30-day device with the digital companion, the inhaler raw materials and the packaging contributed to 96% of the resource depletion impact for the 30-day device without the digital companion. For the 90-day device without the digital companion, packaging contributed between 42% and 62% of the impact across all categories
Since the focus of our study was to assess the environmental impact associated with the Breezhaler® device, the impact of APIs and improvements in the clinical outcomes of asthma were not included in this evaluation. Further studies are needed to assess the impact of these factors on the environment.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/su13126657/s1. Table S1: Life-cycle impact assessment of the Breezhaler® using the ReCiPe 2016 v1.04 method. Table S2: Life-cycle inventory analysis. Table S3: Results for the 30-day device (with and without digital companion) and 90-day device for each impact category per FU. Figure S1: Life-cycle stage heat maps of the three devices in the four markets.
Author Contributions
All authors contributed to the conceptualization, methodology, formal analysis, investigation, data curation, reviewing and editing of the draft, and project administration. All authors have read and agreed to the published version of the manuscript.
Funding
The study was sponsored by Novartis Pharma AG, Basel, Switzerland.
Acknowledgments
The authors thank Anupama Boddupalli and Phani Dantu (Novartis Healthcare Pvt. Ltd. Hyderabad, India) for providing medical writing support/editorial support, which was funded by Novartis, in accordance with Good Publication Practice (GPP3) guidelines (http://www.ismpp.org/gpp3) (accessed on 10 April 2021).
Conflicts of Interest
Brett Fulford and Karen Mezzi are employees of Novartis, Pharma AG, Basel, Switzerland. Simon Aumônier is a partner at ERM, and Andy Whiting is a senior consultant at ERM. ERM is a global sustainability consulting business that works widely on a broad range of assignments for clients across all sectors of the economy. Its work has included pro bono contributions and consulting assignments for the Sustainable Healthcare Coalition and consulting engagements with many pharmaceutical and medical device companies, including Novartis Pharma AG.
Abbreviations
| AP | Acidification potential |
| CFP | Carbon footprint |
| CTUe | Comparative toxic units |
| DPI | Dry powder inhaler |
| EU | European union |
| FU | Functional unit |
| GHG | Greenhouse gas |
| GWP | Global warming potential |
| ILCD | International reference life-cycle data |
| IND/GLY/MF | Indacaterol/glycopyrronium/mometasone furoate |
| IND/MF | Indacaterol/mometasone furoate |
| LCA | Life-cycle assessment |
| LCI | Life-cycle inventory |
| LCIA | Life-cycle impact assessment |
| pMDI | Pressurized metered-dose inhalers |
| UK | United Kingdom |
References
- Mercer, C. How health care contributes to climate change. Can. Med. Assoc. J. 2019, 191, E403–E404. [Google Scholar] [CrossRef] [PubMed]
- Janson, C.; Henderson, R.; Löfdahl, M.; Hedberg, M.; Sharma, R.; Wilkinson, A.J. Carbon footprint impact of the choice of inhalers for asthma and COPD. Thorax 2020, 75, 82–84. [Google Scholar] [CrossRef] [PubMed]
- Van Hove, M.; Leng, G. A more sustainable NHS. BMJ 2019, 366, l4930. [Google Scholar] [CrossRef] [PubMed]
- Hillman, T.; Mortimer, F.; Hopskin, N.S. Inhaled drugs and global warming: Time to shift to dry powder inhalers. BMJ 2013, 346. [Google Scholar] [CrossRef] [PubMed]
- National Institute for Health and Care Excellence. Patient Decision Aid. Inhalers for Asthma. 2019. Available online: https://www.nice.org.uk/guidance/ng80/resources/inhalers-for-asthma-patientdecision-aid-pdf-6727144573 (accessed on 18 May 2021).
- Jeswani, H.K.; Azapagic, A. Environmental impacts of healthcare and pharmaceutical products: Influence of product design and consumer behaviour. J. Clean. Prod. 2020, 253, 119860. [Google Scholar] [CrossRef]
- Wynes, S.; Nicholas, K.A. The climate mitigation gap: Education and government recommendations miss the most effective individual actions. Environ. Res. Lett. 2017, 12, 074024. [Google Scholar] [CrossRef]
- Wilkinson, A.J.K.; Braggins, R.; Steinbach, I.; Smith, J. Costs of switching to low global warming potential inhalers. An economic and carbon footprint analysis of NHS prescription data in England. BMJ Open 2019, 9, e028763. [Google Scholar] [CrossRef] [PubMed]
- Atectura Breezhaler®: Summary of Product Characteristics. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/atectura-breezhaler (accessed on 18 May 2021).
- Enerzair Breezhaler®: Summary of Product Characteristics. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/enerzair-breezhaler (accessed on 18 May 2021).
- Novartis Press Release 7 July 2020. Available online: https://www.novartis.com/news/media-releases/novartis-receives-ec-approval-enerzair-breezhaler-including-first-digital-companion-sensor-and-app-can-be-prescribed-alongside-treatment-uncontrolled-asthma-eu (accessed on 18 May 2021).
- Aumônier, S.; Whiting, A.; Norris, S.; Collins, M.; Coleman, T.; Fulford, B.; Breitmayer, E. Carbon Footprint Assessment of Breezhaler® Dry Powder Inhaler; Drug Delivery to the Lungs: Edinburgh, UK, 2020; Volume 31. [Google Scholar]
- Environmental Management—Life Cycle Assessment—Principles and Framework, 2nd ed.; International Organization for Standardization: Geneva, Switzerland, 2006.
- International Organisation for Standardization. ISO 14044:2006, Environmental Management—Life Cycle Assessment—Principles and Framework; ISO: Geneva, Switzerland, 2006; Available online: https://www.iso.org/standard/37456.html (accessed on 18 May 2021).
- ReCIPE Analysis. Available online: https://www.rivm.nl/en/life-cycle-assessment-lca/recipe (accessed on 31 March 2021).
- IPCC. Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Stocker, T.F., Qin, D., Plattner, G.-K., Tignor, M., Allen, S.K., Boschung, J., Nauels, A., Xia, Y., Bex, V., Midgley, P.M., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2013; 1535p. [Google Scholar]
- Seppälä, J.; Posch, M.; Johansson, M.; Hettelingh, J.P. Country-dependent characterisation factors for acidification and terrestrial eutrophication based on accumulated exceedance as an impact category indicator. Int. J. Life Cycle Assess. 2006, 11, 403–416. [Google Scholar] [CrossRef]
- Posch, M.; Seppälä, J.; Hettelingh, J.P.; Johansson, M.; Margni, M.; Jolliet, O. The role of atmospheric dispersion models and ecosystem sensitivity in the determination of characterisation factors for acidifying and eutrophying emissions in LCIA. Int. J. Life Cycle Assess. 2008, 13, 477–486. [Google Scholar] [CrossRef]
- Scientific Assessment of Ozone Depletion. World Meteorological Organization Global Ozone Research and Monitoring Project-Report No. 44. Available online: https://library.wmo.int/doc_num.php?explnum_id=7304 (accessed on 12 March 2021).
- Van Oers, L.; de Koning, A.; Guinée, J.B.; Huppes, G. Abiotic Resource Depletion in LCA—Improving Characterisation Factors for Abiotic Depletion as Recommended in the New Dutch LCA Handbook; Road and Hydraulic Engineering Institute: Amsterdam, The Netherlands, 2002. [Google Scholar]
- How Far Do People Travel to Collect Their Prescriptions. Available online: https://www.pharmacy2u.co.uk/news/health/news/how-far-do-people-travel-to-collect-their-prescriptions/ (accessed on 13 May 2021).
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).